Abstract
Context:
α-mangostin, one of the xanthone derivative compounds isolated from Garcinia mangostana L. peel extract, has an excellent anticancer efficacy. However, α-mangostin has a lack of site specificity, poor cells selectivity, and low aqueous solubility. Polymeric nanoparticles formulation can be used to solve these problems.
Aim:
Therefore, the main aim of this study was to develop polymeric nanoparticles of α-mangostin-based chitosan (αM-Ch) coated by sodium alginate (αM-Ch/Al), sodium silicate (αM-Ch/Si), and polyethylene glycol 6000 (αM-Ch/PEG).
Materials and Methods:
Polymeric nanoparticles were prepared by ionic gelation method with the spray pyrolysis technique. Optimized formula was characterized by scanning electron microscopy, particle size, entrapment efficiency, drug loading, Fourier transform infrared, X-ray diffraction (XRD), and differential scanning calorimetry (DSC).
Results:
αM-Ch/Al, αM-Ch/Si, and αM-Ch/PEG Nanoparticles were successfully prepared with the range of particle size approximately 200–400nm. The XRD patterns and DSC thermograms of αM-Ch/Al showed an amorphous state, whereas αM-Ch/Si and αM-Ch/PEG indicated low crystalline forms. In addition, αM-Ch/Al had the highest entrapment efficiency (98.33% ± 0.06%) compared to αM-Ch/Si (70.46% ± 8.93%), and αM-Ch/PEG (92.24% ± 10.98%).
Conclusion:
These results suggest that αM-Ch/Al has the potential to enhance the physicochemical properties of α-mangostin for further formulation as an anticancer agent.
Keywords: Alginate, chitosan, α-mangostin, polyethylene glycol, polymeric nanoparticle, silicate
Introduction
α-Mangostin is a major xanthone derivative compound, which is isolated from mangostin pericarp extract.[1] Previous study showed that α-mangostin had an excellent anticancer activity with the inhibition concentration 50% (IC50 value from 5 to 10 µM and was effective in inducing the apoptosis mechanism of leukemic cell lines.[2] However, it has a poor aqueous solubility, a lack of site specificity, and a low selectivity in cancer cells, which may cause problems in its biopharmaceutical performance. To overcome these problems, polymeric nanoparticle drug delivery system can be applied as the primary consideration.[3]
Polymeric nanoparticle is an approaches of nanotechnology that has been used in medicine (nanomedicine). It was consist of biopolymers or synthetic polymers as delivery carrier. Several studies have been proved that the polymeric nanoparticle can increase the solubility of drugs, provide controlled release, and targted drug delivery system.[4,5] Biopolymers become a primary consideration for choosing nanoparticles formulation due to their nontoxic, biodegradable, and biocompatible properties.[6]
Nowadays, chitosan is known as a multifunctional biopolymer in polymeric nanoparticles. Several studies proved that chitosan was able to provide a controlled release system.[7,8] By its cationic charges in the molecular structure, chitosan is able to bind with anionic charges on the surface of cell membrane, this is corresponded to the mucoadhesive properties of the chitosan.[9] In addition, chitosan has the high-affinity property to interact with the cancer cells. In previous studies, polymeric nanoparticle-based chitosan was successfully prepared as a targeted drug delivery system for dextran–doxorubicin. The results suggested that chitosan minimized the side effects, increased the efficacy of dextran–doxorubicin,[10] and provided the super tumor-homing capacity into SCC7 tumor.[11] However, in oral administration, this activity may decrease due to protonation of the amine groups in chitosan structure and degradation.[12]
An enteric-coated nanoparticle is often used as a drug delivery system in oral administration, which can protect the reaction of degradation, prevention of toxic effects, and drug localization. Enteric-coated nanoparticle for 5-fluorouracil represents a potential drug delivery approach for effective delivery of the active pharmaceutical ingredient by the oral route of administration.[12] Also, an enteric-coated nanoparticle was successfully used as a drug delivery carrier for insulin in oral delivery, which can provide relative bioavailability to be approximately 20%.[13] In addition, greatest anti-glioma activity of 16-hydroxy-cleroda-3,13-dien-16,15-olide (HCD) was achieved by enteric-coated nanoparticle.[14]
Therefore, the main object of this study was to prepare and characterize α-mangostin chitosan polymeric nanoparticle using ionic gelation method, then to coat with various types of enteric polymers such as sodium alginate, sodium silicate, and polyethylene glycol (PEG).
Materials and Methods
Materials
α Mangostin was purchased from Chengdu Biopurify Phytochemicals (Sichuan, China). Chitosan as the primary base polymer was isolated with a purity of 70%, sodium tripolyphosphate as cross-linker and the coating polymers sodium alginate were purchased from Wako Pure Chemical Industries (Tokyo, Japan), sodium silicate and PEG 6000 from Sigma Aldrich (Missouri, USA), acetate acid solvent from PT. Brataco (West Java, Indonesia), ethanol from CV. Kristata (West Java, Indonesia), ethyl acetate from PT. Evonik (West Java, Indonesia), and potassium bromide (KBr) from PT. Merck (West Java, Indonesia).
Methods
Preparation of polymeric nanoparticle α-mangostin: The polymeric nanoparticle, which consists of α-mangostin, chitosan, and the cross-linker sodium tripolyphosphate (αM-Ch) was prepared by ionic gelation methods. Briefly, 20 mg α-mangostin was diluted in 20 mL ethanol and mixed with chitosan solution (0.1% wt/vol) in 200 mL of acetate acid (1% vol/vol) under constant mixing until suspension system was formed. Then, the system was ultrasonicated for reducing the particle droplets in suspension system.
In coating process, suspension system containing α-mangostin, chitosan, and sodium tripolyphosphate was dropped into variants of enteric polymer concentrations. In this study, three variants of polymer concentrations were formulated for each polymer, which are F1, F2, and F3 for sodium alginate (αM-Ch/Al); F4, F5, and F6 for sodium silicate (αM-Ch/Si); and F7, F8, and F9 for PEG 6000 (αM-Ch/PEG) [Table 1]. In this step, coating process was conducted by microvolume flow titration method. Then, the nanoparticles powder were obtained by drying the suspension solutions using spray pyrolysis, with temperature system of 80-100oC and airflow (5L/min).[13,14]
Table 1.
α-Mangostin polymeric nanoparticle formulation
| Formulation | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| α-mangostin (mg) | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Chitosan (mg) | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 |
| Tripolyphosphate (mg) | 28 | 28 | 28 | 28 | 28 | 28 | 28 | 28 | 28 |
| Alginate (mg) | 50 | 70 | 90 | 0 | 0 | 0 | 0 | 0 | 0 |
| Silicate (%vol/vol) | 0 | 0 | 0 | 1 | 2.5 | 5 | 0 | 0 | 0 |
| PEG 6000 (mg) | 0 | 0 | 0 | 0 | 0 | 0 | 30 | 75 | 120 |
Tpp = Sodium tripolyphosphate or Tripolyphosphate
Characterization of nanoparticles
Scanning electron microscopy
The surface morphology of polymeric nanoparticles was studied by scanning electron microscopy (SEM) (Model SU3500 SEM; Hitachi, Tokyo, Japan), the nanoparticles were put into the stub and were made conductive by coating with platinum (30s, 10 mA). The photomicrographs of polymeric nanoparticle were observed at a voltage (10kV) with differentials magnification (×10,000–50,000), which was followed by converting the data using software Image J (Wisconsin-Madison, USA) and Origin (version 8.5, Massachustts, USA) to obtain the average of particles size distribution.[15]
Entrapment efficiency
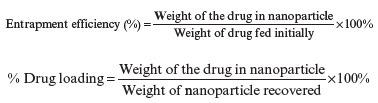
Polymeric nanoparticles (25 mg) after spray pyrolysis were added to ethyl acetate solvent and continued to centrifuge (3000rpm, 10min), then the supernatant was collected and measured at lambda of 261nm in double beam UV-visible spectrophotometer to obtain the amount of free α-mangostin (unencapsulated). Then the residue was diluted in an appropriate volume of ethanol to determine the drugs that were encapsulated to find the total amount of α-mangostin. The entrapment efficiency and drug loading of α-mangostin in polymeric nanoparticles were determined by the following equations[16]:
Fourier transform infrared spectroscopy
The interactions of functional groups of α-mangostin with other ingredients in polymeric nanoparticle system were investigated by Fourier transform infrared (FTIR) spectroscopy (Model IR Prestige-21; Shimadzu, Kyoto, Japan). Polymeric nanoparticles (2 mg) were mixed with 200 mg KBr, then the pellets were molded with vacuum pressure (60kN within 15min), and measured at 4000–400 cm–1. The interaction was represented by comparing functional groups of the polymeric nanoparticle system with the functional groups of pure α-mangostin.[17]
X-ray diffraction
X-ray diffraction (XRD) was performed to study the molecular arrangements of all raw materials and polymeric nanoparticle system using an X-pert MPD diffractometer type (Rigaku International, Tokyo, Japan). The XRD patterns were collected over an angular range 2θ (5°–60°), with 30 mA, and 40kV generator setting.[18]
Differential scanning calorimetry
Thermal behavior of α-mangostin in polymeric nanoparticle system was studied by differential scanning calorimetry (DSC-60; Mettler Toledo, Barcelona, Spain). The samples were analyzed using TA-60WS software (Mettler Toledo, Barcelona, Spain), running at a range of temperatures from 30°C to 300°C, with a heating rate of 20°C/min under nitrogen flow. The same condition was used for raw material analysis.[19]
Data analysis
Statistical analysis was performed by SPSS software version 16.0 (Chicago, USA), using one-way ANOVA test (for all formulas) and t-test independent (for comparison between two formulas). The standard error mean (SEM) was expressed for quantitative data
Results and Discussion
Preparation of polymeric nanoparticle α-mangostin
All of the components in the polymeric nanoparticle system (active pharmaceutical ingredients and polymers) concentration were chosen based on the consideration from literature, and optimization processes were determined in the article. Briefly, the ratio of α-mangostin:chitosan (1:10), sodium tripolyphosphate:chitosan (1:7), and enteric polymers were optimized by various concentrations [Table 1]. The spray pyrolysis condition system such as temperatures, airflow, mixing, and duration of ultrasonication was also optimized and validated.
Characterization of nanoparticles
Scanning electron microscopy, particle size, entrapment efficiency, and drug loading
On morphological study of each polymeric nanoparticles formula, it was observed that it had basic spherical shapes. However, the αM-Ch/Al and αM-Ch/PEG formulas showed some agglomeration and some spherical shapes in these systems [Figure 1]. The particle size, entrapment efficiency, and drug loading of polymeric nanoparticles are shown in [Table 2]. The data of αM-Ch/Al formulas show that the concentrations of sodium alginate are not significantly affecting its particle size and entrapment efficiency value. The αM-Ch/Si formulas showed that the particle size and entrapment efficiency were decreased along with the increase in the concentration of sodium silicate, whereas the αM-Ch/PEG formulas showed that the PEG concentration was directly proportional with the particle size and inversely with the entrapment efficiency value.
Figure 1.
Photomicrograph of nanoparticles; F1-F3 for (αM-Ch/Al), F4-F6 for (αM-Ch/Si), and F7-F9 for (αM-Ch/PEG)
Table 2.
Results of particle size, entrapment efficiency, and drug loading
| Polymeric nanoparticles | Formulas | Particle size (nm) | Entrapment efficiency (%) | Drug loading (%) |
|---|---|---|---|---|
| αM-Ch/Al | F1 | 422.45 ± 146.33 | 98.39 | 6.60 |
| F2 | 386.60 ± 57.91 | 98.43 | 6.19 | |
| F3 | 439.63 ± 59.67 | 98.47 | 5.83 | |
| αM-Ch/Si | F4 | 434.62 ± 216.85 | 80.00 | 6.42 |
| F5 | >500.00 ± 00.0 | 69.08 | 5.52 | |
| F6 | 267.12 ± 161.07 | 62.31 | 4.93 | |
| αM-Ch/PEG | F7 | 271.43 ± 66.55 | 89.80 | 6.46 |
| F8 | 364.45 ± 117.24 | 95.84 | 5.93 | |
| F9 | 411.19 ± 174.89 | 79.58 | 4.33 |
Considering the morphology particle size and entrapment efficiency of the polymeric nanoparticles, we conducted further experiments by selecting one of the best formulas for each polymer. Herein we had taken F1, F6, and F7 to represent αM-Ch/Al, αM-Ch/Si, and αM-Ch/PEG formulas, respectively.
Fourier transform infrared spectroscopy
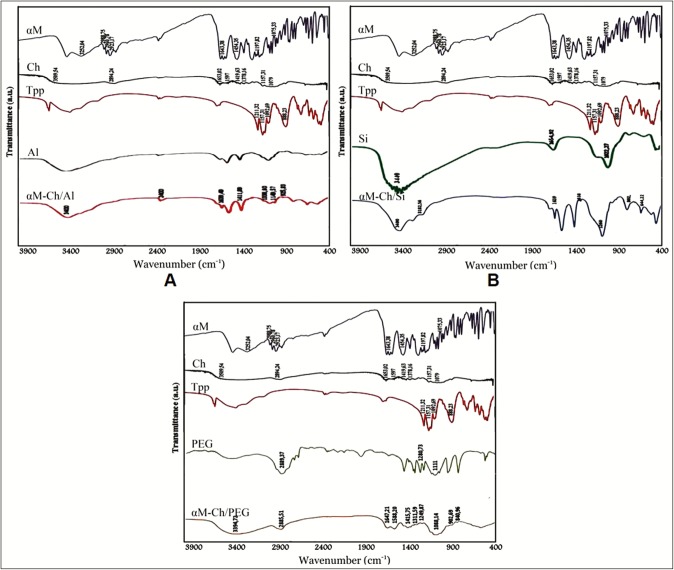
FTIR spectroscopy was studied to observe the interaction of each component of ingredients in polymeric nanoparticles system, specifically the interactions between each functional group, or the interactions that cause the formation of new functional groups. The results of FTIR analysis are shown in Figure 2. Herein the functional group of α-mangostin shows the presence of O–H stretch at wave number 3420.81 and 3252.04 cm–1, C–H stretch at wave number 2988.75, 2960.78, and 2923.17 cm–1, C=O at wave number 1643.38 cm–1, C–C at wave number 1454.35 cm–1, ortho-OCH3 stretch at wave number 1197.82 cm–1, and C–O–C stretch at wave number 1075.33 cm–1. For the chitosan biopolymer, the presence of O–H and N–H stretch at 3509.54 cm–1, C–H stretch at 2894.24 cm–1, C=O stretch at 1653.02 cm–1, N–H bend at 1597 cm–1, C–H bend at 1419.63 cm–1, C–N 1378.16 cm–1, C–O–C stretch at 1157.31 cm–1, and C–O at 1079 cm–1 was observed. The cross-linker sodium tripolyphosphate shows peaks of νasP=O at 1211.32 cm–1, νsO–P=O at 1157.31 cm–1, νasPO3 at 1092.69 cm–1, and νasP–O–P at 888.23 cm–1. The biopolymer sodium alginate shows functional groups C–O–C at 1030.00 cm–1 and νas-sCOO at 1617.20 and 1417.00 cm–1, respectively. Polymer sodium silicate shows H–Si–Si–H stretch at 1990.54 cm–1, –OH bend at 1654.92 cm–1 from silanol (≡Si–OH), Si–O bend νas at 1022.27 cm–1 from siloxane (≡Si–O–Si≡), Si–O bend νs at 771.53 cm–1 at siloxane (≡Si–O–Si≡), and siloxane (≡Si–O–Si≡) bend at 606.65 cm–1 and 466.77 cm–1. For PEG 6000, we found the presence of O-H stretch at 3483.44 cm–1, C-H stretch 2889.50 cm–1, C–O stretch 1280.73 cm–1, and C–O–C at 1111.00 cm–1.
Figure 2.
Fourier transform infrared spectroscopy. (A) αM-Ch/Al. (B) αM-Ch/Si. (C) αM-Ch/PEG
αM-Ch/Al polymeric nanoparticle [Figure 2A] shows similar transmission peaks with pure sodium alginate, such as responses of νas-s COO at wave number 1617.20 and 1417.00 cm–1 with increasing the intensity caused by overlapping with the C=O and C–C at 1643.38 and 1454.35 cm–1, respectively, from α-mangostin. In addition, the wave numbers range of 3600–3300 cm–1 showed peaks of amine groups from α-mangostin compound.
FTIR results of αM-Ch/PEG polymeric nanoparticle formula showed the presence of C=O and C-C from α-mangostin compound at 1643.38 cm-1 and 1454.35 cm-1, respectively [Figure 2B]. In addition, the signal transmissions of α-mangostin’s functional groups especially for C=O and C-C presence at 1600.38 and 1454.35 cm-1 in αM-Ch/Si polymeric nanoparticle formula[Figure 2B]. The data indicates that the α-mangostin was successfully loaded into nanoparticles system
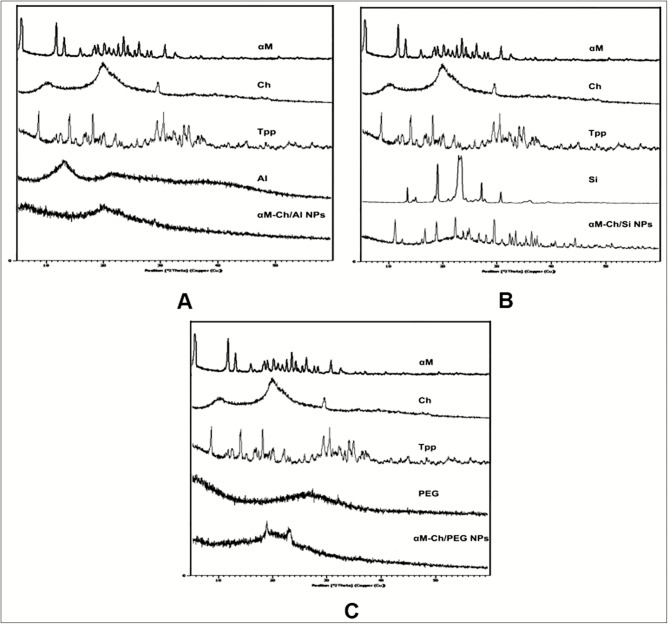
X-ray diffraction
The XRD study was performed to analyze the crystallography of molecular compounds by the intervention of X-ray radiation. The result was shown as 2θ and intensity, which depends strongly on intermolecular electron binding in the molecular compound. The results of this study are shown in Figure 3, where α-mangostin had shown a sharp multiples peak at 2θ 7°–32°, which is indicated as a crystalline pattern, as well as sodium tripolyphosphate and sodium silicate also showed a crystalline pattern. Besides, alginate and chitosan showed a semicrystalline pattern with minor intensity at 2θ 13° and 19.72°, respectively, whereas PEG had shown an amorphous pattern. The XRD results of αM-Ch/Si polymeric nanoparticle formula demonstrated a crystalline form and similar to the diffractogram of sodium silicate [Figure 3B]. However, the crystallinity pattern had a wider range than sodium silicate, which can be assumed to be crystalline responses from α-mangostin and sodium tripolyphosphate compounds. Besides, αM-Ch/PEG formula had shown an amorphous pattern with the presence of minor crystal intensity at 2θ 18° and 22° [Figure 3C].
Figure 3.
X-ray diffraction analysis. (A) αM-Ch/Al. (B) αM-Ch/Si. (C) αM-Ch/PEG
Differential scanning calorimetry
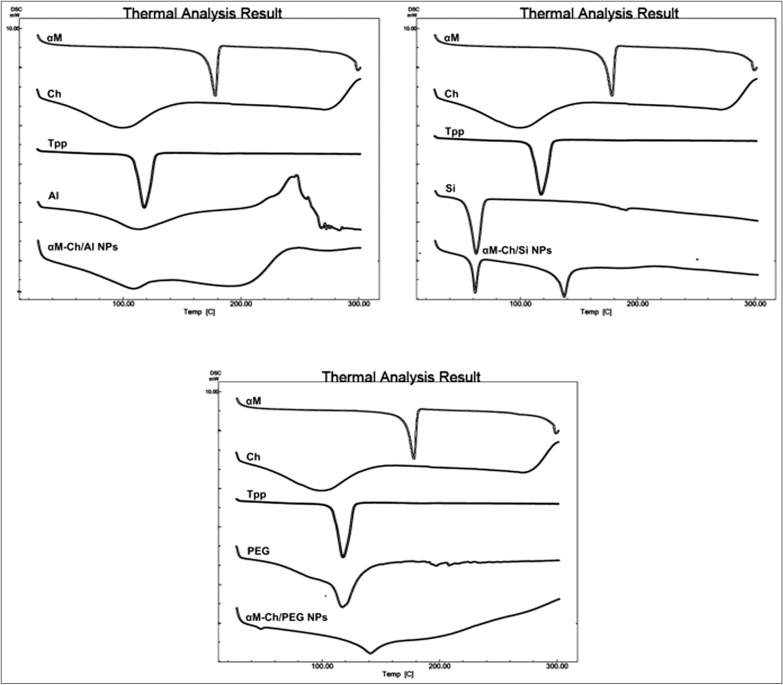
The results of thermal behavior analysis are shown in Figure 4. The DSC curve of α-mangostin, sodium tripolyphosphate, and sodium silicate was shown corresponding to their melting point or endothermic phase at 178.00°C, 120.06°C, and 63.34°C, respectively. The data were correlated with XRD analysis, where three of these ingredients were in crystalline forms. Besides, chitosan and alginat have glass transition pattern, as we know that glass transition becomes the specific characteristic of polymers. Moreover, both of them shows wider peaks around 100oC which assumed that to be the evaporation process of water molecules from polymers. Impressively, polymeric nanoparticle system of αM-Ch/Al [Figure 4A] has shown that the changes of thermal behavior, which is the endothermic pattern of α-mangostin, did not appear in αM-Ch/Al formula, which means that the α-mangostin had been molecularly dispersed in the matrix of polymeric nanoparticles. On the other hand αM-Ch/Si formula showed the presence of endothermic peaks at 60.41 and 136.59oC, indicating the melting point of sodium silicate and the melting point of the interaction between sodium tripolyphosphate with α-mangostin. The αM-Ch/PEG formula [Figure 4C] shows the endothermic pressure from the polymeric nanoparticle system, it revealed a minor peak at 142.65°C, which was assumed to be a peak of interaction between α-mangostin with sodium tripolyphosphate. Based on DSC data, we can conclude that the α-mangostin was not perfectly loaded into the αM-Ch/Si and αM-Ch/PEG polymeric nanoparticles system. In conclusion, this study proved that the αM-Ch/Al has better characteristics compared to other formulas. The data also directly correlated with the XRD analysis.
Figure 4.
Differential scanning calorimetry. (A) αM-Ch/Al. (B) αM-Ch/Si. (C) αM-Ch/PEG
Discussion
Basic spherical shapes of polymeric nanoparticle which were found in this study proved that spherical was a stable form than the others. The formation of spherical shapes is influenced by the laws of thermodynamic particles, hydrostatic equilibrium, angular momentum, and so on.[20,21,22] These formations occur because of the attraction to the core point of the particle.[23] In this study, we also found that some particles agglomeration occurred in αM-Ch/Al and αM-Ch/PEG polymeric nanoparticles. These results probably induced by the high surface energy of nanoparticle, adhesive and affinity properties of polymers for absorbing water molecule.[24] Those properties were affected on hydrostatic equilibrium and on inducing agglomeration.[21] Also, using polymers with different physicochemical properties could be one of the causes inducing the agglomeration phenomena. The absence of clear influence between polymer concentration with particle size on αM-Ch/Al formula can be caused by several factors during preparation process.[25] Generally, the particle size is affected by the amount of ingredient concentration, where the particle size will be larger for formula that contains a greater amount of ingredients[26] as shown by the αM-Ch/PEG formula. In some cases, the result can be different as shown on αM-Ch/Si polymeric nanoparticle, where the use of silica as coating agent on this system can stabilize and prevent agglomeration of the nanoparticle.[27] On the basis of particle size characterization, α-mangostin was successfully prepared with mean particle size around 200–400nm and the entrapment efficiency value of >60%. Furthermore, FTIR study confirmed that the α-mangostin had successfully loaded into the nanoparticles system with the presence of C=O functional groups from α-mangostin and the presence of the specific spectrum of coating agents, such as νas-s COO at wave number 1617.20 and 1417.00 cm–1 in αM-Ch/Al formula, OH bend from silanol (≡Si–OH) at 1022.27 cm-1, Si-O stretch from siloxane (≡Si-O-Si≡) at 771.57, 606.65, and 466.77 cm-1 of sodium silicate in αM-Ch/Si formula, and C-CH of PEG at 2885 cm-1 in αM-Ch/PEG formula. It can be concluded that the polymeric nanoparticle formulas were successfully prepared with α-mangostin in polymeric nanoparticle system. To understand more clearly about the characteristics, XRD and DSC analyses were performed. αM-Ch/Al formula perfectly showed a solid-state transformation from crystalline to an amorphous form, resembling the pattern of sodium alginate [Figure 3A]. Moreover, the semicrystalline transformation to amorphous form of chitosan was induced by disconnection of amine and hydroxyl groups, which cause amorphous complex forms with sodium alginate.[28,29] Comparing with the pure sodium alginate, the semicrystalline transformation was shown in αM-Ch/Al formula. The transformation was caused by disconnection of Na+ from sodium alginate molecule,[30] whereas the presence of wider range of crystalline pattern in αM-Ch/Si and minor crystal intensity at 2θ 18° and 22° in αM-Ch/PEG concluded that α-mangostin had not wholly dispersed in the matrix nanoparticle system. DSC showed similar result with XRD study. A glass transition pattern and absence of melting point signal of α-mangostin or sodium tripolyphosphate were observed in αM-Ch/Al, which means that the system had an amorphous pattern. It can be concluded for αM-Ch/Si and αM-Ch/PEG, where those formulas show an imperfectly encapsulation of α-mangostin compound marked by the presence of strong signal of melting point on the DSC pattern. These results suggested that αM-Ch/Al was the best formula based on its physicochemical characteristics.
Conclusion
In this study, α-mangostin was successfully encapsulated in chitosan polymeric nanoparticles by ionic gelation method using the spray pyrolysis technique with the particle size range of 200–400nm. On the basis of characterization data, we suggest that αM-Ch/Al may be a promising modification for enhancing physicochemical properties and performance of α-mangostin.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
This work was supported by the Fundamental Research Grant (Penelitian Dasar) 2019 no. 1373b/UN6.O/LT/2019, Ministry of Research and Higher Education, Republic of Indonesia.
References
- 1.Ghasemzadeh A, Jaafar H, Baghdadi A, Tayebi-Meigooni A. Alpha-mangostin-rich extracts from mangosteen pericarp: optimization of green extraction protocol and evaluation of biological activity. Molecules. 2018;23:1852. doi: 10.3390/molecules23081852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsumoto K, Akao Y, Kobayashi E, Ohguchi K, Ito T, Tanaka T, et al. Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J Nat Prod. 2003;66:1124–7. doi: 10.1021/np020546u. [DOI] [PubMed] [Google Scholar]
- 3.Loganathan P, Kalmouni M, Al Hosani S, Magzoub MM. pH sensitive peptide functionalized high stability polymeric nanoparticles for mitochondria targeted cancer drug delivery. Biophys J. 2019;116:465a. [Google Scholar]
- 4.Guiot P. Polymeric nanoparticles and microspheres: 0. USA: CRC Press; 2018. [Google Scholar]
- 5.Masood F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater Sci Eng C. 2016;60:569–78. doi: 10.1016/j.msec.2015.11.067. [DOI] [PubMed] [Google Scholar]
- 6.Elgadir MA, Uddin MS, Ferdosh S, Adam A, Chowdhury AJK, Sarker MZI. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: a review. J Food Drug Anal. 2015;23:619–29. doi: 10.1016/j.jfda.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altaani BM, Al-Nimry SS, Haddad RH, Abu-Dahab R. Preparation and characterization of an oral norethindrone sustained release/controlled release nanoparticles formulation based on chitosan. AAPS Pharmscitech. 2019;20:54. doi: 10.1208/s12249-018-1261-3. [DOI] [PubMed] [Google Scholar]
- 8.Kashyap S, Singh A, Mishra A, Singh V. Enhanced sustained release of furosemide in long circulating chitosan-conjugated PLGA nanoparticles. Res Pharm Sci. 2019;14:93–106. doi: 10.4103/1735-5362.253356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pauluk D, Padilha AK, Khalil NM, Mainardes RM. Chitosan-coated zein nanoparticles for oral delivery of resveratrol: formation, characterization, stability, mucoadhesive properties and antioxidant activity. Food Hydrocoll. 2019;94:411–417. [Google Scholar]
- 10.Mitra S, Gaur U, Ghosh PC, Maitra AN. Tumour targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrier. J Control Release. 2001;74:317–23. doi: 10.1016/s0168-3659(01)00342-x. [DOI] [PubMed] [Google Scholar]
- 11.Saravanakumar G, Min KH, Min DS, Kim AY, Lee CM, Cho YW, et al. Hydrotropic oligomer-conjugated glycol chitosan as a carrier of paclitaxel: synthesis, characterization, and in vivo biodistribution. J Control Release. 2009;140:210–7. doi: 10.1016/j.jconrel.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Nogueira F, Gonçalves IC, Martins MC. Effect of gastric environment on Helicobacter pylori adhesion to a mucoadhesive polymer. Acta Biomater. 2013;9:5208–15. doi: 10.1016/j.actbio.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Lim HR, Jung SJ, Hwang TY, Lee J, Kim KH, Cho HB, et al. Electromagnetic wave absorption properties of Fe/MgO composites synthesized by a simple ultrasonic spray pyrolysis method. Appl Surf Sci. 2019;473:1009–13. [Google Scholar]
- 14.Gomathi T, Sudha PN, Florence JAK, Venkatesan J, Anil S. Fabrication of letrozole formulation using chitosan nanoparticles through ionic gelation method. Int J Biol Macromol. 2017;104:1820–32. doi: 10.1016/j.ijbiomac.2017.01.147. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein JI, Newbury DE, Michael JR, Ritchie NWM, Scott JHJ, Joy DC. Scanning electron microscopy and X-ray microanalysis. New York, USA: Springer; 2017. [Google Scholar]
- 16.Song X, Zhao Y, Hou S, Xu F, Zhao R, He J, et al. Dual agents loaded PLGA nanoparticles: systematic study of particle size and drug entrapment efficiency. Eur J Pharm Biopharm. 2008;69:445–53. doi: 10.1016/j.ejpb.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Smith BC. Fundamentals of Fourier transform infrared spectroscopy. USA: CRC Press; 2011. [Google Scholar]
- 18.Zak AK, Majid WHA, Abrishami ME, Yousefi R. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Solid State Sci. 2011;13:251–6. [Google Scholar]
- 19.Sarmento B, Ferreira D, Veiga F, Ribeiro A. Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohydr Polym. 2006;66:1–7. [Google Scholar]
- 20.Defay R, Prigogine I. Chemical thermodynamics. London, UK: Longmans; 1965. [Google Scholar]
- 21.Bekenstein JD. Hydrostatic equilibrium and gravitational collapse of relativistic charged fluid balls. Phys Rev D. 1971;4:2185. [Google Scholar]
- 22.Zare RN. Angular momentum: understanding spatial aspects in chemistry and physics. Wiley-Interscience; 2013. [Google Scholar]
- 23.Kramb RC. The effects of particle shape, size, and interaction on colloidal glasses and gels. 2011.
- 24.Nanda KK, Maisels A, Kruis FE, Fissan H, Stappert S. Higher surface energy of free nanoparticles. Phys Rev Lett. 2003;91:106102. doi: 10.1103/PhysRevLett.91.106102. [DOI] [PubMed] [Google Scholar]
- 25.Sinha B, Müller RH, Möschwitzer JP. Bottom-up approaches for preparing drug nanocrystals: formulations and factors affecting particle size. Int J Pharm. 2013;453:126–41. doi: 10.1016/j.ijpharm.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 27.Koh JJ, Qiu S, Zou H, Lakshminarayanan R, Li J, Zhou X, et al. Rapid bactericidal action of alpha-mangostin against MRSA as an outcome of membrane targeting. Biochim Biophys Acta. 2013;1828:834–44. doi: 10.1016/j.bbamem.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Ghaffari A, Navaee K, Oskoui M, Bayati K, Rafiee-Tehrani M. Preparation and characterization of free mixed-film of pectin/chitosan/Eudragit RS intended for sigmoidal drug delivery. Eur J Pharm Biopharm. 2007;67:175–86. doi: 10.1016/j.ejpb.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Thakral NK, Ray AR, Majumdar DK. Eudragit S-100 entrapped chitosan microspheres of valdecoxib for colon cancer. J Mater Sci Mater Med. 2010;21:2691–9. doi: 10.1007/s10856-010-4109-2. [DOI] [PubMed] [Google Scholar]
- 30.Li P, Dai YN, Zhang JP, Wang AQ, Wei Q. Chitosan-alginate nanoparticles as a novel drug delivery system for nifedipine. Int J Biomed Sci. 2008;4:221. [PMC free article] [PubMed] [Google Scholar]