Abstract
Background:
Advances in genomics and epidemiology can foster the implementation of a risk-based approach to current age-based breast cancer screening programs. This personalized approach would challenge the trajectory for women in the healthcare system by adding both a risk-assessment step (including a genomic test) and screening options.
Objective:
The aim of this study is to explore, from an organizational perspective, the acceptability of different proposals for each step of the trajectory for women in the healthcare system should a personalized approach be implemented in the province of Quebec.
Methods:
We interviewed 20 professional stakeholders who are either involved in the current breast cancer screening program in Quebec or who are likely to play a role in the future implementation of a personalized risk-based approach.
Results and discussion:
Preferences are split between proposals supporting self-management by the women themselves (e.g., solicitation through media campaign, self-collection of information and sample and results provided by letter) and proposals prioritizing more interaction between women and healthcare providers (e.g., solicitation by health professionals, collection of information and samples by a nurse and results provided by health professionals).
Abstract
Contexte:
Les avancées de la science en génomique et en épidémiologie pourraient favoriser l'implantation d'une approche basée sur le risque dans les programmes de dépistage du cancer du sein qui sont actuellement basés sur l'âge. Cette approche personnalisée poserait des défis à la trajectoire des femmes dans le système de santé en ajoutant à la fois une étape d'évaluation du risque (incluant un test génomique) et des options de dépistage.
Objectif:
L'objectif de cette étude est d'explorer, dans une perspective organisationnelle, l'acceptabilité de différentes propositions pour chaque étape de la trajectoire des femmes dans le système de santé si une telle approche était implantée dans la province de Québec.
Méthode:
Nous avons mené des interviews auprès de 20 acteurs du milieu de la santé qui sont impliqués dans le programme actuel de dépistage du cancer du sein au Québec ou qui seraient appelés à jouer un rôle advenant l'implantation d'une approche personnalisée basée sur le risque.
Résultats et discussion:
Les préférences des interviewés sont partagées entre les propositions qui favorisent l'autogestion par les femmes elles-mêmes (p. ex. sollicitation par des campagnes médiatiques, collecte autonome d'information et d'échantillon, résultat transmis par lettre) et les propositions qui favorisent plus d'interactions entre les femmes et les fournisseurs de soins (p. ex. sollicitation par des professionnels de la santé, collecte d'information et d'échantillon par une infirmière et résultat transmis par un professionnel de la santé).
Introduction
Several countries are running breast cancer screening programs that typically offer annual or biannual mammography for women 50 years and older. To mitigate the potential harms of these age-based programs (e.g., overdiagnosis and false positives; Klarenbach et al. 2018), researchers are building evidence supporting a more personalized approach. This approach would adjust mammography frequency according to personal risk level, instead of using age as the only criterion. Such personalization could thereby allocate screening and healthcare resources to women at higher risk, including those under 50 years old (Davies 2017).
Such a personalized approach relies on genomics and clinical, familial and lifestyle factors to identify women most likely to benefit from tailored screening (Lee et al. 2019; Rudolph et al. 2018). Such screening could include more frequent mammograms or additional imaging technologies such as magnetic resonance imaging (Gagnon et al. 2016). This approach is expected to inform current screening strategies (Shieh et al. 2017; van Veen et al. 2018), be more cost effective (Pashayan et al. 2018; Schousboe et al. 2011) and improve the harms–benefits ratio, including decreasing overdiagnosis (Pashayan et al. 2018).
Future inclusion of this personalized risk-based approach to current screening programs has been described as “a radically new approach to prevention” (Dent et al. 2013, p. 94). This approach is in line with the precision public health model, which merges a population focus with an individual-centred perspective, enabling public health programs to provide “the right intervention to the right population at the right time” (Khoury et al. 2016, p. 398).
Despite the interest raised by this personalized approach, its implementation raises organizational issues (Chowdhury et al. 2013; Hall et al. 2013; Lévesque et al. 2018a; Rainey et al. 2018). Our previous work with stakeholders revealed that the implementation of the personalized approach in the province of Quebec would raise organizational challenges with regard to many steps of the screening trajectory in the healthcare system (Figure 1; Dalpé et al. 2017; Hagan et al. 2016; Lévesque et al. 2018b).
Figure 1.

Steps in the screening trajectory likely to raise organizational challenges
At each step, challenges could arise from the addition of a genomic test, the complexity of the risk estimation process and the inclusion of a choice of screening pathway following personalized risk assessment. Current limitations to financial resources in publicly funded healthcare systems and to human resources (e.g., overworked health professionals) and the sheer scale of the group to reach (i.e., thousands of women each year) drove innovative proposals to address these challenges (Hagan et al. 2016; Lévesque et al. 2018b). These include, for instance: reduction of the reliance on health professionals to provide women with the information necessary to make an informed consent before risk assessment, consideration of self-collection of biological samples and extension of the duties of nurses to include the communication of the results of risk calculations and to advise on screening options.
The aim of our study is to explore, from an organizational point of view, the acceptability of different proposals for each step of the trajectory for women in the healthcare system if the personalized approach were to be implemented in the province of Quebec. Because stakeholders' opinions will likely influence the adoption of the personalized approach (Phillips et al. 2016), we conducted semi-structured interviews with stakeholders from the Quebec healthcare system. To our knowledge, this is the first qualitative study to analyze stakeholders' perceptions on the organizational issues of risk-based screening in the Canadian context.
Background
Our study is part of a broader project called PERSPECTIVE (Personalized Risk Stratification for Prevention and Early Detection of Breast Cancer). The PERSPECTIVE project aims to develop tools for the implementation of a personalized approach to breast cancer screening in the Canadian context, including a calculation algorithm, a genomic test, an economic simulation model and screening policies (https://www.genomecanada.ca/en/personalized-risk-stratification-prevention-and-early-detection-breast-cancer). The approach of the PERSPECTIVE project is to then inform screening options by running the individual risk assessment on asymptomatic women aged 30 years and older through the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA) calculation algorithm (Figure 2).
Figure 2.
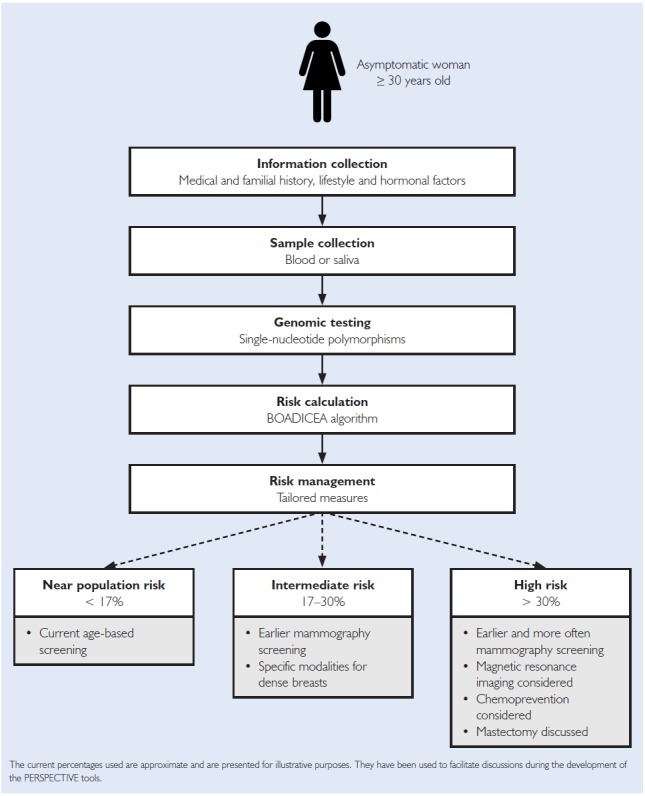
Risk assessment approach in the PERSPECTIVE project
The BOADICEA algorithm (https://www.canrisk.org/) provides personalized risk assessment using multiple risk factors as well as the results of a genomic test involving hundreds of single-nucleotide polymorphisms, each factor increasing the risk slightly (Lee et al. 2019; Mavaddat et al. 2018; Rudolph et al. 2018). At the end of this process, women would be provided with tailored screening and prevention measures depending on which one of the three risk categories they fall into (Gagnon et al. 2016). Our interviews with stakeholders are grounded on these scientific parameters of the PERSPECTIVE project.
Given that the PERSPECTIVE project had already developed clinical recommendations to manage risk results in the specific context of Quebec's breast cancer screening program (Gagnon et al. 2016), the Quebec program was seen as an appropriate focus for our study. As with other Canadian screening programs, this program invites women between 50 and 69 years old for a mammogram every two years. Upon receipt of an invitation letter at home, Quebec women can decide to access an imaging facility for a mammogram covered by the publicly funded healthcare system. In 2016, about 65% of the invited women participated in this program in Quebec (Institut National de Santé Publique du Québec 2018).
Methods
An experienced interviewer with a background in sociology (JH) conducted semi-structured interviews with 20 purposefully selected stakeholders (Patton 2005). Potential interviewees were reached via e-mail, and our response rate was approximately 20%. This is most likely due to the fact that we recruited respondents through our network of collaborators and via snowball sampling in addition to “cold calls” to respondents identified via public documents available on the Internet. The stakeholders selected would have a role at the organizational level in a future implementation of the personalized approach in Quebec because they are currently involved in the organization of the provision of oncology services, the conception/implementation of cancer screening programs or health professionals' policy frameworks or the management of the current breast cancer screening program. We sought a balance between professionals involved at the provincial and regional levels, working in hospitals, in professional organizations or for the Ministry of Health (Table 1).
TABLE 1.
Participants' characteristics
| Characteristics | Total | ||
|---|---|---|---|
| Gender | Female: 13 | Male: 7 | |
| Background | Physician: 10 | Nurse: 6 | Other: 4* |
| Direct involvement in the current breast cancer screening program | Yes: 8 | No: 12 | |
| Role level | Provincial: 9 | Regional: 7 | Professional organization: 4 |
One is a health professional, and three participants did not disclose their background.
The interviews aimed to address the acceptability of organizational proposals for the different steps of the trajectory for women in a personalized approach. The steps and associated proposals for reform were identified in our previous study (Dalpé et al. 2017; Hagan et al. 2016; Lévesque et al. 2018b). The original design of the interviews comprised open-ended, close-ended and ranking questions (see Appendix A, available here). However, because many interviewees explained that their ranking would depend on the availability of resources, the interviewer (JH) chose to focus on the rationale supporting their preferences rather than on ranking questions. The one-hour interviews were conducted from September 2016 to February 2017 in French, either in person or by telephone. Analysis was conducted in the original language of the interviews, and only quotes included in this article were translated by the researchers. At the beginning, interviewees were informed of the core elements of the personalized approach, including a clarification that the genomic test covers only single-nucleotide polymorphisms that slightly increase risk – not genes associated with a substantial increase in familial risk (such as BRCA1/2) because these genes are already tested by genetics clinics, based on family history.
All interviews were audio recorded and transcribed. The interview transcripts were then analyzed thematically using the NVivo software. A first analyst (JH) developed the initial codes and coded the first half of the interviews. A second analyst (DES) coded the second half of the interviews, and in doing so refined the initial coding. The codes and categories identified by each analyst were then compared and discussed following the principles of thematic analysis (Attride-Stirling 2001; Braun and Clarke 2006). As the analysis progressed, new themes and codes were added to reflect the content that transcended the questions asked during the interviews (e.g., when the interviewees articulated the reasoning behind their answers). In doing so, the analysts were able to interpret the interviewees' responses from a more theoretical point of view (Boyatzis 1998). The project was approved by the ethics review boards of the CHU de Québec–Université Laval and McGill University.
Results
Table 2, available online at click here, summarizes the proposals preferred by the interviewees for the management of the organizational challenges in the trajectory for women.
Invitation and Information
Implementation of the personalized approach would require proceeding to large-scale invitation of all asymptomatic women within an age range to have an initial risk assessment. Three main options for inviting women and offering them information on risk assessment emerged from the responses of the participants: postal letter, media information campaign and an encounter with a health professional.
Use of a postal letter was generally perceived as an accessible, manageable and feasible option. Many participants appeared confident in the effectiveness of such a method, given its current use within the Quebec screening program. Nevertheless, some interviewees pointed out the high cost of this option, given the scale of the population to be contacted, and the fact that postal letters tend to miss their target with regard to vulnerable women.
A second option favoured by participants was a media information campaign (traditional and social media). Some interviewees mentioned that such campaigns could be used in conjunction with postal letters as a means of publicizing the changes in screening practices. The interest of using social media was specifically mentioned in consideration of the younger targeted population (i.e., starting at 30 years). The third option preferred by interviewees was an appointment with a health professional, yet some recognized the impracticality of such a method (i.e., not all women have a primary care provider in Quebec).
Informed Consent
In a personalized approach, consent to risk assessment would need to include specific information not currently provided by screening programs. Additional information on the distinct features of this approach (e.g., a genomic test and the use of a computer algorithm for a risk estimation), on potential insurability issues, on the limits of the genomic test (e.g., genes not covered) and on the accuracy of the estimation over time (i.e., changes in risk factors may modify the risk level) would need to be provided. This information is more complex than that provided in current age-based screening programs. Two main options were favoured in the interviews for the support of informed consent: the use of paper information tools and a conversation with a health professional.
Interviewees underlined the convenience of paper tools that could be sent along with the invitation letter. For some participants, such a handout could also be used in combination with a website. However, interviewees recognized that the scope of such handouts is limited by the complex notions of risk estimation and genetics that would need to be explained.
Indeed, other interviewees considered it a necessity to offer women the option to have a discussion with a trained physician or a nurse. For a number of interviewees, only this one-to-one framework would allow women “to thoroughly appraise pros and cons” and ask questions. Furthermore, this would allow each woman to properly understand the information regardless of her level of health literacy. Such professional support would not need, however, to be systematically offered to each woman but to those who feel the need to have a discussion. Moreover, such a discussion would not need to take place in person. Remote communication (e.g., phone or telemedicine) could actually be an option according to many participants who favoured discussion with a health professional:
I think that it is really important that, for the women who have a bit more difficulty, that they can call a 1-800 service or something, with somebody at the end of the line who can explain, reassure them on aspects they worry about. (Public Servant, Provincial Level 1)
Other options were discussed by interviewees but not favoured, such as the use of a website and an explanatory video to guide informed consent.
Information Collection
Under a personalized approach, risk assessment would be made by providing information on extended risk factors (such as clinical and family history as well as lifestyle information) to the Web-based BOADICEA risk calculation tool. As for the best ways to collect this information, two main tracks emerged in participants' responses: by a nurse – in person or remotely – and by the woman herself. Most interviewees considered a mix of methods with nurse involvement and self-collection as the best option. Only a few interviewees regarded self-collection alone as the best method.
Many participants expressed some level of concern with the self-collection of information pertaining to risk factors. Their concerns include the complexity of information that women would need to provide (e.g., breast density assessed by mammography), how their literacy level might distort responses and the possible omission of key data. To address these issues, some interviewees suggested to “isolate a part of it that people are capable of answering for sure” and “try to have most options possible to favour the [women's] participation.” According to a majority of participants, a nurse, either in person or via a phone centre, could ensure “validation” of the self-collected information, while helping to complete the more complex questions.
Meeting with a physician, a gynecologist or a pharmacist was not considered appropriate at this step, given the current organization of the Quebec healthcare system.
Biological Sample Collection
The genomic test used in the approach developed by PERSPECTIVE would use either a saliva or a capillary blood sample. With regard to sample collection, interviewees were split between self-collection and the involvement of a health professional to support each individual.
Self-collection was described by most participants as the most suitable option for a large-scale program. For most of them, self-collection would be better suited for a saliva sample than for a capillary blood sample. Women could get their self-collection kit either by mail or at a local healthcare facility. As for capillary blood sampling, some interviewees were worried that it might discourage part of the population and suggested “to have another option available for people who are going to feel uneasy to go that route” or for those who have disabilities. Many interviewees thought that the ideal approach for capillary blood sampling would be collection assisted by a nurse to assure not only proper sampling but also adequate storage and transportation.
Concerns were raised with regard to how “lay” conditions for storage and transportation could degrade the quality of self-collected samples. One of the interviewees also claimed that the genomic test must be highly reliable if self-collection were to be implemented:
[…] I can guarantee you that for this screening test to work, it has to be so simple that it is a no-brainer. The great difficulty is having an extremely strong test, [strong enough] to resist poor manipulations. (Professional Organization Representative 1)
Despite these concerns, the involvement of a physician in the collection of biological samples was not considered appropriate, as the nurse remained the most acceptable alternative to self-collection for a majority of respondents.
Return of Results
The interviewees' answers about the best way to communicate the results of the risk assessment to women are clearly differentiated by risk category. Participants largely approved sending a postal letter as the best way to communicate results to women ranking in the near to population risk category. However, some interviewees mentioned that a professional resource should also be made available if needed, for instance, via a call centre:
[…] We cannot imagine that we are going to meet everybody who has a risk near the general population individually. It is not feasible. I see rather making a mailing [saying that]: ‘your risk is near the general population and therefore when you turn 50-years-old, you will be reminded to have a screening every two years. If you have further questions, call this number, (Public Servant, Provincial Level 2)
Meeting in person with a health professional, whether a nurse or a physician, was not considered appropriate to return results in the near to population risk category.
Interviewees' preferences change significantly when it comes to communicating results to women in the intermediate- and high-risk categories, who would be advised to have earlier and/or more frequent mammography in respect to the clinical recommendations presented to the interviewees (Figure 1). This appears to mark a decisive turning point. Mailing results were seen as clearly unsatisfactory in such cases. As soon as “there are things to be done,” as one of them said, an encounter with a health professional becomes necessary. Others explained their preference by the complexity surrounding risk assessment results and the importance to have a thorough discussion about the advantages and disadvantages of the screening options recommended. Regarding the type of health professional who should discuss the screening options with women in the intermediate-risk category, the interviewees were divided between the need to see a physician and the acceptability of seeing a nurse trained in risk assessment. For those open to a nurse having such a role, they mentioned that she should be able to refer women to specialized services. As for women in the high-risk category, interviewees showed a clear consensus on the need for an appointment with a physician rather than with a nurse. Their arguments relate to the therapeutic options and their implications, and physician credibility:
When we begin to speak of preventive mastectomy or preventive pharmacotherapy, we start having interventions which are not commonplace, they are invasive and far removed from the two-year mammogram everyone knows. (Professional Organization Representative 2)
Many mentioned that such physicians would need specific training in breast cancer risk assessment and risk communication.
Discussion
Interviewees' views on the organizational challenges for the trajectory for women in the healthcare system reveal two different approaches:
a relational approach emphasizing dialogue and shared decision-making with the close involvement of a healthcare professional; and
a self-management approach relying on the initiative of women regarding self-collection of information and sample.
The latter approach shares similarities with the “proactive approach” in women's decision-making process that was prioritized by the health professionals studied by Rainey et al. (2018).
Table 3 provides a comparison of these approaches to the organizational trajectory. The results presented in Table 3 merit discussion on three aspects: the tension between the focus of clinical ethics and public health ethics, the inherent constraints of a publicly funded healthcare system and the need for training healthcare providers.
TABLE 3.
Comparison of the two approaches for each step of the trajectory for women
| Relational approach | Self-management approach | |
|---|---|---|
| 1. Invitation and information | Postal letter or discussion (nurse/physician) | Postal letter, media campaign or social media |
| 2. Informed consent | Information document or discussion (nurse/physician) | Information document or online information |
| 3. Information collection | Nurse | Self-collection (paper or online) |
| 4. Biological sample collection | Nurse | Self-collection |
| 5. Return of results | ||
| Near to population risk | Nurse or physician | Letter |
| Intermediate risk | Nurse or physician | Letter or nurse |
| High risk | Physician | Nurse or physician |
Tensions Between the Focus on Clinical Ethics or on Public Health Ethics
The two approaches favoured by the interviewees reflect the tensions between the focus on clinical ethics or on public health ethics. The relational approach favours interaction with health professionals and is more in line with the perspective of clinical ethics that places the needs of the individual at the centre of its concerns (Callahan 2003; Kass 2004; Wardrope 2014). In contrast, the self-management approach is closer to public health ethics, which focuses on collective needs (Childress et al. 2002; Schmidt 2015).
The strongest proponents of the relational approach were interviewees who work more closely with patients or representatives from organizations responsible for the oversight of the professional practice of healthcare providers. Their stance seems grounded in the classical principles of clinical ethics such as beneficence (e.g., mitigating women's anxiety), autonomy (e.g., fostering informed consent) and non-maleficence (e.g., ensuring that results are not mishandled; Beauchamp and Childress 2013).
The stakeholders who favoured the self-management approach were predominantly those involved in the management of public health services or clinical services. Oftentimes, the rationale for privileging self-management is that this method put less strain on the limited resources of the healthcare system. As such, their arguments were in line with the considerations of public health ethics such as maximization of benefits over costs (Childress et al. 2002), resource allocation (Kass 2004) and program effectiveness (Kass 2001).
However, some proponents of the relational approach came to adhere to the views of the interviewees who prefer the self-management approach once they considered the practicability of the implementation within a publicly funded population-based program. This reasoning evokes priority setting and allocation of scarce resources, which are core aspects of the public health ethics framework (Abbasi et al. 2018).
Constraints Inherent to a Healthcare System with Limited Resources
Resource limitations (e.g., human, financial and technological) are a major issue in implementing genetic services in public health (Cornel and van El 2017) – even more so in a publicly funded healthcare system (Severin et al. 2015). When human resources – especially when specialized – are limited, allocation issues might become as relevant as allocation of financial resources.
Many interviewees expressed concerns about the capacity of the Quebec healthcare system to provide the appropriate human resources for the personalized approach. Preferences of interviewees about how to address the organizational challenges are grounded in these concerns. Many of those who prefer the self-management approach explained their choice by the necessity to minimize physician involvement due to resource constraints. However, some interviewees pointed out that leveraging nurse competencies to address this issue could also yield the same challenge considering the large workload of nurses and the important shortage of specialized nurse practitioners in Quebec (Gentile 2018).
One emerging screening option that could help minimize the overall use of resources is the reduction of mammography frequency for women at very low risk. For instance, women at a level of risk lower than the general population could be offered less frequent mammography. Howell et al. (2012) estimated that the detection ratio could remain the same if screening intervals were doubled for women at very low risk. This emerging screening option was not presented to our interviewees because it was not part of the PERSPECTIVE project approach. But it could be an option to consider in the future (Hall et al. 2013; Rainey et al. 2018), although its acceptability by women could be challenging (Henneman et al. 2011).
Training Needs
Interviewees – whether they favoured the relational approach or the self-management approach – were concerned about the lack of knowledge of healthcare providers to support women with the social, clinical and legal issues raised by the personalized approach. This confirms the literature showing that the lack of specialized competencies among healthcare providers represents one of the main barriers for a successful implementation of personalized screening programs and precision medicine in general (Chowdhury et al. 2013, 2015; Cornel and van El 2017). Chowdhury et al. (2015) recommended conducting a formal education and training needs assessment to determine current gaps in genetics competencies before implementation. Collins et al. (2014) mentioned that health professionals report a lack of knowledge to implement a personalized approach and considered that appropriate support decision tools could fill this gap. Rainey et al. (2018) identified the “substantial knowledge deficit” of healthcare providers as a challenge to be addressed and suggested educational programs to improve competence in risk communication and screening options. Based on the answers of the interviewees, mastering risk communication skills would also be paramount in Quebec.
Many participants pointed to electronic communications as a solution for professional training (e.g., online training) as well as for interactions with women. Some interactions with women could occur remotely, via phone or through telegenetics, including for genetic counselling (Buchanan et al. 2015). Indeed, there is growing evidence that telehealth can provide professional support to patients without compromising the quality of services (Casey et al. 2017; Melton et al. 2017; Pruthi et al. 2013).
Limitations
Our study focuses on the Quebec healthcare system; as such, results may not be readily generalizable. Nevertheless, our results might be informative for healthcare contexts with similarities to that of the province of Quebec (e.g., publicly funded healthcare system, thousands of women to be invited per year and limited number of specialized health professionals). Another limitation of our study is inherent to the use of purposive sampling in implementation research. Palinkas et al. (2015) have highlighted the trade-off between breadth and depth of qualitative data when relying on purposive sampling strategies. Selecting potential interviewees “on the basis of their role in the implementation process or who have a specific experience […] may fail to capture the experiences or activities of other groups playing other roles in the process” (Palinkas et al. 2015, p. 539). However, selecting according to criteria such as role, experience and knowledge may provide a deeper understanding of the implementation process.
Conclusion
As Dent et al. (2013) explained, a risk-based screening program “is more complex to set up and manage than the current programs based on age eligibility” (p. 98). Our research offers insight into the organizational challenges anticipated in the context of a future implementation of the personalized approach in Quebec. The results show that stakeholders' preferences to address such challenges are split between a relational approach and a self-management approach. The two approaches reflect tensions between clinical ethics and public health ethics. In turn, these tensions highlight the delicate issue of resource allocation in publicly funded healthcare systems and shed light on the need to provide specialized training to the health professional workforce. Although our results focused on the Quebec healthcare system, the literature suggests that the issues identified are relevant to other healthcare systems. These issues should be carefully considered by health authorities before implementing the personalized approach for breast cancer screening in a publicly funded healthcare system.
Acknowledgements
This study was conducted with the financial support of the Quebec Breast Cancer Foundation, the Government of Canada (through Genome Canada and the Canadian Institutes of Health Research) and the Ministère de l'Économie et de l'Innovation du Québec (through Genome Québec and the PSR-SIIRI-949 program).
Contributor Information
Daphne Esquivel-Sada, Sociologist, Centre of Genomics and Policy, Department of Human Genetics, Faculty of Medicine, McGill University, Montreal, QC.
Emmanuelle Lévesque, Lawyer and Academic Associate, Centre of Genomics and Policy, Department of Human Genetics, Faculty of MedicineMcGill University, Montreal, QC.
Julie Hagan, PhD Candidate, Academic Associate, Centre of Genomics and Policy, Department of Human Genetics, Faculty of Medicine McGill University, Montreal, QC.
Bartha Maria Knoppers, Professor, Department of Human Genetics, Faculty of Medicine, McGill University, Montreal, QC, Director, Centre of Genomics and Policy, Department of Human Genetics, Faculty of Medicine, McGill University, Montreal, QC.
Jacques Simard, Professor, Department of Molecular Medicine, Faculty of Medicine, Université Laval, Québec City, QC.
References
- Abbasi M., Majdzadeh R., Zali A., Karimi A., Akrami F. 2018. The Evolution of Public Health Ethics Frameworks: Systematic Review of Moral Values and Norms in Public Health Policy. Medicine, Health Care and Philosophy 21(3): 387–402. < 10.1007/s11019-017-9813-y>. [DOI] [PubMed] [Google Scholar]
- Attride-Stirling J. 2001. Thematic Networks: An Analytic Tool for Qualitative Research. Qualitative Research 1(3): 385–405. <http://psycnet.apa.org/doi/10.1177/146879410100100307>. [Google Scholar]
- Beauchamp T.L., Childress J.F. 2013. Principles of Biomedical Ethics (7th ed.). Oxford, United Kingdom: Oxford University Press. [Google Scholar]
- Boyatzis R.E. 1998. Transforming Qualitative Information: Thematic Analysis and Code Development. Cleveland, OH: Sage. [Google Scholar]
- Braun V., Clarke V. 2006. Using Thematic Analysis in Psychology. Qualitative Research in Psychology 3(2): 7–101. < 10.1191/1478088706qp063oa>. [DOI] [Google Scholar]
- Buchanan A.H., Datta S.K., Skinner C.S., Hollowell G.P., Beresford H.F., Freeland T. et al. 2015. Randomized Trial of Telegenetics vs. In-Person Cancer Genetic Counseling: Cost, Patient Satisfaction and Attendance. Journal of Genetic Counseling 24(6): 961–70. < 10.1007/s10897-015-9836-6>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan D. 2003. Individual Good and Common Good: A Communitarian Approach to Bioethics.” Perspectives in Biology and Medicine 46(4): 496–507. < 10.1353/pbm.2003.0083>. [DOI] [PubMed] [Google Scholar]
- Casey R.G., Powell L., Braithwaite M., Booth C.M., Sizer B., Corr J.G. 2017. Nurse-Led Phone Call Follow-Up Clinics Are Effective for Patients with Prostate Cancer. Journal of Patient Experience 4(3): 114–20. < 10.1177/2374373517706613>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress J.F., Faden R.R., Gaare R.D., Gostin L.O., Kahn J., Bonnie R.J. et al. 2002. Public Health Ethics: Mapping the Terrain. The Journal of Law, Medicine & Ethics 30(2): 170–78. < 10.1111/j.1748-720X.2002.tb00384.x>. [DOI] [PubMed] [Google Scholar]
- Chowdhury S., Dent T., Pashayan N., Hall A., Lyratzopoulos G., Hallowell N. et al. 2013. Incorporating Genomics into Breast and Prostate Cancer Screening: Assessing the Implications. Genetics in Medicine 15(6): 423 < 10.1038/gim.2012.167>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S., Henneman L., Dent T., Hall A., Burton A., Pharoah P. et al. 2015. Do Health Professionals Need Additional Competencies for Stratified Cancer Prevention Based on Genetic Risk Profiling? Journal of Personalized Medicine 5(2): 191–212. <https://dx.doi.org/10.3390%2Fjpm5020191>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins I.M., Steel E., Mann G.B., Emery J.D., Bickerstaffe A., Trainer A. et al. 2014. Assessing and Managing Breast Cancer Risk: Clinicians' Current Practice and Future Needs. The Breast 23(5): 644–50. < 10.1016/j.breast.2014.06.014>. [DOI] [PubMed] [Google Scholar]
- Cornel M.C., van El C.G. 2017. Barriers and Facilitating Factors for Implementation of Genetic Services: A Public Health Perspective. Frontiers in Public Health 5: 195 < 10.3389/fpubh.2017.00195>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalpé G., Ngueng Feze I., Salman S., Joly Y., Hagan J., Lévesque E. et al. 2017. Breast Cancer Risk Estimation and Personal Insurance: A Qualitative Study Presenting Perspectives from Canadian Patients and Decision Makers. Frontiers in Genetics 8: 128 < 10.3389/fgene.2017.00128>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S.C. 2017. Annual Report of the Chief Medical Officer 2016: Generation Genome, London, United Kingdom: Department of Health; Retrieved August 11, 2018. <https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/631043/CMO_annual_report_generation_genome.pdf>. [Google Scholar]
- Dent T., Jbilou J., Rafi I., Segnan N., Törnberg S., Chowdhury S. et al. 2013. Stratified Cancer Screening: The Practicalities of Implementation. Public Health Genomics 16(3): 94–99. < 10.1159/000345941>. [DOI] [PubMed] [Google Scholar]
- Gagnon J., Lévesque E., the Clinical Advisory Committee on Breast Cancer Screening and Prevention , Knoppers B.M., Simard J. 2016. Recommendations on Breast Cancer Screening and Prevention in the Context of Implementing Risk Stratification: Impending Changes to Current Policies. Current Oncology 23(6): e615 <https://dx.doi.org/10.3747%2Fco.23.2961>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile D. 2018. Pénurie d'infirmières: des hôpitaux du Québec retournent recruter en France. Radio Canada. Retrieved August 14, 2018. <https://ici.radio-canada.ca/nouvelle/1098339/penurie-infirmieres-etablissements-quebecois-recrute-france>.
- Hagan J., Lévesque E., Knoppers B. M. 2016. Influence des facteurs organisationnels sur l'implantation d'une approche personnalisée de dépistage du cancer du sein. Santé Publique 28(3): 353–61. < 10.3917/spub.163.0353>. [DOI] [PubMed] [Google Scholar]
- Hall A.E., Chowdhury S., Hallowell N., Pashayan N., Dent T., Pharoah P. et al. 2013. Implementing Risk-Stratified Screening for Common Cancers: A Review of Potential Ethical, Legal and Social Issues. Journal of Public Health 36(2): 285–91. < 10.1093/pubmed/fdt078>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman L., Timmermans D.R., Bouwman C.M., Cornel M.C., Meijers-Heijboer H. 2011. ‘A Low Risk is Still a Risk’: Exploring Women's Attitudes towards Genetic Testing for Breast Cancer Susceptibility in Order to Target Disease Prevention. Public Health Genomics 14(4–5): 238–47. < 10.1159/000276543>. [DOI] [PubMed] [Google Scholar]
- Howell A., Astley S., Warwick J., Stavrinos P., Sahin S., Ingham S. et al. 2012. Prevention of Breast Cancer in the Context of a National Breast Screening Programme. Journal of Internal Medicine 271(4): 321–30. < 10.1111/j.1365-2796.2012.02525.x>. [DOI] [PubMed] [Google Scholar]
- Institut national de santé publique du Québec (INSPQ). 2018. Indicateurs de performance du PQDCS. 2018. Retrieved August 14, 2018. <https://www.inspq.qc.ca/sites/default/files/documents/pqdcs/tableaubordpqdcs.pdf>.
- Kass N.E. 2001. An Ethics Framework for Public Health. American Journal of Public Health 91(11): 1776–82. < 10.2105/AJPH.91.11.1776>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass N.E. 2004. Public Health Ethics from Foundations and Frameworks to Justice and Global Public Health. The Journal of Law, Medicine & Ethics 32(2): 232–42. < 10.1111/j.1748-720X.2004.tb00470.x>. [DOI] [PubMed] [Google Scholar]
- Khoury M.J., Iademarco M.F., Riley W. T. 2016. Precision Public Health for the Era of Precision Medicine. American Journal of Preventive Medicine 50(3): 398 < 10.1016/j.amepre.2015.08.031>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarenbach S., Sims-Jones N., Lewin G., Singh H., Thériault G., Tonelli M. et al. 2018. Recommendations on Screening for Breast Cancer in Women Aged 40–74 Years Who Are Not at Increased Risk for Breast Cancer. The Canadian Medical Association Journal 190(49): E1441–51. < 10.1503/cmaj.180463>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Mavaddat M., Wilcox A.N., Cunningham A.P., Carver T., Hartley S. et al. 2019. BOADICEA: A Comprehensive Breast Cancer Risk Prediction Model Incorporating Genetic and Nongenetic Risk Factors. Genetics in Medicine 21(8): 1708–18. < 10.1038/s41436-018-0406-9>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque E., Kirby E., Bolt I., Knoppers B.M., de Beaufort I., Pashayan N. et al. 2018a. Ethical, Legal, and Regulatory Issues for the Implementation of Omics-Based Risk Prediction of Women's Cancer: Points to Consider. Public Health Genomics 20(6): 1–8. < 10.1159/000492663>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque E., Hagan J., Knoppers B.M., Simard J. 2018b. Organizational Challenges to Equity in the Delivery of Services within a New Personalized Risk-Based Approach to Breast Cancer Screening. New Genetics and Society 38(1): 38–59. < 10.1080/14636778.2018.1549477>. [DOI] [Google Scholar]
- Mavaddat N., Michailidou K., Dennis J., Lush M., Fachal L., Lee A. et al. 2018. Polygenic Risk Scores for Prediction of Breast Cancer and Breast Cancer Subtypes. The American Journal of Human Genetics 104(1): 21–34. < 10.1016/j.ajhg.2018.11.002>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton L., Brewer B., Kolva E., Joshi T., Bunch M. 2017. Increasing Access to Care for Young Adults with Cancer: Results of a Quality-Improvement Project Using a Novel Telemedicine Approach to Supportive Group Psychotherapy. Palliative & Supportive Care 15(2): 176–80. < 10.1017/S1478951516000572>. [DOI] [PubMed] [Google Scholar]
- Palinkas L.A., Horwitz S.M., Green C.A., Wisdom J.P., Duan N., Hoagwood K. 2015. Purposeful Sampling for Qualitative Data Collection and Analysis in Mixed Method Implementation Research. Administration and Policy in Mental Health 42(5): 533–44. 10.1007/s10488-013-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashayan N., Morris S., Gilbert F.J., Pharoah P.D. 2018. Cost-Effectiveness and Benefit-to-Harm Ratio of Risk-Stratified Screening for Breast Cancer: A Life-Table Model. JAMA Oncology 4(11): 1504–10. < 10.1001/jamaoncol.2018.1901>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton M.Q. 2005. Qualitative Research. In Everitt B., Howell D.C., eds., Encyclopedia of Statistics in Behavioral Science. Hoboken, New Jersey: John Wiley & Sons; < 10.1002/0470013192.bsa514>. [DOI] [Google Scholar]
- Phillips K.A., Steel E.J., Collins I., Emery J., Pirotta M., Mann G.B. et al. 2016. Transitioning to Routine Breast Cancer Risk Assessment and Management in Primary Care: What Can We Learn from Cardiovascular Disease? Australian Journal of Primary Health 22(3): 255–61. < 10.1071/PY14156>. [DOI] [PubMed] [Google Scholar]
- Pruthi S., Stange K.J., Malagrino G.D., Jr, Chawla K.S., LaRusso N.F., Kaur J. S. 2013. Successful Implementation of a Telemedicine-Based Counseling Program for High-Risk Patients with Breast Cancer. Mayo Clinic Proceedings 88(1): 68–73. < 10.1016/j.mayocp.2012.10.015>. [DOI] [PubMed] [Google Scholar]
- Rainey L., van der Waal D., Donnelly L.S., Evans D.G., Wengström Y., Broeders M. 2018. Women's Decision-Making Regarding Risk-Stratified Breast Cancer Screening and Prevention from the Perspective of International Healthcare Professionals. PloS One 13(6). < 10.1371/journal.pone.0197772>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph A., Song M., Brook M.N., Milne R.L., Mavaddat N., Michailidou K. et al. 2018. Joint Associations of a Polygenic Risk Score and Environmental Risk Factors for Breast Cancer in the Breast Cancer Association Consortium. International Journal of Epidemiology 47(2): 526–36. < 10.1093/ije/dyx242>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt V.H. 2015. Public Health Ethics. Problems and Suggestions. Public Health Ethics 8(1): 18–26. < 10.1093/phe/phu040>. [DOI] [Google Scholar]
- Schousboe J.T., Kerlikowske K., Loh A., Cummings S.R. 2011. Personalizing Mammography by Breast Density and Other Risk Factors for Breast Cancer: Analysis of Health Benefits and Cost-Effectiveness. Annals of Internal Medicine 155(1): 10–20. <https://dx.doi.org/10.7326%2F0003-4819-155-1-201107050-00003>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severin F., Borry P., Cornel M.C., Daniels N., Fellmann F., Hodgson S.V. et al. 2015. Points to Consider for Prioritizing Clinical Genetic Testing Services: A European Consensus Process Oriented at Accountability for Reasonableness. European Journal of Human Genetics 23(6): 729 < 10.1038/ejhg.2014.190>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh Y., Eklund M., Madlensky L., Sawyer S.D., Thompson C.K., Stover Fiscalini A. et al. 2017. Breast Cancer Screening in the Precision Medicine Era: Risk-Based Screening in a Population-Based Trial. Journal of the National Cancer Institute 109(5). < 10.1093/jnci/djw290>. [DOI] [PubMed] [Google Scholar]
- van Veen E.M., Brentnall A.R., Byers H., Harkness E.F., Astley S.M., Sampson S. et al. 2018. Use of Single-Nucleotide Polymorphisms and Mammographic Density Plus Classic Risk Factors for Breast Cancer Risk Prediction. JAMA Oncology 4(4): 476–82. < 10.1001/jamaoncol.2017.4881>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardrope A. 2014. Relational Autonomy and the Ethics of Health Promotion. Public Health Ethics 8(1): 50–62. < 10.1093/phe/phu025>. [DOI] [Google Scholar]


