Abstract
BACKGROUND:
While perinatal depression is one of the most common complications of pregnancy, there is an insufficient understanding of the mechanistic underpinnings of disease. While an association between peripheral inflammatory cytokines and major depressive disorder has been demonstrated, cytokines cannot freely cross the blood-brain barrier, and thus, they give little insight into alternations in brain function. Because the brain is in direct communication with the cerebrospinal fluid, assessment of inflammation in the cerebrospinal fluid may be more directly related to the biologic markers of affective change.
OBJECTIVE:
Our objectives were to examine the association between perinatal depression and inflammatory cytokines in plasma, the association between perinatal depression and inflammatory cytokines in cerebrospinal fluid, and the correlations between plasma and cerebrospinal fluid inflammatory cytokines.
STUDY DESIGN:
This was a prospective, observational study of women with a singleton gestation at term undergoing a scheduled cesarean delivery. Women were screened for depression and those with depressive symptomatology preferentially enrolled. The Mini-International Neuropsychiatric Interview was administered to confirm the clinical diagnosis of depression. Maternal plasma and cerebrospinal fluid were collected preoperatively and cytokines measured via flow cytometry. Bivariable and multivariable analyses were used to determine the association between each cytokine and perinatal depression. Correlations were measured between the cytokines in plasma and cerebrospinal fluid.
RESULTS:
Of the 117 women who met inclusion criteria, 76 (65%) screened positive for depression, 15 (20%) of whom met the clinical diagnostic criteria for depression. There were no significant associations between any of the plasma cytokines and perinatal depression in our sample. Conversely, in multivariable analyses, higher cerebrospinal fluid interleukin-1β (adjusted odds ratio, 232.7, 95% confidence interval, 5.9–9148.5), interleukin-23 (adjusted odds ratio, 22.1, 95% confidence interval, 1.7–294.5), and interleukin-33 (adjusted odds ratio, 1.7, 95% confidence interval, 1.1–2.6) concentrations were significantly associated with increased odds of perinatal depression. The plasma and cerebrospinal fluid cytokine concentrations were not strongly correlated.
CONCLUSION:
Higher concentrations of cerebrospinal fluid cytokines were associated with perinatal depression. These cerebrospinal fluid cytokines were not strongly correlated with plasma cytokines, and accordingly, plasma cytokines were not significantly associated with perinatal depression. Central neuroinflammation, as opposed to peripheral inflammation, may represent a mechanistic pathway that contributes to perinatal depression.
Keywords: antenatal depression, cerebrospinal fluid, cytokines, interleukin-1 beta, interleukin-23, interleukin-33, plasma
Depression is becoming increasingly recognized as a disorder of immune hyperactivation.1–8 Patients with major depressive disorder have increased plasma inflammatory biomarkers, most consistently interleukin(IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and C-reactive protein.9,10 Blockade of the production of these cytokines is associated with reduced depressive symptoms.11–14 Studies in the perinatal period have suggested that postpartum depression is associated with increased plasma inflammatory biomarkers, most consistently IL-1β, soluble IL-1 receptor antagonist, and IL-6.15–18
While peripheral cytokines are able to cross the blood-brain barrier1, equilibrium between cerebrospinal fluid (CSF) and plasma is not established and peripheral blood markers may not reflect the intracerebral environment milieu.19 Although cytokines are large molecules, a variety of mechanisms facilitate transport between plasma and CSF. These include the following: (1) leaky regions of the blood-brain barrier, (2) active transport via various facilitative molecules, (3) activation of cells surrounding the cerebral vasculature, and (4) binding of cytokine receptors on peripheral afferent nerve fibers.1
When inflammatory cytokines are present in the CSF, they can cause damage to existing nerve cells as well as inhibition of neural cell growth in the hippocampus, amygdala, prefrontal cortex, anterior cingulate, and basal ganglia, which leads to symptoms of depression.20 There is conflicting reported evidence between depressive symptomatology and CSF inflammatory biomarkers (Table 1). Heterogeneity in research methodology, including indications and circumstances for undergoing a lumbar puncture, may explain some of these reported differences.
TABLE 1.
Prior studies examining the association between depression and cerebrospinal fluid inflammatory biomarkers outside pregnancy
| Author | Comparison | CSF changes |
|---|---|---|
| Janelidze et al48 | Suicide attempters with anxiety vs healthy controls | IL-8 decreased |
| Bay-Richter et al49 | Suicide attempters vs healthy controls | IL-6 increased |
| Kern et al50 | Geriatric women with and without depression | IL-6 and IL-8 increased |
| Lindqvist et al51 | Parkinson’s disease with and without depression | CRP and MCP-1 increased |
| Erhardt et al52 | Suicide attempters vs healthy controls | IL-6 increased |
| Sasayama et al53 | People with MDD vs healthy controls | IL-6 increased |
| Isung et al54 | Suicide attempters vs healthy controls | IL-8 decreased |
| Martinez et al55 | People with MDD vs healthy controls | IL-1, IL-6, and TNF-α increased |
| Lindqvist et al19 | Suicide attempters vs healthy controls | IL-6 increased |
| Raison et al56 | People receiving IFN with and without depression | IL-6 and MCP-1 increased |
| Carpenter et al57 | MDD vs healthy controls | No change in IL-6 levels |
| Levine et al58 | MDD vs healthy controls | IL-1β increased, IL-6 decreased, no change in TNF-α |
| Stubner et al59 | Geriatric individuals with and without MDD | IL-6 decreased |
| Hestad et al60 | People with neurologic complaints with and without depression | No changes in CSF cytokines |
CRP, C-reactive protein; CSF, cerebrospinal fluid; IFN, interferon; IL, interleukin; MCP, monocyte chemotactic protein; MDD, major depressive disorder; TNF, tumor necrosis factor.
Pregnancy further complicates the inflammatory assessment because normal pregnancy represents a state of immunologic changes. As pregnancy progresses, increases in peripheral phagocytic (monocytes and granulocytes) and dendritic cells have been observed that are partially offset by decreases in CD4, CD8, B, and NK cells.21 Correspondingly, plasma cytokines and chemokines involved in phagocytic recruitment, such as TNFα, increase, whereas plasma inflammatory molecules, such as vascular endothelial growth factor and interferon (IFN)-γ decrease throughout gestation.22
Despite these changes, perinatal depression has been shown to be associated with increased plasma inflammatory cytokines, similar to major depressive disorder outside pregnancy.15–18 The association between depressive symptomatology and CSF inflammatory cytokines has not been well established in pregnant patients. A positive correlation between CSF IL-6 and TNFα and scores on the Edinburgh Postnatal Depression Scale has been reported in parturients; however, the authors did not account for the potential underlying impact of labor itself or establish clinical diagnoses of major depressive disorder.23
Perinatal depression, or depression that begins either during pregnancy or within 12 months postpartum, is one of the most common complications of pregnancy affecting approximately 1 in 7 women.24,25 However, the majority of women with depression are neither identified nor treated.26,27 This disparity between clinical need and care provision is not due to a lack of access to the health care system in general because contact with health care professionals is typically increased in the perinatal period. The unmet need reflects barriers to mental health care.27 Furthermore, current treatments are not often fully effective, and only a minority of women achieve a therapeutic response.26,27
Defining the mechanistic underpinnings of perinatal depression, including the role of inflammation, is critical to developing more effective interventions. To address this gap in knowledge, we examined the association between both plasma and CSF inflammatory cytokines and perinatal depression. We hypothesized that women with perinatal depression would exhibit higher concentrations of inflammatory cytokines in the plasma and CSF compared with euthymic women.
Materials and Methods
Overview
This prospective observational study aimed to compare inflammatory cytokines in simultaneous plasma and CSF samples from women at term with and without an antenatal major depressive episode. A second aim was to determine whether inflammatory cytokines in the plasma or CSF correlate with later postpartum depressive symptomatology. The third aim was to assess the correlation between CSF and maternal plasma inflammatory cytokines. The Northwestern University Institutional Review Board approved this study, and all women provided written informed consent.
Subjects
Women with singleton, term gestations who were undergoing a scheduled cesarean delivery were eligible for participation. Women in labor (either clinically diagnosed, self-reported to have regular painful contractions, or endorsing leakage of fluid) were excluded because of the potential of the parturition process to alter markers of inflammation obtained at the time of epidural or spinal placement. Similarly, women with diabetes or preeclampsia were excluded because of their potential to confound observed associations, given their relationship with depression28,29 and their inflammatory underpinnings.30 Women also were excluded if they were younger than 18 years old, had diagnosed anomalies in their fetuses, were living with HIV, or were taking antiinflammatory medications (eg, steroids, nonsteroidal antiinflammatory drugs) within 2 weeks of delivery. For this analysis, women treated with antidepressants also were excluded because of the antiinflammatory effects of these medications.31–35
Measures and procedures
Women who presented for a scheduled cesarean delivery were approached upon admission for surgery and, after consent was obtained, screened for depression using the Inventory of Depressive Symptomatology-Self-Report (IDS-SR30). The IDS-SR30 is a depression screen with excellent psychometric properties,36 with a reported sensitivity of 78% and specificity of 76% for prenatal depression.37
The IDS-SR30 asks questions about depressive symptoms over the preceding week and is a valid screen for antenatal depression when administered prior to delivery. The IDS-SR30 was used to identify women with moderate to severe depressive symptoms to enrich the sample for women with clinical evidence of a major depressive episode. One screen-negative (IDS-SR<18) woman was enrolled for every 2 screen-positive women (IDS-SR≥18) who were identified. An IDS-SR score above 11 signifies mild depression, whereas a score above 23 signifies moderate depression.
The choice of 18 as a cutoff was made to enrich the sample with women who had more significant symptomatology to ensure an adequate sample of women with clinical major depressive disorder for analysis. A member of the research team, trained and supervised by a perinatal psychologist, administered the Mini-International Neuropsychiatric Interview (MINI, version 5.0.0) in all enrolled women as the gold standard used to confirm the clinical diagnosis of an active antenatal major depressive episode as well as to identify any other Diagnostic and Statistical Manual of Mental Disorders, fourth edition, psychiatric diagnoses.
While establishing intravenous access, 15 mL of maternal blood was drawn; this sample was centrifuged at −4°C to obtain plasma, which was immediately stored at −80°C. At the time of spinal analgesia administration, 2 mL of CSF was aspirated prior to anesthetic injection and immediately stored at −80°C. Both plasma and CSF samples were assayed in duplicate using flow cytometry and a multiplex bead-based assay panel for 13 inflammatory cytokines/chemokines including IL-1β, IFN-α, IFN-γ, TNF-α, monocyte chemotactic protein (MCP)-1, IL-6, IL-8, IL-10, IL-12p70, IL-17A, IL-18, IL-23, and IL-33 (LEGENDplex; BioLegend, San Diego, CA).
This panel was chosen because it includes cytokines previously associated with major depressive disorder or perinatal depression in the existing literature.9,10,23 Characteristics of the assay, including sensitivity and cross-reactivity are available online.38 The minimum detectable concentration in serum has been reported to be between 0.6 and 1.9 pg/mL for each of the examined cytokines. Each sample was tested in triplicate and each sample was run twice in independent assays. The assay used monoclonal antibodies that have been well characterized and have no known cross-reactivity.
On postpartum day 1, self-reported surveys were administered to collect sociodemographic characteristics. In addition, members of the research team abstracted the medical and obstetric history from the electronic medical records with quality control via spot checks performed by another investigator (E.S.M.). An assessment of perceived stress, measured using Cohen’s Perceived Stress Scale (PSS), was obtained,39 given the established relationship between stress and inflammatory cytokines.40,41 Women were contacted via electronic mail between 4 and 8 weeks postpartum, at which time they were asked to complete a follow-up IDS-SR30.
Statistical analyses
Women were stratified by the diagnosis of a current antenatal major depressive episode (MDE). Sociodemographic and clinical characteristics, as well as PSS scores, were compared across these 2 groups using χ2, Fisher exact, or Mann-Whitney U tests as appropriate. Plasma and CSF cytokine concentrations were compared between women with and without an MDE using Mann-Whitney U tests. Plasma and CSF cytokines that differed between women with and without an MDE at the 20% level of significance (ie, P < .20) were entered into multivariable logistic regression models for the outcome of MDE. Potential confounders were identified using the change in estimate criteria of 10%, in which variables that altered the unadjusted exposure-outcome association by 10% or more were included in multivariable models to establish whether each assayed plasma or CSF cytokine was independently associated with the presence of an MDE.
Analyses further involved Spearman’s sample correlation coefficients of the entire cohort to evaluate a potential relationship between cytokine concentrations and antenatal depressive symptomatology (IDS-SR score). A subscale of the IDS-SR allowed for assessment of the presence of symptoms ascribed to the inflammatory subtype of depression (atypical depression),13 and Spearman’s correlations were used to estimate the association between cytokine concentrations and atypical depressive symptoms. Spearman’s correlations were used to estimate the associations between the cytokine concentrations and the postpartum IDS-SR score. Finally, Spearman’s correlations between CSF and maternal plasma inflammatory cytokines were also estimated. Analyses were performed using Stata version 14.0 (StataCorp, College Station, TX). Tests were all 2 tailed and, unless otherwise specified, assumed a 5% level of statistical significance. We did not adjust P values to account for multiple hypothesis tests.
Results
Cohort characteristics
Of the 1251 women approached for eligibility, 390 consented to participate. After excluding those who did not have biospecimen obtained or who were using an selective serotonin reuptake inhibitor, 117 participants were enrolled between March 2014 and June 2016 in the final sample (Figure). Of the 76 women who endorsed at least moderate depressive symptomatology on the IDS-SR30 (IDS-SR30≥18), 15 (20%) had an MDE based on the MINI evaluation. Of these 15 women, 14 (93%) had a comorbid anxiety disorder, and 6 (40%) had a history suggestive of possible bipolar disorder based on their MINI evaluation.
FIGURE.
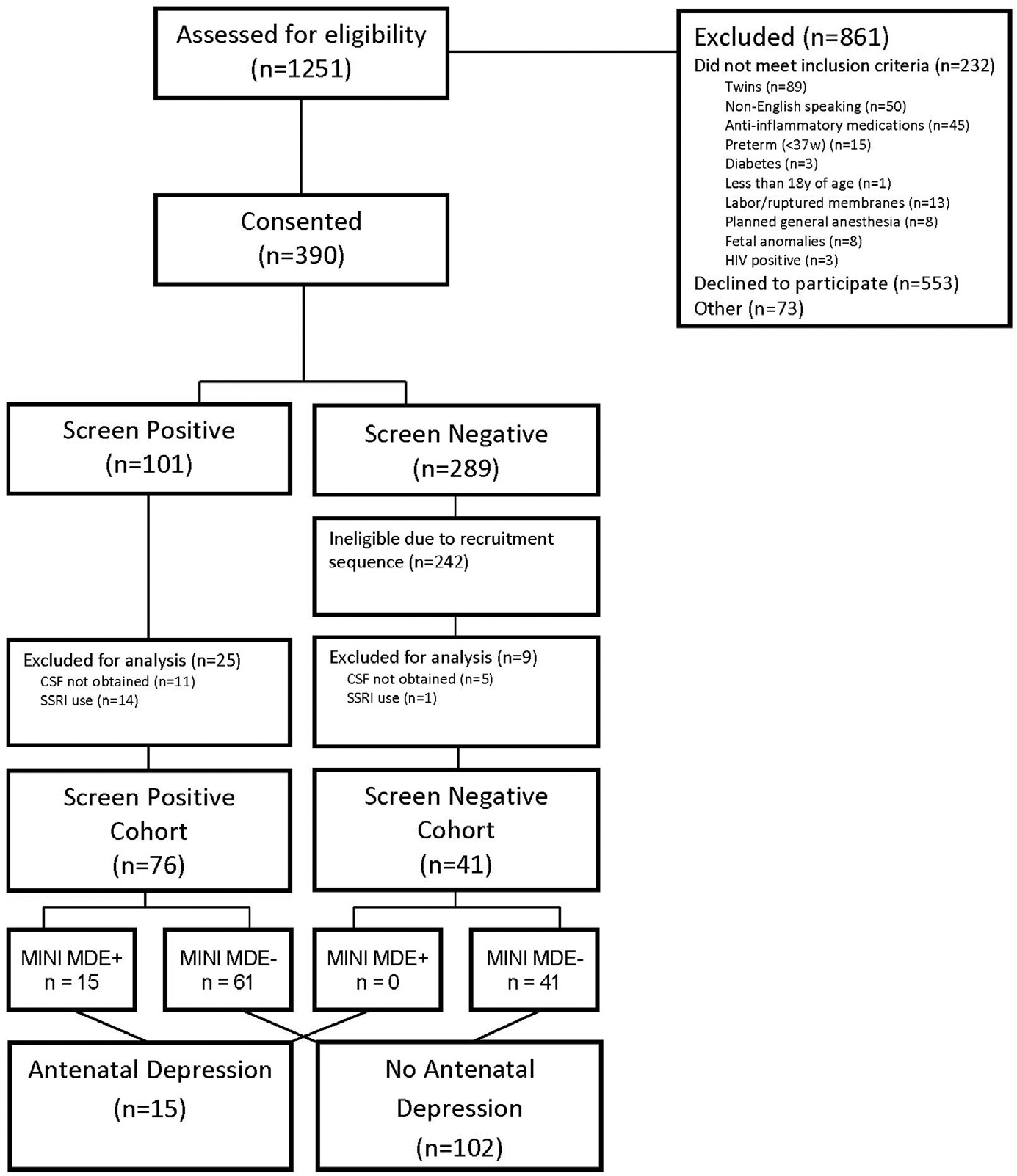
Study flow chart
Of the 61 screen-positive women who did not have an MDE, 11 (18%) had an active anxiety disorder and 4 (5%) had a history of possible bipolar disorder based on their MINI evaluation. The remainder of women had subclinical depressive symptoms, given the absence of diagnostic criteria for a major depressive episode.
Baseline characteristics of the cohort stratified by the presence of an MDE determined by the MINI are shown in Table 2. Compared with women not experiencing an MDE, women with an MDE tended to be younger, have a lower household income, have a higher body mass index at delivery, have a lower level of education, and were more likely to be non-Hispanic black. Women with an MDE also had higher levels of perceived stress.
TABLE 2.
Sociodemographic and clinical characteristics of the cohort, stratified by depression status
| Characteristics | MDE negative (n = 102) | MDE positive (n = 15) | P value |
|---|---|---|---|
| Maternal age, y | 35.3 ± 3.7 | 33.1 ± 5.5 | .045 |
| Race/ethnicity | .014 | ||
| Non-Hispanic white | 75 (73.5%) | 8 (53.3%) | |
| Non-Hispanic black | 8 (7.8%) | 6 (40.0%) | |
| Hispanic | 14 (13.7%) | 1 (6.7%) | |
| Other/unknown | 5 (4.9%) | 0 (0.0%) | |
| Education | .031 | ||
| Completed college | 90 (88.2%) | 10 (66.7%) | |
| Some college/trade school | 10 (9.8%) | 3 (20.0%) | |
| High school or less | 2 (2.0%) | 3 (13.3%) | |
| Employed | 90 (88.2%) | 12 (80.0%) | .407 |
| Married | 93 (91.2%) | 11 (73.3%) | .063 |
| Household income | .006 | ||
| <$40,000 | 9 (8.8%) | 1 (6.7%) | |
| $40,000–79,000 | 5 (4.9%) | 5 (33.3%) | |
| ≥$80,000 | 88 (86.3%) | 9 (60.0%) | |
| Nulliparous | 22 (21.6%) | 1 (6.7%) | .297 |
| BMI at delivery, kg/m2 | 31.1 (27.3–34.0) | 33.6 (29.2–43.2) | .026 |
| Gestational weight gain, lb | 33.7 ± 10.8 | 35.9 ± 10.3 | .464 |
| Gestational weight gain >35 lb | 38 (37.3%) | 9 (60.0%) | .093 |
| Tobacco use (ever) | 18 (17.7%) | 4 (26.7%) | .478 |
| GA at delivery | 39.3 (39.0–39.6) | 39.0 (39.0–39.3) | .084 |
| ≥39 wks at delivery | 90 (88.2%) | 14(93.3%) | .557 |
| Indication for cesarean delivery | .747 | ||
| Repeat | 76 (74.5%) | 13 (86.7%) | |
| Fetal malposition | 12 (11.8%) | 1 (6.7%) | |
| Placenta previa | 1 (1.0%) | 0 (0.0%) | |
| Other | 13 (12.8%) | 1 (6.7%) | |
| PSS score | 19 ± 7 | 30 ± 7 | < .001 |
Data are reported as mean ± SD, n (percentage), or median (interquartile range).
BMI, body mass index; GA, gestational age; MDE, major depressive episode; PSS, Perceived Stress Scale.
Antenatal depression and cytokines
The standard curve for each cytokine was 2–10,000 pg/mL. Prior to testing any clinical samples, recombinant IL-6, IL-8, and IFN gamma were purchased and titered into 3 independent plasma and CSF samples for assay. The calculated curves ([plasma or CSF + cytokine] − [plasma or CSF alone]) for all 3 cytokines were as expected, indicating that the assay performed as described in the presence of plasma or CSF. No cross-reactivity was detected.
Empiric distributions of plasma and CSF cytokines were highly skewed (assessed using the Shapiro-Wilk normality test) despite attempted transformations (including cubic, square, square root, log, 1/square root, inverse, 1/square, and 1/cubic transformations); therefore, analyses involved nonparametric methods. IL-1β and IL-33 were below the limits of detection in the plasma. There were no significant differences in plasma cytokines between women with and without an MDE (Table 3). Aside from IL-6, point estimates for all measured CSF inflammatory cytokines were higher in women experiencing an MDE compared with those without an MDE, although none of these differences reached statistical significance (Table 3). A post hoc power calculation, using an alpha of 0.05, demonstrated that we had 80% power to identify a 1.8-, 1.3-, and 1.4-fold change in CSF IL-1β, IL-6, and TNF-α, respectively, associated with a major depressive episode.
TABLE 3.
Bivariable analyses of plasma and cerebrospinal fluid cytokines stratified by perinatal depression status
| Variables | Plasma | CSF | ||||
|---|---|---|---|---|---|---|
| MDE negative | MDE positive | P value | MDE negative | MDE positive | P value | |
| IL-1β | — | — | — | 0.49 (0.32–0.79) | 0.79 (0.49–1.00) | .062 |
| IL-6 | 3.05 (1.81–6.39) | 1.81 (1.74–6.14) | .283 | 2.32 (1.89–2.97) | 2.43 (1.60–2.67) | .481 |
| IL-8 | 2.28 (1.76–3.73) | 2.28 (1.30–5.59) | .955 | 110.91 (91.72–131.74) | 100.49 (95.50–114.94) | .268 |
| IL-10 | 1.92 (1.58–2.34) | 1.92 (1.58–2.34) | .795 | — | — | — |
| IL-12p70 | 1.95 (1.55–2.44) | 1.95 (0.65–2.44) | .990 | — | — | — |
| IL-17a | 2.53 (1.60–6.36) | 2.53 (1.60–4.89) | .922 | — | — | — |
| IL-18 | 167.74 (119.24–242.81) | 140.26 (110.76–239.46) | .660 | 0.90 (0.63–1.10) | 1.10 (0.63–1.10) | .167 |
| IL-23 | 2.60 (2.16–6.94) | 2.60 (2.16–5.39) | .589 | 1.01 (0.97–1.04) | 1.04 (0.97–1.07) | .161 |
| IL-33 | — | — | — | 4.19 (1.92–5.67) | 4.33 (4.06–5.67) | .179 |
| IFN-α | 2.07 (1.95–4.01) | 2.10 (1.95–6.55) | .813 | — | — | — |
| IFN-γ | 4.93 (3.18–9.49) | 4.53 (3.18–9.49) | .784 | 1.62 (1.45–2.54) | 2.25 (1.01–4.42) | .700 |
| TNF-α | 1.82 (1.45–2.22) | 1.82 (1.75–2.22) | .925 | 0.93 (0.33–1.15) | 1.04 (0.88–1.15) | .290 |
| MCP-1 | 267.49 (193.70–367.30) | 247.89 (173.36–68.24) | .594 | 971.80 (800.81–1135.92) | 1094.16 (745.67–1203.80) | .361 |
Data are presented as median (interquartile range) in picograms per milliliter.
CSF, cerebrospinal fluid; IFN, interferon; IL, interleukin; MCP, monocyte chemotactic protein; MDE, major depressive episode; TNF, tumor necrosis factor.
Because CSF IL-1β, IL-18, IL-23, and IL-33 were associated with an MDE at a significance level less than .2, they were included in the planned multivariable analyses. IL-1β, IL-23, and IL-33 all were significantly associated with an MDE after controlling for potential confounders (Table 4).
TABLE 4.
Multivariable analyses for the outcome of a major depressive episode
| Variables | aORa | 95% CI |
|---|---|---|
| CSF IL-1β | 232.7 | 5.9–9148.5 |
| Non-Hispanic black race | 17.8 | 1.9–169.0 |
| Nulliparous | 0.03 | 0.01–0.05 |
| Gestational age at delivery, wks | 0.4 | 0.1–1.3 |
| PSS score | 1.4 | 1.2–1.6 |
| CSF IL-23b | 22.1 | 1.7–294.5 |
| BMI at delivery, kg/m2 | 1.2 | 1.0–1.3 |
| PSS score | 1.3 | 1.1–1.5 |
| CSF IL-33b | 1.7 | 1.1–2.6 |
| Maternal age, y | 0.9 | 0.7–1.0 |
| Nulliparous | 0.2 | 0.1–2.0 |
| PSS score | 1.3 | 1.1–1.5 |
aOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; CSF, cerebrospinal fluid; IL, interleukin; PSS, Perceived Stress Scale.
Adjusted for the listed potential confounders;
represents 3 separate multivariable analyses.
Antenatal depressive symptomatology and cytokines
There were no significant correlations between antenatal IDS-SR scores and any plasma or CSF cytokine (Table 5).
TABLE 5.
Correlations between cerebrospinal fluid cytokines and antenatal depressive symptomatology
| Variables | Antenatal IDS-SR score | |
|---|---|---|
| Spearman’s rho | P value | |
| Plasma IL-6 | 0.01 | .925 |
| Plasma IL-8 | 0.14 | .127 |
| Plasma IL-10 | 0.03 | .783 |
| Plasma IL-12p70 | 0.06 | .542 |
| Plasma IL-17a | 0.05 | .634 |
| Plasma IL-18 | 0.10 | .277 |
| Plasma IL-23 | −0.01 | .895 |
| Plasma IFN-α | 0.01 | .972 |
| Plasma IFN-γ | 0.16 | .084 |
| Plasma TNF-α | 0.05 | .573 |
| Plasma MCP-1 | 0.15 | .118 |
| CSF IL-1β | 0.01 | .890 |
| CSF IL-6 | 0.06 | .533 |
| CSF IL-8 | 0.01 | .910 |
| CSF IL-18 | −0.06 | .496 |
| CSF IL-23 | 0.01 | .888 |
| CSF IL-33 | 0.02 | .792 |
| CSF IFN-γ | 0.08 | .398 |
| CSF TNF-α | 0.03 | .719 |
| CSF MCP-1 | 0.07 | .439 |
CSF, cerebrospinal fluid; IDS-SR, Inventory of Depressive Symptomatology-Self Report; IFN, interferon; IL, interleukin; MCP, monocyte chemotactic protein; TNF, tumor necrosis factor.
Atypical depression
There was a weak correlation between atypical depression and plasma IFN-γ but no significant correlation between any of the other plasma or CSF cytokines and the IDS-SR30 atypical depression scores (Table 6). These findings were unchanged when women with other psychiatric comorbidities were excluded from analysis (data not shown).
TABLE 6.
Correlations between plasma cerebrospinal fluid cytokines and depressive symptomatology
| Variables | Antenatal atypical depression score | Postpartum IDS-SR score | ||
|---|---|---|---|---|
| Spearman’s rho | P value | Spearman’s rho | P value | |
| Plasma IL-6 | −0.05 | 0.598 | −0.11 | .288 |
| Plasma IL-8 | 0.18 | 0.054 | 0.01 | .909 |
| Plasma IL-10 | 0.11 | 0.240 | 0.22 | .037 |
| Plasma IL-12p70 | 0.20 | 0.031 | 0.14 | .197 |
| Plasma IL-17a | −0.01 | 0.961 | 0.15 | .161 |
| Plasma IL-18 | 0.11 | 0.234 | 0.02 | .836 |
| Plasma IL-23 | −0.04 | 0.689 | −0.18 | .085 |
| Plasma IFNα | −0.09 | 0.368 | −0.05 | .628 |
| Plasma IFNγ | 0.21 | 0.024 | 0.17 | .109 |
| Plasma TNFα | −0.06 | 0.501 | −0.05 | .673 |
| Plasma MCP-1 | 0.09 | 0.318 | 0.06 | .570 |
| CSF IL-1β | 0.08 | 0.390 | −0.04 | .724 |
| CSF IL-6 | −0.04 | 0.707 | 0.07 | .529 |
| CSF IL-8 | −0.02 | 0.797 | 0.05 | .672 |
| CSF IL-18 | −0.10 | 0.302 | 0.02 | .828 |
| CSF IL-23 | 0.06 | 0.545 | 0.01 | .924 |
| CSF IL-33 | −0.03 | 0.766 | 0.11 | .316 |
| CSF IFNγ | 0.01 | 0.960 | −0.05 | .621 |
| CSF TNFα | −0.03 | 0.787 | 0.18 | .086 |
| CSF MCP-1 | 0.01 | 0.926 | 0.04 | .678 |
CSF, cerebrospinal fluid; IDS-SR, Inventory of Depressive Symptomatology-Self-Report; IFN, interferon; IL, interleukin; MCP, monocyte chemotactic protein; TNF, tumor necrosis factor.
Postpartum depressive symptomatology
Of the 117 women who met inclusion criteria, 90 (77%) completed a postpartum IDS-SR30: 46 (51%) with no, 34 (38%) with mild, 9 (10%) with moderate, and 1 (1%) with severe depressive symptoms. There was an observed weak correlation between plasma IL-10 and postpartum depressive symptomatology, but no other significant correlations between postpartum IDS-SR scores and the measured plasma or CSF inflammatory cytokines (Table 6).
Correlations between plasma and CSF cytokines
Plasma IL-6, IL-8, IFN-γ, and MCP-1 were not significantly correlated with their corresponding CSF cytokines. While IL-18, IL-23, and TNF-α did exhibit statistically significant correlations between plasma and CSF concentrations, these relationships were all weak (Table 7).
TABLE 7.
Correlations between cerebrospinal fluid and plasma cytokines
| Variables | Spearman’s rho | P value |
|---|---|---|
| IL-6 | −0.03 | .751 |
| IL-8 | −0.01 | .950 |
| IL-18 | 0.37 | < .001 |
| IL-23 | 0.31 | < .001 |
| IFNγ | 0.07 | .465 |
| TNFα | 0.21 | .026 |
| MCP-1 | −0.05 | .598 |
IL, interleukin; IFN, interferon; MCP, monocyte chemotactic protein; TNF, tumor necrosis factor.
Comment
Findings and proposed pathophysiology
In term pregnant women, CSF concentrations of IL-1β, IL-23, and IL-33 are increased in the setting of an active major depressive episode. IL-1β and IL-33 are both in the IL-1 family of cytokines and may be mechanistically linked with antenatal depression via associated alterations in tryptophan metabolism.42
Tryptophan is the precursor to serotonin, a monoamine whose production is critical to mood stability. Th1-cytokines, such as the IL-1 family of cytokines, induce expression of indolamine-2,3-dioxygenase, which degrades tryptophan into its catabolites (ie, kynurenine and quinolinic acid). Not only does the breakdown of tryptophan yield a state of relative serotonin depletion, but these catabolites have also been shown to independently induce depressive and anxiety symptoms.
IL-23 has received less study in the pathogenesis of depression. IL-23 is a proinflammatory cytokine involved in the differentiation of Th17 lymphocytes.43 In this role, IL-23 has recently been recognized as essential in the development of autoimmunity, and trials of antibodies to IL-23 for the treatment of psoriasis, rheumatoid arthritis, and Crohn’s disease are underway.44 Based on our results along with the promising preliminary findings in other disease states with a dysregulated inflammatory system, further investigation of the role of Th17 lymphocytes and the IL-23 immune axis in the pathophysiology of depression is warranted.
Comparison to existing literature
In contrast to the CSF findings, plasma concentrations of inflammatory cytokines were not associated with depressive symptomatology, atypical depressive symptomatology, or an active major depressive episode. These findings differ from previous reports.15–18 One reason for this discrepancy might be that this investigation excluded women who have experienced antenatal inflammatory conditions such as preterm labor, preeclampsia, or diabetes. Perhaps by systematically excluding women with these complications, we selected a subgroup of antenatal depression that did not have an inflammatory phenotype.
To the best of our knowledge, there is only 1 prior manuscript assessing CSF cytokines and postpartum depressive symptomatology. Boufidou et al23 demonstrated a positive association between the two CSF cytokines examined, IL-6 and TNF-α, and depressive mood symptoms in the first 4 days postpartum. While we did not identify significant correlations between these cytokines and postpartum depressive symptomatology, as described in the previous text, our restrictive inclusion criteria may have masked an association that may be present in a more generalized population.
Strengths and limitations
The results of our study are strengthened by the use of the MINI to achieve a diagnosis of perinatal depression. Another strength of this study is the exclusion of women in labor, which avoided potential parturitional alterations in inflammation. While this strengthens our confidence in the observed association between inflammatory markers and perinatal depression, our findings may not be generalizable. A significant number of approached women (553, 44%) declined to participate. Furthermore, because inflammatory cytokines are increased in women who undergo spontaneous preterm labor, our inclusion criteria (women who had not entered spontaneous labor and were at term) may have selected a subset of women with less systemic inflammation.
Each of these characteristics of the study sample may limit the generalizability of the findings when applied to the broader obstetric population. In addition, we do not have data on the serum/CSF albumin ratio, which has been postulated to impact CSF concentrations of cytokines45 and may be influenced by inflammatory mechanisms.46
Another limitation is that, given the relatively small sample size of women with an active MDE, bivariable analyses between cytokines and perinatal depression were not significant and our multivariable models may be over-saturated. While this should raise some caution when interpreting the results, the consistent association between IL-1β, IL-23, and IL-33 concentrations and MDE in both bivariable and multivariable analyses supports the veracity of this relationship.
Similarly, because of the relatively small sample size, convergence could not be achieved when attempting to fit the data into a log-linear model, and thus, a logistic regression with its associated odds ratio is reported. Consequently, the magnitude of the observed associations in the multivariable analyses may be slightly lower than those reflected in the reported odds ratios.
Finally, given their antiinflammatory effects,31–35 this study excluded women with perinatal depression taking antidepressant medications, and the results may not apply to them. However, because the majority of women with perinatal depression in the United States do not receive treatment,27,47 we believe that this exclusion results in applicability to many women with major depressive disorder and is more reflective of the pathophysiology underlying major depressive disorder.
Depressive symptomatology
We were unable to identify a dose-response relationship between antenatal depressive symptomatology and CSF inflammatory cytokines, which raises caution in interpreting the observed associations. One explanation could be a threshold effect of inflammation that yields overt depression (defined by the MINI) and that subclinical depressive symptoms (ie, women with a positive IDS-SR30 but a negative screen for a major depressive episode on the MINI) represent a separate pathophysiology.
Alternatively, as evidenced by the discordance between the IDS-SR screen results and the diagnostic findings on the MINI, the heterogeneity of diagnoses uncovered by a positive IDS-SR (eg, anxiety, bipolar disorder) biases correlations between overt depressive symptomatology and inflammatory cytokines toward the null. In addition, there was not a strong correlation between postpartum depressive symptomatology and any of the measured plasma or CSF inflammatory cytokines. This suggests that any observed cytokine alterations are a transient finding and not a harbinger for future depressive symptoms.
Implications of the findings
Notably, we identified a lack of strong correlation between plasma and CSF inflammatory cytokines. The discrepancy between plasma and CSF cytokines emphasizes the critical need for paired CSF and plasma for biomarker development research and novel therapeutic development in the field of perinatal depression. Specific focus should be paid to the pathophysiologic mechanisms that may link CSF IL-1β, IL-23, and IL-33 concentrations with development of a MDE.
Conclusion
In conclusion, CSF concentrations of the inflammatory cytokines IL-1β, IL-23, and IL-33 are independently associated with an MDE in pregnant women at term. These findings support a neuroinflammatory mechanism for perinatal depression. If confirmed, these findings would support future research on adjunctive antiinflammatory agents to reduce depressive symptomatology during pregnancy.
AJOG at a Glance.
Why was this study conducted?
Major depression outside pregnancy can be a disorder of immune hyperactivation. Peripheral cytokines are large molecules that cannot freely cross the blood-brain barrier and may not reflect the intracerebral milieu. Assessment of inflammation in the cerebrospinal fluid (CSF) is more directly related to biologic markers of affective change and may inform the pathophysiology of perinatal depression.
Key findings
In multivariable analyses, higher CSF concentrations of interleukin (IL)-1β, IL-23, and IL-33 were associated with an increased risk for perinatal depression.
What does this study add to what is known?
Plasma and CSF inflammatory cytokines are not correlated in pregnant women. While plasma inflammatory cytokines are not associated, CSF IL-1β, IL-23, and IL-33 are associated with perinatal depression. Further exploration of the neurobiologic mechanisms of perinatal depression is necessary to facilitate biomarker development and identify novel treatment targets.
Acknowledgments
The funding sources had no role in the study design, data collection, analysis or interpretation, writing the report, or submitting the article for publication.
This study was supported by National Institute of Child and Human Development grant K12 HD050121-09, the Friends of Prentice Foundation, and National Institutes of Health’s National Center for Advancing Translational Sciences grant UL1TR001422.
Footnotes
The authors report no conflicts of interest.
Presented as an oral presentation at the annual meeting of the Society for Maternal-Fetal Medicine, Dallas, Texas, Feb. 2, 2018.
References
- 1.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009;65:732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 2013;246:199–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woiciechowsky C, Schoning B, Lanksch WR, Volk HD, Docke WD. Mechanisms of brain-mediated systemic anti-inflammatory syndrome causing immunodepression. J Mol Med (Berl) 1999;77:769–80. [DOI] [PubMed] [Google Scholar]
- 4.Szelenyi J, Vizi ES. The catecholamine cytokine balance: interaction between the brain and the immune system. Ann N Y Acad Sci 2007;1113:311–24. [DOI] [PubMed] [Google Scholar]
- 5.Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev 2005;29:891–909. [DOI] [PubMed] [Google Scholar]
- 6.Maes M, Berk M, Goehler L, et al. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med 2012;10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Soczynska JK, Kennedy SH. Inflammatory biomarkers in depression: an opportunity for novel therapeutic interventions. Curr Psychiatry Rep 2011;13:316–20. [DOI] [PubMed] [Google Scholar]
- 8.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 2006;27: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010;67:446–57. [DOI] [PubMed] [Google Scholar]
- 10.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009;71:171–86. [DOI] [PubMed] [Google Scholar]
- 11.Kohler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 2014;71:1381–91. [DOI] [PubMed] [Google Scholar]
- 12.Abbott R, Whear R, Nikolaou V, et al. Tumour necrosis factor-alpha inhibitor therapy in chronic physical illness: a systematic review and meta-analysis of the effect on depression and anxiety. J Psychosom Res 2015;79:175–84. [DOI] [PubMed] [Google Scholar]
- 13.Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 2013;70:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet 2006;367:29–35. [DOI] [PubMed] [Google Scholar]
- 15.Corwin EJ, Johnston N, Pugh L. Symptoms of postpartum depression associated with elevated levels of interleukin-1 beta during the first month postpartum. Biol Res Nurs 2008;10:128–33. [DOI] [PubMed] [Google Scholar]
- 16.Maes M, Lin AH, Ombelet W, et al. Immune activation in the early puerperium is related to postpartum anxiety and depressive symptoms. Psychoneuroendocrinology 2000;25:121–37. [DOI] [PubMed] [Google Scholar]
- 17.Maes M, Ombelet W, De Jongh R, Kenis G, Bosmans E. The inflammatory response following delivery is amplified in women who previously suffered from major depression, suggesting that major depression is accompanied by a sensitization of the inflammatory response system. J Affect Disord 2001;63: 85–92. [DOI] [PubMed] [Google Scholar]
- 18.Maes M, Verkerk R, Bonaccorso S, Ombelet W, Bosmans E, Scharpe S. Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sci 2002;71: 1837–48. [DOI] [PubMed] [Google Scholar]
- 19.Lindqvist D, Janelidze S, Hagell P, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry 2009;66:287–92. [DOI] [PubMed] [Google Scholar]
- 20.Maes M, Yirmyia R, Noraberg J, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis 2009;24:27–53. [DOI] [PubMed] [Google Scholar]
- 21.Kraus TA, Engel SM, Sperling RS, et al. Characterizing the pregnancy immune phenotype: results of the viral immunity and pregnancy (VIP) study. J Clin Immunol 2012;32:300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraus TA, Sperling RS, Engel SM, et al. Peripheral blood cytokine profiling during pregnancy and post-partum periods. Am J Reprod Immunol 2010;64:411–26. [DOI] [PubMed] [Google Scholar]
- 23.Boufidou F, Lambrinoudaki I, Argeitis J, et al. CSF and plasma cytokines at delivery and postpartum mood disturbances. J Affect Disord 2009;115:287–92. [DOI] [PubMed] [Google Scholar]
- 24.Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evidence Rep Technol Assess 2005:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wisner KL, Sit DK, McShea MC, et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry 2013;70:490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett IM, Marcus SC, Palmer SC, Coyne JC. Pregnancy-related discontinuation of antidepressants and depression care visits among Medicaid recipients. Psychiatr Serv 2010;61:386–91. [DOI] [PubMed] [Google Scholar]
- 27.Vesga-Lopez O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry 2008;65:805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kharaghani R, Geranmaye M, Janani L, et al. Preeclampsia and depression: a case-control study in Tehran. Arch Gynecol Obstet 2012;286:249–53. [DOI] [PubMed] [Google Scholar]
- 29.Kozhimannil KB, Pereira MA, Harlow BL. Association between diabetes and perinatal depression among low-income mothers. JAMA 2009;301:842–7. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Bao W, Liu J, et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2013;36:166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frommberger UH, Bauer J, Haselbauer P, Fraulin A, Riemann D, Berger M. Interleukin-6 (IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci 1997;247:228–33. [DOI] [PubMed] [Google Scholar]
- 32.Kenis G, Maes M. Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol 2002;5:401–12. [DOI] [PubMed] [Google Scholar]
- 33.Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 2000;22: 370–9. [DOI] [PubMed] [Google Scholar]
- 34.Sluzewska A, Rybakowski JK, Laciak M, Mackiewicz A, Sobieska M, Wiktorowicz K. Interleukin-6 serum levels in depressed patients before and after treatment with fluoxetine. Ann N Y Acad Sci 1995;762:474–6. [DOI] [PubMed] [Google Scholar]
- 35.Tuglu C, Kara SH, Caliyurt O, Vardar E, Abay E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology 2003;170:429–33. [DOI] [PubMed] [Google Scholar]
- 36.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med 1996;26:477–86. [DOI] [PubMed] [Google Scholar]
- 37.Brunoni AR, Benute GR, Fraguas R, et al. The self-rated Inventory of Depressive Symptomatology for screening prenatal depression. Int J Gynaecol Obstet 2013;121: 243–6. [DOI] [PubMed] [Google Scholar]
- 38.BioLegend. LEGENDplex multi-analyte flow assay kit: human inflammation panel. San Diego (CA): BioLegend; 2018. [Google Scholar]
- 39.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. [PubMed] [Google Scholar]
- 40.Christian LM. Psychoneuroimmunology in pregnancy: immune pathways linking stress with maternal health, adverse birth outcomes, and fetal development. Neurosci Biobehav Rev 2012;36:350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shapiro GD, Fraser WD, Frasch MG, Seguin JR. Psychosocial stress in pregnancy and preterm birth: associations and mechanisms. J Perinat Med 2013;41: 631–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry 2011;35: 702–21. [DOI] [PubMed] [Google Scholar]
- 43.Toussirot E The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases. Inflamm Allergy Drug Targets 2012;11: 159–68. [DOI] [PubMed] [Google Scholar]
- 44.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 2014;14: 585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isgren A, Jakobsson J, Palsson E, et al. Increased cerebrospinal fluid interleukin-8 in bipolar disorder patients associated with lithium and antipsychotic treatment. Brain Behav Immun 2015;43:198–204. [DOI] [PubMed] [Google Scholar]
- 46.Yao Y, Tsirka SE. Monocyte chemoattractant protein-1 and the blood-brain barrier. Cell Mol Life Sci 2014;71:683–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayes RM, Wu P, Shelton RC, et al. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies. Am J Obstet Gynecol 2012;207:49–e1–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janelidze S, Suchankova P, Ekman A, et al. Low IL-8 is associated with anxiety in suicidal patients: genetic variation and decreased protein levels. Acta Psychiatr Scand 2015;131:269–78. [DOI] [PubMed] [Google Scholar]
- 49.Bay-Richter C, Linderholm KR, Lim CK, et al. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain Behav Immun 2015;43:110–7. [DOI] [PubMed] [Google Scholar]
- 50.Kern S, Skoog I, Borjesson-Hanson A, et al. Higher CSF interleukin-6 and CSF interleukin-8 in current depression in older women. Results from a population-based sample. Brain Behav Immun 2014;41:55–8. [DOI] [PubMed] [Google Scholar]
- 51.Lindqvist D, Hall S, Surova Y, et al. Cerebrospinal fluid inflammatory markers in Parkinson’s disease–associations with depression, fatigue, and cognitive impairment. Brain Behav Immun 2013;33:183–9. [DOI] [PubMed] [Google Scholar]
- 52.Erhardt S, Lim CK, Linderholm KR, et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology 2013;38:743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sasayama D, Hattori K, Wakabayashi C, et al. Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J Psychiatr Res 2013;47:401–6. [DOI] [PubMed] [Google Scholar]
- 54.Isung J, Aeinehband S, Mobarrez F, et al. Low vascular endothelial growth factor and interleukin-8 in cerebrospinal fluid of suicide attempters. Transl Psychiatry 2012;2:e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinez JM, Garakani A, Yehuda R, Gorman JM. Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depression Anxiety 2012;29:32–8. [DOI] [PubMed] [Google Scholar]
- 56.Raison CL, Borisov AS, Majer M, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry 2009;65:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carpenter LL, Heninger GR, Malison RT, Tyrka AR, Price LH. Cerebrospinal fluid interleukin (IL)-6 in unipolar major depression. J Affect Disord 2004;79:285–9. [DOI] [PubMed] [Google Scholar]
- 58.Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology 1999;40:171–6. [DOI] [PubMed] [Google Scholar]
- 59.Stubner S, Schon T, Padberg F, et al. Interleukin-6 and the soluble IL-6 receptor are decreased in cerebrospinal fluid of geriatric patients with major depression: no alteration of soluble gp130. Neurosci Lett 1999;259: 145–8. [DOI] [PubMed] [Google Scholar]
- 60.Hestad KA, Engedal K, Whist JE, et al. Patients with depression display cytokine levels in serum and cerebrospinal fluid similar to patients with diffuse neurological symptoms without a defined diagnosis. Neuropsychiatr Dis Treat 2016;12:817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


