Abstract
Introduction:
Drawing on the seminal work of DeLong, Albin, and Young, we have now entered an era of basal ganglia neuromodulation. Understanding, re-evaluating, and leveraging the lessons learned from neuromodulation will be crucial to facilitate an increased and improved application of neuromodulation in human disease cohorts.
Methods:
We will focus on deep brain stimulation (DBS) – the most common form of basal ganglia neuromodulation – however, similar principles can apply to other neuromodulation modalities. We start with a brief review of DBS for Parkinson’s disease, essential tremor, dystonia, and Tourette syndrome. We then review hallmark studies on basal ganglia circuits and electrophysiology resulting from decades of experience in neuromodulation. The organization and content of this paper follow Dr. Okun’s Lecture from the 2018 Parkinsonism and Related Disorders World Congress.
Results:
Information gained from neuromodulation has led to an expansion of the basal ganglia rate model, an enhanced understanding of nuclei dynamics, an emerging focus on pathological oscillations, a revision of the tripartite division of the basal ganglia, and a redirected focus toward individualized symptom-specific stimulation. Though there have been many limitations of the basal ganglia “box model,” the construct provided the necessary foundation to advance the field. We now understand that information in the basal ganglia is encoded through complex neural responses that can be reliably measured and used to infer disease states for clinical translation.
Conclusions:
Our deepened understanding of basal ganglia physiology will drive new neuromodulation strategies such as adaptive DBS or cell-specific neuromodulation through the use of optogenetics.
Keywords: basal ganglia, neuromodulation, deep brain stimulation, optogenetics
Introduction
Once considered a black box, the basal ganglia are becoming increasingly well-characterized through the application of clinical and research modalities. Consisting of both input and output nuclei, the basal ganglia receive information predominately through a corticostriatal pathway (Figure 1, see caption). The classic box-and-arrow models of the direct and indirect pathways derived by DeLong, Albin, and Young have provided a foundation for hypotheses and mechanistic explanations of basal ganglia function and dysfunction[1,2]. Stemming from this framework, the success of brain ablation studies and preliminary work applying electrical stimulation ultimately led the French neurosurgeon Alim Benabid to implant a lead within the thalamus to provide continuous delivery of electricity through a modern deep brain stimulation (DBS) system[3–5]. Basal ganglia neuromodulation is now established for the treatment of specific movement and neuropsychiatric disorders. In turn, this ongoing era of neuromodulation has expanded our understanding of basal ganglia circuitry and electrophysiology, and this has motivated improved neuromodulation techniques.
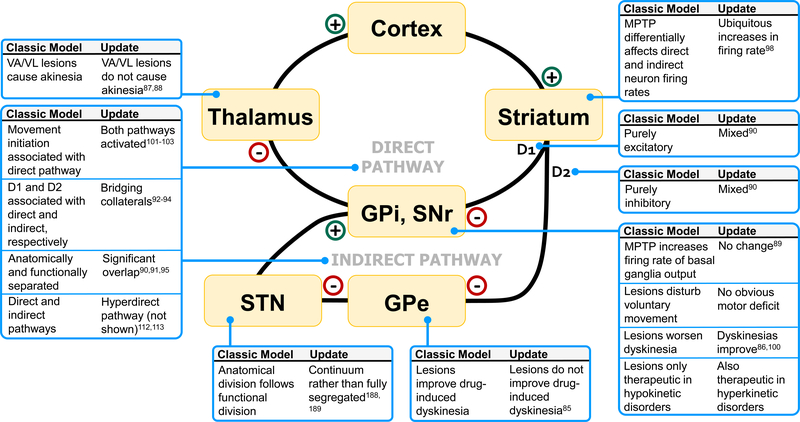
Figure 1. Limitations of the rate model.
GPi, globus pallidus internus; SNr, substantia nigra pars reticulata; GPe, globus pallidus externus; D, dopamine. Plus signs indicate glutamatergic projections and minus signs indicate GABAergic projections. Classic basal ganglia “box” models include a direct and indirect pathway. The direct and indirect pathways are classically associated with D1 and D2 receptors, respectively. The direct pathway disinhibits the thalamus via inhibition of the GPi through a direct striatal-pallidal-thalamic loop. In contrast, the indirect pathway sends inhibitory projections first to the GPe, leading to less inhibition of the STN, and subsequently more activation of the GPi. Firing rates can be measured along these pathways and are commonly used to investigate pathological under- or over-activity of these nuclei in specific diseases. Although the original rate model proved pivotal for motivating neurosurgical efforts in the treatment of basal ganglia diseases, recent observations have demonstrated its many limitations. Here, we depict a subset of examples, indicating for each what the classic model would predict and what new evidence has demonstrated.
In this paper, we will review data drawn from neuromodulation studies of Parkinson’s disease (PD), essential tremor (ET), dystonia, and Tourette syndrome (TS). The content will follow directly from the lecture given by Dr. Michael S. Okun at the 2018 International Association of Parkinsonism and Related Disorders World Congress in Lyon, France. Namely, we will recount notable lessons learned about basal ganglia neuromodulation, including lesioning and stimulation, from both clinical and research perspectives. These efforts have led to an expansion of the basal ganglia rate model, an enhanced understanding of nuclei dynamics beyond simple excitation and inhibition, an emerging focus on pathological oscillations, and a revision of the tripartite division of the basal ganglia. We will also address the upcoming era of patient-specific neuromodulation.
Neuromodulating basal ganglia pathologies
Currently, DBS and its experimental indications remain in early stages of development. Here, we briefly review four current and growing indications of DBS, namely PD, ET, dystonia, and TS, with focused background information relating the relevant basal ganglia pathology to underlying circuitry. We will also comment briefly on the surgical targets for these indications and their potential clinical advantages.
Parkinson’s disease
Though it is known that PD is underpinned by neurodegeneration of substantia nigra (SN) dopaminergic neurons and by widespread progressive brain pathology, dysfunction at the neural circuit level remains uncertain. In PD, there is decreased globus pallidus externus (GPe) activity and increased subthalamic nucleus (STN), globus pallidus internus (GPi), and SN pars reticulata (SNr) activity. Thus, PD symptoms conceptually arise from an aberrant increase of indirect pathway output via the GPe and a decrease in direct pathway activity via the GPi (Figure 1), in which both mechanisms lead to over-inhibition of the thalamus and an under-activation of the cortex.
To date, several controlled clinical trials have demonstrated that PD-DBS applied in either the STN or GPi can improve both motor symptoms and quality of life[6–10]. Some studies even suggest an improvement in non-motor symptoms (e.g. anxiety and pain)[11,12]. However, there can be appreciable differences in responses based on target choice. Though stimulating both targets similarly impact many of the primary motor outcomes, STN-DBS may lead to higher rates of adverse cognitive outcomes[13,14]. The STN is however the target associated with more medication reduction[14], whereas the GPi may be preferable for dyskinesia control and when long-term flexibility in medication management is required[13]. Both STN- and GPi-DBS are not effective in addressing motor symptoms unresponsive to dopaminergic therapies (with the exception of tremor), including voice, cognition, gait, and balance difficulties[13].
Other brain targets have been explored in PD, including the centromedian nucleus (CM)[15,16], the zona incerta (ZI)[17], the SN[18], and the pedunculopontine nucleus (PPN)[19]. The PPN is highly underactive in the PD state due to the increased inhibitory projections from the GPi, thus gait and balance problems may subside if this inhibitory outflow is interrupted[20]. The PPN in particular may be useful for axial motor symptoms[19,21], but further studies are warranted due to inconsistent results[21–23].
Essential Tremor
The pathophysiological basis of ET is emerging[24–26] and high-frequency stimulation (HFS) of the thalamus has been shown efficacious by several randomized trials[27–32]. The pathologic basis of ET has begun to emerge with post-mortem evidence supporting cerebellar degeneration[33–35] and with clinical studies demonstrating abnormal cerebellar function including tandem gait and balance problems[36,37]. One study reported brainstem Lewy bodies, especially within the locus coeruleus, where neurons synapse with Purkinje cells[33]. This reduced inhibition from Purkinje cells could result in increased disinhibition of deep cerebellar neurons, thus over-activating the cerebello-thalamo-cortical network.
Presumably to combat this over-activation, stimulation directed to the ventralis intermedius (VIM) nucleus of the thalamus (the cerebellar recipient nucleus[27,28]) is highly effective. Other targets such as the caudal ZI (cZI) or posterior subthalamic area (PSA) have also been investigated[38–44]. Stimulation to these areas are postulated to have similar or possibly more advantageous effects on proximal tremor. Results from retrospective studies that analyzed differences in outcomes among various targets have been mixed[45,46]. Recently, one prospective, randomized trial comparing the VIM and PSA found no difference in tremor control. However, this trial demonstrated that PSA-DBS had similar clinical efficacy with lower stimulation amplitudes, potentially lessening the chance for stimulation-induced adverse effects and the rare occurrence of tolerance[47]. Overall, there is a paucity of evidence suggesting superiority of either the VIM, PSA, or cZI, and larger, prospective trials will be needed. The main issue affecting the outcome of ET-DBS has been disease progression despite neuromodulation[48], which contrasts to what is observed in PD-DBS where tremor can be suppressed long-term[49].
Dystonia
Dystonia is a hyperkinetic movement disorder characterized by involuntary sustained or sporadic movements possibly arising from decreased cortical inhibition and increased long-term potentiation of synaptic plasticity within the motor cortex[50,51]. Studies in non-human primates have suggested that dystonia arises from increased inhibition of the STN and GPi by inputs from the GPe, leading to reduced inhibition to the thalamus and increased excitation to the cortex[52]. Corroborating these results are studies reporting reduced discharge rates from the pallidum [53–56] and hyperactivity of the basal ganglia’s direct pathway[57] in dystonia.
Although there are reports of STN- and thalamic-DBS for dystonia, the most common target has remained the GPi[58]; however, growing evidence suggests the STN may be equally suitable[59,60]. The sustained clinical effectiveness of DBS has been demonstrated most clearly in primary generalized and segmental dystonia[61–64], but other forms of dystonia have also been successfully treated with DBS[65–67]. Tardive dystonia specifically has a more rapid response to neuromodulation compared to other forms of dystonia where response is delayed and may evolve over weeks to months. Consequently, not all dystonia subtypes respond favorably to DBS and more research is needed to better characterize targets and applications of DBS into a largely heterogeneous population.
Tourette syndrome
Involuntary tics are thought to arise from reduced inhibition of the thalamocortical circuitry and excessive activity of frontocortical areas[68] as well as from changes in striosomes[69–71]. More specifically, research suggests that a specific set of striatal neurons may become pathologically active resulting in the inhibition of a specific set of GPi neurons. This excessive inhibition of the GPi leads to disinhibition of the thalamus and over-activation of the cortex, resulting in involuntary movement[72], similar in some respects to the mechanism reported in dystonia. Stemming from the success of ablative procedures, TS-DBS was introduced in 1999[73,74]. There are many comorbidities in TS patients and the optimal approach and target(s) for DBS remain unknown. Since most centers lack the volume to accomplish a randomized clinical trial, a recent international registry has been created to gather cases and outcomes[75].
Brain targets for neuromodulation have included the pallidum[76–78], the nucleus accumbens, the anterior limb of the internal capsule[79,80], the STN[81], and the centromedian thalamic region[74,82–84]. For every target explored, clinical evidence has demonstrated mixed results, but overall improvements in motor and vocal tics have been reported to be approximately 30–40%[85].
Moving beyond the rate model
Despite empirical support for the rate model in explaining the pathophysiology of these diseases, more recent neuromodulation studies have uncovered numerous paradoxes and limitations that have motivated further investigation (Figure 1). For instance, under the rate model, excessive and deficient thalamic output is associated with hyperkinesia and hypokinesia, respectively. It is therefore paradoxical that lesions of the GPi, leading to thalamic inhibition, could be therapeutic for PD dyskinesias[86,87] or dystonia and that thalamic ventral lateral and ventral anterior lesions actually do not cause akinesia[88,89]. Similarly, it would be expected that PD is associated with heightened activity in basal ganglia output nuclei[2], yet some 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) primate models have found no change in either GPi or SNr firing rates[90]. Furthermore, it is now known that there exists bi-directional and collateral connectivity within the basal ganglia, and in contrast to simplistic models, D1 and D2 receptors are not strictly associated with excitation and inhibition or even associated with the direct and indirect pathway, respectively[91–96].
Despite these observations, firing rate alone has helped explain disease pathology and subsequent lesion-induced disease improvement, where lesions act as a mean of inhibition. However, neuromodulation cannot be solely understood within the framework of the rate model, as it falls short when explaining complex patterns induced by stimulation (Figure 2). We now know that while stimulation and lesioning may lead to similar clinical outcomes, their underlying mechanisms likely differ. One example was a MPTP primate experiment which revealed that STN-DBS increased the firing rate of the GPi[97]. Another surprising result was observed during GPe stimulation in a MPTP primate model, which unexpectedly improved bradykinesia[98]. According to the rate model, if GPe was inhibited during HFS, PD symptoms would be worsened, which was observed in GPe lesional studies[98]. MPTP was also expected to differentially affect direct and indirect neuronal firing rates within the striatum, although, newer evidence suggests ubiquitous increases[99,100]. Similarly, the rate model does not directly explain several phenomena such as dyskinesia[86,87,101], akinesia[88,89], and movement initiation[102–104] (Figure 1).
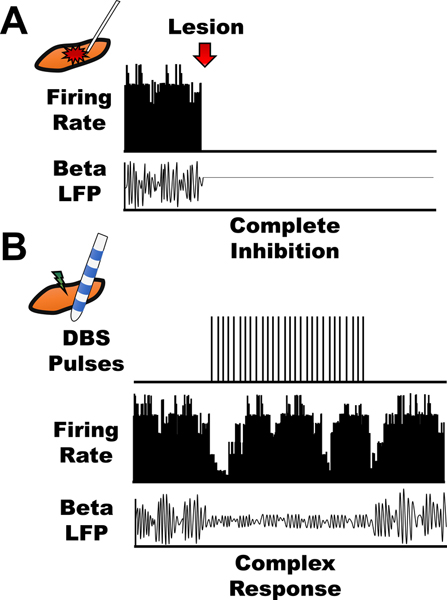
Figure 2. Lesion versus stimulation.
Once thought to be an informational lesion, we have now learned that stimulation causes complex neuronal responses within the brain, both in the stimulated and downstream nuclei. Lesions cause complete inhibition of the targeted nucleus, thus, causing a cessation of firing rate and local field potentials (LFPs) (A). In contrast, stimulation causes excitation, inhibition, or both during and after the stimulation pulses. Stimulation can also affect LFPs within target and distant areas. For example, in PD patients undergoing DBS, stimulation is known to cause a decrease in pathological beta oscillations throughout the basal ganglia (B).
Overall, we now know that firing rate alone cannot account for variability in outcomes witnessed during experimental and clinical DBS. Moving beyond the rate model, researchers have begun to examine patterns of stimulation and to employ more sophisticated electrophysiological measures (Figure 3).
Figure 3. From rate to rhythm.
Classic models of the basal ganglia were developed under the premise that neural information is encoded through firing rate extracted from single-cell traces (A). Increasingly, firing patterns, especially local field potential oscillations (B), are being characterized with meaningful clinical translation. For instance, a pathologically elevated beta rhythm can be extracted and used for closed-loop neuromodulation to drive dynamic and personalized stimulation approaches (C). Adapted from Eisinger et al., 2018. Front. Neurosci. - https://doi.org/10.3389/fnins.2018.00385 [119] with permission.
Neural responses: Excitation, inhibition, and temporal considerations
Rather than simply increasing or decreasing basal ganglia output, the therapeutic effect of neuromodulation may lie in more complex downstream or upstream effects. For example, STN-DBS was originally thought to inhibit STN output, but follow-up studies have demonstrated that HFS can drive output[97]. Still, cases have emerged that demonstrate a reduction in STN output during HFS, suggesting that the temporal sequence of modulation in firing rate could be an important consideration[105]. In fact, STN stimulation in PD primate models can elicit both excitatory and inhibitory effects on the pallidum at different time intervals following stimulation pulses[97,106].
Neural responses have also been examined in various disease models using GPi-DBS. For instance, GPi stimulation in MPTP primates caused an overall inhibition of thalamic neurons, thus increasing GPi output[107]. A separate study demonstrated a specificity of this phenomenon within high-firing rate neurons in the thalamus of a dystonia patient[108], while another study reported an increase in neuronal activity of the thalamus during the inter-stimulus interval of GPi-DBS in a patient with both dystonia and tremor[109]. Additionally, important temporal patterns, such as clustering within the inter-pulse interval, were observed in the spiking activity of neurons within the pallidum of a TS animal model[110]. It is suspected that this temporal locking phenomenon suppresses the transmission of aberrant information in the basal ganglia. GPi-DBS has been shown to induce both excitation and inhibition within the GPi and GPe[110,111], subsequently demonstrating the complex responses elicited from GPi-DBS. Complex excitation, inhibition and their related temporal sequence in the period following stimulation could be a result of bi-directional interactions between the STN and GPi and the neural pathways between the GPi and GPe[97,112].
Neuromodulation has thus taught us that both local and downstream nuclei can be differentially modulated. Beyond STN and GPi stimulation, studies have also shown mixed excitation and inhibition in the basal ganglia in response to cortical stimulation[112]. There has also been a resurgence of interest in examining upstream or retrograde effects as well, especially with regards to the hyperdirect pathway from the cortex to the STN[113–115]. Taken together, these findings have therefore added another layer of complexity to the basal ganglia network, suggesting that complex responses to stimulation likely propagate throughout the entire cortico-basal ganglia-thalamo-cortical loop.
Staying in rhythm: Oscillations of the basal ganglia
Beyond firing rate, experimental DBS studies have taught us that applied electrical current affects brain oscillations. Unlike studies of excitation and inhibition, brain oscillations are observed at the neuronal-population level[116] and can be measured through local field potentials (LFPs) recorded from DBS electrodes. These oscillations are defined by different frequency bands, namely delta (1.5–4 Hz), theta (4–10 Hz), beta (10–30 Hz), and gamma (30–80 Hz) oscillations[116]. Although oscillatory activity can coincide with firing rates, oscillatory activity results largely from subthreshold activity and depends heavily on cell geometry[117,118]. Oscillations may reflect states of cognitive, behavioral, and motor function[119–122], and some oscillations have been identified as pathological[123]. DBS surgery has afforded the opportunity to study these brain rhythms throughout the basal ganglia, particularly in response to neuromodulation. Many studies have also reported oscillations at distant sites outside the basal ganglia, as oscillations may be important for long-range brain communication[97,119,124–127].
Pathological oscillations are now being delineated from normal oscillations through extensive characterization across different diseases (Figure 4). For example, in PD patients off-medication, excessive beta rhythms and decreased gamma rhythms that correlate with disease severity have been observed in the basal ganglia and cortex[128,129] (Figure 4a). In these patients, dopaminergic treatment subsequently decreases pathologically elevated STN-GP beta coherence[126], and can shift this synchronization to the gamma range[128]. These beta oscillations have also been characterized by their location within the STN, with more occurring near the dorsolateral region[130,131]. In the case of dystonia patients, abnormally decreased GPi beta power and increased theta power have been observed[132], as well as excessive 3–18 Hz synchronization within the GPi during pathological spasms unlike voluntary and rest conditions[133] (Figure 4b). Pallidal peak theta activity has also been shown to significantly correlate with preoperative symptom severity in cervical dystonia subjects[134]. Conversely, studies have reported increased theta band activity at rest in dystonia patients[135]. Similarly, TS patients reportedly have increased activity within both the 2–7 Hz and 8–13 Hz band frequencies[136,137] (Figure 4c). One study found distinct peaks in theta from both pallidal and thalamic recordings that correlated with motor tic severity, as well as significant theta and beta coherence between oscillations of the thalamus and pallidum[138]. However, several case studies have reported increased beta synchrony as well as theta and alpha frequencies within the GPi of TS patients, suggesting that more work is needed to truly characterize LFPs in TS[139,140]. Overall, various disease-specific rhythms may be a key aspect of underlying pathology in the basal ganglia, but further investigation is needed.
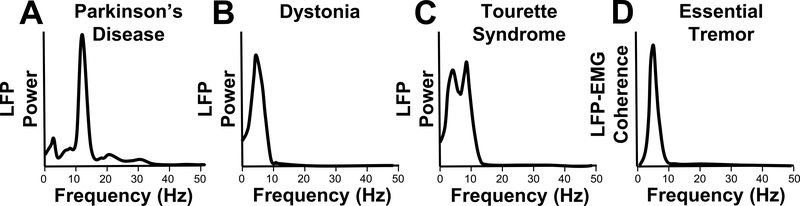
Figure 4. Pathological oscillations.
Pathological oscillations within and outside the basal ganglia can manifest in several ways. In PD, pathological oscillations are known to occur in the beta (11–30 Hz) frequency range (A) and have been recorded in several areas in the basal ganglia and even cortex. In dystonic patients, pathological oscillations mostly occur in the lower frequency range, especially theta (4–7 Hz). These oscillations have been recorded within the basal ganglia, including GPi, but also downstream in muscles during dystonic movements (B). Similarly, TS patients have increased activity in 2–7 Hz and in alpha (8–13 Hz) frequencies within the basal ganglia (C). In ET patients, oscillations within the thalamus and cortex have been coherent with peripheral tremor frequency (D). In most cases, pathological oscillations occur in low frequencies.
Apart from simple amplitude analysis, studies have also focused on the length of pathological bursts within oscillations. For instance, in PD patients longer duration beta bursts are significantly higher during levodopa off conditions, whereas shorter bursts are correlated with on levodopa periods[141]. Additionally, higher amounts of shorter bursts tend to be negatively correlated with clinical impairment and conversely, higher amounts of longer bursts are positively correlated[141,142]. In TS subjects, the length of theta bursts within the pallidum and thalamus significantly correlates with pre-operative tic severity scores, especially extended theta bursts[138].
Beyond the basal ganglia, pathological oscillations can also be linked to muscular activity through coherence analyses. In PD, significant coherence was calculated during rest tremor between the primary motor cortex (M1) in a magnetoencephalography (MEG) recording and electromyography (EMG), however, M1 MEG contributed to coherence observed at double the tremor frequency[143]. Similar results were observed in coherence analyses between STN-LFP and contralateral EMG in tremor dominant PD patients during tremor epochs[144]. In dystonia, both cortico-muscular and muscular-muscular coherence demonstrates pathological synchronization in lower frequency bands (4–12 Hz) during involuntary contractions[134,145–147]. Oscillatory patterns within ET patients usually manifest as increased power at peripheral tremor frequency (4–12 Hz). Additionally, cortico-muscular and thalamo-muscular coherence at tremor frequencies in ET patients have been extensively described[148–150] (Figure 4d). Such studies provide the basis for linking the central and peripheral nervous systems, ultimately contributing to our expanded understanding of disease pathology and our understanding of how to leverage these pathological correlates for next-generation, patient-specific neuromodulatory therapies.
Patient-specific neuromodulation
The diseases we modulate are heterogeneous, and thus in order to achieve the most clinically efficacious outcomes, our neuromodulation should be designed from the perspective of a precision medicine paradigm. Future neuromodulation therapies will need to focus on patient-specific approaches that are less disease-centric and more symptom-specific.
How do we stimulate?
An important area of next-generation neuromodulation is closed-loop or adaptive stimulation, which is a form of neuromodulation that relies on simultaneous sensing and stimulation (Figure 3C). Studies have tested whether pathological oscillations, recorded from either an implanted DBS lead, an electrocorticography strip, or from wearable sensors, can be utilized as the control signal for closed-loop algorithms. For instance, Little and colleagues first demonstrated in 8 PD patients that the power of the STN-LFP beta band was successful at controlling not only the timing but also the amplitude of DBS[151]. Additionally, Cagnan et al. used an acceleration signal recorded from the tremulous hand to deliver stimulation at specific phases within the tremor cycle. This concept was applied to DBS therapy to decouple tremor oscillators and it was proven effective in ET but not dystonic tremor patients[152]. Adaptive DBS has even been shown to have a selective effect on burst duration by shifting the phenomenon from longer to shorter bursts, whereas conventional DBS has not been shown to change the distribution of burst durations[142]. Other forms of closed-loop DBS are currently under investigation for a variety of indications, and if perfected, these techniques could possibly be more clinically efficacious than conventional DBS[153–158]. Ultimately, identifying pathological markers of diseases will provide an input for these algorithms, however it also remains unclear what the appropriate stimulation output during each indication (i.e., disease or symptom) should be.
Stimulation parameters (frequency, amplitude, pulse width) are an important consideration for optimizing outcomes. Different diseases and basal ganglia targets require tailored stimulation settings. As such, basic and clinical investigations have shown differential effects with varying frequencies. Liu and colleagues demonstrated that increasing frequency stimulation (LFS) of the GPi resulted in decreased neuronal activity within the nucleus, with HFS (> 50 Hz) silencing neuronal firing[159]. Depending on disease and symptoms, both LFS and HFS can be favorable. Pedrosa and colleagues reported a significant reduction in ET intention tremor during HFS compared to both LFS and to no stimulation conditions[160]. Although HFS has been shown useful for ET, LFS in ET results in entrainment effects rather than tremor suppression[160–163]. Similarly in PD, STN-LFS can exacerbate specific symptoms, especially akinesia[164]. However within the PPN, LFS is considered to be more clinically efficacious than HFS[19,165]. Additionally, STN-LFS has shown preliminary success in improving cognitive control in patients with PD[166]. On the contrary, the optimal stimulation parameters in dystonia have been even more variable. In cases of STN and GPi dystonia patients, HFS was more favorable[167,168], with LFS studies reporting exacerbation rather than suppression of symptoms[169]. However, LFS has been preferable in other cohorts and may be superior in younger DYT1 subjects[170–172]. Nonetheless, individualizing stimulation parameters will require substantial time, skill, and patience, and moving forward, the success of neuromodulation approaches will likely hinge on tailoring the therapy.
Another stimulation strategy is the use of reduced pulse widths, which can limit current spread and potentially offer a larger therapeutic window. In STN-DBS patients, the therapeutic window increased twofold when using a pulse width of 30 μsec compared to the standard setting of 60 μsec[173,174]. Additionally, shorter pulse widths required an increase in current delivered, but the total charge required for full control of rigidity decreased, suggesting that shorter pulse widths offered less risks of adverse effects and improved energy efficiency.
A more complex technique that has been explored is coordinated reset (CR), which involves brief, high frequency and low intensity stimulation through different contacts of the electrode to desynchronize pathological activity. Early studies with MPTP primate models demonstrated that low intensity CR-DBS had sustained after-effects on motor function, which were not elicited after CR with standard DBS intensity or classical DBS[175]. In the first human cohort, 6 STN-DBS PD patients underwent CR-DBS that resulted in cumulative after-effects on beta LFP oscillations, which were associated with an improvement in motor symptoms[176]. CR-DBS after-effects have been reported to persist up to two weeks after treatment cessation[177]. Although the results have been promising, larger clinical trials are needed.
Where do we stimulate?
The discovery that the basal ganglia is organized somatotopically based on behavioral functions — motor, associative, and limbic[178–181] — catapulted an era of clinical interventions for basal ganglia diseases (Figure 5). Support for this segregation is drawn from a vast body of evidence[97,182,183]. This tripartite model was an elegant demonstration of the possibility for precise neurosurgical targeting of specific dysfunctional pathways. It further offered explanations for specific symptoms experienced across a range of disorders. For instance, a functional map of the STN can be obtained using microelectrode recordings during DBS implantation surgeries in patients with PD. This mapping can differentially label cells associated with leg and arm movement. Specifically, the dorsolateroposterior area of the STN tends to be arm responsive, whereas the leg responsive area tends to be located more ventromedioanteriorly, supporting the further segregation of the sensorimotor region within these nuclei[184]. Somatotopic segregation has also been demonstrated using neuromodulation, such as one report describing hypomania resulting from anteromedial STN-DBS[185]. Another demonstration of the multifaceted nature of the STN and its numerous roles has been observed with neuropsychological studies of STN-DBS both in the on and off state. For example, impulsivity is a common non-motor effect of STN-DBS and is thought to stem from impaired decision-making through unintentional off-target neuromodulation[119,186]. These changes may underlie the onset of sub-clinical impulsivity or impulse control disorders observed after DBS[187]. Such observations motivate the need for further understanding of basic anatomy and physiology.
Figure 5. Towards a symptom-segregated model.
The tripartite, or segregated, model of the basal ganglia describes the anatomical separation of sensorimotor, limbic, and associative pathways. It is now recognized that the division of labor in the basal ganglia may not be so distinct and can instead be considered a continuum with overlapping boundaries. Nonetheless, there is now a shift towards a symptom-segregated model, in which specific brain targets at the millimeter or submillimeter scale are chosen to address specific predominant symptoms. Adapted from Eisinger et al., 2018. Front. Neurosci. - https://doi.org/10.3389/fnins.2018.00385 [119] with permission.
Recent studies have questioned this tripartite hypothesis and demonstrate that the parallel pathways within the basal ganglia may not be as segregated as once thought[188–190]. Some experts are now viewing these pathways as a continuum rather than entities with distinct borders[191,192]. Nevertheless, precise targeting and post-operative DBS lead localization with tractography-based imaging, combined with neuromodulation experiments, can provide vital information to guide therapy towards a symptom-segregated model (Figure 5). Within the dorsolateral portion of the STN, which is one optimal target for STN-DBS[193], further ideal targets could be potentially identified for specific symptoms. For instance, it appears that STN connectivity with primary motor cortex predicts tremor suppression, STN connectivity with supplementary motor cortex predicts improvement in bradykinesia and rigidity, and STN connectivity to the prefrontal cortex predicts improvement in rigidity[194]. As another example, one study showed that LFS through ventral contacts worsened intention tremor, but had little influence on postural tremor[160].
Even more selectivity can be found in specific cells within these nuclei. For instance, differential cell-specific modulations within the GPe induce similar responses: excitation of parvalbumin-GPe neurons and inhibition of Lim homeobox 6-GPe neurons reversed pathological SN burst firing in dopamine-depleted mice[195] (Figure 6), demonstrating the elegance and specificity in the structure of the basal ganglia. Such results have opened a new line of work, specifically optogenetically-inspired DBS. Conventional DBS generally has a non-specific mechanism of action, affecting both nearby and distant circuitry. Optogenetics enables the possibility for targeted neuromodulation of particular genetically-defined neurons that are engineered to express light-sensitive ion channels. Preliminary work demonstrates the clinical effectiveness of this stimulation modality as applied to animal models[195,196], but optogenetics remains largely a research tool. Through both research and clinical potential, we believe optogenetics could catapult the individualization of brain neuromodulation. In parallel, it will undoubtedly raise new and interesting challenges for the road ahead.
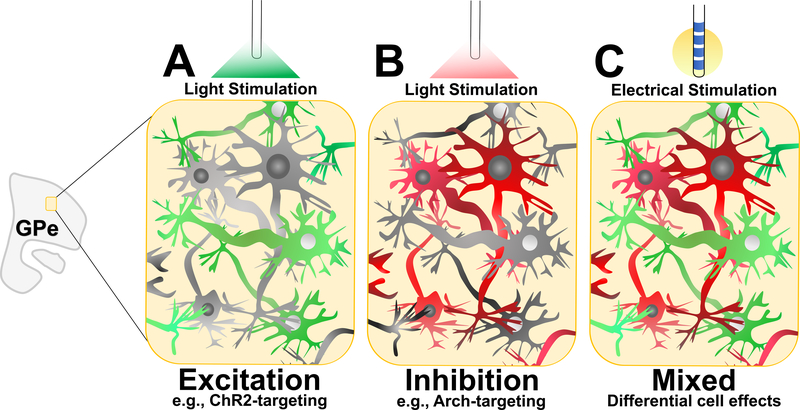
Figure 6. Optogenetically-inspired stimulation.
Cell-specific neuromodulation through optogenetics has inspired meticulous investigation of the basic mechanisms of neuromodulatory therapies. For instance, symptomatic control in PD animals can be achieved through excitation of parvalbumin-GPe neurons (A) and inhibition of Lim homeobox 6-GPe neurons (B), which can be optogenetically stimulated through ChR2 and Arch, respectively. Rather than being a non-specific stimulation modality, moving forward, new electrical stimulation approaches can aim to have specific intended differential effects at the cellular level (C).
Despite advanced understanding of nuclei somatotopy and cell content, clinical efficacy across a range of anatomical targets and diseases is still under active investigation. Within a target, current steering has also been employed to optimize therapy[197,198]. This approach can involve new DBS lead designs[199], multiple independent current sources for the DBS contacts[200], and novel pulse shapes[201,202]. Recently, current shaping studies have focused on modulating the pulse-width waveform. This type of modulation was proven to offer selective activation of specific neuronal populations in computational models, specifically with asymmetric, charged-balanced biphasic stimulation pulses[203,204]. Clinically, a square-biphasic waveform (active rather than passive charge-balancing phase) has been investigated in recent small trials[201,202,205]. Square-biphasic pulse widths were well tolerated in dystonia and tremor, suggesting that nonconventional waveforms would be successful in ameliorating pathological symptoms while potentially reducing adverse effects. Larger and longer clinical trials are needed to validate the efficacy. Subsequently, the ability to reprogram the firmware on the device will offer new treatment options to patients without the need for further surgery.
The era ahead: clinical considerations and lessons learned
The era of basal ganglia neuromodulation is at its genesis. While much has been learned about the brain through DBS studies, we have also learned that neuromodulation systems have yet to be fully optimized. Clinically, side effects remain a limiting factor in the success of DBS due to unintended and unspecific current spread[206,207]. Neuromodulation devices have not changed much since their introduction, and the near-term future will likely harness many hardware improvements along with more tailored stimulation paradigms. One-third of PD-DBS failures today result from inappropriate patient selection and half from suboptimal placement of the DBS lead[208]. Therefore, refining brain targets and target populations within each disease model will require further improvements.
We have learned that successful neuromodulation requires a multidisciplinary approach through joint efforts across multiple clinical, research and engineering specialties partnered with industry, federal representatives, and ethicists[209]. The overarching success of neuromodulation as a treatment has spurred a list of difficult questions that remain unanswered, especially those involving the underlying mechanisms of DBS. Additionally, the optimal stimulation targets and parameters will need refinement and DBS will likely evolve to be a more symptom-based rather than disease-based therapy. At the same time, advancing accuracy for neurosurgical targeting is critical[208,210]. Stimulation-related adverse effects are common to many DBS targets and may be overcome with higher fidelity targeting procedures[211,212]. As little as 1–2 millimeters of targeting error can be the difference between clinical success and failure[213]. Failure can translate into revision surgery or rescue leads.
Ultimately, surgery is only the first step in a long path towards optimizing therapy. Patients require meticulous and careful postoperative DBS programming, a process that continues for many months. This is a large undertaking that requires necessary infrastructure and clinician experience. Overall, neuromodulation needs to be a patient-specific, individualized therapy tailored to the target and to the symptoms. Successful neuromodulation demands careful examination of every patient with thoughtful consideration of the clinical syndrome, surgical risk factors, and long-term prognosis.
Conclusion
Recent observations of basal ganglia physiology have acted as a springboard for rapid advancement of neuromodulation. Not only have we soared beyond the rate model by analyzing the complex behavior of the basal ganglia circuitry, we have also characterized oscillation-level activity and are beginning to use it for tailored therapy. Initially thought of as an informational lesion, stimulation has profound impacts on basal ganglia physiology. Continuous updates of basal ganglia models will be necessary as the classical model has provided invaluable infrastructure but contains many inconsistencies. The expansion to neuropsychiatric conditions will require careful considerations of neuroethics, informed consent and regulatory oversight. The future is likely to facilitate neuromodulation spreading across a wide range of brain areas, and the many lessons learned from basal ganglia neuromodulation will undoubtedly enable these efforts.
Acknowledgements:
We would like to thank Jackson Cagle for helpful comments and suggestions and Leora Lieberman for assistance with figures. We thank the Parkinson’s Foundation Center of Excellence at the University of Florida, the Tourette Association of America Center of Excellence and Tyler’s Hope for a Dystonia Cure. This work is supported by the NIH/NCATS Clinical and Translational Science Awards to the University of Florida UL1TR001427, KL2TR001429, and TL1TR001428.
References
- [1].Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 1989;12:366–75. doi: 10.1016/0166-2236(89)90074-X. [DOI] [PubMed] [Google Scholar]
- [2].DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 1990;13:281–5. doi: 10.1016/0166-2236(90)90110-V. [DOI] [PubMed] [Google Scholar]
- [3].Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-Year Follow-up of Bilateral Stimulation of the Subthalamic Nucleus in Advanced Parkinson’s Disease. N Engl J Med 2003;349:1925–34. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- [4].Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol 1987;50:344–6. [DOI] [PubMed] [Google Scholar]
- [5].Okun MS. Deep-Brain Stimulation — Entering the Era of Human Neural-Network Modulation. N Engl J Med 2014;371:1369–73. doi: 10.1056/NEJMp1408779. [DOI] [PubMed] [Google Scholar]
- [6].Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, et al. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: The COMPARE Trial. Ann Neurol 2009;65:586–95. doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, et al. A Randomized Trial of Deep-Brain Stimulation for Parkinson’s Disease. N Engl J Med 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- [8].Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, et al. Bilateral Deep Brain Stimulation vs Best Medical Therapy for Patients With Advanced Parkinson Disease: A Randomized Controlled Trial. JAMA 2009;301:63. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol 2010;9:581–91. doi: 10.1016/S1474-4422(10)70093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schuepbach WMM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, et al. Neurostimulation for Parkinson’s Disease with Early Motor Complications. N Engl J Med 2013;368:610–22. doi: 10.1056/NEJMoa1205158. [DOI] [PubMed] [Google Scholar]
- [11].Witt K, Daniels C, Reiff J, Krack P, Volkmann J, Pinsker MO, et al. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study. Lancet Neurol 2008;7:605–14. doi: 10.1016/S1474-4422(08)70114-5. [DOI] [PubMed] [Google Scholar]
- [12].Witjas T, Kaphan E, Régis J, Jouve E, Chérif AA, Péragut J-C, et al. Effects of chronic subthalamic stimulation on nonmotor fluctuations in Parkinson’s disease. Mov Disord 2007;22:1729–34. doi: 10.1002/mds.21602. [DOI] [PubMed] [Google Scholar]
- [13].Ramirez-Zamora A, Ostrem JL. Globus Pallidus Interna or Subthalamic Nucleus Deep Brain Stimulation for Parkinson Disease. JAMA Neurol 2018;75:367. doi: 10.1001/jamaneurol.2017.4321. [DOI] [PubMed] [Google Scholar]
- [14].Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, et al. Pallidal versus Subthalamic Deep-Brain Stimulation for Parkinson’s Disease. N Engl J Med 2010;362:2077–91. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- [15].Peppe A, Gasbarra A, Stefani A, Chiavalon C, Pierantozzi M, Fermi E, et al. Deep brain stimulation of CM/PF of thalamus could be the new elective target for tremor in advanced Parkinson’s Disease? Parkinsonism Relat Disord 2008;14:501–4. doi: 10.1016/j.parkreldis.2007.11.005. [DOI] [PubMed] [Google Scholar]
- [16].Stefani A, Peppe A, Pierantozzi M, Galati S, Moschella V, Stanzione P, et al. Multi-target strategy for Parkinsonian patients: The role of deep brain stimulation in the centromedian–parafascicularis complex. Brain Res Bull 2009;78:113–8. doi: 10.1016/j.brainresbull.2008.08.007. [DOI] [PubMed] [Google Scholar]
- [17].Plaha P, Ben-Shlomo Y, Patel NK, Gill SS. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in improving contralateral parkinsonism. Brain 2006;129:1732–47. doi: 10.1093/brain/awl127. [DOI] [PubMed] [Google Scholar]
- [18].Scholten M, Klemt J, Heilbronn M, Plewnia C, Bloem BR, Bunjes F, et al. Effects of Subthalamic and Nigral Stimulation on Gait Kinematics in Parkinson’s Disease. Front Neurol 2017;8:543. doi: 10.3389/fneur.2017.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Plaha P, Gill SS. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson’s disease. Neuroreport 2005;16:1883–7. [DOI] [PubMed] [Google Scholar]
- [20].Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson’s disease. Brain 2000;123:1767–83. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]
- [21].Thevathasan W, Silburn PA, Brooker H, Coyne TJ, Khan S, Gill SS, et al. The impact of low-frequency stimulation of the pedunculopontine nucleus region on reaction time in parkinsonism. J Neurol Neurosurg Psychiatry 2010;81:1099–104. doi: 10.1136/jnnp.2009.189324. [DOI] [PubMed] [Google Scholar]
- [22].Ferraye MU, Debu B, Fraix V, Goetz L, Ardouin C, Yelnik J, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson’s disease. Brain 2010;133:205–14. doi: 10.1093/brain/awp229. [DOI] [PubMed] [Google Scholar]
- [23].Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain 2007;130:1596–607. doi: 10.1093/brain/awl346. [DOI] [PubMed] [Google Scholar]
- [24].Louis ED. Essential tremor then and now: How views of the most common tremor diathesis have changed over time. Parkinsonism Relat Disord 2018;46:S70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Louis ED. The evolving definition of essential tremor: What are we dealing with? Parkinsonism Relat Disord 2018;46:S87–91. doi: 10.1016/j.parkreldis.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Louis ED. Essential tremor and the cerebellum. Handb. Clin. Neurol., vol. 155, 2018, p. 245–58. doi: 10.1016/B978-0-444-64189-2.00016-0. [DOI] [PubMed] [Google Scholar]
- [27].Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet (London, England) 1991;337:403–6. [DOI] [PubMed] [Google Scholar]
- [28].Benabid AL, Pollak P, Gao D, Hoffmann D, Limousin P, Gay E, et al. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg 1996;84:203–14. doi: 10.3171/jns.1996.84.2.0203. [DOI] [PubMed] [Google Scholar]
- [29].Alesch F, Pinter MM, Helscher RJ, Fertl L, Benabid AL, Koos WT. Stimulation of the ventral intermediate thalamic nucleus in tremor dominated Parkinson’s disease and essential tremor. Acta Neurochir (Wien) 1995;136:75–81. [DOI] [PubMed] [Google Scholar]
- [30].Koller WC, Lyons KE, Wilkinson SB, Troster AI, Pahwa R. Long-term safety and efficacy of unilateral deep brain stimulation of the thalamus in essential tremor. Mov Disord 2001;16:464–8. [DOI] [PubMed] [Google Scholar]
- [31].Schuurman PR, Bosch DA, Bossuyt PMM, Bonsel GJ, van Someren EJW, de Bie RMA, et al. A Comparison of Continuous Thalamic Stimulation and Thalamotomy for Suppression of Severe Tremor. N Engl J Med 2000;342:461–8. doi: 10.1056/NEJM200002173420703. [DOI] [PubMed] [Google Scholar]
- [32].Pahwa R, Lyons KE, Wilkinson SB, Simpson RK, Ondo WG, Tarsy D, et al. Long-term evaluation of deep brain stimulation of the thalamus. J Neurosurg 2006;104:506–12. doi: 10.3171/jns.2006.104.4.506. [DOI] [PubMed] [Google Scholar]
- [33].Louis ED, Faust PL, Vonsattel J-PG, Honig LS, Rajput A, Robinson CA, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 2007;130:3297–307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- [34].Axelrad JE, Louis ED, Honig LS, Flores I, Ross GW, Pahwa R, et al. Reduced Purkinje Cell Number in Essential Tremor. Arch Neurol 2008;65:101–7. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Louis ED, Faust PL, Ma KJ, Yu M, Cortes E, Vonsattel J-PG. Torpedoes in the Cerebellar Vermis in Essential Tremor Cases vs. Controls. The Cerebellum 2011;10:812–9. doi: 10.1007/s12311-011-0291-0. [DOI] [PubMed] [Google Scholar]
- [36].Stolze H, Petersen G, Raethjen J, Wenzelburger R, Deuschl G. The gait disorder of advanced essential tremor. Brain 2001;124:2278–86. doi: 10.1093/brain/124.11.2278. [DOI] [PubMed] [Google Scholar]
- [37].Louis ED, Rios E, Rao AK. Tandem gait performance in essential tremor: clinical correlates and association with midline tremors. Mov Disord 2010;25:1633–8. doi: 10.1002/mds.23144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kitagawa M, Murata J, Kikuchi S, Sawamura Y, Saito H, Sasaki H, et al. Deep brain stimulation of subthalamic area for severe proximal tremor. Neurology 2000;55:114–6. doi: 10.1212/WNL.55.1.114. [DOI] [PubMed] [Google Scholar]
- [39].Murata J, Kitagawa M, Uesugi H, Saito H, Iwasaki Y, Kikuchi S, et al. Electrical stimulation of the posterior subthalamic area for the treatment of intractable proximal tremor. J Neurosurg 2003;99:708–15. doi: 10.3171/jns.2003.99.4.0708. [DOI] [PubMed] [Google Scholar]
- [40].Plaha P, Patel NK, Gill SS. Stimulation of the subthalamic region for essential tremor. J Neurosurg 2004;101:48–54. doi: 10.3171/jns.2004.101.1.0048. [DOI] [PubMed] [Google Scholar]
- [41].Blomstedt P, Sandvik U, Tisch S. Deep brain stimulation in the posterior subthalamic area in the treatment of essential tremor. Mov Disord 2010;25:1350–6. doi: 10.1002/mds.22758. [DOI] [PubMed] [Google Scholar]
- [42].Plaha P, Khan S, Gill SS. Bilateral stimulation of the caudal zona incerta nucleus for tremor control. J Neurol Neurosurg Psychiatry 2008;79:504–13. doi: 10.1136/jnnp.2006.112334. [DOI] [PubMed] [Google Scholar]
- [43].Plaha P, Javed S, Agombar D, O’ Farrell G, Khan S, Whone A, et al. Bilateral caudal zona incerta nucleus stimulation for essential tremor: outcome and quality of life. J Neurol Neurosurg Psychiatry 2011;82:899–904. doi: 10.1136/jnnp.2010.222992. [DOI] [PubMed] [Google Scholar]
- [44].Fytagoridis A, Sandvik U, Åström M, Bergenheim T, Blomstedt P. Long term follow-up of deep brain stimulation of the caudal zona incerta for essential tremor. J Neurol Neurosurg Psychiatry 2012;83:258–62. doi: 10.1136/jnnp-2011-300765. [DOI] [PubMed] [Google Scholar]
- [45].Eisinger RS, Wong J, Almeida L, Ramirez-Zamora A, Cagle JN, Giugni JC, et al. Ventral Intermediate Nucleus Versus Zona Incerta Region Deep Brain Stimulation in Essential Tremor. Mov Disord Clin Pract 2018;5:75–82. doi: 10.1002/mdc3.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sandvik U, Koskinen L-O, Lundquist A, Blomstedt P. Thalamic and Subthalamic Deep Brain Stimulation for Essential Tremor. Neurosurgery 2012;70:840–6. doi: 10.1227/NEU.0b013e318236a809. [DOI] [PubMed] [Google Scholar]
- [47].Barbe MT, Reker P, Hamacher S, Franklin J, Kraus D, Dembek TA, et al. DBS of the PSA and the VIM in essential tremor: A randomized, double-blind, crossover trial. Neurology 2018. doi: 10.1212/WNL.0000000000005956. [DOI] [PubMed] [Google Scholar]
- [48].Favilla CG, Ullman D, Wagle Shukla A, Foote KD, Jacobson CE, Okun MS. Worsening essential tremor following deep brain stimulation: disease progression versus tolerance. Brain 2012;135:1455–62. doi: 10.1093/brain/aws026. [DOI] [PubMed] [Google Scholar]
- [49].Wong JK, Cauraugh JH, Ho KWD, Broderick M, Ramirez-Zamora A, Almeida L, et al. STN vs. GPi deep brain stimulation for tremor suppression in Parkinson disease: A systematic review and meta-analysis. Parkinsonism Relat Disord 2018. doi: 10.1016/J.PARKRELDIS.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ruge D, Tisch S, Hariz MI, Zrinzo L, Bhatia KP, Quinn NP, et al. Deep brain stimulation effects in dystonia: Time course of electrophysiological changes in early treatment. Mov Disord 2011;26:1913–21. doi: 10.1002/mds.23731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hallett M Pathophysiology of dystonia. J Neural Transm Suppl 2006:485–8. [DOI] [PubMed] [Google Scholar]
- [52].Mitchell IJ, Luquin R, Boyce S, Clarke CE, Robertson RG, Sambrook MA, et al. Neural mechanisms of dystonia: Evidence from a 2-deoxyglucose uptake study in a primate model of dopamine agonist-induced dystonia. Mov Disord 1990;5:49–54. doi: 10.1002/mds.870050113. [DOI] [PubMed] [Google Scholar]
- [53].Nambu A, Chiken S, Shashidharan P, Nishibayashi H, Ogura M, Kakishita K, et al. Reduced Pallidal Output Causes Dystonia. Front Syst Neurosci 2011;5:89. doi: 10.3389/fnsys.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Starr PA, Rau GM, Davis V, Marks WJ, Ostrem JL, Simmons D, et al. Spontaneous Pallidal Neuronal Activity in Human Dystonia: Comparison With Parkinson’s Disease and Normal Macaque. J Neurophysiol 2005;93:3165–76. doi: 10.1152/jn.00971.2004. [DOI] [PubMed] [Google Scholar]
- [55].Tang JKH, Moro E, Mahant N, Hutchison WD, Lang AE, Lozano AM, et al. Neuronal Firing Rates and Patterns in the Globus Pallidus Internus of Patients With Cervical Dystonia Differ From Those With Parkinson’s Disease. J Neurophysiol 2007;98:720–9. doi: 10.1152/jn.01107.2006. [DOI] [PubMed] [Google Scholar]
- [56].Vitek JL, Chockkan V, Zhang JY, Kaneoke Y, Evatt M, DeLong MR, et al. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol 1999;46:22–35. [DOI] [PubMed] [Google Scholar]
- [57].Simonyan K, Cho H, Hamzehei Sichani A, Rubien-Thomas E, Hallett M. The direct basal ganglia pathway is hyperfunctional in focal dystonia. Brain 2017;140:3179–90. doi: 10.1093/brain/awx263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ostrem JL, Starr PA. Treatment of dystonia with deep brain stimulation. Neurotherapeutics 2008;5:320–30. doi: 10.1016/j.nurt.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ostrem JL, San Luciano M, Dodenhoff KA, Ziman N, Markun LC, Racine CA, et al. Subthalamic nucleus deep brain stimulation in isolated dystonia. Neurology 2017;88:25–35. doi: 10.1212/WNL.0000000000003451. [DOI] [PubMed] [Google Scholar]
- [60].Kleiner-Fisman G, Lin Liang GS, Moberg PJ, Ruocco AC, Hurtig HI, Baltuch GH, et al. Subthalamic nucleus deep brain stimulation for severe idiopathic dystonia: impact on severity, neuropsychological status, and quality of life. J Neurosurg 2007;107:29–36. doi: 10.3171/JNS-07/07/0029. [DOI] [PubMed] [Google Scholar]
- [61].Coubes P, Roubertie A, Vayssiere N, Hemm S, Echenne B. Treatment of DYT1-generalised dystonia by stimulation of the internal globus pallidus. Lancet 2000;355:2220–1. doi: 10.1016/S0140-6736(00)02410-7. [DOI] [PubMed] [Google Scholar]
- [62].Kupsch A, Benecke R, Müller J-UJ, Trottenberg T, Schneider G-H, Poewe W, et al. Pallidal Deep-Brain Stimulation in Primary Generalized or Segmental Dystonia. N Engl J Med 2006;355:1978–90. doi: 10.1056/NEJMoa063618. [DOI] [PubMed] [Google Scholar]
- [63].Vidailhet M, Vercueil L, Houeto J-L, Krystkowiak P, Lagrange C, Yelnik J, et al. Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol 2007;6:223–9. doi: 10.1016/S1474-4422(07)70035-2. [DOI] [PubMed] [Google Scholar]
- [64].Volkmann J, Wolters A, Kupsch A, Müller J, Kühn AA, Schneider G-H, et al. Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia: 5-year follow-up of a randomised trial. Lancet Neurol 2012;11:1029–38. doi: 10.1016/S1474-4422(12)70257-0. [DOI] [PubMed] [Google Scholar]
- [65].Trottenberg T, Volkmann J, Deuschl G, Kuhn AA, Schneider G-H, Muller J, et al. Treatment of severe tardive dystonia with pallidal deep brain stimulation. Neurology 2005;64:344–6. doi: 10.1212/01.WNL.0000149762.80932.55. [DOI] [PubMed] [Google Scholar]
- [66].Limotai N, Go C, Oyama G, Hwynn N, Zesiewicz T, Foote K, et al. Mixed results for GPi-DBS in the treatment of cranio-facial and cranio-cervical dystonia symptoms. J Neurol 2011;258:2069–74. doi: 10.1007/s00415-011-6075-0. [DOI] [PubMed] [Google Scholar]
- [67].Gruber D, Trottenberg T, Kivi A, Schoenecker T, Kopp UA, Hoffmann KT, et al. Long-term effects of pallidal deep brain stimulation in tardive dystonia. Neurology 2009;73:53–8. doi: 10.1212/WNL.0b013e3181aaea01. [DOI] [PubMed] [Google Scholar]
- [68].Mink JW. Basal ganglia dysfunction in Tourette’s syndrome: a new hypothesis. Pediatr Neurol 2001;25:190–8. [DOI] [PubMed] [Google Scholar]
- [69].Mink JW. Neurobiology of basal ganglia and Tourette syndrome: basal ganglia circuits and thalamocortical outputs. Adv Neurol 2006;99:89–98. [PubMed] [Google Scholar]
- [70].Leckman JF, Bloch MH, Smith ME, Larabi D, Hampson M. Neurobiological substrates of Tourette’s disorder. J Child Adolesc Psychopharmacol 2010;20:237–47. doi: 10.1089/cap.2009.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Leckman JF. Tourette’s syndrome. Lancet 2002;360:1577–86. doi: 10.1016/S0140-6736(02)11526-1. [DOI] [PubMed] [Google Scholar]
- [72].Mink JW. Basal ganglia dysfunction in Tourette’s syndrome: a new hypothesis. Pediatr Neurol 2001;25:190–8. doi: 10.1016/S0887-8994(01)00262-4. [DOI] [PubMed] [Google Scholar]
- [73].Hassler R, Dieckmann G. [Stereotaxic treatment of tics and inarticulate cries or coprolalia considered as motor obsessional phenomena in Gilles de la Tourette’s disease]. Rev Neurol (Paris) 1970;123:89–100. [PubMed] [Google Scholar]
- [74].Vandewalle V, van der Linden C, Groenewegen HJ, Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet (London, England) 1999;353:724. [DOI] [PubMed] [Google Scholar]
- [75].Martinez-Ramirez D, Jimenez-Shahed J, Leckman JF, Porta M, Servello D, Meng F-G, et al. Efficacy and Safety of Deep Brain Stimulation in Tourette Syndrome. JAMA Neurol 2018;75:353. doi: 10.1001/jamaneurol.2017.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cannon E, Silburn P, Coyne T, O’Maley K, Crawford JD, Sachdev PS. Deep Brain Stimulation of Anteromedial Globus Pallidus Interna for Severe Tourette’s Syndrome. Am J Psychiatry 2012;169:860–6. doi: 10.1176/appi.ajp.2012.11101583. [DOI] [PubMed] [Google Scholar]
- [77].Dehning S, Mehrkens J-H, Norbert Müller N, Bötzel K, Norbert Müller K, Bötzel K. Therapy-refractory tourette syndrome: Beneficial outcome with globus pallidus internus deep brain stimulation. Mov Disord 2008;23:1300–2. doi: 10.1002/mds.21930. [DOI] [PubMed] [Google Scholar]
- [78].Martínez-Fernández R, Zrinzo L, Aviles-Olmos I, Hariz M, Martinez-Torres I, Joyce E, et al. Deep brain stimulation for Gilles de la Tourette syndrome: A case series targeting subregions of the globus pallidus internus. Mov Disord 2011;26:1922–30. doi: 10.1002/mds.23734. [DOI] [PubMed] [Google Scholar]
- [79].Flaherty AW, Williams ZM, Amirnovin R, Kasper E, Rauch SL, Cosgrove GR, et al. Deep brain stimulation of the anterior internal capsule for the treatment of tourette syndrome: technical case report. NeurosurgeryONS 2005;57. doi: 10.1227/01.NEU.0000176854.24694.95. [DOI] [PubMed] [Google Scholar]
- [80].Kühn J, Lenartz D, Mai JK, Huff W, Lee S-H, Koulousakis A, et al. Deep brain stimulation of the nucleus accumbens and the internal capsule in therapeutically refractory Tourette-syndrome n.d. doi: 10.1007/s00415-006-0404-8. [DOI] [PubMed] [Google Scholar]
- [81].Martinez-Torres I, Hariz MI, Zrinzo L, Foltynie T, Limousin P. Improvement of tics after subthalamic nucleus deep brain stimulation. Neurology 2009;72:1787–9. doi: 10.1212/WNL.0b013e3181a60a0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Servello D, Porta M, Sassi M, Brambilla A, Robertson MM. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatry 2008;79:136–42. doi: 10.1136/jnnp.2006.104067. [DOI] [PubMed] [Google Scholar]
- [83].Porta M, Servello D, Zanaboni C, Anasetti F, Menghetti C, Sassi M, et al. Deep brain stimulation for treatment of refractory Tourette syndrome: long-term follow-up. Acta Neurochir (Wien) 2012;154:2029–41. doi: 10.1007/s00701-012-1497-8. [DOI] [PubMed] [Google Scholar]
- [84].Okun MS, Foote KD, Wu SS, Ward HE, Bowers D, Rodriguez RL, et al. A Trial of Scheduled Deep Brain Stimulation for Tourette Syndrome. JAMA Neurol 2013;70:85. doi: 10.1001/jamaneurol.2013.580. [DOI] [PubMed] [Google Scholar]
- [85].Baldermann JC, Schüller T, Huys D, Becker I, Timmermann L, Jessen F, et al. Deep Brain Stimulation for Tourette-Syndrome: A Systematic Review and Meta-Analysis. Brain Stimul 2016;9:296–304. doi: 10.1016/j.brs.2015.11.005. [DOI] [PubMed] [Google Scholar]
- [86].Laitinen L V, Bergenheim AT, Hariz MI. Leksell’s posteroventral pallidotomy in the treatment of Parkinson’s disease. J Neurosurg 1992;76:53–61. doi: 10.3171/jns.1992.76.1.0053. [DOI] [PubMed] [Google Scholar]
- [87].Munhoz RP, Cerasa A, Okun MS. Surgical treatment of dyskinesia in Parkinson’s disease. Front Neurol 2014;5:65. doi: 10.3389/fneur.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Burchiel KJ. Thalamotomy for movement disorders. Neurosurg Clin N Am 1995;6:55–71. [PubMed] [Google Scholar]
- [89].Canavan AG, Nixon PD, Passingham RE. Motor learning in monkeys (Macaca fascicularis) with lesions in motor thalamus. Exp Brain Res 1989;77:113–26. [DOI] [PubMed] [Google Scholar]
- [90].Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp Brain Res 1999;125:397–409. [DOI] [PubMed] [Google Scholar]
- [91].Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci 2014;17:1022–30. doi: 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- [92].Kress GJ, Yamawaki N, Wokosin DL, Wickersham IR, Shepherd GMG, Surmeier DJ. Convergent cortical innervation of striatal projection neurons. Nat Neurosci 2013;16:665–7. doi: 10.1038/nn.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Nadjar A, Brotchie JM, Guigoni C, Li Q, Zhou S-B, Wang G-J, et al. Phenotype of Striatofugal Medium Spiny Neurons in Parkinsonian and Dyskinetic Nonhuman Primates: A Call for a Reappraisal of the Functional Organization of the Basal Ganglia. J Neurosci 2006;26:8653–61. doi: 10.1523/JNEUROSCI.2582-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Cazorla M, de Carvalho FD, Chohan MO, Shegda M, Chuhma N, Rayport S, et al. Dopamine D2 Receptors Regulate the Anatomical and Functional Balance of Basal Ganglia Circuitry. Neuron 2014;81:153–64. doi: 10.1016/j.neuron.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Parent A, Sato F, Wu Y, Gauthier J, Lévesque M, Parent M. Organization of the basal ganglia: the importance of axonal collateralization. Trends Neurosci 2000;23:S20–7. doi: 10.1016/S1471-1931(00)00022-7. [DOI] [PubMed] [Google Scholar]
- [96].Lee HJ, Weitz AJ, Bernal-Casas D, Kravitz A V, Kreitzer AC, Hyung J, et al. Activation of Direct and Indirect Pathway Medium Spiny Neurons Drives Distinct Brain-wide Responses In Activation of Direct and Indirect Pathway Medium Spiny Neurons Drives Distinct Brain-wide Responses. Neuron 2016;91:412–24. doi: 10.1016/j.neuron.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci 2003;23:1916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Vitek JL, Zhang J, Hashimoto T, Russo GS, Baker KB. External pallidal stimulation improves parkinsonian motor signs and modulates neuronal activity throughout the basal ganglia thalamic network. Exp Neurol 2012;233:581–6. doi: 10.1016/j.expneurol.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Liang L, DeLong MR, Papa SM. Inversion of dopamine responses in striatal medium spiny neurons and involuntary movements. J Neurosci 2008;28:7537–47. doi: 10.1523/JNEUROSCI.1176-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Rothblat DS, Schneider JS. Response of caudate neurons to stimulation of intrinsic and peripheral afferents in normal, symptomatic, and recovered MPTP-treated cats. J Neurosci 1993;13:4372–8. doi: 10.1523/JNEUROSCI.13-10-04372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Blanchet PJ, Boucher R, Bédard PJ. Excitotoxic lateral pallidotomy does not relieve L-DOPA-induced dyskinesia in MPTP parkinsonian monkeys. Brain Res 1994;650:32–9. doi: 10.1016/0006-8993(94)90203-8. [DOI] [PubMed] [Google Scholar]
- [102].Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 2013;494:238–42. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Jin X, Tecuapetla F, Costa RM. Basal ganglia subcircuits distinctively encode the parsing and concatenation of action sequences. Nat Neurosci 2014;17:423–30. doi: 10.1038/nn.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Isomura Y, Takekawa T, Harukuni R, Handa T, Aizawa H, Takada M, et al. Reward-Modulated Motor Information in Identified Striatum Neurons. J Neurosci 2013;33:10209–20. doi: 10.1523/JNEUROSCI.0381-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Welter M-L, Houeto J-L, Bonnet A-M, Bejjani P-B, Mesnage V, Dormont D, et al. Effects of High-Frequency Stimulation on Subthalamic Neuronal Activity in Parkinsonian Patients. Arch Neurol 2004;61:89. doi: 10.1001/archneur.61.1.89. [DOI] [PubMed] [Google Scholar]
- [106].Bar-Gad I, Elias S, Vaadia E, Bergman H. Complex Locking Rather Than Complete Cessation of Neuronal Activity in the Globus Pallidus of a 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Treated Primate in Response to Pallidal Microstimulation. J Neurosci 2004;24:7410–9. doi: 10.1523/JNEUROSCI.1691-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Anderson ME, Postupna N, Ruffo M. Effects of High-Frequency Stimulation in the Internal Globus Pallidus on the Activity of Thalamic Neurons in the Awake Monkey. J Neurophysiol 2003;89:1150–60. doi: 10.1152/jn.00475.2002. [DOI] [PubMed] [Google Scholar]
- [108].Pralong E, Debatisse D, Maeder M, Vingerhoets F, Ghika J, Villemure J-G. Effect of deep brain stimulation of GPI on neuronal activity of the thalamic nucleus ventralis oralis in a dystonic patient. Neurophysiol Clin 2003;33:169–73. [DOI] [PubMed] [Google Scholar]
- [109].Montgomery E Jr Effects of GPi stimulation on human thalamic neuronal activity. Clin Neurophysiol 2006;117:2691–702. doi: 10.1016/j.clinph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- [110].McCairn KW, Iriki A, Isoda M. Deep Brain Stimulation Reduces Tic-Related Neural Activity via Temporal Locking with Stimulus Pulses. J Neurosci 2013;33:6581–93. doi: 10.1523/JNEUROSCI.4874-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Dostrovsky JO, Levy R, Wu JP, Hutchison WD, Tasker RR, Lozano AM. Microstimulation-Induced Inhibition of Neuronal Firing in Human Globus Pallidus. J Neurophysiol 2000;84:570–4. doi: 10.1152/jn.2000.84.1.570. [DOI] [PubMed] [Google Scholar]
- [112].Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, et al. Excitatory Cortical Inputs to Pallidal Neurons Via the Subthalamic Nucleus in the Monkey. J Neurophysiol 2000;84:289–300. doi: 10.1152/jn.2000.84.1.289. [DOI] [PubMed] [Google Scholar]
- [113].Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal “hyperdirect” pathway. Neurosci Res 2002;43:111–7. [DOI] [PubMed] [Google Scholar]
- [114].Monakow KH, Akert K, Künzle H. Projections of the precentral motor cortex and other cortical areas of the frontal lobe to the subthalamic nucleus in the monkey. Exp Brain Res 1978;33:395–403. [DOI] [PubMed] [Google Scholar]
- [115].Anderson RW, Farokhniaee A, Gunalan K, Howell B, McIntyre CC. Action potential initiation, propagation, and cortical invasion in the hyperdirect pathway during subthalamic deep brain stimulation. Brain Stimul 2018;11:1140–50. doi: 10.1016/j.brs.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Buzsaki G, Draguhn A. Neuronal Oscillations in Cortical Networks. Science (80-) 2004;304:1926–9. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- [117].Eccles JC. Interpretation of action potentials evoked in the cerebral cortex. Electroencephalogr Clin Neurophysiol 1951;3:449–64. [DOI] [PubMed] [Google Scholar]
- [118].Kühn AA, Trottenberg T, Kivi A, Kupsch A, Schneider G-H, Brown P. The relationship between local field potential and neuronal discharge in the subthalamic nucleus of patients with Parkinson’s disease. Exp Neurol 2005;194:212–20. doi: 10.1016/j.expneurol.2005.02.010. [DOI] [PubMed] [Google Scholar]
- [119].Eisinger RS, Urdaneta ME, Foote KD, Okun MS, Gunduz A. Non-motor Characterization of the Basal Ganglia: Evidence From Human and Non-human Primate Electrophysiology. Front Neurosci 2018;12:385. doi: 10.3389/fnins.2018.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 1996;273:1868–71. [DOI] [PubMed] [Google Scholar]
- [121].Tsodyks M, Kenet T, Grinvald A, Arieli A. Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science 1999;286:1943–6. [DOI] [PubMed] [Google Scholar]
- [122].Herculano-Houzel S, Munk MH, Neuenschwander S, Singer W. Precisely synchronized oscillatory firing patterns require electroencephalographic activation. J Neurosci 1999;19:3992–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kühn AA, Kempf F, Brucke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, et al. High-Frequency Stimulation of the Subthalamic Nucleus Suppresses Oscillatory Activity in Patients with Parkinson’s Disease in Parallel with Improvement in Motor Performance. J Neurosci 2008;28:6165–73. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Uhlhaas PJ, Singer W. Neural Synchrony in Brain Disorders: Relevance for Cognitive Dysfunctions and Pathophysiology. Neuron 2006;52:155–68. doi: 10.1016/J.NEURON.2006.09.020. [DOI] [PubMed] [Google Scholar]
- [125].Harris AZ, Gordon JA. Long-range neural synchrony in behavior. Annu Rev Neurosci 2015;38:171–94. doi: 10.1146/annurev-neuro-071714-034111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Brown P, Mazzone P, Oliviero A, Altibrandi MG, Pilato F, Tonali PA, et al. Effects of stimulation of the subthalamic area on oscillatory pallidal activity in Parkinson’s disease. Exp Neurol 2004;188:480–90. [DOI] [PubMed] [Google Scholar]
- [127].de Hemptinne C, Swann NC, Ostrem JL, Ryapolova-Webb ES, San Luciano M, Galifianakis NB, et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci 2015;18:779–86. doi: 10.1038/nn.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J Neurosci 2001;21:1033–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kühn AA, Tsui A, Aziz T, Ray N, Brücke C, Kupsch A, et al. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol 2009;215:380–7. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- [130].Accolla EA, Herrojo Ruiz M, Horn A, Schneider G-H, Schmitz-Hübsch T, Draganski B, et al. Brain networks modulated by subthalamic nucleus deep brain stimulation. Brain 2016;139:2503–15. doi: 10.1093/brain/aww182. [DOI] [PubMed] [Google Scholar]
- [131].Zaidel A, Spivak A, Grieb B, Bergman H, Israel Z. Subthalamic span of oscillations predicts deep brain stimulation efficacy for patients with Parkinson’s disease. Brain 2010;133:2007–21. doi: 10.1093/brain/awq144. [DOI] [PubMed] [Google Scholar]
- [132].Wang DD, de Hemptinne C, Miocinovic S, Ostrem JL, Galifianakis NB, San Luciano M, et al. Pallidal Deep-Brain Stimulation Disrupts Pallidal Beta Oscillations and Coherence with Primary Motor Cortex in Parkinson’s Disease. J Neurosci 2018;38:4556–68. doi: 10.1523/JNEUROSCI.0431-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Liu X, Wang S, Yianni J, Nandi D, Bain PG, Gregory R, et al. The sensory and motor representation of synchronized oscillations in the globus pallidus in patients with primary dystonia. Brain 2008;131:1562–73. doi: 10.1093/brain/awn083. [DOI] [PubMed] [Google Scholar]
- [134].Neumann W-J, Horn A, Ewert S, Huebl J, Brücke C, Slentz C, et al. A localized pallidal physiomarker in cervical dystonia. Ann Neurol 2017;82:912–24. doi: 10.1002/ana.25095. [DOI] [PubMed] [Google Scholar]
- [135].Barow E, Neumann W-J, Brücke C, Huebl J, Horn A, Brown P, et al. Deep brain stimulation suppresses pallidal low frequency activity in patients with phasic dystonic movements. Brain 2014;137:3012–24. doi: 10.1093/brain/awu258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Marceglia S, Servello D, Foffani G, Porta M, Sassi M, Mrakic-Sposta S, et al. Thalamic single-unit and local field potential activity in Tourette syndrome. Mov Disord 2010;25:300–8. doi: 10.1002/mds.22982. [DOI] [PubMed] [Google Scholar]
- [137].Bour LJ, Ackermans L, Foncke EMJ, Cath D, van der Linden C, Visser Vandewalle V, et al. Tic related local field potentials in the thalamus and the effect of deep brain stimulation in Tourette syndrome: Report of three cases. Clin Neurophysiol 2015;126:1578–88. doi: 10.1016/J.CLINPH.2014.10.217. [DOI] [PubMed] [Google Scholar]
- [138].Neumann W-J, Huebl J, Brücke C, Lofredi R, Horn A, Saryyeva A, et al. Pallidal and thalamic neural oscillatory patterns in tourette’s syndrome. Ann Neurol 2018;84:505–14. doi: 10.1002/ana.25311. [DOI] [PubMed] [Google Scholar]
- [139].Zauber SE, Ahn S, Worth RM, Rubchinsky LL. Oscillatory neural activity of anteromedial globus pallidus internus in Tourette syndrome. Clin Neurophysiol 2014;125:1923–4. doi: 10.1016/j.clinph.2014.01.003. [DOI] [PubMed] [Google Scholar]
- [140].Jimenez-Shahed J, Telkes I, Viswanathan A, Ince NF. GPi Oscillatory Activity Differentiates Tics from the Resting State, Voluntary Movements, and the Unmedicated Parkinsonian State. Front Neurosci 2016;10:436. doi: 10.3389/fnins.2016.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Tinkhauser G, Pogosyan A, Tan H, Herz DM, Kühn AA, Brown P. Beta burst dynamics in Parkinson’s disease OFF and ON dopaminergic medication. Brain 2017;140:2968–81. doi: 10.1093/brain/awx252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Tinkhauser G, Pogosyan A, Little S, Beudel M, Herz DM, Tan H, et al. The modulatory effect of adaptive deep brain stimulation on beta bursts in Parkinson’s disease. Brain 2017;140:1053–67. doi: 10.1093/brain/awx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Timmermann L, Gross J, Dirks M, Volkmann J, Freund H-J, Schnitzler A. The cerebral oscillatory network of parkinsonian resting tremor. Brain 2003;126:199–212. doi: 10.1093/brain/awg022. [DOI] [PubMed] [Google Scholar]
- [144].Florin E, Pfeifer J, Visser-Vandewalle V, Schnitzler A, Timmermann L. Parkinson subtype-specific Granger-causal coupling and coherence frequency in the subthalamic area. Neuroscience 2016;332:170–80. doi: 10.1016/J.NEUROSCIENCE.2016.06.052. [DOI] [PubMed] [Google Scholar]
- [145].Grosse P, Edwards M, Tijssen MAJ, Schrag A, Lees AJ, Bhatia KP, et al. Patterns of EMG-EMG coherence in limb dystonia. Mov Disord 2004;19:758–69. doi: 10.1002/mds.20075. [DOI] [PubMed] [Google Scholar]
- [146].Liu X, Griffin IC, Parkin SG, Miall RC, Rowe JG, Gregory RP, et al. Involvement of the medial pallidum in focal myoclonic dystonia: A clinical and neurophysiological case study. Mov Disord 2002;17:346–53. [DOI] [PubMed] [Google Scholar]
- [147].Tijssen MAJ, Münchau A, Marsden JF, Lees A, Bhatia KP, Brown P. Descending control of muscles in patients with cervical dystonia. Mov Disord 2002;17:493–500. doi: 10.1002/mds.10121. [DOI] [PubMed] [Google Scholar]
- [148].Raethjen J, Govindan RB, Kopper F, Muthuraman M, nther Deuschl G. Cortical involvement in the generation of essential tremor. J Neuro-Physiol 2007;97:3219–28. doi: 10.1152/jn.00477.2006. [DOI] [PubMed] [Google Scholar]
- [149].Pedrosa DJ, Quatuor E-L, Reck C, Pauls KAM, Huber CA, Visser-Vandewalle V, et al. Thalamomuscular Coherence in Essential Tremor: Hen or Egg in the Emergence of Tremor? J Neurosci 2014;34:14475–83. doi: 10.1523/JNEUROSCI.0087-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Marsden JF, Ashby P, Limousin-Dowsey P, Rothwell JC, Brown P. Coherence between cerebellar thalamus, cortex and muscle in man Cerebellar thalamus interactions. vol. 123 2000. [DOI] [PubMed] [Google Scholar]
- [151].Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol 2013;74:449–57. doi: 10.1002/ana.23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Cagnan H, Pedrosa D, Little S, Pogosyan A, Cheeran B, Aziz T, et al. Stimulating at the right time: phase-specific deep brain stimulation. Brain 2017;140:132–45. doi: 10.1093/brain/aww286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Shute JB, Okun MS, Opri E, Molina R, Rossi PJ, Martinez-Ramirez D, et al. Thalamocortical network activity enables chronic tic detection in humans with Tourette syndrome. NeuroImage Clin 2016;12:165–72. doi: 10.1016/j.nicl.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Swann NC, de Hemptinne C, Thompson MC, Miocinovic S, Miller AM, Gilron R, et al. Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J Neural Eng 2018;15:046006. doi: 10.1088/1741-2552/aabc9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Swann NC, de Hemptinne C, Miocinovic S, Qasim S, Wang SS, Ziman N, et al. Gamma Oscillations in the Hyperkinetic State Detected with Chronic Human Brain Recordings in Parkinson’s Disease. J Neurosci 2016;36:6445–58. doi: 10.1523/JNEUROSCI.1128-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Herron JA, Thompson MC, Brown T, Chizeck HJ, Ojemann JG, Ko AL. Chronic electrocorticography for sensing movement intention and closed-loop deep brain stimulation with wearable sensors in an essential tremor patient. J Neurosurg 2016:1–8. doi: 10.3171/2016.8.JNS16536. [DOI] [PubMed] [Google Scholar]
- [157].Malekmohammadi M, Herron J, Velisar A, Blumenfeld Z, Trager MH, Chizeck HJ, et al. Kinematic Adaptive Deep Brain Stimulation for Resting Tremor in Parkinson’s Disease. Mov Disord 2016;31:426–8. doi: 10.1002/mds.26482. [DOI] [PubMed] [Google Scholar]
- [158].Arlotti M, Marceglia S, Foffani G, Volkmann J, Lozano AM, Moro E, et al. Eight-hours adaptive deep brain stimulation in patients with Parkinson disease. Neurology 2018;90:e971–6. doi: 10.1212/WNL.0000000000005121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Liu LD, Prescott IA, Dostrovsky JO, Hodaie M, Lozano AM, Hutchison WD. Frequency-dependent effects of electrical stimulation in the globus pallidus of dystonia patients. J Neurophysiol 2012;108:5–17. doi: 10.1152/jn.00527.2011. [DOI] [PubMed] [Google Scholar]
- [160].Pedrosa DJ, Auth M, Eggers C, Timmermann L. Effects of low-frequency thalamic deep brain stimulation in essential tremor patients. Exp Neurol 2013;248:205–12. doi: 10.1016/j.expneurol.2013.06.009. [DOI] [PubMed] [Google Scholar]
- [161].Barnikol UB, Popovych O V, Hauptmann C, Sturm V, Freund H-J, Tass PA. Tremor entrainment by patterned low-frequency stimulation. Philos Trans R Soc A Math Phys Eng Sci 2008;366:3545–73. doi: 10.1098/rsta.2008.0104. [DOI] [PubMed] [Google Scholar]
- [162].Milosevic L, Kalia SK, Hodaie M, Lozano AM, Popovic MR, Hutchison WD. Physiological mechanisms of thalamic ventral intermediate nucleus stimulation for tremor suppression. Brain 2018. doi: 10.1093/brain/awy139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Constantoyannis C, Kumar A, Stoessl AJ, Honey CR. Tremor induced by thalamic deep brain stimulation in patients with complex regional facial pain. Mov Disord 2004;19:933–6. doi: 10.1002/mds.20047. [DOI] [PubMed] [Google Scholar]
- [164].Timmermann L, Wojtecki L, Gross J, Lehrke R, Voges J, Maarouf M, et al. Ten-Hertz stimulation of subthalamic nucleus deteriorates motor symptoms in Parkinson’s disease. Mov Disord 2004;19:1328–33. doi: 10.1002/mds.20198. [DOI] [PubMed] [Google Scholar]
- [165].Nosko D, Ferraye MU, Fraix V, Goetz L, Chabardès S, Pollak P, et al. Low-frequency versus high-frequency stimulation of the pedunculopontine nucleus area in Parkinson’s disease: a randomised controlled trial. J Neurol Neurosurg Psychiatry 2015;86:674–9. doi: 10.1136/jnnp-2013-307511. [DOI] [PubMed] [Google Scholar]
- [166].Scangos KW, Carter CS, Gurkoff G, Zhang L, Shahlaie K. A pilot study of subthalamic theta frequency deep brain stimulation for cognitive dysfunction in Parkinson’s disease. Brain Stimul 2018;11:456–8. doi: 10.1016/j.brs.2017.11.014. [DOI] [PubMed] [Google Scholar]
- [167].Ostrem JL, Markun LC, Glass GA, Racine CA, Volz MM, Heath SL, et al. Effect of frequency on subthalamic nucleus deep brain stimulation in primary dystonia. Parkinsonism Relat Disord 2014;20:432–8. doi: 10.1016/j.parkreldis.2013.12.012. [DOI] [PubMed] [Google Scholar]
- [168].Velez-Lago FM, Oyama G, Foote KD, Hwynn N, Zeilman P, Jacobson C, et al. Low-Frequency Deep Brain Stimulation for Dystonia: Lower is Not Always Better. Tremor Other Hyperkinet Mov (N Y) 2012;2. doi: 10.7916/D85X27PH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Kupsch A, Klaffke S, Kühn AA, Meissner W, Arnold G, Schneider GH, et al. The effects of frequency in pallidal deep brain stimulation for primary dystonia. J Neurol 2003;250:1201–5. doi: 10.1007/s00415-003-0179-0. [DOI] [PubMed] [Google Scholar]
- [170].Alterman RL, Miravite J, Weisz D, Shils JL, Bressman SB, Tagliati M. Sixty hertz pallidal deep brain stimulation for primary torsion dystonia. Neurology 2007;69:681–8. doi: 10.1212/01.wnl.0000267430.95106.ff. [DOI] [PubMed] [Google Scholar]
- [171].Sarva H, Miravite J, Swan MC, Deik A, Raymond D, Severt WL, et al. A Case of Myoclonus-Dystonia Responding to Low-frequency Pallidal Stimulation. Tremor Other Hyperkinet Mov (N Y) 2017;7:460. doi: 10.7916/D82Z1BS4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Alterman RL, Shils JL, Miravite J, Tagliati M. Lower stimulation frequency can enhance tolerability and efficacy of pallidal deep brain stimulation for dystonia. Mov Disord 2007;22:366–8. doi: 10.1002/mds.21274. [DOI] [PubMed] [Google Scholar]
- [173].Reich MM, Steigerwald F, Sawalhe AD, Reese R, Gunalan K, Johannes S, et al. Short pulse width widens the therapeutic window of subthalamic neurostimulation. Ann Clin Transl Neurol 2015;2:427–32. doi: 10.1002/acn3.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Steigerwald F, Timmermann L, Kühn A, Schnitzler A, Reich MM, Kirsch AD, et al. Pulse duration settings in subthalamic stimulation for Parkinson’s disease. Mov Disord 2018;33:165–9. doi: 10.1002/mds.27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Tass PA, Qin L, Hauptmann C, Dovero S, Bezard E, Boraud T, et al. Coordinated reset has sustained aftereffects in Parkinsonian monkeys. Ann Neurol 2012;72:816–20. doi: 10.1002/ana.23663. [DOI] [PubMed] [Google Scholar]
- [176].Adamchic I, Hauptmann C, Barnikol UB, Pawelczyk N, Popovych O, Barnikol TT, et al. Coordinated Reset Neuromodulation for Parkinson’s Disease: Proof-of-Concept Study n.d. doi: 10.1002/mds.25923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Wang J, Nebeck S, Muralidharan A, Johnson MD, Vitek JL, Baker KB. Coordinated Reset Deep Brain Stimulation of Subthalamic Nucleus Produces Long-Lasting, Dose-Dependent Motor Improvements in the 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine Non-Human Primate Model of Parkinsonism. Brain Stimul 2016;9:609–17. doi: 10.1016/J.BRS.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].DeLong MR. Activity of pallidal neurons during movement. J Neurophysiol 1971;34:414–27. doi: 10.1152/jn.1971.34.3.414. [DOI] [PubMed] [Google Scholar]
- [179].DeLong MR. Activity of basal ganglia neurons during movement. Brain Res 1972;40:127–35. [DOI] [PubMed] [Google Scholar]
- [180].DeLong MR, Georgopoulos AP, Crutcher MD. Cortico-basal ganglia relations and coding of motor performance. Exp Brain Res 1983;49:30–40. [Google Scholar]
- [181].Delong MR, Georgopoulos AP, Crutcher MD, Mitchell SJ, Richardson RT, Alexander GE. Functional organization of the basal ganglia: contributions of single-cell recording studies. Ciba Found Symp 1984;107:64–82. [DOI] [PubMed] [Google Scholar]
- [182].Sinclair NC, McDermott HJ, Bulluss KJ, Fallon JB, Perera T, Xu SS, et al. Subthalamic nucleus deep brain stimulation evokes resonant neural activity. Ann Neurol 2018;83:1027–31. doi: 10.1002/ana.25234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Haynes WIA, Haber SN. The Organization of Prefrontal-Subthalamic Inputs in Primates Provides an Anatomical Substrate for Both Functional Specificity and Integration: Implications for Basal Ganglia Models and Deep Brain Stimulation. J Neurosci 2013;33:4804–14. doi: 10.1523/JNEUROSCI.4674-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Romanelli P, Heit G, Hill BC, Kraus A, Hastie T, Brontë-Stewart HM. Microelectrode recording revealing a somatotopic body map in the subthalamic nucleus in humans with Parkinson disease. J Neurosurg 2004;100:611–8. doi: 10.3171/jns.2004.100.4.0611. [DOI] [PubMed] [Google Scholar]
- [185].Mallet L, Schupbach M, N’Diaye K, Remy P, Bardinet E, Czernecki V, et al. Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. Proc Natl Acad Sci 2007;104:10661–6. doi: 10.1073/pnas.0610849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Seymour B, Barbe M, Dayan P, Shiner T, Dolan R, Fink GR. Deep brain stimulation of the subthalamic nucleus modulates sensitivity to decision outcome value in Parkinson’s disease. Sci Rep 2016;6:32509. doi: 10.1038/srep32509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Rossi PJ, Gunduz A, Okun MS. The Subthalamic Nucleus, Limbic Function, and Impulse Control. Neuropsychol Rev 2015;25:398–410. doi: 10.1007/s11065-015-9306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Rektor I, Bareš M, Brázdil M, Kaňovský P, Rektorová I, Sochurková D, et al. Cognitive- and movement-related potentials recorded in the human basal ganglia. Mov Disord 2005;20:562–8. doi: 10.1002/mds.20368. [DOI] [PubMed] [Google Scholar]
- [189].Sieger T, Serranová T, Růžička F, Vostatek P, Wild J, Šťastná D, et al. Distinct populations of neurons respond to emotional valence and arousal in the human subthalamic nucleus. Proc Natl Acad Sci 2015;112:3116–21. doi: 10.1073/pnas.1410709112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Justin Rossi P, Peden C, Castellanos O, Foote KD, Gunduz A, Okun MS. The human subthalamic nucleus and globus pallidus internus differentially encode reward during action control. Hum Brain Mapp 2017;38:1952–64. doi: 10.1002/hbm.23496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Nambu A Somatotopic organization of the primate Basal Ganglia. Front Neuroanat 2011;5:26. doi: 10.3389/fnana.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Parent A, Coˆté P-Y, Lavoie B. Chemical anatomy of primate basal ganglia. Prog Neurobiol 1995;46:131–97. doi: 10.1016/0301-0082(95)80010-6. [DOI] [PubMed] [Google Scholar]
- [193].Herzog J, Fietzek U, Hamel W, Morsnowski A, Steigerwald F, Schrader B, et al. Most effective stimulation site in subthalamic deep brain stimulation for Parkinson’s disease. Mov Disord 2004;19:1050–4. doi: 10.1002/mds.20056. [DOI] [PubMed] [Google Scholar]
- [194].Akram H, Sotiropoulos SN, Jbabdi S, Georgiev D, Mahlknecht P, Hyam J, et al. Subthalamic deep brain stimulation sweet spots and hyperdirect cortical connectivity in Parkinson’s disease. Neuroimage 2017;158:332–45. doi: 10.1016/j.neuroimage.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Mastro KJ, Zitelli KT, Willard AM, Leblanc KH, Kravitz A V, Gittis AH. Cell-specific pallidal intervention induces long-lasting motor recovery in dopamine-depleted mice 2017;20. doi: 10.1038/nn.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Kravitz A V, Freeze BS, Parker PRL, Kay K, Thwin MT, Deisseroth K, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 2010;466:622–6. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Barbe MT, Dembek TA, Becker J, Raethjen J, Hartinger M, Meister IG, et al. Individualized current-shaping reduces DBS-induced dysarthria in patients with essential tremor. Neurology 2014;82:614–9. doi: 10.1212/WNL.0000000000000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Ramirez-Zamora A, Kahn M, Campbell J, DeLaCruz P, Pilitsis JG. Interleaved programming of subthalamic deep brain stimulation to avoid adverse effects and preserve motor benefit in Parkinson’s disease. J Neurol 2015;262:578–84. doi: 10.1007/s00415-014-7605-3. [DOI] [PubMed] [Google Scholar]
- [199].Steigerwald F, Müller L, Johannes S, Matthies C, Volkmann J. Directional deep brain stimulation of the subthalamic nucleus: A pilot study using a novel neurostimulation device. Mov Disord 2016;31:1240–3. doi: 10.1002/mds.26669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Valldeoriola F, Muñoz E, Rumià J, Roldán P, Cámara A, Compta Y, et al. Simultaneous low-frequency deep brain stimulation of the substantia nigra pars reticulata and high-frequency stimulation of the subthalamic nucleus to treat levodopa unresponsive freezing of gait in Parkinson’s disease: A pilot study. Parkinsonism Relat Disord 2018. doi: 10.1016/J.PARKRELDIS.2018.09.008. [DOI] [PubMed] [Google Scholar]
- [201].Almeida L, Martinez-Ramirez D, Ahmed B, Deeb W, De Jesus S, Skinner J, et al. A pilot trial of square biphasic pulse deep brain stimulation for dystonia: The BIP dystonia study. Mov Disord 2017. doi: 10.1002/mds.26906. [DOI] [PubMed] [Google Scholar]
- [202].De Jesus S, Almeida L, Shahgholi L, Martinez-Ramirez D, Roper J, Hass CJ, et al. Square biphasic pulse deep brain stimulation for essential tremor: The BiP tremor study. Park Relat Disord 2018;46:41–6. doi: 10.1016/j.parkreldis.2017.10.015. [DOI] [PubMed] [Google Scholar]
- [203].McIntyre CC, Grill WM. Extracellular Stimulation of Central Neurons: Influence of Stimulus Waveform and Frequency on Neuronal Output. J Neurophysiol 2002;88:1592–604. doi: 10.1152/jn.2002.88.4.1592. [DOI] [PubMed] [Google Scholar]
- [204].McIntyre CC, Grill WM. Selective Microstimulation of Central Nervous System Neurons. Ann Biomed Eng 2000;28:219–33. doi: 10.1114/1.262. [DOI] [PubMed] [Google Scholar]
- [205].Akbar U, Raike RS, Hack N, Hess CW, Skinner J, Martinez-Ramirez D, et al. Randomized, Blinded Pilot Testing of Nonconventional Stimulation Patterns and Shapes in Parkinson’s Disease and Essential Tremor: Evidence for Further Evaluating Narrow and Biphasic Pulses Neuromodulation: Technology at the Neural Interface 2016;19:343–56. doi: 10.1111/ner.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Anderson DN, Osting B, Vorwerk J, Dorval AD, Butson CR. Optimized programming algorithm for cylindrical and directional deep brain stimulation electrodes. J Neural Eng 2018;15:026005. doi: 10.1088/1741-2552/aaa14b. [DOI] [PubMed] [Google Scholar]
- [207].Butson CR, McIntyre CC. Current steering to control the volume of tissue activated during deep brain stimulation. Brain Stimul 2008;1:7–15. doi: 10.1016/j.brs.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Okun MS, Tagliati M, Pourfar M, Fernandez HH, Rodriguez RL, Alterman RL, et al. Management of Referred Deep Brain Stimulation Failures. Arch Neurol 2005;62:1250. doi: 10.1001/archneur.62.8.noc40425. [DOI] [PubMed] [Google Scholar]
- [209].Ramirez-Zamora A, Giordano JJ, Gunduz A, Brown P, Sanchez JC, Foote KD, et al. Evolving Applications, Technological Challenges and Future Opportunities in Neuromodulation: Proceedings of the Fifth Annual Deep Brain Stimulation Think Tank. Front Neurosci 2018;11:734. doi: 10.3389/fnins.2017.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Gross RE, McDougal ME. Technological advances in the surgical treatment of movement disorders. Curr Neurol Neurosci Rep 2013;13:371. doi: 10.1007/s11910-013-0371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Buhmann C, Huckhagel T, Engel K, Gulberti A, Hidding U, Poetter-Nerger M, et al. Adverse events in deep brain stimulation: A retrospective long-term analysis of neurological, psychiatric and other occurrences. PLoS One 2017;12:e0178984. doi: 10.1371/journal.pone.0178984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Patel DM, Walker HC, Brooks R, Omar N, Ditty B, Guthrie BL. Adverse Events Associated With Deep Brain Stimulation for Movement Disorders. Neurosurgery 2015;11 Suppl 2:1. doi: 10.1227/NEU.0000000000000659. [DOI] [PubMed] [Google Scholar]
- [213].Montgomery EB. Microelectrode targeting of the subthalamic nucleus for deep brain stimulation surgery. Mov Disord 2012;27:1387–91. doi: 10.1002/mds.25000. [DOI] [PubMed] [Google Scholar]