Abstract
OBJECTIVES
Community-acquired pneumonia (CAP) is a potentially lethal lower respiratory tract infection for children. For this reason, early recognition and appropriate treatment is essential. In addition, we need to determine which patient will be hospitalized or not hospitalized. Here, we aimed to evaluate the plasma endocan level to determine whether it is effective in making the decision on hospitalization and the assessment of the response to treatment in patients with CAP.
MATERIALS AND METHODS
This prospective case-control study was conducted between November 2015 and May 2016 at Erciyes University School of Medicine. Fifty-three patients diagnosed as CAP with clinical and radiological findings were enrolled in the study. The patients were divided into various subgroups, such as inpatient, outpatient, complicated, non-complicated, dead patients, etc., and the levels of endocan were compared between the control group and those various groups.
RESULTS
A total of 53 children with a diagnosis of CAP and 55 healthy children were enrolled in the study. Patients were divided into two groups: hospitalized patients and outpatients. There was no statistically significant difference between these groups’ serum endocan levels on the 1st day and serum endocan levels on the 4th day (p=0.783, p=0.419).
CONCLUSION
Serum endocan level had no significant value in predicting patients’ hospitalization. On the other hand, high serum endocrine levels may be important in predicting the severity and prognosis of the disease.
Keywords: Community-acquired pneumonia, endocan, hospitalization
INTRODUCTION
Community-acquired pneumonia (CAP) is defined as a clinical diagnosis of pneumonia caused by an infection acquired outside hospital in a previously healthy child [1]. CAP is a potentially lethal lower respiratory tract infection, affecting children all over the world [2]. For this reason, early recognition and appropriate treatment is essential as well as determining the patients to be hospitalized on the basis of clinical findings; however, these depend on the clinician’s experience. Sometimes, clinical symptoms may be difficult to detect in busy emergency departments and hospitals. Although markers such as ESR (erythrocyte sedimentation ratio), CRP (C-reactive protein), PCT (procalcitonin), cytokines, and leukocyte counts are commonly measured, none of them are specific to pneumonia screening or to the decision to hospitalize a patient. Serum levels may increase in other inflammatory events and infections. Studies have shown that the serum PCT levels and some cytokines (IL-6) are associated with the prognosis of the disease [3]. However, there are currently no specific markers for identifying disease severity and in the hospitalization decision of children with CAP [4].
Endocan, also known as endothelial cell-specific molecule-1 (ECM-1), is a peptidoglycan synthesized in endothelial cells. It is a sign of endothelial activation, as well as of endothelial-associated pathogens [5]. Endocan is secreted from activated endothelial cells, particularly from the lung and less frequently from renal vessels and tumor endothelial cells. The control of the secretion of endocan is mediated by cytokines and growth factors. Factors such as IL-8, TNF-α, IL-1β, e-selectin, and vascular endothelial growth factor increase the secretion of endocan, but IFN-gamma inhibits its secretion [6,7]. There is approximately 1 ng/mL of endocan in the blood of healthy people, but serum levels are increased in cases of infection such as septic shock and in cancer diseases [8].
In this study, the aim was to evaluate plasma endocan levels at the time of admission, on the 4th day of treatment in children with CAP, and to examine its relationship with commonly used markers, such as CRP, white blood cell (WBC), and the neutrophil count. Furthermore, value of endocan, that is secreted in the lung tissue and increases in infections, in determination of disease severity, effects of hospitalization, and the assessment of the response to treatment in patients with CAP was evaluated.
MATERIAL AND METHODS
This prospective case-control study was conducted between November 2015 and May 2016 in the Erciyes University School of Medicine, Department of pediatric pulmonology. Fifty-three patients, diagnosed with CAP with clinical and radiological findings, who had not been treated previously and aged between 3 months and 18 years of were included in the study. CAP, diagnosed by a pediatrician, was defined by clinical symptoms (i.e., fever >38.0°C, cough, dyspnea, tachypne, and pleuritic chest pain), physical examination findings (i.e., crackles [rales], retractions, and rhonchus), and chest X-ray [9]. If there were unclear radiological findings, a pediatric radiologist was consulted.
The patients were separated into two groups of outpatients and hospitalized patients according to the Clinical Practice Guidelines of the Paediatric Infectious Diseases Society of America [4]. Patients were treated using the most recent guideline and they were discharged from the hospital upon clinical improvement. Patients, who were treated without hospitalization, were checked on the 4th day of treatment and 2 weeks later. All the patients’ routine examinations were done. These patients did not have any problems during follow-up, and treatment was successful in all. Eighty patients with CAP were initially included; however, 27 patients, who did not come for follow up on the 4th day, were excluded from the study.
Exclusion criteria were as follows: age <3 months or >18 years, cystic fibrosis, bronchiectasis, tuberculosis, immotile cilia syndrome, sickle cell anemia, Down syndrome, cerebral palsy, acute/chronic renal insufficiency, acute/chronic liver failure, congenital heart disease, patients who were previously treated at other locations, those with multiple antiepileptic and immunosuppressive treatment, chemical pneumonia, hospital-acquired pneumonia, and ventilator associated pneumonia. Fifty-five healthy children who came to the hospital for routine examinations were enrolled in the study as the control group.
Blood samples for endocan were taken at the time of diagnosis and on the 4th day of treatment. They were placed in EDTA tubes and then centrifuged for 10 minutes at 2,000 g. Then, the plasma samples were stored at −80°C until the time of analysis. Hemogram, CRP, ESR, and blood cultures were performed and measured the same day. The levels of endocan were determined in duplicate by using the enzyme-linked immunos or bent assay kits (Yehua brand ELISA kit cat no: YHB1079HU). CRP was determined using a Siemens brand BN-II device via the immunonephelometric method in Erciyes University Hospital, Central Laboratories.
All procedures were approved by the institutional review board of the Erciyes University (number: 96681246/192). All patients and control groups were informed about the steps of the study, and written informed consent was obtained.
Statistical Analysis
Statistical analysis was performed using the Statistical Package for Science Studies version 22.0 for Windows (SPSS IBM Corp.; Armonk, NY, USA). Variables were stated as means with maximum-minimum, or median with the 25th and 75th percentile. The Pearson Chi-Squared test was used to compare qualitative data as well as descriptive statistical methods (mean, standard deviation, and frequency). The distribution of the data was evaluated by histogram, q-q graphs, and the Shapiro-Wilk test. The difference between the two groups without normal distribution was compared with the Mann-Whitney test. The Wilcoxon Signed Ranks test was used for comparison of two dependent groups for variables without normal distribution. The Kruskal-Wallis test was used to compare the medians when the number of cases in the groups was not equal. A p-value ≤0.05 was considered to be statistically significant. The Dunn-Bonferroni correction was applied for multiple comparisons. The receiver operating characteristic curve (ROC) was used to assess the sensitivity and specificity of the markers. The area under the curve (AUC) was used to calculate the predictive value of the markers for pneumonia. The data were evaluated using programs R3.2.2 (www.r-project.org) and easy ROC (www.biosoft.hacettepe.edu.tr /easy ROC).
RESULTS
Fifty-three children with CAP and 55 healthy children were enrolled in the study. The demographic data of the patients are summarized in Table 1. There was no statistically significant difference in age and gender between the patient and control groups (p=0.429 for age, and p=0.546 for gender). Thirty-eight patients (71.6%) were hospitalized and treated; 15 patients (28.4%) received medical treatment at home. Complications developed in 15 (28.3%) patients, which were hospitalized, and three (5.7%) died. Twelve (22.6%) of the complicated cases were of pleural effusion, there was one case of hemolytic uremic syndrome, and two cases of abscess. Six (11.3%) patients were admitted to the intensive care unit because of mechanical ventilation (MV) requirements. One of the patients was followed up by non-invasive MV and 5 by invasive MV. There was no statistically significant difference between the serum endocan levels on the 1st day of the patient and control group (p=0.400; Table 2).
Table 1.
Demographic data of patient and control groups
| Patients n (%) |
Control group n (%) |
||
|---|---|---|---|
| Gender | Male | 31 (58.5%) | 29 (52.7%) |
| Female | 22 (41.5%) | 26 (47.3%) | |
| Age (month) | Mean (minimum–maximum) | 52 (7–192) | 56 (5–192) |
| First day serum endocan level (ng/mL) (median) | 0.52 (0.19–2.98)* | 0.54 (0.18–1.77)* | |
No statistically significant difference was determined between the CAP and control groups with respect to age and gender.
p>0.05,
There was no statistically significant difference between the 1st day serum endocan level in the CAP and control groups
Table 2.
Clinical features of patient group and comparison of the endocan levels
| Endocan level of patient groups; median (min–max) (ng/mL) | 1st day | 4th day | p | |
|---|---|---|---|---|
| 0.52 (0.19–2.98) | 0.52 (0.17–2.47) | >0.05 | ||
| Follow-up n (%) | Outpatients 15, (28.4%) | 0.66 (0.35–1.69) | 0.58 (0.34–1.77) | >0.05 |
| Hospitalization 38, (71.6%) | 0.43 (0.28–1.06) | 0.41 (0.29–1.13) | >0.05 | |
| Complication n (%) | No-Complication 35, (66%) | 0.55 (0.19– 2.98) | 0.49 (0.17–2.47) | >0.05 |
| Complication 15, (28.3%) | 0.34 (0.20–2.66) | 0.39 (0.19–2.28) | >0.05 | |
| Dead patients 3, (5.7%) | 0.32 (0.25–0.36) | 0.71 (0.69–1.34) | >0.05 | |
| Mechanical ventilation (MV) n (%) | Non-MV 47, (88.7%) | 0.52 (0.19–2.98) | 0.43 (0.17–2.47) | >0.05 |
| MV 6, (11.3%) | 0.80 (0.19–2.65) | 1.58 (0.68–1.96) | ||
| Culture | Positive 6, (11.3%) | 0.75 (0.19–2.48) | 1.62 (0.29–2.28) | >0.05 |
| Negative 47, (88.7%) | 0.52 (0.19–2.89) | 0.48 (0.17–2.47) | ||
Plasma endocan levels of the patient group were measured on the 1st and 4th days. There was no statistically significant difference between the plasma endocan levels (p=0.155; Table 2). There was no statistically significant difference between the hospitalized or outpatient groups serum endocan levels on the 1st day and serum endocan levels on the 4th day (p=0.783, p=0.419; Table 2). When we assessed the endocan levels according to the clinical status of the patients, serum endocan levels were higher in the patients who died than the other patients on the 4th day (1st day 0.32 [0.25–0.36] ng/mL, 4th day 0.71 [0.69–1.34] ng/mL, versus 0.54 [0.33–1.30] ng/mL, 0.48 [0.29–1.3] ng/mL). In addition, the patients who needed MV had serum endocan levels higher than those of other patients (1st day 0.80 [0.19–2.65] ng/mL, 4th day 1.58 [0.68–1.96] ng/mL, versus 0.52 [0.19–2.98] ng/mL, 0.43 [0.17–2.47] ng/mL). Microorganisms were detected in the blood cultures of 6 patients. The median serum endocan levels of these 6 patients were 0.75 (0.19–2.48) ng/mL on the 1st day, 1.62 (0.29–2.28) on the 4th day, and 0.52 (0.19–2.89) ng/mL on the 1st day, 0.48 (0.17–2.47) on the 4th day in blood culture negative patients (Table 2), respectively.
The WBC count was calculated as median 12,840 (8800–17310), and the neutrophil count was 8450 (4220–12,685) cells/mm3. CRP was evaluated in 53 patients; the median was 33.2 (5.32–151) mg/L. Plasma endocan levels were positively correlated with WBC and neutrophil count, but were not correlated with CRP (respectively, p=0.01, r=0.434; p=0.01, r=0.340; p=0.804, r=−0.035).
In this study, the ROC analysis was evaluated to determine the diagnostic role of endocan in the diagnosis of patients with pneumonia, and endocan levels remained below the acceptable limits (AUC=0.547). Based on the ROC curve, an optimal cut-off value for pneumonia diagnosis was set at 1.626 ng/mL of endocan. The positive predictive value was calculated as 92.3%, and the negative predictive value as 56.8%. When the plasma endocan levels on the 1st day of the patient group and plasma endocan levels of the control group were evaluated, the sensitivity and specificity were found as 22.64% and 98.18% for pneumonia (Figure 1).
Figure 1.
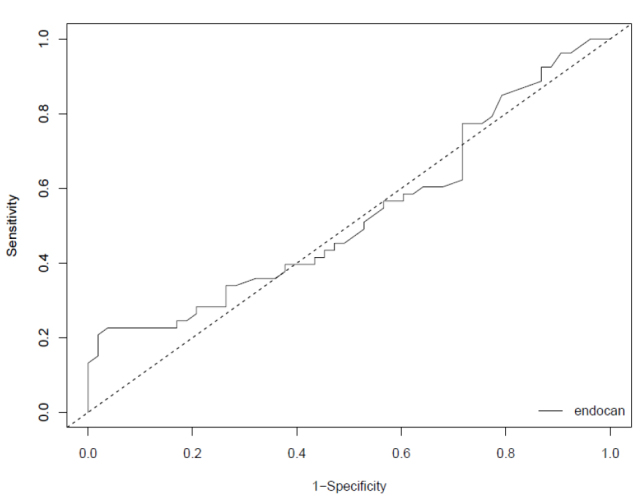
ROC analyses of endocan, in patients with CAP
ROC: receiver operating characteristic; CAP: community-acquired pneumonia
DISCUSSION
In our study, there was no statistically significant difference between the serum endocan levels of the patient and control groups. The study also did not find a meaningful result when we compared the serum endocan levels of hospitalized patients and outpatient groups. Our study showed that endocan is not consistent in determining hospitalization according to the Infectious Diseases Society of America (IDSA). There are very few studies in the related literature on this subject to evaluate the relationship between the serum endocan levels and CAP in children. However, a different aspect of our study from other studies was that we compared the serum endocan level of hospitalized patients with outpatients. There was no statistical difference in the serum endocan levels between the 1st and 4th day in patients with CAP. According to our study results, endocan was not considered appropriate in the evaluation of the treatment response. However, there were groups of patients (culture positive, requiring MV, and those who died) whose serum endocan levels did not decrease despite treatment. Their results were not statistically significant because of the number of these patients, but their serum endocan levels were higher than in the control groups. Patients with elevated serum endocan levels need to be more carefully monitored. Further research is required on this subject.
There are few studies in the literature evaluating the relationship between pneumonia and endocan. In the study performed by Paketci et al. [10] the serum endocan levels of children with pneumonia were found to be statistically higher than those of the control group. The results of our study were not consistent with these studies. In our study, 6 patients had severe pneumonia defined as need of MV, but Paketci et al. [10] had 29 severe pneumonia patients in their study. For this reason, there may be a difference between that study and our study. Severe pneumonia criteria of the British Thoracic Society include more severe clinical findings than those of the IDSA hospitalization criteria [4,11]. In our study, patients, who needed MV, had higher serum endocan levels than other patients. These findings suggest that high serum endocan levels are associated with pneumonia severity, but we have not been able to identify a relationship with hospitalization. In a study conducted by Kao et al. [12] with adult pneumonia patients, serum endocan levels were found to be higher than in the control group. This discrepancy may have been due to the higher-than-average age, co-morbidity, or more severe pneumonia of their patients than in our study.
There are diffirent studies in the literature with serum endocan levels, beside pneumonia. There are more than pneumonia studies endocan has been shown to be an important and potent marker of organ failure and mortality in sepsis. In studies investigating better ways to determine the clinical course of sepsis, endocan levels were found to be high in patients with sepsis. In addition, high endocan levels were found to be associated with disease severity in terms of shock development and mortality [13]. In a study by Saldir et al. [14] in septic newborns, endocan and IL-6 levels were higher than in non-septic patients. It has been suggested that this is a landmark study for the early recognition of septic newborns and for their differentiation from non-septic infants. When these studies are evaluated, it is understood that the median serum endocan level is increased in infective cases and related with the severity of the disease. In our study, microorganisms were detected in blood cultures of 6 patients. The median serum endocan level was 0.75 (0.19–2.84) ng/mL in these 6 blood culture-positive patients and 0.52 (0.19–2.89) ng/mL in 47 blood culture-negative patients. Although the median serum endocan level was high at the time of diagnosis of the culture positive patients, it was not statistically significant (p=0.902). In the study by Seo et al. [15] serum endocan levels in patients with bacterial pneumonia were shown to be higher in bacteremic patients than in non-bacteremic patients, and no correlation was found between serum endocan levels, CRP, or PCT. In our study, the endocan level was not correlated with the CRP level, but it was correlated with WBC and neutrophils. The results of our study are similar to those by Seo et al [15]. Endothelial cells form a multifunctional cell lining that covers the entire inner surface of blood vessels, and they regulate several important physiological and pathological processes [16]. We know that endocan is secreted by endothelial cells in response to proinflammatory cytokines, lipopolysaccharide, and angiogenic factors. These studies showed that if infection is unrestricted and spreads, endothelial cells become activated, and the level of serum endocan is increased [17]. This may be a sign that the prognosis of the disease will worsen.
In our study, the ROC analysis was used to determine the diagnostic role of endocan in the diagnosis of patients with pneumonia. Endocan levels remained under the acceptable limits (AUC=0.547). In the study performed by Paketci et al. [10] the endocan level AUC (0.769) value was not found to be significant for pneumonia, similar to our study. These results might not be clinically significant because the sensitivity and specificity of endocan is not found to be higher when compared to old and new markers such as CRP, PCT, and copeptin, as mentioned in a recently published meta-analysis [18]. A limitation to our study is the fact that the number of patients was low, and we did not discriminate between viral and bacterial pneumonia. In addition, patients could be grouped according to the disease severity score.
According to the results of our study, endocan was not predictive of hospitalization in CAP patients. However, high serum endocan levels may be important in predicting the severity and prognosis of the disease. Further research is required to inspect the effects of endocan on CAP.
MAIN POINTS.
Community-acquired pneumonia is one of the most common causes of death under five years old worldwide and there is none of marker specific to pneumonia screening.
Endocan is a peptidoglycan synthesized in endothelial cells particularly from the lungs.
Endocan levels are not predictive for hospitalization in children with Community-acquired pneumonia
Higher endocan levels may be associated with the disease severity and prognosis.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Erciyes University (number: 96681246/192).
Informed Consent: Written informed consent was obtained from patients and patients’ parents who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.K.; Design - M.K., M.H.; Supervision - M.K., M.H.; Materials -D.Ö.; Data Collection and/or Processing - D.Ö., D.B.K.; Analysis and/or Interpretation - M.H., M.K.; Literature Search - M.H.; Writing Manuscript - M.H., M.K.; Critical Review - M.K., M.H.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: This study was funded by the Scientific Research Projects Unit of Erciyes University (Project code TTU-2016-6541).
REFERENCES
- 1.Kocabaş E, Ersoz D, Karakoç F, et al. Turkish Thoracic Society Childhood Community Acquired Pneumonia Diagnosis and Treatment Report. In: Umut S, Soysal SB, editors. Turkish Thoracic Journal İstanbul Aves Yayıncılık. 2009. pp. 1–24. [Google Scholar]
- 2.Rudan I, Boschi-Pinto C, Biloglav Z, et al. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408–16. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michelow IC, Katz K, McCracken GH, et al. Systemic cytokine profile in children with community-acquired pneumonia. Pediatr Pulmonol. 2007;42:640–5. doi: 10.1002/ppul.20633. [DOI] [PubMed] [Google Scholar]
- 4.Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia (CAP) in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society (PIDS) and the Infectious Diseases Society of America (IDSA) Clin Infect Dis. 2011;53:25–76. doi: 10.1093/cid/cir625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lassalle P, Molet S, Janin A, et al. ESM-1 is a novel human endothelial cell-specific molecule expressed in lung and regulated by cytokines. J Biol Chem. 1996;271:20458–64. doi: 10.1074/jbc.271.34.20458. [DOI] [PubMed] [Google Scholar]
- 6.Dieterich LC, Mellberg S, Langenkamp E, et al. Transcriptional profiling of human glioblastoma vessels indicates a key role of VEGF-A and TGFβ2 in vascular abnormalization. J Pathol. 2012;228:378–90. doi: 10.1002/path.4072. [DOI] [PubMed] [Google Scholar]
- 7.Delehedde M, Devenyns L, Maurage CA, et al. Endocan in cancers: a lesson from a circulating dermatan sulfate proteoglycan. Int J Cell Biol. 2013;2013:705027. doi: 10.1155/2013/705027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bechard D, Meignin V, Scherpereel A, et al. Characterization of the secreted form of endothelial-cell-specific molecule 1 by specific monoclonal antibodies. J Vasc Res. 2000;37:417–25. doi: 10.1159/000025758. [DOI] [PubMed] [Google Scholar]
- 9.Cherian T, Mulholland EK, Carlin JB, et al. 2005 Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ. 2005;83:353–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Paketci C, Paketci A, Erdede O, et al. Serum endocan levels in children with community-acquired pneumonia. Pediatr Allergy Immunol Pulmonol. 2017 doi: 10.1089/ped.2017.0744. [DOI] [Google Scholar]
- 11.Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66:1–23. doi: 10.1136/thoraxjnl-2011-200598. [DOI] [PubMed] [Google Scholar]
- 12.Kao SJ, Chuang CY, Tang CH, et al. Plasma endothelial cell-specific molecule-1 (ESM-1) in management of community-acquired pneumonia. Clin Chem Lab Med. 2014;52:445–51. doi: 10.1515/cclm-2013-0638. [DOI] [PubMed] [Google Scholar]
- 13.Scherpereel A, Depontieu F, Grigoriu B, et al. Endocan, a new endothelial marker in human sepsis. Crit Care Med. 2006;34:532–7. doi: 10.1097/01.CCM.0000198525.82124.74. [DOI] [PubMed] [Google Scholar]
- 14.Saldir M, Tunc T, Cekmez F, et al. Endocan and soluble triggering receptor expressed on myeloid cells-1 as novel markers for neonatal sepsis. Pediatr Neonatol. 2015;56:415–21. doi: 10.1016/j.pedneo.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Seo K, Kitazawa T, Yoshino Y, et al. Characteristics of serum endocan levels in infection. PLoS One. 2015;10:e0123358. doi: 10.1371/journal.pone.0123358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 17.Scherpereel A, Depontieu F, Grigoriu B, et al. Endocan, a new endothelial marker in human sepsis. Crit Care Med. 2006;34:532–7. doi: 10.1097/01.CCM.0000198525.82124.74. [DOI] [PubMed] [Google Scholar]
- 18.Viasus D, Del Rio-Pertuz G, Simonetti AF, et al. Biomarkers for predicting short-term mortality in community-acquired pneumonia: A systematic review and meta-analysis. J Infect. 2016;7:273–82. doi: 10.1016/j.jinf.2016.01.002. [DOI] [PubMed] [Google Scholar]


