Abstract
Objective:
Kidney stones (nephrolithiasis) is one of the kidney diseases in the form of stones that contain crystal and organic matrix components. It is one of the most common diseases of the urinary tract. Calcium stone is the most important type of stone (80%) found in the case of kidney stones. Celery (Apium graveolens L.) is a plant rich in flavonoids, which can break down calcium crystals. Apigenin is considered to be one of the main flavonoids because of its presence and abundance in celery. This research aimed to compare the anticalculi effect of apigenin with that of celery extract.
Materials and Methods:
Wistar albino rats were given ethylene glycol 0.75% (vol/vol) and ammonium chloride 2% (wt/vol) orally for 7 days in all groups to induce hyperoxaluria and Rats treated by Apigenin at doses 1.2, 2.4, and 4.8 mg/kg of rat body weight and celery extract at doses of 200, 400, and 600 mg/kg of rat body weight as anticalculi. Measurements of calcium levels in the kidneys and urine of rats was obtained using atomic absorption spectroscopy. Data obtained were statistically analyzed with the IBM SPSS by ANOVA Method version 21.0 probability value < 0.05 was considered significant.
Result:
The results showed that both apigenin and celery extracts caused kidney stone to decay. From the data Apigenin and celery showed that calcium level in urine there were significant differences (p value < 0.05) in treated group from negative control group but calcium level in kidney there were not significant differences (p value > 0.05).
Conclusion:
Celery extract has better ability to break down kidney stones than apigenin.
Keywords: Anticalculi, apigenin, calcium, celery extract, nephrolithiasis
Introduction
Nephrolithiasis is a disease characterized by the formation of crystal stones in the pelvis or calyx of the kidneys. This disease is the most common cause of abnormalities in the urinary tract.[1] The formation of kidney stone is composed of intrinsic and extrinsic factors. Intrinsic factors are age, sex, and genetic, whereas extrinsic factors are geographical conditions, climate, eating habits, substances contained in urine, and activity.[2] Increased kidney stone disease also directly increases the prevalence of urinary system abnormalities, affecting 12% of the world’s population.[1]
Various drugs given orally are also used to treat kidney stones. However, long-term drug use is limited by severe side effects and lack of tolerance by patients. Therefore, many alternative treatment options, including herbal medicines, have been used to treat urinary stones since hundreds of years without dangerous side effects.[3] Celery belongs to a group of plants, which potentially treats kidney stones.[4]
On the basis of the data,[5] it was known that ethanol extract of celery herbs (Apium graveolens L.) at doses of 20 mg/100g body weight (BW) of rats has an anticalculi effect. The compound, which has an anticellular effect, is the flavonoid called apigenin. The previous study showed that Sonchus arvensis L. leaves prevent the development of stone weight by 8.12% and the inhibition of the development stone weights 14,02%,[6] 7-O-glucosida active as anticalculi in rat at dose 0.15 mg/kgBW with effective inhibition about 51.62% (P ≤ 0.05), suppression effect about 3.77%[7] and quercetin as antiurolithiasis.[8]
The aim of this study was to compare the anticalculi activity of apigenin with ethanol extract of celery herbs at higher doses from previous study. Parameter for anticalculi studies were the kidneys weight ratio, calcium levels in kidneys and calcium levels in urine.
Materials and Methods
This was an experimental study with an in vivo method using Wistar strain male rats. This study was carried out by obtaining the activity of apigenin compounds and extracts of celery compared with that of the positive control. The study was conducted by randomly dividing into six groups for apigenin and six groups for celery herb extract. The formula for calculate minimum number of rats based on Frederer’s (t-1) (n-1)≥15, which is amount of repetitions in this study ≥4 for each treatment. Total of rats are used in research 48. This research received ethical approval from the research ethics commission at Padjadjaran University in Bandung, Sumedang, Indonesia, number 233 and 449/UN6.KEP/EC 2019.
Materials
The materials used in this study were distilled water (H2O), ammonium chloride (NH4Cl), apigenin obtained from Chengdu Biopurify Phytochemicals (Shinchuan, China) (purity 98%), ethylene glycol (C2H6O2), carbon dioxide (CO2), Pulvis Gummi Arabici (PGA), batugin elixir, formaldehyde 10% (CH2O), and ethanol extract (96%) of celery herbs from Ciwidey, Indonesia.
Instruments
The tools used were mouse drinking equipment, metabolic cage, atomic absorption spectrophotometer (PerkinElmer, Inc Ekanal Analyst 400 AA), analytical scales, rat scales (Harnic HL-4350), syringes, oral sonde, and a set of surgical tools.
Procedure
Rats were divided into six groups for apigenin test and six groups for 96% ethanolic celery extract. Rats were houses in cages at laboratory padjadjaran university. Acclimatization of animals during 7 days and before treatment were fasted 16 hours until 18 hours but still given drink. Group II, III, IV, V, and VI animasl received 0.75% (vol/vol) and ammonium chloride 2% (wt/vol) from the first day until the seventh day. Rats were placed individually in a metabolic cage for 24h to collect urine. The 24-h urine sample was collected on the 7th and 14th day before the rats were killed. All animals test sacrificed at the fifteenth day to take the kidney and storage in 10% formaldehyde solution and weighed the kidney to determine kidney characteristic. Kidney obtained from the rats were dried in oven (100°C) for 24 hours and then heated in 540°C for eight hours and then diluted with nitric acid and demineralized water, so sample ready to read by AAS (Atomic Absorption Spectrscopy)
Statistical analysis
The experimental results were analyzed statistically using the Statistical Package for the Social Sciences (SPSS) software, version 21.0. The normality of the research data was determined by using the Kolmogorov–Smirnov method and its homogeneity with the Levene method. If these two tests met the requirement, then it was preceded with a one-way analysis of variance test to observe differences between the groups. If there were differences, then it was followed by the post hoc Newman–Keuls test to observe the significant differences between the treatment groups. If the normality and homogeneity tests did not match or one of the two tests was not fulfilled, then it was preceded with the Kruskal–Wallis test to observe the differences between the groups.
Results
Kidney body weight ratio and calcium level in kidney
This study compared the results of the apigenin test with ethanol extract (96%) of celery herbs as anticalculi in vivo in Wistar strain rats. The results of the study were observed by renal weight ratio, calcium levels in the kidneys, and calcium levels in the urine. All kidneys in the test animal group were brownish red and shaped like peanuts. Shape of the kidney is shown in Figure 1.
Figure 1.

Shape of kidney
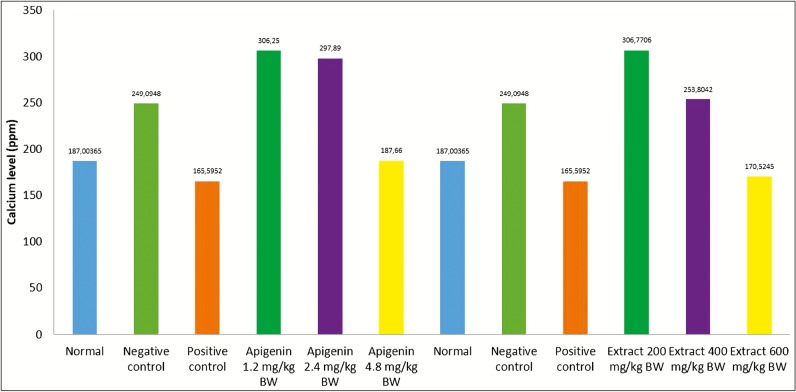
The kidney weight ratio showed that the negative control for the apigenin group and the extract group had the highest kidney weight ratio, which was 0.80 and 0.79. An increase in kidney weight was induced by ethylene glycol, which increases oxalate levels in the kidneys; it occurred because ethylene glycol in the body is metabolized to oxalic acid. The addition of ammonium chloride causes acceleration of crystal formation in the kidney. High level of oxalate was known to increase the bond formation between calcium and oxalate to form crystals. The condition of hyperoxaluria causes an increase in the deposition of calcium oxalate crystals in the kidneys, thereby increasing rat’s kidney weight.[9] The calcium levels decreased when treated with ethanol extract of celery (A. graveolens L) and apigenin. The treatment with ethanol extract dose of 600 mg/kg BW and apigenin dose of 4.8 mg/kg BW as in Table 1 decreased the calcium levels compared to negative control. These results indicated that ethanol extract of celery (A. graveolens L) and apigenin treatment show improvement in renal function compared to Group II control negative, but statistically the result was not significant (P >0.05). The results of comparison of the analysis of renal weight ratio and calcium levels in the kidneys are present in Table 2.
Table 1.
Procedure test For Animal Groupings
| Apigenin group | Apigenin group | Extract celery group | Extract celery group |
|---|---|---|---|
| Group I | Normal control and rat not induced | Group I | Normal control and rat not induced |
| Group II | Negative control group, rats were induced and given 2% PGA suspension orally | Group II | Negative control group, rats were induced and given 2% PGA suspension orally |
| Group III | Positive control group, rats were induced and given batugin elixir orally | Group III | Positive control group, rats were induced and given batugin elixir orally |
| Group IV | Test group 1, rats were induced and given apigenin 1.2 mg/kg BW in PGA 2% suspension orally | Group IV | Test group 1, rats were induced and given celery extract dose 200 mg/kg BW in PGA 2% suspension orally |
| Group V | Test group 2, rats were induced and given apigenin 2.4 mg/kg BW in PGA 2% suspension orally | Group V | Test group 2, rats were induced and given celery extract dose 400 mg/kg BW in PGA 2% suspension orally |
| Group VI | Test group 3, rats were induced and given apigenin 4.8 mg/kg BW in PGA 2% suspension orally | Group VI | Test group 3, rats were induced and given celery extract dose 600 mg/kg BW in PGA 2% suspension orally |
Table 2.
Biochemical parameter results in kidney
| Apigenin Group I | Kidney weight ratio (g/100g) | Calcium level in kidney (ppm) | Celery extract Group II | Kidney weight ratio (g/100g) | Calcium level in kidney (ppm) |
|---|---|---|---|---|---|
| Normal | 0.65 ± 0.117 | 187.0037 ± 36.03 | Normal | 0.65 ± 0.117 | 187.0037 ± 36.03 |
| Negative control | 0.80 ± 0.065 | 249.09 ± 124.10 | Negative control | 0.80 ± 0.065 | 249.0948 ± 124.1 |
| Positive control | 0.72 ± 0.079 | 165.5952 ± 64.65 | Positive control | 0.72 ± 0.079 | 165.5952 ± 64.65 |
| Apigenin 1.2 mg/kg BW | 0.71 ± 71.33 | 306.25 ± 71.33 | Extract 200 mg/ kg BW | 0.7027 ± 0.0608 | 306.7706 ± 177.5 |
| Apigenin 2.4 mg/kg BW | 0.76 ± 90.12 | 297.89 ± 90.12 | Extract 400 mg/ kg BW | 0.7536 ± 0.0330 | 253.8042 ± 62.65 |
| Apigenin 4.8 mg/kg BW | 0.74 ± 41.28 | 187.66 ± 41.28 | Extract 600 mg/ kg BW | 0.7156 ± 0.0848 | 170.5245 ± 18.62 |
Values are mean ± standard deviation for rats in each group (n = 4)
Increased calcium levels in kidney tissue can be caused by increased bioavailability of nitric oxide (NO), which can activate cGMP (Cyclic guanosine monophosphate) and can control the increase of intracellular calcium levels. The literature shows that NO donors can control the increase in intracellular calcium levels.[10]
Effect on the urinary parameters in urine
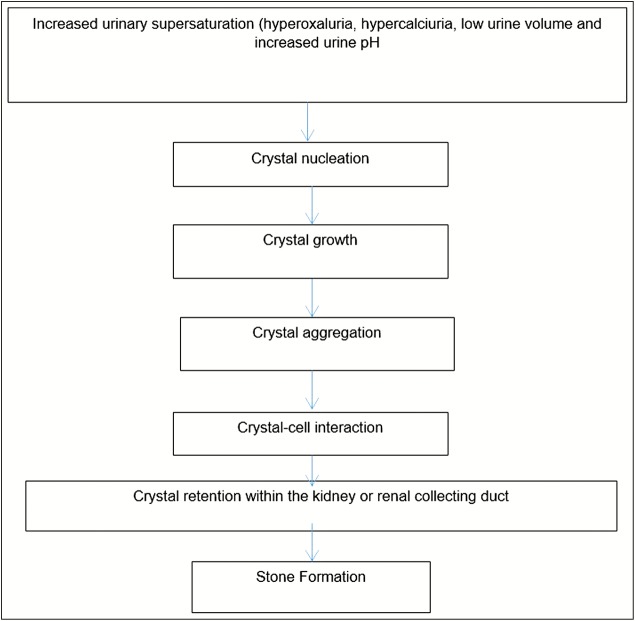
The formation of urinary tract stones is a result of increase in urinary supersaturation and the subsequent formation of crystalline materials. The mechanism of formation of crystalline particles in the urine is based on the thermodynamic state of the urine chemistry. The natural progression of the urine chemistry leading to stone development is urine saturation, urine supersaturation, crystal nucleation, aggregation, the retention of crystals by the urothelium, and the continued growth of the stone on the retained crystals.[11] Mechanism of kidney stone formation is shown in Figure 2.
Figure 2.
Mechanism of kidney stone formation
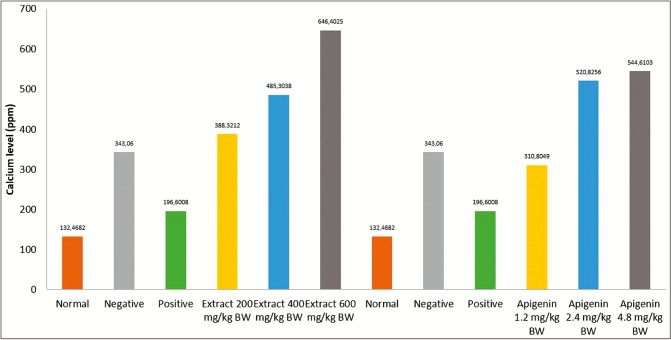
Celery extract at dose 600 mg/kg BW had calcium decay activity higher than other doses but still under batugin elixir drug as positive control like previous study,[12] meaning that compared to Apigenin, extract celery has higher calcium decay activity.
High doses of apigenin and celery extract have the best calcium decay activity compared to other test groups. The results of calcium levels in urine after administration of treatment are measured against various groups as shown in Table 3.
Table 3.
Average of calcium level in urine
| Groups | Calcium level (ppm) | Groups | Calcium level (ppm) |
|---|---|---|---|
| Normal | 132.46 ± 80.15 | Normal | 132.46 ± 80.15 |
| Negative control | 343.06 ± 259.08 | Negative control | 343.06 ± 259.08 |
| Positive control | 196.6 ± 43.79 | Positive control | 196.6 ± 43.79 |
| Apigenin, 1.2 mg/kg BW | 310.8049 ± 67.81 | EHS, 200 mg/kg BW | 388.3212 ± 99.54 |
| Apigenin, 2.4 mg/kg BW | 520.8256 ± 251.7 | EHS, 400 mg/kg BW | 485.3038 ± 294.8 |
| Apigenin, 4.8 mg/kg BW | 544.6103 ± 136.4 | EHS, 600 mg/kg BW | 646.4025 ± 215.6 |
Values are mean± standard deviation for rats in each group (n = 4), P < 0.05 values are significantly different compare to control groupEHS = Celery herbal extract.
As per Table 3, a significant difference was observed between the calcium levels in the urine of the normal control group compared to the group induced by ethylene glycol and ammonium chloride.
From Figures 3 and 4, it was observed that the apigenin test solution dose of 4.8 mg/kg BW and extract test solution dose of 600 mg/kg BW provide the best decay activity compared to the application of apigenin or extract at other doses. Apigenin and Celery extract have decay kidney stone activity. The decay activity of kidney stones by apigenin and celery extract on urine calcium levels increased with increasing dose of apigenin and extract given, statistically, the result showed a significant difference (P < 0.05) for calcium level in urine. Kidney stones decay activity of apigenin is believed to be related to its ability as an anti-inflammatory, antioxidant, and antimicrobial.[13] Factor to dissolve calcium oxalate is flavonoids,[14] and Apigenin was included flavonoid.
Figure 3.
Calcium level in kidney
Figure 4.
Calcium level in urine
Conclusion
Result from anticalculi test can be concluded that Apigenin and ethanol extract 96% of Celery (Apium graveolens L) has the activity as anticalculi. In vivo test result showed decreased calcium level in the kidneys on 0.75% ethylene glycol with 2% ammonium chloride–induced nephrolithiasis male Wistar rats.
Acknowledgement
We would like to thank the Chancellor of the Universitas Padjadjaran, Sumedang Indonesia for supporting the funding of this research through the Academic Leadership Grant (ALG) program and we would like to thank the minister of the ministry of research, technology and higher education for supporting the funding of this research through the doctoral grant 2018 program.
Financial support and sponsorship
This research was supported by Universitas Padjadjaran, Bandung Sumedang Indonesia and ministry of research, technology and higher education Indonesia.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chauhan CK, Joshi MJ. Growth inhibition of struvite crystals in the presence of juice of Citrus medica Linn. Urol Res. 2008;36:265–73. doi: 10.1007/s00240-008-0154-4. [DOI] [PubMed] [Google Scholar]
- 2.Purnomo BB. The Basic of Urology. 2nd ed. Jakarta, Indonesia: Sagung Seto; 2003. [Google Scholar]
- 3.Zhang H, Li N, Li K, Li P. Protective effect of Urtica dioica methanol extract against experimentally induced urinary calculi in rats. Mol Med Rep. 2014;10:3157–62. doi: 10.3892/mmr.2014.2610. [DOI] [PubMed] [Google Scholar]
- 4.Bahmani M, Baharvand-Ahmadi B, Tajeddini P, Rafieian-Kopaei M, Naghdi N. Identification of medicinal plants for the treatment of kidney and urinary stones. J Renal Inj Prev. 2016;5:129–33. doi: 10.15171/jrip.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Agency Of Drug and Food Control. Celery (Apium graveolens L.). The lates scientific data series on medicinal plant. Jakarta, Indonesia: National agency of drug and food control; 2007. [Google Scholar]
- 6.Dhianawaty D, Padmawinata K, Soediro I, Andreanus A, Soemardji. A, Soemardji. Isolation, Characterization and Activity anticalculi of apigenin 7-O-glukosida from Sonchus arvensis L. in rats by glycolic acid matrix method. J. Indonesian Natural Ingridients. 2004;3:162–4. [Google Scholar]
- 7.Dhianawaty D, Padmawinata K, Soediro I, Andreanus A, Soemardji Isolation, characterization and activity anticalculi of luteolin 7-O-glukosida from Sonchus arvensis L in rats by glycolic acid matrix method. Bionatura. 2003. p. 5. Available from: http://jurnal.unpad. ac.id/bionatura/article/view/5927 . [Last accessed on May, 16 2018].
- 8.Dinnimath BM, Jalalpure SS, Patil UK. Antiurolithiatic activity of natural constituents isolated from Aerva lanata. J Ayurveda Integr Med. 2017;8:226–32. doi: 10.1016/j.jaim.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin B, Feriz HM, Hariri AT, Meybodi NT, Hosseinzadeh H. Protective effects of the aqueous extract of Crocus sativus against ethylene glycol induced nephrolithiasis in rats. EXCLI J. 2015;14:411–22. doi: 10.17179/excli2014-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saha S, Verma RJ. Antinephrolithiatic and antioxidative efficacy of Dolichos biflorus seeds in a lithiasic rat model. J Pharm Biol. 2015;53:16–30. doi: 10.3109/13880209.2014.909501. [DOI] [PubMed] [Google Scholar]
- 11.Ratkalkar VN, Kleinman JG. Mechanisms of stone formation. Clin Rev Bone Miner Metab. 2011;9:187–97. doi: 10.1007/s12018-011-9104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anggraini T. Potency of citrus (Citrus aurantium) water as inhibitor calcium lithogenesis on urinary tract. J Major. 2015;4:99–104. [Google Scholar]
- 13.Sen P, Sahu PK, Haldar R, Sahu KK, Prasad P, Roy A. Apigenin naturally occurring flavonoids: occurrence and bioactivity. UK J Pharm Biosci. 2016;4:56–68. [Google Scholar]
- 14.Mentari aisyah. Antinephrolithiasis effect of ethanol extract of phaleria macrocarpa (Scheff.) Boerl in male Wistar Rat. J Inn Pharm Biosci (JIPBS) 2018;5:42–45. [Google Scholar]