Abstract
The current study provides a comprehensive overview and analysis of the lignan literature. Data for the current study were extracted from the electronic Web of Science Core Collection database via the search string TOPIC = (“lignan*”) and processed by the VOSviewer software. The search yielded 10,742 publications. The ratio of original articles to reviews was 14.6:1. Over 80% of the analyzed papers have been published since the year 2000 and nearly 50% since the year 2010. Many of the publications were focused on pharmacology, chemistry, and plant sciences. The United States and Asian countries, such as China, Japan, South Korea, and India, were the most productive producers of lignan publications. Among the 5 most productive institutions was the University of Helsinki in Finland, the country that ranked 9th. Nineteen journals collectively published 3,607 lignan publications and were considered as core journals. Their impact factor did not correlate with the proportion of uncited papers. Highly cited publications usually mentioned phytoestrogen, isoflavone, daidzein, enterodiol, enterolactone, equol, genistein, and isoflavonoid. Cancer (e.g., breast cancer), cardiovascular disease, and antioxidation were the major themes. Clinical trials were estimated to contribute to 0.2–1.1% of the analyzed body of literature, so more of them should be conducted in the future to substantiate the beneficial effects and optimal dose of lignan intake in humans. Moreover, researchers can refer to these findings for future research directions and collaborations.
Keywords: lignans, pharmacology, chemistry, plant science, cancer, citation analysis, VOSviewer, Web of Science
Introduction
The current study aimed to perform a quantitative analysis on the literature of lignans to unveil the major contributors in terms of institutions, countries/regions, and journals. By analyzing the publication and citation data, the major research themes present in the lignan literature were identified and further discussed.
Lignans are 1,4-diarylbutan compounds derived from the shikimic acid biosynthetic pathway (Lewis and Davin, 1999; Imai et al., 2006). In the 1970s, it was still commonly believed that lignans were synthesized in plants only (Hartwell, 1976). It was only in the 1980s when scientists identified lignans produced by microbes living in humans and animals (Axelson et al., 1982). Geographically, the intakes are greater in the European population relative to the Asian population (Bhakta et al., 2006). The main common dietary lignans are secoisolariciresinol, lariciresinol, matairesinol, pinoresinol, medioresinol, and syringaresinol (Durazzo et al., 2018); the range of components is very wide and efforts on isolation of new compounds are being carried out (Eklund and Raitanen, 2019; Xiao et al., 2019). Plant lignans are metabolized to enterodiol and enterolactone, called enterolignans or mammalian lignans (Landete, 2012).
The recent work of Durazzo et al. (2018) well summarized the occurrence of lignans in food groups and existing lignan databases at European level. As reported by Durazzo et al. (2018), the main sources of dietary lignans are oilseeds such as flax, soy, rapeseed, and sesame; whole-grain cereals such as wheat, oats, rye, and barley; legumes; various vegetables and fruits (particularly berries); beverages (i.e., coffee, tea, and wine); and, recently, lignans are also determined in dairy products, meat, and fish (Valsta et al., 2003; Milder et al., 2005a; Milder et al., 2005b; Peñalvo et al., 2005; Thompson et al., 2006; Penalvo et al., 2007; Kuhnle et al., 2008a; Kuhnle et al., 2008b; Durazzo et al., 2009; Kuhnle et al., 2009a; Kuhnle et al., 2009b; Smeds et al., 2009; Moreno-Franco et al., 2011; Smeds et al., 2012; Durazzo et al., 2013a; Durazzo et al., 2013b; Mulligan et al., 2013; Durazzo et al., 2014b; Turfani et al., 2017; Angeloni et al., 2018; Angeloni et al., 2019).
Within the bioactive compounds realm (Santini et al., 2018; Santini and Novellino, 2018; Daliu et al., 2019; Durazzo et al., 2019), the class of lignans is of interest for their potential biological activities, i.e., estrogenic and antiestrogenic, antioxidant, anti-inflammatory, metabolism-modulating, anti-proliferative, and anticancerogenic properties (Baumgartner et al., 2011; Teponno et al., 2016; Wang et al., 2016; Linder et al., 2019; Zálešák et al., 2019). Moreover, it is worth mentioning that the spectrum of biological activities attributed to lignans is being enlarged, i.e., related to newly discovered compounds belonged to this group (Zhang et al., 2014; Gnabre et al., 2015; Su and Wink, 2015; Hongthong et al., 2016; Azam et al., 2019; Zhuang et al., 2019).
Several studies showed that consumption of lignan-rich diets, which contain vegetables, fruits, and whole grain products, may protect against chronic diseases, particularly hormone-dependent cancer and cardiovascular diseases (Ward et al., 2009; Peterson et al., 2010; Buck et al., 2011; Guglielmini et al., 2012; Penalvo and López-Romero, 2012; Zamora-Ros et al., 2012; Lowcock et al., 2013; Durazzo et al., 2014a; Rodríguez-García et al., 2019). Proper evaluation of adherence, efficacy, and communication aspects should be taken into account as well as the retrospective analysis of databases as per recent studies in the field (Iolascon et al., 2016; Scala et al., 2016; Guerriero et al., 2017; Menditto et al., 2018).
The overview presented in the current study should be helpful to readers in better understanding the lignan research community, identifying potential research directions and collaboration partners, and conducting more in-depth literature searches of chemicals/chemical classes of interest.
Materials and Methods
In July 2019, we queried the Web of Science (WoS) Core Collection online database, owned by Clarivate Analytics, to identify lignan publications with the following search string: TOPIC = (“lignan*”). This search identified publications mentioning the word “lignan” or its derivatives in the title, abstract, or keywords. No additional filters were placed on the search.
Data Extraction
Several aspects of each publication identified from the search were recorded, namely: (1) publication year; (2) institutions; (3) countries/regions of the institutions; (4) journal title; (5) WoS journal category; (6) type of publication; (7) language; and (8) number of total citations received. By using the “Export Records to File” function of WoS, full records and cited references of the identified publications were exported as “tab-delimited text files” to VOSviewer for additional processing.
The VOSviewer software (v.1.6.11, 2019) was used to analyze the titles and abstracts of publications, by breaking down the paragraphs into words and phrases, associating them with the citation data of the publications, and presenting the results in the form of a bubble map (Van Eck and Waltman, 2009). Default parameters were used for the analyses and visualizations. The size of a bubble represents the frequency of appearance of a term (multiple appearances of a word counted once, single use of the same word in a paper equally weighted). Two bubbles are positioned more closely to each other if the terms co-appeared more often in the analyzed publications. The color represents the averaged citations per publication (CPP). To simplify the bubble map, we analyzed and visualized words that appeared in at least 1% (n = 108) of the publications.
Apart from analyzing the whole dataset, we additionally probed into the articles published by the most prolific journals to see how many of them were uncited. According to Bradford's law of scattering, the core journals for a body of literature are defined as the prolific journals that collectively published 1/3 of the papers (Vickery, 1948). Using the current analyzed dataset, we tested if the core journals had their impact factor negatively correlated to the proportion of uncited papers, which was previously demonstrated in another field (Yeung, 2019). Pearson's correlation test was performed using SPSS 25.0 (IBM, New York, USA). Test results were significant if p < 0.05.
Results
The literature search resulted in 10,742 publications. The earliest publications on lignans indexed in WoS were published in 1970, which isolated new lignans at that time and identified their structures (Corrie et al., 1970). Over 80% of the analyzed papers have been published since the year 2000, and nearly 50% since the year 2010. The numbers of original articles (n = 9,422) and reviews (n = 644) were in the ratio of 14.6:1. Reviews were more cited (CPP = 56.8) than original articles (CPP = 22.5). The majority of the publications were written in English (n = 10,483; 97.6%). Contributions came from 4,748 institutions located in 141 countries/regions and were published in 1,509 journals. The top five contributors with regard to WoS category, journal, institution, and country/region are listed in Table 1. It is worth mentioning that Molecules was the 6th most productive journal, with 208 lignan publications (1.9%) and CPP of 11.9. Nineteen journals collectively published 3,607 lignan publications and were considered as core journals (Table 2). Their impact factor did not correlate with the proportion of uncited papers (r = -0.257, p = 0.289). Though University of Helsinki was among the top 5 most productive institutions, Finland was ranked 9th in terms of countries/regions (n = 437, 4.1%). The 5 most productive countries were all from Asia, except the United States.
Table 1.
The top five contributors in terms of Web of Science category, journal, institution, and country/region of publications concerning lignans.
| Contributor | Publication count (% of total) | Citation per manuscript |
|---|---|---|
| Web of Science category | ||
| Pharmacology pharmacy | 2,522 (23.5%) | 20.5 |
| Chemistry medicinal | 2,520 (23.5%) | 18.9 |
| Plant sciences | 2,050 (19.1%) | 22.9 |
| Biochemistry molecular biology | 1,796 (16.7%) | 24.8 |
| Chemistry multidisciplinary | 1,509 (14.0%) | 16.3 |
| Journal | ||
| Phytochemistry | 587 (5.5%) | 31 |
| Journal of Natural Products | 345 (3.2%) | 27.1 |
| Planta Medica | 332 (3.1%) | 18.9 |
| Chemical and Pharmaceutical Bulletin | 266 (2.5%) | 28.4 |
| Journal of Agricultural and Food Chemistry | 213 (2.0%) | 54.5 |
| Organization | ||
| Chinese Academy of Sciences | 473 (4.4%) | 13.8 |
| University of Helsinki | 246 (2.3%) | 81.9 |
| Chinese Academy of Medical Sciences Peking Union Medical College | 195 (1.8%) | 14.0 |
| Kunming Institute of Botany | 193 (1.8%) | 12.9 |
| Universidade de Sao Paulo | 186 (1.7%) | 18.1 |
| Country/Territory | ||
| China | 2,482 (23.1%) | 13.0 |
| United States | 1,321 (12.3%) | 41.2 |
| Japan | 1,305 (12.1%) | 24.5 |
| South Korea | 691 (6.4%) | 15.5 |
| India | 638 (5.9%) | 16.5 |
Table 2.
Core journals publishing lignan papers.
| Journal | Impact factor | Proportion of uncited lignan papers (in %) |
|---|---|---|
| Phytochemistry | 2.905 | 1.5 |
| Journal of Natural Products | 4.257 | 1.4 |
| Planta Medica | 2.746 | 17.8 |
| Chemical & Pharmaceutical Bulletin | 1.405 | 0.8 |
| Journal of Agricultural and Food Chemistry | 3.571 | 3.8 |
| Molecules | 3.060 | 15.9 |
| Tetrahedron Letters | 2.259 | 1.1 |
| Tetrahedron | 2.379 | 1.8 |
| Natural Product Research | 1.999 | 13.7 |
| Journal of Organic Chemistry | 4.745 | 3.4 |
| Fitoterapia | 2.431 | 13.4 |
| Biochemical Systematics and Ecology | 1.127 | 12.1 |
| Journal of Ethnopharmacology | 3.414 | 2.5 |
| Journal of Asian Natural Products Research | 1.170 | 7.1 |
| Bioorganic & Medicinal Chemistry Letters | 2.448 | 2.8 |
| Food Chemistry | 5.399 | 4.8 |
| Phytochemistry Letters | 1.338 | 15.8 |
| Bioscience, Biotechnology, and Biochemistry | 1.297 | 3.2 |
| Archives of Pharmacal Research | 2.458 | 5.4 |
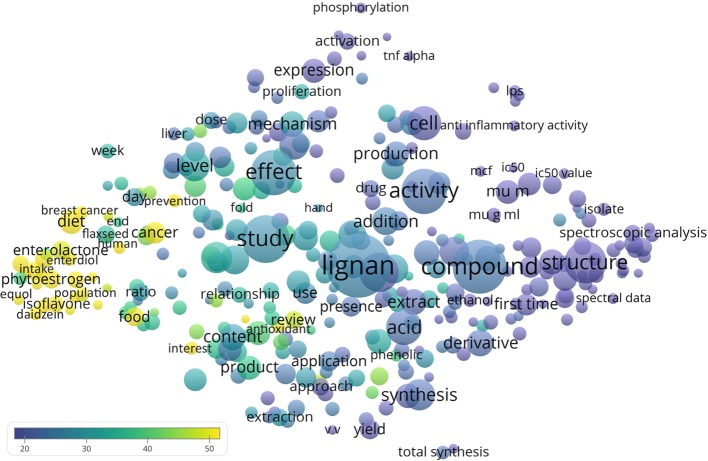
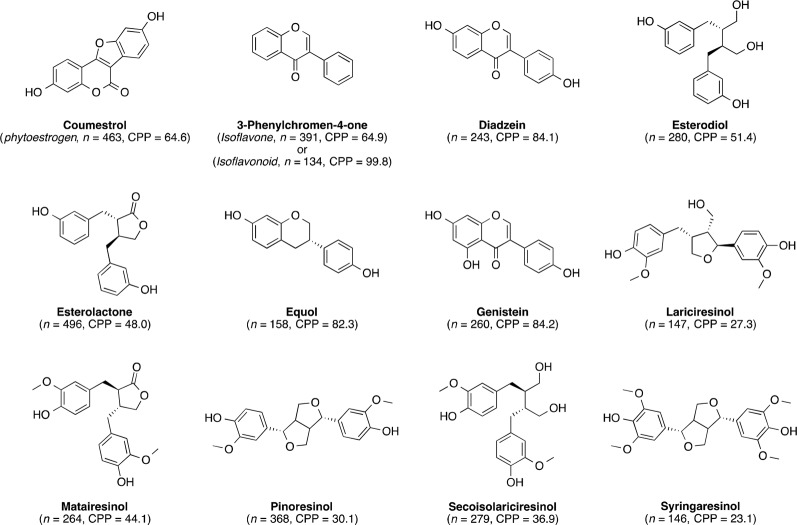
There were 311 terms that appeared in at least 1% (n = 108) of the 10,742 lignan publications (Figure 1). The highly cited publications usually mentioned phytoestrogen (4.3%, n = 463, CPP = 64.6), isoflavone (3.6%, n = 391, CPP = 64.9), or related terms such as daidzein (2.3%, n = 243, CPP = 84.1), enterodiol (2.6%, n = 280, CPP = 51.4), enterolactone (4.6%, n = 496, CPP = 48.0), equol (1.5%, n = 158, CPP = 82.3), genistein (2.4%, n = 260, CPP = 84.2), and isoflavonoid (1.2%, n = 134, CPP = 99.8). These terms were often mentioned together with cancer (6.1%, n = 655, CPP = 51.8), breast cancer (2.2%, n = 237, CPP = 51.8), or cardiovascular disease (1.5%, n = 157, CPP = 61.9). Some of the main common dietary lignans were frequently mentioned, such as lariciresinol (1.4%, n = 147; CPP = 27.3), matairesinol (2.5%, n = 264; CPP = 44.1), pinoresinol (3.4%, n = 368; CPP = 30.1), secoisolariciresinol (2.6%, n = 279; CPP = 36.9), syringaresinol (1.4%, n = 146; CPP = 23.1). The structures of these chemicals are shown in Figure 2. The top 20 recurring terms are listed in Table 3.
Figure 1.
Bubble map visualizing words from titles and abstracts of the 10,742 lignan publications. VOSviewer software was used to evaluate the recurring terms. Only the 311 terms that appeared in at least 1% (n = 108) of the publications were analyzed and visualized. The size of a bubble represents the frequency of appearance of a term (multiple appearances within one publication were treated as one appearance). Two bubbles are positioned more closely to each other if the terms co-appeared more often. The color represents the averaged citations per publication.
Figure 2.
Chemical structures of key single phytochemicals or representatives of chemical classes that were often discussed in the evaluated lignan publications. In parentheses are the cited compound classes (italic), number of publications (n), and citations per publication (CPP) for each chemical or representative chemical class.
Table 3.
The top 20 recurring terms from titles and abstracts.
| Term | Appearance (% of 10,742 publications) |
|---|---|
| Lignan | 5,700 (53.1%) |
| Compound | 3,995 (37.2%) |
| Study | 3,120 (29.0%) |
| Effect | 2,878 (26.8%) |
| Activity | 2,843 (26.5%) |
| Structure | 2,443 (22.7%) |
| Analysis | 2,121 (19.7%) |
| Acid | 1,768 (16.5%) |
| Cell | 1,629 (15.2%) |
| Level | 1,367 (12.7%) |
| Concentration | 1,365 (12.7%) |
| Synthesis | 1,347 (12.5%) |
| Treatment | 1,217 (11.3%) |
| Value | 1,197 (11.1%) |
| Group | 1,186 (11.0%) |
| Plant | 1,144 (10.6%) |
| Addition | 1,085 (10.1%) |
| Data | 1,065 (9.9%) |
| Derivative | 974 (9.1%) |
| Content | 949 (8.8%) |
The keywords listed by authors and WoS (KeyWords Plus) were collectively analyzed. There were 88 keywords that appeared in at least 1% (n = 108) of the lignan publications, and the 20 most common ones are listed in Table 4. The keywords suggested that antioxidation (4.3%) and apoptosis (3.2%) were two frequently investigated themes, and that in vitro (5.0%) studies were prevalent.
Table 4.
The top 20 recurring keywords.
| Keyword | Occurrence (% of 10,742 publications) |
|---|---|
| Lignans | 3,825 (35.6%) |
| Lignan | 1,358 (12.6%) |
| Constituents | 1,217 (11.3%) |
| Derivatives | 536 (5.0%) |
| Neolignans | 536 (5.0%) |
| In-vitro | 535 (5.0%) |
| Flavonoids | 509 (4.7%) |
| Phytoestrogens | 488 (4.5%) |
| Antioxidant activity | 458 (4.3%) |
| Identification | 438 (4.1%) |
| Cells | 435 (4.0%) |
| Leaves | 433 (4.0%) |
| Antioxidant | 399 (3.7%) |
| Glycosides | 388 (3.6%) |
| Flaxseed | 383 (3.6%) |
| Inhibition | 366 (3.4%) |
| Enterolactone | 347 (3.2%) |
| Apoptosis | 345 (3.2%) |
| Acid | 344 (3.2%) |
| Expression | 334 (3.1%) |
To analyze the temporal changes in the keywords, we separately assessed lignan publications in three time periods: 1990s and before, 2000s, and 2010s. The top 20 recurring keywords for each of the three periods are listed in Table 5. Antioxidant activity rose to popularity since the 2000s. Apoptosis, cytotoxicity, and oxidative stress became popular in the 2010s.
Table 5.
The top 20 recurring keywords in each decade.
| 1990s and before | Occurrence (% of 2,144) | 2000s | Occurrence (% of 3,312) |
2010s | Occurrence (% of 5,295) |
|---|---|---|---|---|---|
| Lignans | 593 (27.7) | Lignans | 1,304 (39.4) | Lignans | 1,928 (36.4) |
| Lignan | 192 (9.0) | Lignan | 467 (14.1) | Constituents | 708 (13.4) |
| Constituents | 148 (6.9) | Constituents | 361 (10.9) | Lignan | 699 (13.2) |
| Neolignans | 108 (5.0) | Phytoestrogens | 254 (7.7) | In-vitro | 366 (6.9) |
| Phytoestrogens | 96 (4.5) | Neolignans | 180 (5.4) | Flavonoids | 324 (6.1) |
| Genistein | 86 (4.0) | Derivatives | 179 (5.4) | Antioxidant activity | 313 (5.9) |
| Breast-cancer | 60 (2.8) | Enterolactone | 157 (4.7) | Antioxidant | 307 (5.8) |
| Derivatives | 60 (2.8) | Flaxseed | 152 (4.6) | Cells | 298 (5.6) |
| Identification | 57 (2.7) | In-vitro | 143 (4.3) | Derivatives | 297 (5.6) |
| Cancer | 50 (2.3) | Antioxidant activity | 140 (4.2) | Leaves | 295 (5.6) |
| Women | 48 (2.2) | Flavonoids | 138 (4.2) | Apoptosis | 272 (5.1) |
| Chemistry | 47 (2.2) | Phyto-estrogens | 132 (4.0) | Identification | 266 (5.0) |
| Flavonoids | 47 (2.2) | Breast-cancer | 127 (3.8) | Expression | 253 (4.8) |
| Bark | 46 (2.1) | Inhibition | 119 (3.6) | Glycosides | 252 (4.8) |
| Acid | 45 (2.1) | Metabolism | 119 (3.6) | Neolignans | 248 (4.7) |
| Inhibition | 44 (2.1) | Identification | 115 (3.5) | Phenolic-compounds | 210 (4.0) |
| Podophyllotoxin | 44 (2.1) | Acid | 114 (3.4) | Oxidative stress | 209 (3.9) |
| Diet | 42 (2.0) | Mammalian lignans | 114 (3.4) | Flaxseed | 203 (3.8) |
| Estrogens | 42 (2.0) | Cells | 113 (3.4) | Inhibition | 203 (3.8) |
| Route | 42 (2.0) | Podophyllotoxin | 113 (3.4) | Cytotoxicity | 194 (3.7) |
Discussion
The current literature analysis on lignan publications revealed the large publication shares from Asian countries, which were consistent with related bodies of literature such as antioxidants and curcumin (Yeung et al., 2019b; Yeung et al., 2019c). Examples of some highly cited original research papers recently published by Asian teams in the 2010s, without international collaborations, are discussed here. For instance, a Chinese paper reported results from sesame transcriptomes that provide useful information for understanding the relevant lignan biosynthesis molecular mechanism (Wei et al., 2011). Another Chinese team tested the effects of new lignans and neolignans on inhibiting nitric oxide production in mouse macrophages and against serum deprivation-induced PC12 cell damage (Xiong et al., 2011). These papers received over 100 citations. Meanwhile, Korean teams published the anti-inflammatory effects of several lignans isolated from Schiandra chinensis (Oh et al., 2010), and the hepatoprotective effect of pinoresinol isolated from Forsythiae Fructus (Kim et al., 2010). These papers were cited over 50 times. In Japan, a randomized controlled trial was conducted, and results found that oral intake of flaxseed (Linum usitatissimum L.) lignan could lower blood cholesterol level and risk of hepatic diseases in hypercholesterolemic men (Fukumitsu et al., 2010). Another Japanese team described an efficient synthetic route to synthesize herbindoles as naturally occurring forms (Saito et al., 2012). In India, researchers extracted, separated, and characterized sesame oil lignan (Reshma et al., 2010) and reported a phylogenetic analysis of L. usitatissimum L. (Barvkar et al., 2012). These Japanese and Indian papers had around 40 citations each. All these examples demonstrate the variety of the lignan research field, which ranged from basic sciences to human clinical trials.
Similar to the related research fields of berries, dietary natural products, and functional foods (Yeung et al., 2018a; Yeung et al., 2018b; Yeung et al., 2019d), the bubble map suggested that cancer and cardiovascular diseases were highly cited topics for lignan research. Readers can refer to comprehensive reviews on the relationship between phytoestrogens (such as lignans and isoflavonoids) and Western diseases (such as breast cancer and coronary heart disease) (Adlercreutz and Mazur, 1997; Rietjens et al., 2017). Their modulatory effects on steroid biosynthetic enzymes, hormone concentrations, and cellular events seem to be beneficial against cancer development (Adlercreutz and Mazur, 1997; Rietjens et al., 2017). In the early 1990s, a Finnish-Japanese collaboration probed into the low mortality in hormone-dependent cancer among the Japanese and found that they had high intake of soybean products rich in phytoestrogens, as demonstrated by a high concentration of isoflavonoids (and lignans to a lesser extent) excreted in their urine (Adlercreutz et al., 1991). In the year 1997, a case-control study published in Lancet reported that a high intake of phytoestrogens particularly lignan enterolactone and isoflavone equol could substantially reduce breast cancer risk in women (Ingram et al., 1997). Later, another paper reviewed data on existing epidemiologic studies and suggested that lignans and flavonoids have beneficial effects on cardiovascular diseases and lung cancer, but not other cancers (Arts and Hollman, 2005). The issues of low bioavailability might partly explain the differences in the results obtained between studies using cell/animal models and humans, particularly for the anti-cancer effects (Yang et al., 2001).
In addition, the bubble map can also relate to some of the potential biological activities of lignans, e.g., estrogenic and antiestrogenic, antioxidant, anti-inflammatory, and anticancerogenic properties (Baumgartner et al., 2011; Teponno et al., 2016; Wang et al., 2016; Linder et al., 2019; Zálešák et al., 2019), especially with antioxidant and anti-inflammatory activity being identified as frequently mentioned terms, whereas they were strong interests in phytoestrogen and cancer.
By limiting to “articles” (excluding other publication types such as reviews), a quick query of “clinical trial*” within the analyzed body of literature returned with 50 hits only. After evaluation, we found that there were only 19 randomized clinical trials, which was equivalent to 0.2% of the 10,742 lignan publications. A follow-up search in PubMed database with a query of “lignan*” and limited article type to “Clinical Trial” returned with 121 hits, which was equivalent to 1.1% of the analyzed publications. With such a small ratio of clinical trials in the lignan research literature, we believe that more clinical trials should be conducted to substantiate the beneficial effects and optimal dose of lignan intake on humans. In addition, researchers are currently experiencing common difficulties in estimating the dietary intakes of lignans (and also other non-nutritive substances) because they are not routinely included in the food composition tables, and there exists variability in contents reactive to soil quality, sun exposure, etc. All these complicate the works concerning the dose of lignan intake.
This study inherited some limitations, such as using indexed data based on a single database (WoS). Furthermore, the latest research trends, if any, might remain undetected due to a lack of time to accumulate publication and citation counts. Similar to previous literature analyses on curcumin and resveratrol (Yeung et al., 2019a; Yeung et al., 2019b), we did not analyze the authorship of the lignan publications, as there existed many Chinese authors with similar initials that caused inaccurate counting. Analyzing authorship by authors' full names was also not practical, as many publication records listed author initials only. Moreover, the analysis cannot evaluate the scientific methods used to determine the research findings (e.g., distinguish between in vivo work used to determine mechanistic relationships at a molecular level, and disease associations elucidated from population research). For an analysis of over 10,000 publications, this requires additional automatic labeling of the documents (data tagging), which is currently very limited in the literature databases. For Web of Science, for example, there are only a few publication types, e.g., articles, reviews, editorials. Besides, lignan sub-types and method of action in metabolizers are not analyzed.
Overall, the current report identified the terms and themes in the lignan research literature, being important in terms of publication and citation data. Results revealed several recurring or highly cited themes, implying that the bibliometric analysis was able to quantitatively highlight the topics in the field deemed important by the field experts.
Conclusions
To summarize, a bibliometric analysis was conducted to evaluate publications on lignans. The current findings revealed that the United States and Asian countries, such as China, Japan, South Korea, and India, were the most productive countries. Some productive institutions were based outside these countries, such as the University of Helsinki in Finland. Many of the publications were focused on pharmacology (23.5%), chemistry (23.5%), and plant sciences (19.1%). Over 80% of the analyzed papers have been published since year 2000, and nearly 50% since year 2010. The highly cited publications usually mentioned specific terms such as phytoestrogen, isoflavone, daidzein, enterodiol, enterolactone, equol, genistein, isoflavonoid, cancer, breast cancer, or cardiovascular disease. Some frequently mentioned and discussed main common dietary lignans were lariciresinol, matairesinol, pinoresinol, secoisolariciresinol, and syringaresinol.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding authors.
Author Contributions
AY, AD, AA, and AS conceived the work, performed data collection and analysis, and drafted the manuscript. All authors critically revised the manuscript and approved the submission of the manuscript.
Funding
AA acknowledges the support by the Polish KNOW (Leading National Research Centre) Scientific Consortium “Healthy Animal—Safe Food,” decision of the Ministry of Science and Higher Education No. 05-1/KNOW2/2015. EN and AS acknowledge the support of the research project Nutraceutica come supporto nutrizionale nel paziente oncologico, CUP: B83D18000140007.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Adlercreutz H., Mazur W. (1997). Phyto-oestrogens and Western diseases. Ann. Med. 29, 95–120. 10.3109/07853899709113696 [DOI] [PubMed] [Google Scholar]
- Adlercreutz H., Honjo H., Higashi A., Fotsis T., Hämäläinen E., Hasegawa T., et al. (1991). Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am. J. Clin. Nutr. 54, 1093–1100. 10.1093/ajcn/54.6.1093 [DOI] [PubMed] [Google Scholar]
- Angeloni S., Navarini L., Sagratini G., Torregiani E., Vittori S., Caprioli G. (2018). Development of an extraction method for the quantification of lignans in espresso coffee by using HPLC-MS/MS triple quadrupole. J. mass Spectrom. 53, 842–848. 10.1002/jms.4251 [DOI] [PubMed] [Google Scholar]
- Angeloni S., Navarini L., Khamitova G., Sagratini G., Vittori S., Caprioli G. (2019). Quantification of lignans in 30 ground coffee samples and evaluation of theirs extraction yield in espresso coffee by HPLC-MS/MS triple quadrupole. Int. J. Food Sci. Nutr. 6, 1–8. 10.1080/09637486.2019.1624693 [DOI] [PubMed] [Google Scholar]
- Arts I. C., Hollman P. C. (2005). Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 81, 317S–325S. 10.1093/ajcn/81.1.317S [DOI] [PubMed] [Google Scholar]
- Axelson M., Sjövall J., Gustafsson B., Setchell K. (1982). Origin of lignans in mammals and identification of a precursor from plants. Nature 298, 659–660. 10.1038/298659a0 [DOI] [PubMed] [Google Scholar]
- Azam S., Jakaria M., Kim I.-S., Kim J., Haque M. E., Choi D.-K. (2019). Regulation of toll-like receptor (TLR) signaling pathway by polyphenols in the treatment of age-linked neurodegenerative diseases: focus on TLR4 signaling. Front. In Immunol. 10, 1000. 10.3389/fimmu.2019.01000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barvkar V. T., Pardeshi V. C., Kale S. M., Kadoo N. Y., Gupta V. S. (2012). Phylogenomic analysis of UDP glycosyltransferase 1 multigene family in Linum usitatissimum identified genes with varied expression patterns. BMC Genomics 13, 175. 10.1186/1471-2164-13-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner L., Sosa S., Atanasov A. G., Bodensieck A., Fakhrudin N., Bauer J., et al. (2011). Lignan derivatives from Krameria lappacea roots inhibit acute inflammation in vivo and pro-inflammatory mediators in vitro. J. Natural Prod. 74, 1779–1786. 10.1021/np200343t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakta D., Higgins C. D., Sevak L., Mangtani P., Adlercreutz H., Mcmichael A. J., et al. (2006). Phyto-oestrogen intake and plasma concentrations in South Asian and native British women resident in England. Br. J. Nutr. 95, 1150–1158. 10.1079/BJN20061777 [DOI] [PubMed] [Google Scholar]
- Buck K., Vrieling A., Zaineddin A. K., Becker S., Hüsing A., Kaaks R., et al. (2011). Serum enterolactone and prognosis of postmenopausal breast cancer. J. Clin. Oncol. 29, 3730–3738. 10.1200/JCO.2011.34.6478 [DOI] [PubMed] [Google Scholar]
- Corrie J. E. T., Green G. H., Ritchie E., Taylor W. C.(1970). The chemical constituents of Australian Zanthoxylum species. V. The constituents of Z. pluviatile Hartley; the structures of two new lignans. Aust. J. of Chem. 23, 133–145. 10.1071/CH9700133 [DOI] [Google Scholar]
- Daliu P., Santini A., Novellino E. (2019). From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Rev. Clin. Pharmacol. 12, 1–7. 10.1080/17512433.2019.1552135 [DOI] [PubMed] [Google Scholar]
- Durazzo A., Raguzzini A., Azzini E., Foddai M., Narducci V., Maiani G., et al. (2009). Bioactive molecules in cereals. Tec. Molitoria Int. 60, 150–162. [Google Scholar]
- Durazzo A., Azzini E., Turfani V., Polito A., Maiani G., Carcea M. (2013. a). Effect of cooking on lignans content in whole-grain pasta made with different cereals and other seeds. Cereal Chem. 90, 169–171. 10.1094/CCHEM-05-12-0065-N [DOI] [Google Scholar]
- Durazzo A., Zaccaria M., Polito A., Maiani G., Carcea M. (2013. b). Lignan content in cereals, buckwheat and derived foods. Foods 2, 53–63. 10.3390/foods2010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo A., Carcea M., Adlercreutz H., Azzini E., Polito A., Olivieri L., et al. (2014. a). Effects of consumption of whole grain foods rich in lignans in healthy postmenopausal women with moderate serum cholesterol: a pilot study. Int. J. Food Sci. Nutr. 65, 637–645. 10.3109/09637486.2014.893283 [DOI] [PubMed] [Google Scholar]
- Durazzo A., Turfani V., Narducci V., Azzini E., Maiani G., Carcea M. (2014. b). Nutritional characterisation and bioactive components of commercial carobs flours. Food Chem. 153, 109–113. 10.1016/j.foodchem.2013.12.045 [DOI] [PubMed] [Google Scholar]
- Durazzo A., Lucarini M., Camilli E., Marconi S., Gabrielli P., Lisciani S., et al. (2018). Dietary lignans: definition, description and research trends in databases development. Molecules 23, 3251. 10.3390/molecules23123251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo A., Lucarini M., Souto E. B., Cicala C., Caiazzo E., Izzo A. A., et al. (2019). Polyphenols: a concise overview on the chemistry, occurrence and human health. Phytother. Res. 33, 2221–2243. 10.1002/ptr.6419 [DOI] [PubMed] [Google Scholar]
- Eklund P., Raitanen J.-E. (2019). 9-Norlignans: occurrence, properties and their semisynthetic preparation from hydroxymatairesinol. Molecules 24, 220. 10.3390/molecules24020220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumitsu S., Aida K., Shimizu H., Toyoda K. (2010). Flaxseed lignan lowers blood cholesterol and decreases liver disease risk factors in moderately hypercholesterolemic men. Nutr. Res. 30, 441–446. 10.1016/j.nutres.2010.06.004 [DOI] [PubMed] [Google Scholar]
- Gnabre J., Bates R., Huang R. C. (2015). Creosote bush lignans for human disease treatment and prevention: perspectives on combination therapy. J. Tradit. Complement. Med. 5, 119–126. 10.1016/j.jtcme.2014.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero F., Orlando V., Monetti V. M., Russo V., Menditto E. (2017). Biological therapy utilization, switching, and cost among patients with psoriasis: retrospective analysis of administrative databases in Southern Italy. ClinicoEconomics Outcomes Res. 9, 741–748. 10.2147/CEOR.S147558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmini P., Rubagotti A., Boccardo F. (2012). Serum enterolactone levels and mortality outcome in women with early breast cancer: a retrospective cohort study. Breast Cancer Res. Treat 132, 661–668. 10.1007/s10549-011-1881-8 [DOI] [PubMed] [Google Scholar]
- Hartwell J. L. (1976). Types of anticancer agents isolated from plants. Cancer Treat Rep. 60, 1031–1067. [PubMed] [Google Scholar]
- Hongthong S., Kuhakarn C., Jaipetch T., Piyachaturawat P., Jariyawat S., Suksen K., et al. (2016). A new neolignan, and the cytotoxic and anti-HIV-1 activities of constituents from the roots of Dasymaschalon sootepense. Natural Prod. Commun. 11, 809–813. 10.1177/1934578X1601100628 [DOI] [PubMed] [Google Scholar]
- Imai T., Nomura M., Fukushima K. (2006). Evidence for involvement of the phenylpropanoid pathway in the biosynthesis of the norlignan agatharesinol. J. Plant Physiol. 163, 483–487. 10.1016/j.jplph.2005.08.009 [DOI] [PubMed] [Google Scholar]
- Ingram D., Sanders K., Kolybaba M., Lopez D. (1997). Case-control study of phyto-oestrogens and breast cancer. Lancet 350, 990–994. 10.1016/S0140-6736(97)01339-1 [DOI] [PubMed] [Google Scholar]
- Iolascon G., Gimigliano F., Moretti A., Riccio I., Di Gennaro M., Illario M., et al. (2016). Rates and reasons for lack of persistence with anti-osteoporotic drugs: analysis of the Campania region database. Clin. cases In Mineral Bone Metab. 13, 126–129. 10.11138/ccmbm/2016.13.2.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-Y., Kim J.-K., Choi J.-H., Jung J.-Y., Oh W.-Y., Kim D. C., et al. (2010). Hepatoprotective effect of pinoresinol on carbon tetrachloride–induced hepatic damage in mice. J. Pharmacol. Sci. 112, 105–112. 10.1254/jphs.09234FP [DOI] [PubMed] [Google Scholar]
- Kuhnle G. G., Dell'aquila C., Aspinall S. M., Runswick S. A., Mulligan A. A., Bingham S. A. (2008. a). Phytoestrogen content of beverages, nuts, seeds, and oils. J. Agric. Food Chem. 56, 7311–7315. 10.1021/jf801534g [DOI] [PubMed] [Google Scholar]
- Kuhnle G. G., Dell'aquila C., Aspinall S. M., Runswick S. A., Mulligan A. A., Bingham S. A. (2008. b). Phytoestrogen content of foods of animal origin: dairy products, eggs, meat, fish, and seafood. J. Agric. Food Chem. 56, 10099–10104. 10.1021/jf801344x [DOI] [PubMed] [Google Scholar]
- Kuhnle G. G., Dell'aquila C., Aspinall S. M., Runswick S. A., Mulligan A. A., Bingham S. A. (2009. a). Phytoestrogen content of cereals and cereal-based foods consumed in the UK. Nutr. Cancer 61, 302–309. 10.1080/01635580802567141 [DOI] [PubMed] [Google Scholar]
- Kuhnle G. G., Dell'aquila C., Aspinall S. M., Runswick S. A., Joosen A. M., Mulligan A. A., et al. (2009. b). Phytoestrogen content of fruits and vegetables commonly consumed in the UK based on LC–MS and 13C-labelled standards. Food Chem. 116, 542–554. 10.1016/j.foodchem.2009.03.002 [DOI] [Google Scholar]
- Landete J. M. (2012). Plant and mammalian lignans: a review of source, intake, metabolism, intestinal bacteria and health. Food Res. Int. 46, 410–424. 10.1016/j.foodres.2011.12.023 [DOI] [Google Scholar]
- Lewis N. G., Davin L. B. (1999). “Lignans: biosynthesis and function,” in Comprehensive Natural Products Chemistry, vol. 639-712 . Eds. Barton D., Nakanishi K., Meth-Cohn O. (Amsterdam, the Netherlands: Elsevier; ). 10.1016/B978-0-08-091283-7.00027-8 [DOI] [Google Scholar]
- Linder T., Liu R., Atanasov A. G., Li Y., Geyrhofer S., Schwaiger S., et al. (2019). Leoligin-inspired synthetic lignans with selectivity for cell-type and bioactivity relevant for cardiovascular disease. Chem. Sci. 10, 5815–5820. 10.1039/C9SC00446G [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowcock E. C., Cotterchio M., Boucher B. A. (2013). Consumption of flaxseed, a rich source of lignans, is associated with reduced breast cancer risk. Cancer Causes Control 24, 813–816. 10.1007/s10552-013-0155-7 [DOI] [PubMed] [Google Scholar]
- Menditto E., Cahir C., Aza-Pascual-Salcedo M., Bruzzese D., Poblador-Plou B., Malo S., et al. (2018). Adherence to chronic medication in older populations: application of a common protocol among three European cohorts. Patient Prefer. Adherence 12, 1975–1987. 10.2147/PPA.S164819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milder I. E., Arts I. C., Van De Putte B., Venema D. P., Hollman P. C. (2005. a). Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br. J. Nutr. 93, 393–402. 10.1079/BJN20051371 [DOI] [PubMed] [Google Scholar]
- Milder I. E., Feskens E. J., Arts I. C., De Mesquita H. B. B., Hollman P. C., Kromhout D. (2005. b). Intake of the plant lignans secoisolariciresinol, matairesinol, lariciresinol, and pinoresinol in Dutch men and women. J. Nutr. 135, 1202–1207. 10.1093/jn/135.5.1202 [DOI] [PubMed] [Google Scholar]
- Moreno-Franco B., García-González Ñ., Montero-Bravo A. M., Iglesias-Gutiérrez E., ~beda N., Maroto-Núnez L., et al. (2011). Dietary alkylresorcinols and lignans in the Spanish diet: development of the alignia database. J. Agric. Food Chem. 59, 9827–9834. 10.1021/jf2015446 [DOI] [PubMed] [Google Scholar]
- Mulligan A. A., Kuhnle G. G., Lentjes M. A., Van Scheltinga V., Powell N. A., Mctaggart A., et al. (2013). Intakes and sources of isoflavones, lignans, enterolignans, coumestrol and soya-containing foods in the Norfolk arm of the European Prospective Investigation into Cancer and Nutrition (EPIC-Norfolk), from 7 d food diaries, using a newly updated database. Public Health Nutr. 16, 1454–1462. 10.1017/S1368980012003904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S.-Y., Kim Y. H., Bae D. S., Um B. H., Pan C.-H., Kim C. Y., et al. (2010). Anti-inflammatory effects of gomisin N, gomisin J, and schisandrin C isolated from the fruit of Schisandra chinensis. Biosci. Biotechnol. Biochem. 74, 285–291. 10.1271/bbb.90597 [DOI] [PubMed] [Google Scholar]
- Peñalvo J. L., Haajanen K. M., Botting N., Adlercreutz H. (2005). Quantification of lignans in food using isotope dilution gas chromatography/mass spectrometry. J. Agric. Food Chem. 53, 9342–9347. 10.1021/jf051488w [DOI] [PubMed] [Google Scholar]
- Penalvo J. L., López-Romero P. (2012). Urinary enterolignan concentrations are positively associated with serum HDL cholesterol and negatively associated with serum triglycerides in US adults. J. Nutr. 142, 751–756. 10.3945/jn.111.150516 [DOI] [PubMed] [Google Scholar]
- Penalvo J. L., Adlercreutz H., Uehara M., Ristimaki A., Watanabe S. (2007). Lignan content of selected foods from Japan. J. Agric. Food Chem. 56, 401–409. 10.1021/jf072695u [DOI] [PubMed] [Google Scholar]
- Peterson J., Dwyer J., Adlercreutz H., Scalbert A., Jacques P., Mccullough M. L. (2010). Dietary lignans: physiology and potential for cardiovascular disease risk reduction. Nutr. Rev. 68, 571–603. 10.1111/j.1753-4887.2010.00319.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshma M., Balachandran C., Arumughan C., Sunderasan A., Sukumaran D., Thomas S., et al. (2010). Extraction, separation and characterisation of sesame oil lignan for nutraceutical applications. Food Chem. 120, 1041–1046. 10.1016/j.foodchem.2009.11.047 [DOI] [Google Scholar]
- Rietjens I. M., Louisse J., Beekmann K. (2017). The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 174, 1263–1280. 10.1111/bph.13622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-García C., Sánchez-Quesada C., Toledo E., Delgado-Rodríguez M., Gaforio J. J. (2019). Naturally Lignan-rich foods: a dietary tool for health promotion? Molecules 24, 917. 10.3390/molecules24050917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito N., Ichimaru T., Sato Y. (2012). Total synthesis of (–)-herbindoles A, B, and C via transition-metal-catalyzed intramolecular [2 + 2+ 2] cyclization between ynamide and diynes. Org. Lett. 14, 1914–1917. 10.1021/ol300571b [DOI] [PubMed] [Google Scholar]
- Santini A., Novellino E. (2018). Nutraceuticals-shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharmacol. 11, 545–547. 10.1080/17512433.2018.1464911 [DOI] [PubMed] [Google Scholar]
- Santini A., Cammarata S. M., Capone G., Ianaro A., Tenore G. C., Pani L., et al. (2018). Nutraceuticals: opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 84, 659–672. 10.1111/bcp.13496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala D., Menditto E., Armellino M. F., Manguso F., Monetti V. M., Orlando V., et al. (2016). Italian translation and cultural adaptation of the communication assessment tool in an outpatient surgical clinic. BMC Health Serv. Res. 16, 163. 10.1186/s12913-016-1411-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeds A. I., Jauhiainen L., Tuomola E., Peltonen-Sainio P. (2009). Characterization of variation in the lignan content and composition of winter rye, spring wheat, and spring oat. J. Agric. Food Chem. 57, 5837–5842. 10.1021/jf9004274 [DOI] [PubMed] [Google Scholar]
- Smeds A. I., Eklund P. C., Willför S. M. (2012). Content, composition, and stereochemical characterisation of lignans in berries and seeds. Food Chem. 134, 1991–1998. 10.1016/j.foodchem.2012.03.133 [DOI] [PubMed] [Google Scholar]
- Su S., Wink M. (2015). Natural lignans from Arctium lappa as antiaging agents in Caenorhabditis elegans. Phytochemistry 117, 340–350. 10.1016/j.phytochem.2015.06.021 [DOI] [PubMed] [Google Scholar]
- Teponno R. B., Kusari S., Spiteller M. (2016). Recent advances in research on lignans and neolignans. Natural Prod. Rep. 33, 1044–1092. 10.1039/C6NP00021E [DOI] [PubMed] [Google Scholar]
- Thompson L. U., Boucher B. A., Liu Z., Cotterchio M., Kreiger N. (2006). Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr. Cancer 54, 184–201. 10.1207/s15327914nc5402_5 [DOI] [PubMed] [Google Scholar]
- Turfani V., Narducci V., Durazzo A., Galli V., Carcea M. (2017). Technological, nutritional and functional properties of wheat bread enriched with lentil or carob flours. LWT-Food Sci. Technol. 78, 361–366. 10.1016/j.lwt.2016.12.030 [DOI] [Google Scholar]
- Valsta L. M., Kilkkinen A., Mazur W., Nurmi T., Lampi A.-M., Ovaskainen M.-L., et al. (2003). Phyto-oestrogen database of foods and average intake in Finland. Br. J. Nutr. 89, S31–S38. 10.1079/BJN2002794 [DOI] [PubMed] [Google Scholar]
- Van Eck N. J., Waltman L. (2009). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84, 523–538. 10.1007/s11192-009-0146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery B. C. (1948). Bradford's law of scattering. J. Doc. 4, 198–203. 10.1108/eb026133 [DOI] [Google Scholar]
- Wang L., Ladurner A., Latkolik S., Schwaiger S., Linder T., HošEk J., et al. (2016). Leoligin, the major lignan from edelweiss (Leontopodium nivale subsp. alpinum), promotes cholesterol efflux from THP-1 macrophages. J. Natural Prod. 79, 1651–1657. 10.1021/acs.jnatprod.6b00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward H. A., Kuhnle G. G., Mulligan A. A., Lentjes M. A., Luben R. N., Khaw K.-T. (2009). Breast, colorectal, and prostate cancer risk in the European prospective investigation into cancer and nutrition–norfolk in relation to phytoestrogen intake derived from an improved database. Am. J. Clin. Nutr. 91, 440–448. 10.3945/ajcn.2009.28282 [DOI] [PubMed] [Google Scholar]
- Wei W., Qi X., Wang L., Zhang Y., Hua W., Li D., et al. (2011). Characterization of the sesame (Sesamum indicum L.) global transcriptome using Illumina paired-end sequencing and development of EST-SSR markers. BMC Genomics 12, 451. 10.1186/1471-2164-12-451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H.-H., Lv J., Mok D., Yao X.-S., Wong M.-S., Cooper R. (2019). NMR applications for botanical mixtures: the use of HSQC data to determine lignan content in Sambucus williamsii. J. Natural Prod. 82, 1733–1740. 10.1021/acs.jnatprod.8b00891 [DOI] [PubMed] [Google Scholar]
- Xiong L., Zhu C., Li Y., Tian Y., Lin S., Yuan S., et al. (2011). Lignans and neolignans from Sinocalamus affinis and their absolute configurations. J. Natural Prod. 74, 1188–1200. 10.1021/np200117y [DOI] [PubMed] [Google Scholar]
- Yang C. S., Landau J. M., Huang M.-T., Newmark H. L. (2001). Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 21, 381–406. 10.1146/annurev.nutr.21.1.381 [DOI] [PubMed] [Google Scholar]
- Yeung A. W. K., Aggarwal B., Barreca D., Battino M., Belwal T., Horbańczuk O., et al. (2018. a). Dietary natural products and their potential to influence health and disease including animal model studies. Anim. Sci. Pap. Rep. 36, 345–358. [Google Scholar]
- Yeung A. W. K., Mocan A., Atanasov A. G. (2018. b). Let food be thy medicine and medicine be thy food: a bibliometric analysis of the most cited papers focusing on nutraceuticals and functional foods. Food Chem. 269, 455–465. 10.1016/j.foodchem.2018.06.139 [DOI] [PubMed] [Google Scholar]
- Yeung A. W. K., Aggarwal B. B., Orhan I. E., Horbańczuk O. K., Barreca D., Battino M., et al. (2019. a). Resveratrol, a popular dietary supplement for human and animal health: quantitative research literature analysis - a review. Anim. Sci. Pap. Rep. 37, 103–118. [Google Scholar]
- Yeung A. W. K., Horbańczuk M., Tzvetkov N. T., Mocan A., Carradori S., Maggi F., et al. (2019. b). Curcumin: total-scale analysis of the scientific literature. Molecules 24, 1393. 10.3390/molecules24071393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. W. K., Tzvetkov N. T., El-Tawil O. S., Bungǎu S. G., Abdel-Daim M. M., Atanasov A. G. (2019. c). Antioxidants: scientific literature landscape analysis. Oxid. Med. Cell. Longevity 2019, 8278454. 10.1155/2019/8278454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. W. K., Tzvetkov N. T., Zengin G., Wang D., Xu S., Mitrović G., et al. (2019. d). The berries on the top. J. Berry Res. 9, 125–139. 10.3233/JBR-180357 [DOI] [Google Scholar]
- Yeung A. W. K. (2019). Higher impact factor of neuroimaging journals is associated with larger number of articles published and smaller percentage of uncited articles. Front. In Hum. Neurosci. 12, 523. 10.3389/fnhum.2018.00523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zálešák F., Bon D.J.-Y.D., Pospíšil J. (2019). Lignans and Neolignans: plant secondary metabolites as a reservoir of biologically active substances. Pharmacol. Res. 146, 104284. 10.1016/j.phrs.2019.104284 [DOI] [PubMed] [Google Scholar]
- Zamora-Ros R., Agudo A., Luján-Barroso L., Romieu I., Ferrari P., Knaze V., et al. (2012). Dietary flavonoid and lignan intake and gastric adenocarcinoma risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am. J. Clin. Nutr. 96, 1398–1408. 10.3945/ajcn.112.037358 [DOI] [PubMed] [Google Scholar]
- Zhang C., Mao X., Zhao X., Liu Z., Liu B., Li H., et al. (2014). Gomisin N isolated from Schisandra chinensis augments pentobarbital-induced sleep behaviors through the modification of the serotonergic and GABAergic system. Fitoterapia 96, 123–130. 10.1016/j.fitote.2014.04.017 [DOI] [PubMed] [Google Scholar]
- Zhuang W., Li Z., Dong X., Zhao N., Liu Y., Wang C., et al. (2019). Schisandrin B inhibits TGF-β1-induced epithelial-mesenchymal transition in human A549 cells through epigenetic silencing of ZEB1. Exp. Lung Res. 45, 157–166. 10.1080/01902148.2019.1631906 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding authors.