Abstract
Tumor recurrences or metastases remain a major hurdle in improving overall cancer survival. In the perioperative period, the balance between the ability of the cancer to seed and grow at the metastatic site and the ability of the patient to fight against the tumor (i.e. the host antitumor immunity) may determine the development of clinically evident metastases and influence the patient outcome. Up to 80% of oncological patients receive anesthesia and/or analgesia for diagnostic, therapeutic or palliative interventions. Therefore, anesthesiologists are asked to administer drugs such as opiates and volatile or intravenous anesthetics, which may determine different effects on immunomodulation and cancer recurrence. For instance, some studies suggest that intravenous drugs, such as propofol, may inhibit the host immunity to a lower extent as compared to volatile anesthetics. Similarly, some studies suggest that analgesia assured by local anesthetics may provide a reduction of cancer recurrence rate; whilst on the opposite side, opioids may exert negative consequences in patients undergoing cancer surgery, by interacting with the immune system response via the modulation of the hypothalamic-pituitary-adrenal axis and autonomic nervous system, or directly through the opioid receptors on the surface of immune cells. In this review, we summarize the main findings on the effects induced by different drugs on immunomodulation and cancer recurrence.
Keywords: anesthesia, anesthetic drugs, anesthetic technique, oncologic surgery, immunosuppression, cancer recurrence
Introduction
The hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS) are both stimulated by oncological surgery and anesthesia. HPA axis was shown to suppress the host immune response through the release of catecholamines, prostaglandin E2, cytokines and cortisol, and further other neuroendocrine mediators, such as the vascular endothelial growth factor, interleukins (IL) 6 and 8, and matrix metalloproteinases.1,2 These latter play a major role in the regulation of tumor growth and angiogenesis, prompting an eventual re-activation of micrometastasis already disseminated at the time of the surgery and potentially unresponsive to adjuvant therapies.2 This scenario is further complicated by the impairment of cell-mediated immunity and the release of tumoral cells from the primary lesions into the systemic circulation, secondary to the surgical manipulation. Therefore, if, on one hand, oncologic surgery is of paramount importance in the management of solid tumors since a definitive resection can be totally curative, on the other hand, it might potentially increase the risk for micro-dissemination and for further clinically evident metastases.3 Noteworthy, the development of clinically evident metastases also depends on the balance between the ability of the cancer to seed and grow at the metastatic site and the ability of the patient to fight against the tumor (i.e. the host antitumor immunity).
Mechanisms of Anesthetic and Analgesic Drugs on Immune Response and Cancer Recurrence
Up to 80% of patients with cancer receive anesthesia for diagnostic, therapeutic or palliative intervention.4 In particular, patients undergoing oncological surgery of the digestive tract often require general anesthesia, which must guarantee analgesia, amnesia, sedation and neuromuscular block during surgery. To get these purposes, anesthesiologists use drugs such as opiates and volatile or intravenous anesthetics. Furthermore, anesthesiologists are also asked to provide an optimal postoperative analgesic plan adjusted on the basis of adequacy of pain relief and presence of adverse events, with a multimodal analgesia, including also morphine or other opiates.5
The first data suggesting a role of anesthesia in immunomodulation and cancer recurrent were published few decades ago. In 1977, a large retrospective study including 1358 patients receiving surgery for breast cancer reported for the first time that the survival rate was higher in patients receiving halothane anesthesia, compared to those receiving ether.6 At that time, the authors already suggested that the mechanism of anesthetic effects on survival rate should be researched in alterations of the HPA axis, intraoperative carcinemia, immunity of tumor cell and growth of metastases.6 In 1981, Shapiro and coworkers investigated the effect of four anesthetic agents (i.e. thiopental, ketamine, halothane and N2O) on the postoperative growth of cancer in mice.7 The authors reported an accelerated growth of lung metastases and development of secondary cancers in organs not otherwise commonly associated with metastases.7 Further data from animal studies suggest that anesthetic drugs modify the biology of cancer and immune cell lines by direct activation of the cellular receptors and cell signaling pathways, and by alteration of cellular kinetics and gene transcription.8,9 We have therefore conducted this literature review focusing solely on the effects induced by different anesthetic drugs on immunomodulation and cancer recurrence.
Intravenous Anesthetic Drugs
Intravenous anesthetic drugs produce different and multiple effects on the immunity system (see Table 1). Melamed et al administered thiopental, ketamine and propofol via different routes in a rat model of breast cancer with radiolabeled cells to assess the effects of drugs with respect to the lung tumor retention and number of lung metastases, as indicated by the ratio of radioactivity between the lung and injected tumor cell suspension.10 Twenty-four hours after cell inoculation, thiopental and ketamine, while not propofol, were shown to significantly increase the lung retention of tumoral cells and lung metastasis, due to a reduced activity of the Natural Killer (NK) cells.10 Furthermore, thiopental specifically and differentially induces a heat shock response and it mediates the cytoprotection and reduces the apoptosis in human T lymphocytes,11 whereas ketamine, in a concentration-dependent manner, induces human lymphocyte apoptosis via the mitochondrial pathway.12 It should also be mentioned that both thiopental and ketamine suppress the immune system, the former (i.e. thiopental) through the suppression of the activation of nuclear factor kappa B (NF-κB) and through inhibition of the neutrophil function, the latter (i.e. ketamine) by decreasing the levels of IL-6 and tumor necrosis factor-α (TNF-α), that are pro-inflammatory cytokines. The inhibition of NF-κB leads to the suppression of the activity of NF-κB-driven reporter gene, which regulates the expression of IL-2, IL-6, IL-8 and IFN-γ and the activation of T-lymphocyte.13
Table 1.
Effects of Anesthetic Agents on Activity of Different Actors on Cancer Recurrence and Immunomodulation
| Lung Cell Retention | Cell Growth | Stress Response | NK Activity | Lymph Activity | Lymph Apoptosis | Th1/Th2 | NF-κB | TNFα | IL-2 | IL-6 | IL-8 | IFNγ | EGF | HIF-1α | MAPK | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intravenous drugs | ||||||||||||||||
| Propofol | = | = | + | = | - | - | - | |||||||||
| Barbiturates | + | - | + | - | - | - | - | - | - | - | - | |||||
| Ketamine | + | - | - | - | ||||||||||||
| Volatile agents | ||||||||||||||||
| Halothane | - | - | - | |||||||||||||
| Isoflurane | - | + | - | + | ||||||||||||
| Sevoflurane | - | - | - | + | - | |||||||||||
| Desflurane | = | + | ||||||||||||||
| Opioids | ||||||||||||||||
| Morphine | - | - | - | - | - | - | ||||||||||
| Fentanyl | - | - | ||||||||||||||
| Alfentanyl | - | - | ||||||||||||||
| Sufentanyl | - | - | ||||||||||||||
| Local anesthetics | - | - | - |
Notes: Modifications of different actors of the immune system and cancer recurrence are reported separately for each drug used in the perioperative period. =, no modifications; + increased activity; -, reduced activity.
Abbreviations: NK, natural killer; Lymph, lymphocytes; Th1/Th2, ratio between lymphocytes T helper 1 and 2; NF-ΚB, nuclear factor kappa B; TNFα, tumor necrosis factor α; IL, Interleukin; IFNγ, interferon γ; EGF, epidermal growth Factor; HIF-1α, hypoxia inducible factor-1α; MAPK, mitogen-activated protein kinase.
If, on one hand, thiopental and ketamine have an anti-inflammatory effect and suppression of the immune system, on the other hand, in a murine thymoma model, propofol significantly suppresses the tumor growth, most likely by enhancing cytotoxic T-lymphocytes activity,14 counteracts the activity of the cyclooxygenases (COX) in macrophages and, via the diminution of the production of prostaglandin E2, which acts on EP4 receptor on NK cell, downregulates the interferon γ (IF-γ), reduces the tumor growth and the tumor evasion of host immune surveillance through the suppression of immune cell functions.15 Furthermore, propofol mitigates the immunosuppression induced by surgery, since it does not affect the T helper 1 (Th1) and T helper 2 (Th2) cell ratios.16
The modifications induced by anesthetic drugs on hypoxia-inducible factor-1α (HIF-1α) are also relevant. Indeed, a high HIF-1α activity is associated with more aggressive phenotypes of cancer and worsened clinical outcome.3 Propofol reversibly inhibits the HIF-1α activity, in a manner proportional to the oxygen concentration of the environment, by reducing the translation of the mRNA into protein.17 Furthermore, propofol also inhibits the activation of HIF-1α induced by the lipo-polysaccharide and suppress the glucose metabolism in macrophages.18 Barbiturates also inhibit the activation of HIF-1α by disruption of mRNA translation.19 In particular, both propofol and barbiturates suppress the activity of Mitogen-Activated Protein Kinase (MAPK), which is an intracellular signal-transducing system in eukaryotes, and it plays a fundamental role in regulating the HIF-1α mRNA translation.19,20 Noteworthy, HIF-1α promotes angiogenesis via the activation of the transcription of the VEGF-A, Ang-1 and Ang-2 genes, and facilitates cell proliferation (through IGF-2 and TGF-α gene transcription) and metastasis (via CXCR4, CXCL-12, LOX and repression of E-cadherin genes).3 Furthermore, clinical concentrations of propofol modulate the GTPase RhoA, guarantying an adjunctive protective effect against the migration and invasion of cancer cells.21
Volatile Anesthetic Drugs
Immune response is also modulated and affected by volatile anesthetics (see Table 1). Markovic et al exposed mice to the administration of halothane or isoflurane. The authors reported that both drugs inhibited the NK cell activity, 67% in the case of exposition to halothane, >90% to isoflurane. Noteworthy, if interferon was given before anesthetic exposure, no inhibition occurred.22 In addition to NK cell activity attenuation, isoflurane induces T-lymphocyte and B-lymphocyte apoptosis and decreases the Th1/Th2 ratio.23 Loop et al reported that isoflurane and sevoflurane induce T-lymphocyte apoptosis, via increased activation of caspase-3 and augmented permeability of the mitochondrial membrane.24 These findings were not confirmed, in the same study, for desflurane.24 Sevoflurane decreases also the lymphocytes and NK cell activity, while it increases leukocyte and neutrophil counts and activity.25 In another study, 32 patients, undergoing elective surgery for primary breast cancer, were randomized to receive general anesthesia with sevoflurane and intravenous opiates or a combination of propofol and paravertebral analgesia.26 Compared to propofol and paravertebral strategy, sevoflurane and opiates increased the levels of pro-tumorigenic cytokines and matrix metalloproteinases.26
Several studies have also shown that some volatile anesthetic drugs (i.e., isoflurane and desflurane) provide cytoprotective properties and upregulate the HIF-1α.3 Isoflurane and desflurane may stimulate a pro-tumorigenic behavior of residual cells and, therefore, they may facilitate the tumor’s recurrence.3 Sevoflurane is also characterized by an upregulation of HIF-1α activity.3 On the opposite, other anesthetic agents (i.e. halothane) downregulate HIF-1α activity, and, in principle, should be preferred for oncologic surgery.27
In mice models, Shapiro et al have shown that halothane may accelerate the metastase progression by the lung carcinoma or by melanoma, and it may induce the appearance of metastases in the liver.7 In another study by Ecimovic et al, sevoflurane was also reported to increase the proliferation and migration of breast cancer, while the invasion was observed only in estrogen-receptor-positive tumors.28 On the opposite, Liang et al reported that sevoflurane could suppress the growth induced by HIF-1α and metastases of lung cancer cells.29
Opioids
Analgesia is one of the components of anesthesia and it is generally assured intraoperatively and post-operatively with drugs like opioids. Although certainly effective analgesics, a growing body of literature and evidence suggests that opioids may exert negative consequences in patients undergoing cancer surgery. Indeed, similar to intravenous and inhalational anesthetic drugs, opioids interact with the immune system response via the modulation of the HPA axis and autonomic nervous system, or directly through the opioid receptors on the surface of immune cells (see Table 1).
One of the greatest effects of opioids on immune system is the inhibition of the proliferation and differentiation of T-lymphocyte, besides promoting their apoptosis, too.30,31 For instance, morphine, fentanyl, alfentanil and sufentanil all decrease the NK cell activity,30–32 while remifentanil was shown to completely overwhelm the lymphocyte proliferation and the NK cell activity in rats.33 In particular, morphine reduces NK cell activity to an extent proportional to the administered dose in rats34 and in healthy volunteers, up to 24 hrs after cessation of intravenous administration.35 In addition, morphine reduces also the phagocytic activity and production of antibodies and cytokines,3 and, in clinically relevant doses, increases the angiogenesis and growth of breast tumors in mice.36 In a recent study published by Qi et al37 192 elderly patients undergoing laparoscopic radical resection of colorectal cancer were randomized to receive anesthesia with sufentanil or remifentanil by target controlled infusion.
Patients randomized to receive remifentanil tended to have higher concentrations of glucose, cortisol and IL-6. In addition, compared to remifentanil, sufentanil had a lower degree of decrease of T-cell subsets and, after the surgery, a shorter recovery of cellular immunity.37 Noteworthy, the interaction between the immune system and opioids is furthermore complex. The administration of morphine also attenuates the tumor-retentive effect of laparotomic surgery,38 and it promotes cell death and apoptosis in an adenocarcinoma cell line.39
Local Anesthetics
Local anesthetics efficiently inhibits the neural signal transmission, by blocking the voltage-gated sodium channels (VGSC), assuring an effective intraoperative anesthesia and postoperative analgesia. In particular, VGSC are transmembrane proteins composed of α (pore-forming) and one or more β-units. Local anesthetics have the advantage: 1) to attenuate the stress response and, therefore, limiting the immunosuppression and preserving the innate immune response and 2) to reduce the need for opioids and volatile anesthetics and limiting their possible negative effects on cancer recurrence. In addition, some evidence suggest that local anesthetics have direct antitumor effects on cancer cells (see Table 1). Some cancers, such as colon, breast and lung, highly express VGSC, which are particularly active in these cells. Indeed, administration of local anesthetics blocks the VGSC, potentially inhibiting the tumor growth.
In an in vitro study, Lucchinetti et al isolated mesenchymal cells from femurs and tibias of mice and they exposed cells to increasing concentrations of local anesthetics (i.e. lidocaine, ropivacaine, and bupivacaine).39 At 100 μM, all local anesthetics reduced the cell growth and proliferation by delaying or arresting the G0/1-S phase transition, decreased the colony formation, impaired the differentiation into osteoblasts, delayed wound healing and increased lactate dehydrogenase release. Subsequent analysis through microarray showed changes in genes controlling the expression of lysosomal genes and sterol metabolism. All these findings suggest a potential role of local anesthetics in the perioperative period in patients with cancer, although they could potentially interfere with the wound healing.40 In addition, clinical concentrations of lidocaine inhibit the epidermal growth factor (EGF) receptor in human cancer cells.41
Another more recent study by Chang et al has shown that both lidocaine and bupivacaine, at clinical concentrations, promote apoptosis in breast cancer cells, either in vitro and in vivo.42 Furthermore, lidocaine diminishes also the formation and function of tubulin micro-tentacles of tumoral cells, reducing their spread into the blood.43 Similar findings were also reported by Mammoto et al;44 indeed, the authors reported that lidocaine blocks the invasion of cancer cells, throughout inhibition of ectodomain shedding of heparin-binding EGF from the cell surface and modulation of the intracellular calcium concentration, which further contributes to this action.44 In contrast, ropivacaine and bupivacaine express their antitumor growth activity at dosages and concentrations higher than those used in clinical settings.45 In addition, in patients undergoing radical prostatectomy, epidural analgesia significantly reduces the stress response (i.e., concentrations of serum cortisol and insulin) while not the inflammatory response, as compared to opioid-based analgesia.46 Similar findings have been also reported in patients undergoing colorectal cancer surgery.47
Nowadays, the evidence is spurred by the publication of several retrospective clinical studies. The most recent systematic review includes 67,577 patients across 28 studies and assesses the relationship between regional anesthesia and cancer.48 After pulled data analysis, the authors report that regional anesthesia does not provide any benefit in terms of patients’ survival; however, some single studies report a benefit in terms of cancer recurrence.48 In the end, they also conclude that further studies are required to draw a final and definitive conclusion, to stress the need for further investigations and knowledge.48
Current Evidence on the Effects of Anesthesia on Short and Long-Term Outcomes After Cancer Surgery
The intraoperative anesthetic management and the postoperative analgesic strategy may play a key role in the dissemination and/or elimination of micrometastasis and residual tumor cells after oncological surgery.1 However, solid data and evidence on patients currently lack. This hypothesis is nowadays sustained only by retrospective studies and meta-analyses, which suggest that general anesthesia and use of some drugs are associated with higher impairment of cell-mediated immunity, leading to an increased cancer recurrence and mortality rates.49 Furthermore, a review conducted in 2015 reports conflicting results and evidence regarding volatile agents.50
For instance, a recent retrospective analysis of 7030 patients undergoing elective oncological surgery has investigated the eventual difference in a 3-year period long-term survival between the cohort of patients having received total intravenous versus volatile inhalational anesthesia strategies.51 Volatile inhalational anesthesia was associated with an increased risk of death, with a hazard ratio of 1.80; this was true also after a propensity-matched analysis of 2607 patients in each group, with similar baseline characteristics.51 Another retrospective analysis of 2838 patients, receiving oncological surgery for breast (1837 patients), colon (695 patients) or rectal (306 patients) cancer, reported an increased survival rate if anesthesia was performed with propofol, rather than volatile anesthetics.52 Noteworthy, a shift over time towards a more frequent use of propofol was reported. In fact, while less than 1% of patients received propofol from 1997 to 2000, more than 85% received it after 2007. This could be therefore a bias to be taken in count.52 Another retrospective analysis of 325 patients, having received surgery for breast cancer, also suggested a possible reduction of cancer recurrence in patients anesthetized with propofol, as opposed to those with volatile agents.53 However, no difference was reported in the overall survival between the two groups.53 Another retrospective study investigated the role of different anesthetic strategy in 156 patients who underwent free flap surgery for head and neck cancer.54 No differences in hospital mortality or length of stay were observed between groups, probably due also to a little sample. However, patients receiving propofol showed a lower rate of postoperative pulmonary complications, as opposed to those receiving inhalational anesthetics.54
Data from prospective randomized controlled trials are nowadays little. In a small randomized controlled trial, 28 consecutive patients undergoing oncological surgery for urinary bladder cancer were randomized to receive or total intravenous anesthesia or balanced inhalational anesthesia.55 Patients receiving total intravenous anesthesia showed a weaker immunity modulation, as opposed to those receiving balanced inhalational anesthesia. In addition, no difference in rate of metastasis after surgery and survival was observed between groups.55 Another prospective trial randomized 48 patients, undergoing Ivor Lewis operation, to receive sevoflurane or total intravenous anesthesia.56 In this population, volatile anesthesia attenuated the increase of IL-6 blood concentration at the end of surgery; however, postoperative pulmonary morbidity was similar between cohorts.56 Another further prospective study compared the effects of propofol and sevoflurane anesthesia with respect to perioperative immune response in 58 patients undergoing laparoscopic radical hysterectomy for cervical cancer.57 Compared to volatile-based anesthesia, a propofol-based anesthesiologic strategy was characterized by a lower immunosuppression during the perioperative period; however, no differences in short-term adverse consequence, such as hospital stay and infection rate, were reported between groups.57
A recent systematic review suggests that total intravenous anesthesia might reduce the overall mortality and improve the recurrence-free survival. However, these evidences are limited to small and low-quality studies, deeming necessary further proper designed randomized controlled trials.58
Another systematic review and meta-analysis, very recently published by Yap et al examine the potential effects of anesthetic strategies for cancer surgery on survival.59 Compared to volatile anesthesia, total intravenous anesthesia was characterized by lower mortality in patients with gastric (Hazard Ratio 0.61 [0.55–0.69]; Z: 8.02; p<0.01), mixed gastrointestinal (Hazard Ratio 0.68 [0.60–0.78]; Z:5.78; p<0.01) and esophageal cancer (hazard ratio 0.63 [0.50–0.81]; Z:3.70; p<0.01), while in not in patients with colon (Hazard Ratio 0.58 [0.23–1.49]; Z:1.13; p=0.26) or rectal cancer (hazard ratio 0.83 [0.52–1.31]; Z:0.79; p=0.43).59
The explanation of these findings might be searched into the effects of single drugs on immune system components.
Future Perspectives
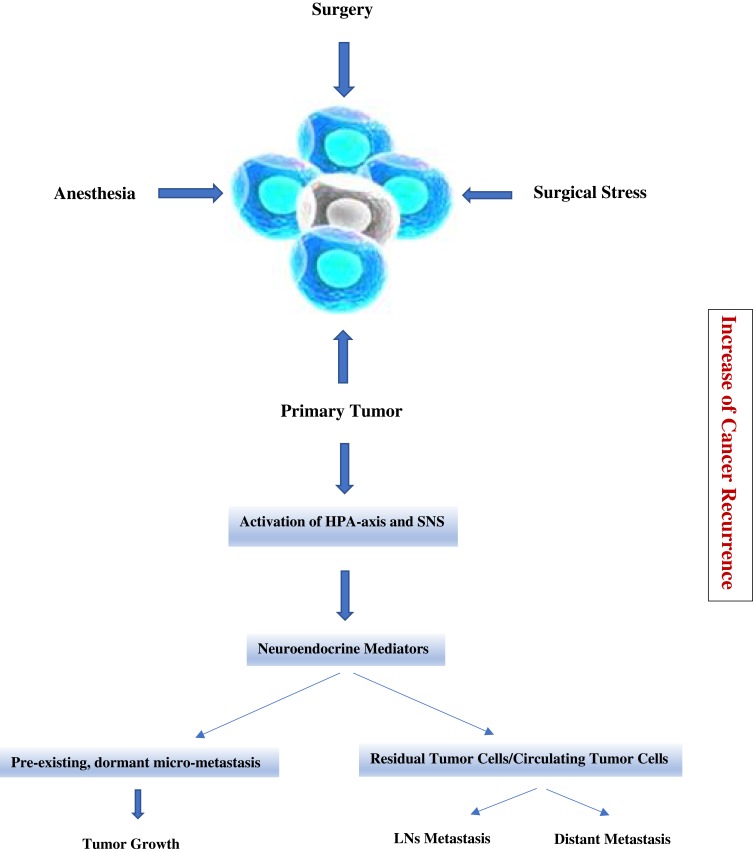
The body of preclinical literature in onco-anesthesia is certainly growing; however, a strong evidence to support one anesthesiologic strategy, rather than another one, currently lacks. In fact, the survival benefit associated with the use of locoregional or blended (general plus locoregional) or propofol-based anesthesia remains uncertain. The hypothetical evidence of superiority of one strategy or another would potentially be of paramount importance, since anesthesia and perioperative care may influence the outcome of patients undergoing cancer surgery. In 2015 a consensus statement highlighted the need for more clinical trials on the effects of different anesthetic and analgesic strategies on postoperative cancer recurrence.60 In 2018, another consensus statement, based on a systematic review, has defined and standardized the endpoint related to research in onco-anesthesia to be assessed by the future trials.61 To date, a certain number of prospective randomized controlled trials in onco-anesthesia research are ongoing (i.e., NCT01975064; NCT03034096; NCT02660411; NCT04074460), with the intent to assess if there is or not a better anesthesia plan and perioperative care to be chosen in perioperative cancer care (Figure 1).
Figure 1.
Cancer metastasis and recurrence caused by surgery and anesthetic induced immunosuppression in the peri-operative period. Surgery, anesthesia and analgesia stimulate HPA-axis and SNS during the peri-operative period. Activated neuroendocrine mediators lead to increases in several immunosuppressive soluble factors that promote tumor progression and metastasis, resulting in an increase of cancer recurrence.
Conclusions
Although sometimes conflicting data have been published, anesthetic drugs may play a major role in the immunomodulation and cancer recurrence. Since the evidence is not completely clear to date, future studies are needed to clarify the role of anesthesia in immunomodulation and cancer recurrence in patients undergoing elective oncological surgery for different types of cancer.
Disclosure
Professor Paolo Navalesi reports grants, personal fees, and non-financial support from Maquet Critical Care, grants and non-financial support from Draeger and Intersurgical, and personal fees from Orion Pharma, Philips, Resmed, MSD, and Novartis, outside the submitted work. The authors report no other relevant affiliation or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
References
- 1.Horowitz M, Neeman E, Sharon E, et al. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol. 2015;12(4):213–226. doi: 10.1038/nrclinonc.2014.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim R. Anesthetic technique for cancer surgery: harm or benefit for cancer recurrence? Eur J Surg Oncol. 2018;44(5):557–558. doi: 10.1016/j.ejso.2018.02.207 [DOI] [PubMed] [Google Scholar]
- 3.Tavare AN, Perry NJ, Benzonana LL, et al. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130(6):1237–1250. doi: 10.1002/ijc.26448 [DOI] [PubMed] [Google Scholar]
- 4.Dubowitz JA, Sloan EK, Riedel BJ. Implicating anaesthesia and the perioperative period in cancer recurrence and metastasis. Clin Exp Metastasis. 2018;35(4):347–358. doi: 10.1007/s10585-017-9862-x [DOI] [PubMed] [Google Scholar]
- 5.Chou R, Gordon DB, de Leon-casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131–157. doi: 10.1016/j.jpain.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 6.Fried IA. The influence of the anaesthetic on survival rates of breast cancer patients after surgery. Int J Cancer. 1977;20(2):213–218. doi: 10.1002/(ISSN)1097-0215 [DOI] [PubMed] [Google Scholar]
- 7.Shapiro J, Jersky J, Katzav S, et al. Anesthetic drugs accelerate the progression of postoperative metastases of mouse tumors. J Clin Invest. 1981;68(3):678–685. doi: 10.1172/JCI110303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishikawa M, Tanaka S, Arai M, et al. Differences in microRNA changes of healthy rat liver between sevoflurane and propofol anesthesia. Anesthesiology. 2012;117(6):1245–1252. doi: 10.1097/ALN.0b013e3182746676 [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto A, Imai J, Nishikawa A, et al. Influence of inhalation anesthesia assessed by comprehensive gene expression profiling. Gene. 2005;356:39–48. doi: 10.1016/j.gene.2005.03.022 [DOI] [PubMed] [Google Scholar]
- 10.Melamed R, Bar-Yosef S, Shakhar G, et al. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg. 2003;97(5):1331–1339. doi: 10.1213/01.ANE.0000082995.44040.07 [DOI] [PubMed] [Google Scholar]
- 11.Roesslein M, Schibilsky D, Muller L, et al. Thiopental protects human T lymphocytes from apoptosis in vitro via the expression of heat shock protein 70. J Pharmacol Exp Ther. 2008;325(1):217–225. doi: 10.1124/jpet.107.133108 [DOI] [PubMed] [Google Scholar]
- 12.Braun S, Gaza N, Werdehausen R, et al. Ketamine induces apoptosis via the mitochondrial pathway in human lymphocytes and neuronal cells. Br J Anaesth. 2010;105(3):347–354. doi: 10.1093/bja/aeq169 [DOI] [PubMed] [Google Scholar]
- 13.Loop T, Liu Z, Humar M, et al. Thiopental inhibits the activation of nuclear factor kappaB. Anesthesiology. 2002;96(5):1202–1213. doi: 10.1097/00000542-200205000-00025 [DOI] [PubMed] [Google Scholar]
- 14.Kushida A, Inada T, Shingu K. Enhancement of antitumor immunity after propofol treatment in mice. Immunopharmacol Immunotoxicol. 2007;29(3–4):477–486. doi: 10.1080/08923970701675085 [DOI] [PubMed] [Google Scholar]
- 15.Inada T, Kubo K, Shingu K. Possible link between cyclooxygenase-inhibiting and antitumor properties of propofol. J Anesth. 2011;25(4):569–575. doi: 10.1007/s00540-011-1163-y [DOI] [PubMed] [Google Scholar]
- 16.Inada T, Yamanouchi Y, Jomura S, et al. Effect of propofol and isoflurane anaesthesia on the immune response to surgery. Anaesthesia. 2004;59(10):954–959. doi: 10.1111/ana.2004.59.issue-10 [DOI] [PubMed] [Google Scholar]
- 17.Takabuchi S, Hirota K, Nishi K, et al. The intravenous anesthetic propofol inhibits hypoxia-inducible factor 1 activity in an oxygen tension-dependent manner. FEBS Lett. 2004;577(3):434–438. doi: 10.1016/j.febslet.2004.10.042 [DOI] [PubMed] [Google Scholar]
- 18.Tanaka T, Takabuchi S, Nishi K, et al. The intravenous anesthetic propofol inhibits lipopolysaccharide-induced hypoxia-inducible factor 1 activation and suppresses the glucose metabolism in macrophages. J Anesth. 2010;24(1):54–60. doi: 10.1007/s00540-009-0829-1 [DOI] [PubMed] [Google Scholar]
- 19.Humar M, Andriopoulos N, Pischke SE, et al. Inhibition of activator protein 1 by barbiturates is mediated by differential effects on mitogen-activated protein kinases and the small G proteins ras and rac-1. J Pharmacol Exp Ther. 2004;311(3):1232–1240. doi: 10.1124/jpet.104.071332 [DOI] [PubMed] [Google Scholar]
- 20.Nagata T, Kansha M, Irita K, et al. Propofol inhibits FMLP-stimulated phosphorylation of p42 mitogen-activated protein kinase and chemotaxis in human neutrophils. Br J Anaesth. 2001;86(6):853–858. doi: 10.1093/bja/86.6.853 [DOI] [PubMed] [Google Scholar]
- 21.Mammoto T, Mukai M, Mammoto A, et al. Intravenous anesthetic, propofol inhibits invasion of cancer cells. Cancer Lett. 2002;184(2):165–170. doi: 10.1016/S0304-3835(02)00210-0 [DOI] [PubMed] [Google Scholar]
- 22.Markovic SN, Knight PR, Murasko DM. Inhibition of interferon stimulation of natural killer cell activity in mice anesthetized with halothane or isoflurane. Anesthesiology. 1993;78(4):700–706. doi: 10.1097/00000542-199304000-00013 [DOI] [PubMed] [Google Scholar]
- 23.Kim R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med. 2018;16(1):8. doi: 10.1186/s12967-018-1389-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loop T, Dovi-Akue D, Frick M, et al. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology. 2005;102(6):1147–1157. doi: 10.1097/00000542-200506000-00014 [DOI] [PubMed] [Google Scholar]
- 25.Pirbudak Cocelli L, Ugur MG, Karadasli H. Comparison of effects of low-flow sevoflurane and desflurane anesthesia on neutrophil and T-cell populations. Curr Ther Res Clin Exp. 2012;73(1–2):41–51. doi: 10.1016/j.curtheres.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deegan CA, Murray D, Doran P, et al. Anesthetic technique and the cytokine and matrix metalloproteinase response to primary breast cancer surgery. Reg Anesth Pain Med. 2010;35(6):490–495. doi: 10.1097/AAP.0b013e3181ef4d05 [DOI] [PubMed] [Google Scholar]
- 27.Itoh T, Hirota K, Hisano T, et al. The volatile anesthetics halothane and isoflurane differentially modulate proinflammatory cytokine-induced p38 mitogen-activated protein kinase activation. J Anesth. 2004;18(3):203–209. doi: 10.1007/s00540-004-0237-5 [DOI] [PubMed] [Google Scholar]
- 28.Ecimovic P, McHugh B, Murray D, et al. Effects of sevoflurane on breast cancer cell function in vitro. Anticancer Res. 2013;33(10):4255–4260. [PubMed] [Google Scholar]
- 29.Liang H, Yang CX, Zhang B, et al. Sevoflurane suppresses hypoxia-induced growth and metastasis of lung cancer cells via inhibiting hypoxia-inducible factor-1alpha. J Anesth. 2015;29(6):821–830. doi: 10.1007/s00540-015-2035-7 [DOI] [PubMed] [Google Scholar]
- 30.Sacerdote P, Bianchi M, Gaspani L, et al. The effects of tramadol and morphine on immune responses and pain after surgery in cancer patients. Anesth Analg. 2000;90(6):1411–1414. doi: 10.1097/00000539-200006000-00028 [DOI] [PubMed] [Google Scholar]
- 31.Das J, Kumar S, Khanna S, et al. Are we causing the recurrence-impact of perioperative period on long-term cancer prognosis: review of current evidence and practice. J Anaesthesiol Clin Pharmacol. 2014;30(2):153–159. doi: 10.4103/0970-9185.129996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shavit Y, Ben-Eliyahu S, Zeidel A, et al. Effects of fentanyl on natural killer cell activity and on resistance to tumor metastasis in rats. Dose and timing study. Neuroimmunomodulation. 2004;11(4):255–260. doi: 10.1159/000078444 [DOI] [PubMed] [Google Scholar]
- 33.Sacerdote P, Gaspani L, Rossoni G, et al. Effect of the opioid remifentanil on cellular immune response in the rat. Int Immunopharmacol. 2001;1(4):713–719. doi: 10.1016/S1567-5769(01)00005-4 [DOI] [PubMed] [Google Scholar]
- 34.Beilin B, Martin FC, Shavit Y, et al. Suppression of natural killer cell activity by high-dose narcotic anesthesia in rats. Brain Behav Immun. 1989;3(2):129–137. doi: 10.1016/0889-1591(89)90013-5 [DOI] [PubMed] [Google Scholar]
- 35.Yeager MP, Colacchio TA, Yu CT, et al. Morphine inhibits spontaneous and cytokine-enhanced natural killer cell cytotoxicity in volunteers. Anesthesiology. 1995;83(3):500–508. doi: 10.1097/00000542-199509000-00008 [DOI] [PubMed] [Google Scholar]
- 36.Gupta K, Kshirsagar S, Chang L, et al. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62(15):4491–4498. [PubMed] [Google Scholar]
- 37.Qi Y, Yao X, Zhang B, et al. Comparison of recovery effect for sufentanil and remifentanil anesthesia with TCI in laparoscopic radical resection during colorectal cancer. Oncol Lett. 2016;11(5):3361–3365. doi: 10.3892/ol.2016.4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page GG, McDonald JS, Ben-Eliyahu S. Pre-operative versus postoperative administration of morphine: impact on the neuroendocrine, behavioural, and metastatic-enhancing effects of surgery. Br J Anaesth. 1998;81(2):216–223. doi: 10.1093/bja/81.2.216 [DOI] [PubMed] [Google Scholar]
- 39.Tegeder I, Grosch S, Schmidtko A, et al. G protein-independent G1 cell cycle block and apoptosis with morphine in adenocarcinoma cells: involvement of p53 phosphorylation. Cancer Res. 2003;63(8):1846–1852. [PubMed] [Google Scholar]
- 40.Lucchinetti E, Awad AE, Rahman M, et al. Antiproliferative effects of local anesthetics on mesenchymal stem cells: potential implications for tumor spreading and wound healing. Anesthesiology. 2012;116(4):841–856. doi: 10.1097/ALN.0b013e31824babfe [DOI] [PubMed] [Google Scholar]
- 41.Sakaguchi M, Kuroda Y, Hirose M. The antiproliferative effect of lidocaine on human tongue cancer cells with inhibition of the activity of epidermal growth factor receptor. Anesth Analg. 2006;102(4):1103–1107. doi: 10.1213/01.ane.0000198330.84341.35 [DOI] [PubMed] [Google Scholar]
- 42.Chang YC, Liu CL, Chen MJ, et al. Local anesthetics induce apoptosis in human breast tumor cells. Anesth Analg. 2014;118(1):116–124. doi: 10.1213/ANE.0b013e3182a94479 [DOI] [PubMed] [Google Scholar]
- 43.Yoon JR, Whipple RA, Balzer EM, et al. Local anesthetics inhibit kinesin motility and microtentacle protrusions in human epithelial and breast tumor cells. Breast Cancer Res Treat. 2011;129(3):691–701. doi: 10.1007/s10549-010-1239-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mammoto T, Higashiyama S, Mukai M, et al. Infiltration anesthetic lidocaine inhibits cancer cell invasion by modulating ectodomain shedding of heparin-binding epidermal growth factor-like growth factor (HB-EGF). J Cell Physiol. 2002;192(3):351–358. doi: 10.1002/(ISSN)1097-4652 [DOI] [PubMed] [Google Scholar]
- 45.Juneja R. Opioids and cancer recurrence. Curr Opin Support Palliat Care. 2014;8(2):91–101. doi: 10.1097/SPC.0000000000000056 [DOI] [PubMed] [Google Scholar]
- 46.Girard N. Evidence appraisal of Haugen AS, Softeland E, Eide GE, et al. Impact of the World Health Organization’s Surgical Safety Checklist on safety culture in the operating theatre: a controlled intervention study. Br J Anaesth. 2013;110(5);807–815. AORN J. 2013;98(6):663–668. doi: 10.1093/bja/aet005 [DOI] [PubMed] [Google Scholar]
- 47.Siekmann W, Eintrei C, Magnuson A, et al. Surgical and not analgesic technique affects postoperative inflammation following colorectal cancer surgery: a prospective, randomized study. Colorectal Dis. 2017;19(6):O186–O195. doi: 10.1111/codi.2017.19.issue-6 [DOI] [PubMed] [Google Scholar]
- 48.Grandhi RK, Lee S, Abd-Elsayed A. The relationship between regional anesthesia and cancer: a metaanalysis. Ochsner J. 2017;17(4):345–361. [PMC free article] [PubMed] [Google Scholar]
- 49.Kim R. Anesthetic technique and cancer recurrence in oncologic surgery: unraveling the puzzle. Cancer Metastasis Rev. 2017;36(1):159–177. doi: 10.1007/s10555-016-9647-8 [DOI] [PubMed] [Google Scholar]
- 50.Byrne K, Levins KJ, Buggy DJ. Can anesthetic-analgesic technique during primary cancer surgery affect recurrence or metastasis? Can J Anaesth. 2016;63(2):184–192. doi: 10.1007/s12630-015-0523-8 [DOI] [PubMed] [Google Scholar]
- 51.Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology. 2016;124(1):69–79. doi: 10.1097/ALN.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 52.Enlund M, Berglund A, Andreasson K, et al. The choice of anaesthetic–sevoflurane or propofol–and outcome from cancer surgery: a retrospective analysis. Ups J Med Sci. 2014;119(3):251–261. doi: 10.3109/03009734.2014.922649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee JH, Kang SH, Kim Y, et al. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: a retrospective study. Korean J Anesthesiol. 2016;69(2):126–132. doi: 10.4097/kjae.2016.69.2.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang YT, Wu CC, Tang TY, et al. Differences between total intravenous anesthesia and inhalation anesthesia in free flap surgery of head and neck cancer. PLoS One. 2016;11(2):e0147713. doi: 10.1371/journal.pone.0147713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sofra M, Fei PC, Fabrizi L, et al. Immunomodulatory effects of total intravenous and balanced inhalation anesthesia in patients with bladder cancer undergoing elective radical cystectomy: preliminary results. J Exp Clin Cancer Res. 2013;32:6. doi: 10.1186/1756-9966-32-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee JJ, Kim GH, Kim JA, et al. Comparison of pulmonary morbidity using sevoflurane or propofol-remifentanil anesthesia in an Ivor Lewis operation. J Cardiothorac Vasc Anesth. 2012;26(5):857–862. doi: 10.1053/j.jvca.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 57.Liu S, Gu X, Zhu L, et al. Effects of propofol and sevoflurane on perioperative immune response in patients undergoing laparoscopic radical hysterectomy for cervical cancer. Medicine (Baltimore). 2016;95(49):e5479. doi: 10.1097/MD.0000000000005479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soltanizadeh S, Degett TH, Gogenur I. Outcomes of cancer surgery after inhalational and intravenous anesthesia: a systematic review. J Clin Anesth. 2017;42:19–25. doi: 10.1016/j.jclinane.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 59.Yap A, Lopez-Olivo MA, Dubowitz J, et al. Anesthetic technique and cancer outcomes: a meta-analysis of total intravenous versus volatile anesthesia. Can J Anaesth. 2019;66(5):546–561. doi: 10.1007/s12630-019-01330-x [DOI] [PubMed] [Google Scholar]
- 60.Buggy DJ, Borgeat A, Cata J, et al. Consensus statement from the BJA workshop on cancer and anaesthesia. Br J Anaesth. 2015;114(1):2–3. doi: 10.1093/bja/aeu262 [DOI] [PubMed] [Google Scholar]
- 61.Buggy DJ, Freeman J, Johnson MZ, et al. Systematic review and consensus definitions for standardised endpoints in perioperative medicine: postoperative cancer outcomes. Br J Anaesth. 2018;121(1):38–44. doi: 10.1016/j.bja.2018.03.020 [DOI] [PubMed] [Google Scholar]