Abstract
Objectives
To investigate pharmacokinetics (PK) and safety (primary objectives) and efficacy (secondary objective) of the investigational monobactam/β-lactamase inhibitor combination aztreonam/avibactam in patients with complicated intra-abdominal infection (cIAI).
Methods
This Phase 2a open-label, multicentre study (NCT02655419; EudraCT 2015-002726-39) enrolled adults with cIAI into sequential cohorts for 5–14 days treatment. Cohort 1 patients received an aztreonam/avibactam loading dose of 500/137 mg (30 min infusion), followed by maintenance doses of 1500/410 mg (3 h infusions) q6h; Cohort 2 received 500/167 mg (30 min infusion), followed by 1500/500 mg (3 h infusions) q6h. Cohort 3 was an extension of exposure at the higher dose regimen. Doses were adjusted for creatinine clearance of 31–50 mL/min (Cohorts 2 + 3). All patients received IV metronidazole 500 mg q8h. PK, safety and efficacy were assessed.
Results
Thirty-four patients (Cohort 1, n = 16; Cohorts 2 + 3, n = 18) comprised the modified ITT (MITT) population. Mean exposures of aztreonam and avibactam in Cohorts 2 + 3 were consistent with those predicted to achieve joint PK/pharmacodynamic target attainment in >90% patients. Adverse events (AEs) were similar between cohorts. The most common AEs were hepatic enzyme increases [n = 9 (26.5%)] and diarrhoea [n = 5 (14.7%)]. Clinical cure rates at the test-of-cure visit overall were 20/34 (58.8%) (MITT) and 14/23 (60.9%) (microbiological-MITT population).
Conclusions
Observed AEs were consistent with the known safety profile of aztreonam monotherapy, with no new safety concerns identified. These data support selection of the aztreonam/avibactam 500/167 mg (30 min infusion) loading dose and 1500/500 mg (3 h infusions) maintenance dose q6h regimen, in patients with creatinine clearance >50 mL/min, for the Phase 3 development programme.
Introduction
Bacterial infections caused by MDR Gram-negative pathogens, including ESBL-producing and carbapenem-resistant Enterobacteriaceae (CRE), are increasing in prevalence and represent a serious threat to public health.1,2 Strains that express MBLs are of particular concern as coexpression of serine β-lactamases and other drug resistance determinants means these strains are often resistant to a range of antibiotics,3–5 resulting in a lack of effective treatment options and a high level of unmet need.
Complicated intra-abdominal infections (cIAIs) are polymicrobial in nature, with pathogens frequently including Enterobacteriaceae, particularly Escherichia coli and Klebsiella spp.; those less frequently isolated include obligate and facultative anaerobes and Gram-positive cocci.6–9 cIAIs are an important cause of morbidity and mortality, particularly if not adequately managed.10,11
The monobactam aztreonam has a well-established safety and efficacy profile and is licensed for the treatment of various serious infections caused by susceptible Gram-negative bacteria.12 Aztreonam has activity against MBL-producing pathogens, but can be hydrolysed and rendered inactive by most Ambler Class A, C and D serine β-lactamases.13,14 Avibactam is a broad-spectrum non-β-lactam β-lactamase inhibitor that inhibits Ambler class A, C and some D β-lactamases.15
The combination of ceftazidime with avibactam is approved in the USA and Europe,16,17 and many other countries worldwide, for the treatment of various serious infections caused by susceptible Gram-negative bacteria. The safety profile of ceftazidime/avibactam across infection types is consistent with the established safety profile of ceftazidime monotherapy, with the addition of avibactam resulting in no clinically relevant safety differences.16,17 In contrast to ceftazidime/avibactam and the newer β-lactam/β-lactamase inhibitors, the combination of avibactam with aztreonam restores the in vitro activity and in vivo efficacy (in preclinical models) of aztreonam against MBL-producing pathogens via inhibition of coexpressed serine β-lactamases,18–21 making it a unique potential treatment option.
A Phase 1, randomized, double-blind study (NCT01689207) showed aztreonam/avibactam to be generally well tolerated, with no evidence of drug–drug interaction between aztreonam and avibactam in healthy subjects, and identified a dosing regimen for further evaluation.22
The current Phase 2a study (REJUVENATE) was the first study of aztreonam/avibactam in a representative population of patients with cIAI, with the aim of selecting the dose regimen for the Phase 3 aztreonam/avibactam development programme. Metronidazole was coadministered with aztreonam/avibactam to provide coverage for anaerobic organisms. The primary objectives were to investigate the pharmacokinetics (PK) and safety of aztreonam/avibactam in patients with cIAI. Assessment of clinical efficacy was a secondary objective.
The study is the first interventional clinical trial conducted within the Innovative Medicines Initiative (IMI)-supported Combatting Bacterial Resistance in Europe - Carbapenem Resistance (COMBACTE-CARE) project. IMI is a joint undertaking between the EU and the European Federation of Pharmaceutical Industries and Associations (EFPIA). COMBACTE-CARE is a consortium of 19 academic and 3 pharmaceutical partners focusing on carbapenem resistance in Europe with the aim of increasing the efficiency of antimicrobial drug development.
Patients and methods
Ethics
The study was conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization (ICH) Good Clinical Practice Guidelines. The study protocol was approved by the relevant Institutional Review Boards and/or Independent Ethics Committees at each study site: CPP sud-ouest et Outre-Mer IV, Cabanis Haut—Centre hospitalier Esquirol, Limoges, France (CPP16-006); Universitaet zu Koeln—Geschaeftsstelle Ethikkommission, Cologne, Germany (15-451); CEI de los Hospitales Universitarios Virgen Macarena y Virgen del Rocío, Seville, Spain. All patients provided written informed consent. If patients were unable to provide informed consent at screening, they could be entered into the study by their guardian or legal representative (if in accordance with national and local regulations and as approved by the institution-specific guidelines) and provide their own written informed consent for continuing to participate in the study as soon as possible on recovery.
Study design and patients
REJUVENATE was a prospective, open-label, single-arm, multicentre study (NCT02655419; EudraCT: 2015-002726-39). Eligible patients were aged 18–90 years with a diagnosis of cIAI and a requirement for surgical intervention within 24 h prior to or after initiation of study treatment. A full list of inclusion and exclusion criteria for the study can be found in Text S1 (available as Supplementary data at JAC Online). Initially, only patients with creatinine clearance (CLCR) >50 mL/min (estimated using the Cockcroft–Gault formula) were eligible for enrolment (Cohort 1). A planned review of all available safety and PK data, by the Scientific Advisory Committee (SAC), occurred once the first 10 patients in Cohort 1 had completed all scheduled assessments. Enrolment was paused during this review. The SAC made recommendations regarding the ongoing conduct of the study. A protocol amendment (see below) broadened the inclusion criteria for Cohorts 2 and 3 to also include patients with CLCR of 31–50 mL/min, with provision for an appropriately adjusted dosing regimen and incorporated a second SAC review of safety and PK data from Cohort 2.
Treatments
Patients were enrolled into three sequential cohorts. Dosage regimens are shown in Table 1. Aztreonam/avibactam dosage regimen for Cohort 1 was supported by population PK modelling and based on the final dosage regimen evaluated in the Phase 1 study by Edeki et al.22 The population PK model was used in Monte Carlo simulations to select a dosing regimen that achieved a joint PTA for aztreonam and avibactam of >90%. The joint PTA was based on PK/pharmacodynamic (PD) targets of free (unbound) concentrations of aztreonam exceeding an MIC of 8 mg/L for ≥60% of a dosing interval (≥60% fT>MIC) and free concentrations of avibactam exceeding a threshold concentration (CT) of 2.5 mg/L for ≥50% of a dosing interval (≥50% fT>CT). The PK/PD targets and joint PTA for aztreonam and avibactam are reported separately.23
Table 1.
Dosing regimen for each cohort and renal function group
| Cohort and CLCR threshold | Aztreonam/avibactam loading dose (30 min IV infusion) | Aztreonam/avibactam extended loading dose | Aztreonam/avibactam maintenance infusion (3 h IV infusion q6h | Metronidazole (1 h IV infusion q8h) |
|---|---|---|---|---|
| Cohort 1 (CLCR >50 mL/min) | 500/137 mg | not applicable | 1500/410 mga | 500 mg |
| Cohorts 2 and 3 (CLCR >50 mL/min) | 500/167 mg | not applicable | 1500/500 mga | 500 mg |
| Cohorts 2 and 3 (CLCR 31–50 mL/min) | 500/167 mg | 1500/500 mg by 3 h IV infusionb | 750/250 mgb | 500 mg |
First maintenance dose administered immediately after completion of loading dose.
Extended loading dose administered immediately after completion of loading dose; first maintenance dose administered 3 h after completion of extended loading dose.
Population PK modelling, simulation and PTA analyses indicated that an increase in the avibactam component of the regimen optimizes joint PTA >90% at an MIC of 8 mg/L.23 In addition, dose regimens were proposed for patients with CLCR of 31–50 mL/min. A protocol amendment therefore provided options for an optimized (i.e. 500 mg q6h avibactam) dosing regimen, as well as exposure-equivalent regimens for patients with CLCR of 31–50 mL/min and a revised discontinuation criterion (CLCR ≤30 mL/min). Selection of the dosing regimen used in Cohorts 2 and 3 was subject to recommendations from the SAC, following planned interim reviews of safety and PK data from Cohorts 1 and 2. Patients in Cohort 2 subsequently received aztreonam/avibactam at the 1500/500 mg q6h avibactam regimen, with Cohort 3 as an extension cohort at this same dose.
For all cohorts, the planned duration of IV treatment with aztreonam/avibactam (plus IV metronidazole for anaerobic coverage) was 5–14 days. If patients had shown significant clinical improvement after a minimum of 5 days IV therapy, all study treatments could be discontinued at the discretion of the investigator. Daily estimation of CLCR was required during treatment to guide appropriate dose adjustment or discontinuation.
Assessments
PK
Blood samples for plasma concentration analysis of aztreonam and avibactam were collected at predefined sampling times on Day 1 and on Day 4 (±1 day). All patients underwent sparse PK sampling (four samples/patient) on Day 1. On Day 4 (±1 day), the first 25 patients enrolled were to undergo intensive PK sampling (11 samples/patient). The remaining 15 patients were to undergo sparse PK sampling on Day 4 (±1 day). Samples were drawn from the opposite arm to that which was used for drug infusions if possible. A central venous catheter (CVC) could be used if peripheral sampling was not possible and a CVC was required for clinical reasons. Aztreonam and avibactam plasma concentrations were determined by Covance Bioanalytical Laboratory (Harrogate, UK) using validated LC–MS/MS methods (described in Text S1). The lower limit of quantification was 0.1 μg/mL for aztreonam and 10 ng/mL for avibactam. Aztreonam and avibactam PK parameters were calculated for each patient with intensive plasma sampling collected on Day 4 (±1 day) using non-compartmental analysis of concentration–time data (Phoenix® WinNonlin 6.4; Certara L.P., St Louis, Missouri, USA).
Safety
The number, causality and severity of adverse events (AEs) were monitored throughout the study, from screening (Visit 1) until the late follow-up (LFU) visit (Day 35 ± 3 days). Vital signs were assessed at screening, at baseline (Visit 2), daily during treatment, at the end of treatment (EOT), at the test-of-cure (TOC) visit (Day 25 ± 3 days) and at LFU. A complete physical examination was performed at screening, EOT, TOC and LFU visits. ECGs were performed in triplicate on each occasion at baseline (prior to dosing), twice on Day 3 and once at EOT. Hepatic enzymes and renal function [AST, ALT, alkaline phosphatase (ALP), GGT, total bilirubin and CLCR] were closely monitored throughout the treatment period and intensified monitoring of liver-related laboratory parameters initiated when specific safety criteria were attained (details provided in Text S1).
Efficacy
Assessments included the number and percentage of patients with investigator-determined clinical cure, clinical failure and indeterminate outcomes at the EOT, TOC and LFU visits (criteria for outcomes shown in Table S1).
Microbiological samples
Specimen collection and culture of isolates for determination of pathogens are described in Text S1.
Statistical methods
Up to 40 patients were to be enrolled. Although the study was not powered to perform statistical tests, assessment of safety and complete PK assessments from at least 30 patients with cIAI were considered adequate to confirm the safety and PK profile of aztreonam/avibactam in a population with a representative burden of disease.
PK were analysed for all patients with ≥1 plasma concentration data assessment available, no fundamental violations of the study enrolment criteria, or protocol violations affecting assessment of PK (PK population). Safety was analysed for the modified ITT (MITT) population, which included all enrolled patients who received any amount of study drug. Efficacy was analysed for the MITT population and the microbiologically MITT (mMITT) population, which included all enrolled patients who had a diagnosis of cIAI and had ≥1 intra-abdominal pathogen isolated at baseline, regardless of susceptibility to aztreonam/avibactam.
Descriptive statistics were used to summarize PK, safety and efficacy continuous variables.
Role of the funding source
The academic and EFPIA IMI-supported COMBACTE-CARE consortium partners were involved in: study design; data collection, analysis and interpretation; and data checking of information provided in the article. Responsibility for opinions, conclusions and data interpretation lies with the authors. All authors had full access to all study data and final responsibility for the decision to submit for publication.
Results
Patients
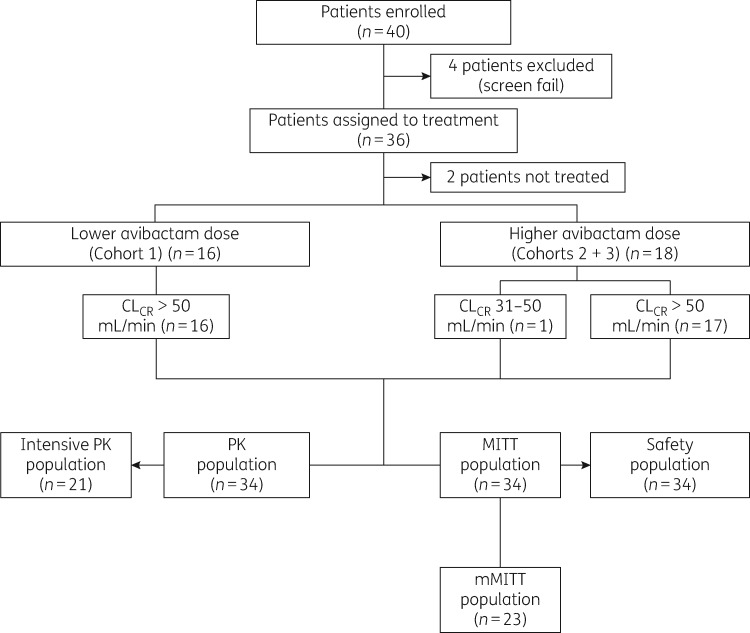
Between 19 May 2016 and 26 October 2017, 40 patients were enrolled across 11 centres located in France, Germany and Spain and, of these, 34 received treatment and thus comprised the MITT population (Cohort 1, n = 16; Cohorts 2 and 3, n = 18); patient disposition is shown in Figure 1. Primary diagnosis relating to appendiceal perforation or peri-appendiceal abscess was at a frequency of 41.2% (Table 2). Only one patient (Cohorts 2 and 3) had CLCR of 31–50 mL/min at baseline. However, this patient, who had acquired acute kidney injury prior to study inclusion, was withdrawn from study treatment on Day 2, owing to further deterioration in renal function meeting the discontinuation criterion. Patient demographics and baseline characteristics were generally well balanced across cohorts (Table 2).
Figure 1.
Patient flow diagram.
Table 2.
Patient demographics and baseline characteristics, including cIAI diagnosis (MITT population)
| Patients, n (%) |
|||
|---|---|---|---|
| Characteristic | Cohort 1 (n = 16) | Cohorts 2 + 3 (n = 18) | total (N = 34) |
| Age (years) | |||
| <65 | 13 (81.3) | 13 (72.2) | 26 (76.5) |
| 65–74 | 3 (18.8) | 5 (27.8) | 8 (23.5) |
| median (range) | 49.0 (31–70) | 55.5 (19–71) | 51.5 (19–71) |
| Sex | |||
| male | 12 (75.0) | 14 (77.8) | 26 (76.5) |
| female | 4 (25.0) | 4 (22.2) | 8 (23.5) |
| Race | |||
| white | 13 (81.3) | 17 (94.4) | 30 (88.2) |
| native Hawaiian or other Pacific Islander | 1 (6.3) | 0 | 1 (2.9) |
| other | 1 (6.3) | 0 | 1 (2.9) |
| unknown | 1 (6.3) | 1 (5.6) | 2 (5.9) |
| Ethnicity | |||
| Hispanic or Latino | 11 (68.8) | 14 (77.8) | 25 (73.5) |
| not Hispanic or Latino | 5 (31.3) | 4 (22.2) | 9 (26.5) |
| CLCR, mL/min, median (range) | 96.5 (54.1–165.0) | 110.1 (40.2–182.4) | 107.7 (40.2–182.4) |
| Height, cm, median (range) | 170.0 (158.0–182.0) | 168.0 (151.0–185.0) | 168.5 (151.0–185.0) |
| Weight, kg, median (range) | 82.5 (60.0–120.0) | 79.0 (55.0–100.0) | 80.0 (55.0–120.0) |
| BMI, kg/m2, median (range) | 26.0 (19.6–44.1) | 27.3 (17.3–31.5) | 26.5 (17.3–44.1) |
| Diagnosis of cIAI | |||
| acute gastric and duodenal perforations (operated on >24 h after perforation) | 0 | 1 (5.6) | 1 (2.9) |
| appendiceal perforation or peri-appendiceal abscess | 7 (43.8) | 7 (38.9) | 14 (41.2) |
| cholecystitis with gangrenous perforation or progression of the infection beyond the gallbladder wall | 2 (12.5) | 5 (27.8) | 7 (20.6) |
| diverticular disease with perforation or abscess | 3 (18.8) | 1 (5.6) | 4 (11.8) |
| intra-abdominal abscess (including of liver or spleen provided that there was extension beyond the organ with evidence of intraperitoneal involvement) | 0 | 3 (16.7) | 3 (8.8) |
| secondary peritonitis (but not spontaneous bacterial peritonitis associated with cirrhosis and chronic ascites | 4 (25.0) | 1 (5.6) | 5 (14.7) |
Values are displayed as n (%), unless specified otherwise.
PK
Four patients (three in Cohort 1 and one in Cohorts 2 and 3) did not complete intensive PK sampling as planned on Day 4, due to early discontinuation of study treatment; therefore, 21 of the planned 25 patients underwent intensive PK sampling.
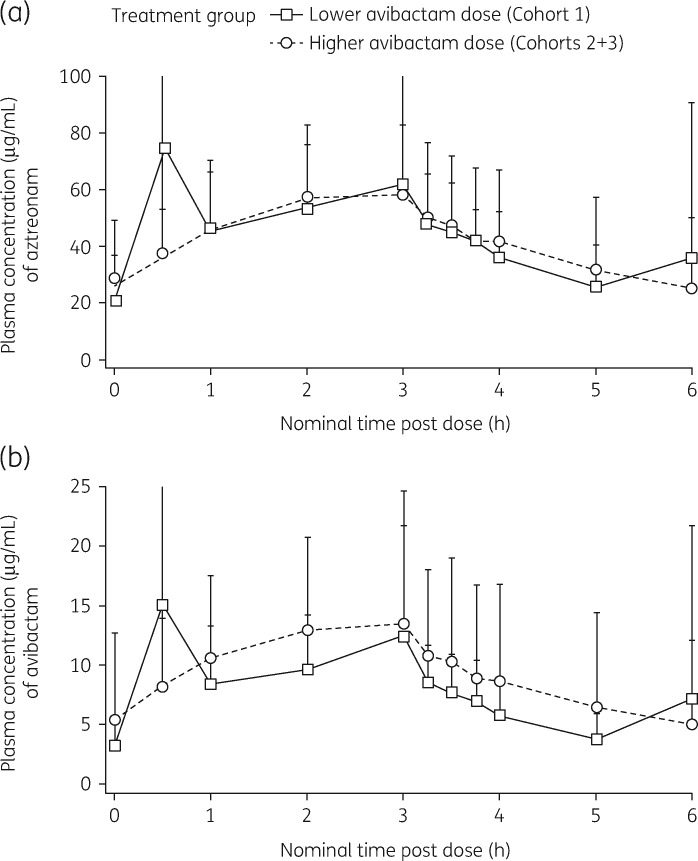
Plasma concentration–time profiles of aztreonam and avibactam following infusion of aztreonam/avibactam on Day 4 are summarized in Figure 2. Table 3 provides a summary of observed PK parameters. On Day 4, geometric mean (geometric coefficient of variance, CV%) steady-state aztreonam area under the plasma concentration–time curve (AUC0–6) was 235.2 μg·h/mL (60.6%) in Cohort 1 and 234.7 μg·h/mL (54.6%) in Cohorts 2 and 3 (Table 3), whereas that of avibactam was 40.4 μg·h/mL (74.0%) in Cohort 1 and 47.5 μg·h/mL (79.2%) in Cohorts 2 and 3 (Table 3). Cmax of both aztreonam and avibactam generally occurred close to the end of infusion (Table 3). Plasma concentrations of both aztreonam and avibactam at predose and 6 h were similar, indicating that steady-state was attained by Day 4 of the maintenance dose regimen (Figure 2). PK parameters of both aztreonam and avibactam were similar between avibactam dose groups, with the exception that avibactam AUC0–6 was higher in Cohorts 2 and 3 than in Cohort 1 in proportion to the increased avibactam dose (Table 3).
Figure 2.
Mean (±SD) plasma concentration–time curves for aztreonam (a) and avibactam (b) following 3 h IV infusion of aztreonam/avibactam on Day 4, intensive PK sampling.
Table 3.
Summary of steady-state aztreonam and avibactam PK parameters following IV infusion of aztreonam/avibactam
| PK parameter | Cohort 1 (n = 16) | Cohort 2 + 3 (n = 18) |
|---|---|---|
| Aztreonam | ||
| AUC0–6 (μg·h/mL) | ||
| n | 13 | 8 |
| geometric mean | 235.2 | 234.7 |
| CV (%) | 60.6 | 54.6 |
| C max (μg/mL) | ||
| n | 13 | 8 |
| geometric mean | 62.5 | 55.4 |
| CV (%) | 146.9 | 42.6 |
| T max (h) | ||
| n | 13 | 8 |
| median | 2.9 | 2.4 |
| (min–max) | 0.5–3.5 | 2.0–3.0 |
| t ½ (h) | ||
| n | 11 | 8 |
| mean (SD) | 2.3 (1.06) | 2.8 (2.05) |
| CL (L/h) | ||
| n | 13 | 8 |
| geometric mean | 6.4 | 6.4 |
| CV (%) | 35.4 | 35.5 |
| V ss (L) | ||
| n | 11 | 8 |
| geometric mean | 20.3 | 19.6 |
| CV (%) | 16.9 | 31.8 |
| V z (L) | ||
| n | 11 | 8 |
| geometric mean | 21.4 | 21.6 |
| CV (%) | 15.3 | 24.1 |
| Avibactam | ||
| AUC0–6 (μg·h/mL) | ||
| n | 13 | 8 |
| geometric mean | 40.4 | 47.5 |
| CV (%) | 74.0 | 79.2 |
| C max (μg/mL) | ||
| n | 13 | 8 |
| geometric mean | 11.6 | 12.1 |
| CV (%) | 164.5 | 61.2 |
| T max (h) | ||
| n | 13 | 8 |
| median (min–max) | 2.9 (0.5–3.8) | 2.8 (2.0–3.3) |
| t ½ (h) | ||
| n | 11 | 8 |
| mean (SD) | 1.8 (0.59) | 2.2 (1.85) |
| CL (L/h) | ||
| n | 13 | 8 |
| geometric mean | 10.1 | 10.5 |
| CV (%) | 42.6 | 41.4 |
| V ss (L) | ||
| n | 11 | 8 |
| geometric mean | 26.0 | 23.7 |
| CV (%) | 22.0 | 29.7 |
| V z (L) | ||
| n | 11 | 8 |
| geometric mean | 28.2 | 27.4 |
| CV (%) | 20.4 | 20.6 |
V z, volume of distribution during the terminal phase after IV administration.
Safety
A total of 55 treatment-emergent AEs were reported in 23 (67.6%) patients overall, [11 (68.8%) in Cohort 1; 12 (66.7%) in Cohorts 2 and 3; Table 4]. The most common AE [Medical Dictionary for Regulatory Activities (MedDRA) preferred term] was ‘hepatic enzyme increased’, occurring in nine (26.5%) patients [seven (43.8%) in Cohort 1 and two (11.1%) in Cohorts 2 and 3]. Of these nine cases, seven (20.6%) were considered related to aztreonam/avibactam, one was related to metronidazole and one was not drug-related. The onset of these AEs ranged from Day 4 to Day 17 (Table S2). All cases were mild or moderate in severity.
Table 4.
Summary of treatment-emergent AEs, treatment-emergent AEs occurring in ≥2 patients and SAEs, by system organ class and preferred term (MITT population)
| Characteristic | Cohort 1 (n = 16) | Cohorts 2 + 3 (n = 18) | Total (N = 34) |
|---|---|---|---|
| Patients with any AE | 11 (68.8) | 12 (66.7) | 23 (67.6) |
| Patients with outcome of death (related and not related) | 0 | 1 (5.6) | 1 (2.9)a |
| Patients with SAEs | 4 (25.0) | 5 (27.8) | 9 (26.5) |
| Patients discontinued from study drug due to AEs and continued study | 2 (12.5) | 2 (11.1) | 4 (11.8) |
| Any AE with severe intensity | 3 (18.8) | 2 (11.1) | 5 (14.7) |
| Any AE related to aztreonam/avibactam | 8 (50.0) | 2 (11.1) | 10 (29.4) |
| Any AE related to metronidazole | 1 (6.3) | 0 | 1 (2.9) |
| AEs by system organ class/MedDRA preferred term, n (%) | |||
| blood and lymphatic system disorders | 2 (12.5) | 2 (11.1) | 4 (11.8) |
| anaemia | 1 (6.3) | 2 (11.1) | 3 (8.8) |
| gastrointestinal disorders | 3 (18.8) | 8 (44.4) | 11 (32.4) |
| abdominal pain lower | 0 | 2 (11.1) | 2 (5.9) |
| diarrhoea | 2 (12.5) | 3 (16.7) | 5 (14.7) |
| nausea | 0 | 2 (11.1) | 2 (5.9) |
| general disorders/administration site conditions | 1 (6.3) | 2 (11.1) | 3 (8.8) |
| oedema | 0 | 2 (11.1) | 2 (5.9) |
| Investigations | 7 (43.8) | 2 (11.1) | 9 (26.5) |
| hepatic enzyme increased | 7 (43.8) | 2 (11.1) | 9 (26.5) |
| SAEs by system organ class/MedDRA preferred term, n (%) | |||
| patients with any SAE | 4 (25.0) | 5 (27.8) | 9 (26.5) |
| gastrointestinal disorders | 0 | 2 (11.1) | 2 (5.9) |
| intra-abdominal haematoma | 0 | 1 (5.6) | 1 (2.9) |
| pancreatitis acute | 0 | 1 (5.6) | 1 (2.9) |
| infections and infestations | 2 (12.5) | 0 | 2 (5.9) |
| abdominal wall infection | 1 (6.3) | 0 | 1 (2.9) |
| sepsis | 1 (6.3) | 0 | 1 (2.9) |
| injury, poisoning and procedural complications | 0 | 2 (11.1) | 2 (5.9) |
| arterial injury | 0 | 1 (5.6) | 1 (2.9) |
| postoperative ileus | 0 | 1 (5.6) | 1 (2.9) |
| neoplasms benign, malignant and unspecified (including cysts and polyps) | 1 (6.3) | 0 | 1 (2.9) |
| colon cancer | 1 (6.3) | 0 | 1 (2.9) |
| renal and urinary disorders | 0 | 1 (5.6) | 1 (2.9) |
| acute kidney injury | 0 | 1 (5.6) | 1 (2.9) |
| respiratory, thoracic and mediastinal disorders | 2 (12.5) | 0 | 2 (5.9) |
| acute respiratory distress syndrome | 1 (6.3) | 0 | 1 (2.9) |
| respiratory distress | 1 (6.3) | 0 | 1 (2.9) |
| skin and subcutaneous tissue disorders | 0 | 1 (5.6) | 1 (2.9) |
| haemorrhage, subcutaneous | 0 | 1 (5.6) | 1 (2.9) |
This event was a post-LFU event. Values are displayed as n (%).
The second most frequent treatment-emergent AE was diarrhoea, occurring in five (14.7%) patients. All cases were of mild severity and not considered related to aztreonam/avibactam or due to Clostridioides difficile infection. Thrombocytosis occurred in six (17.6%) patients who met predefined potentially clinically significant (PCS) criteria [platelets >1.5 × upper limit of normal (ULN) and >100% increase from baseline]. Possible alternative aetiologies were evident in five of these cases and no patient reported a thrombotic event.
Ten AEs considered to be treatment-related were reported in eight patients in Cohort 1 (seven AEs of ‘hepatic enzyme increased’, one of visual hallucinations, one of thrombocytosis and one of hepatitis) and two patients in Cohorts 2 and 3 (one AE each of ‘hepatic enzyme increased’).
Serious AEs (SAEs) were reported in a total of nine (26.5%) patients [Cohort 1, n = 4; Cohorts 2 and 3, n = 5 (Table 4)]. None was considered treatment-related. Four patients (11.8%) were discontinued from the study drug owing to AEs, of which three were due to liver enzyme disorders (two of these were ‘hepatic enzyme increased’ and considered treatment-related and one was an event of hypertransaminasaemia that was assessed as being unrelated to the study drug). The fourth was a patient with worsening of kidney failure unrelated to the study drug.
PCS increases in ALT and/or AST (>3 × ULN and 100% change from baseline), based on laboratory assessment alone, were identified in six patients [17.6%; two patients in Cohort 1 (one patient with AE report of ‘hepatic enzyme increased’ and one with AE of hepatitis) and four in Cohorts 2 and 3 (one patient with AE of ‘hepatic enzyme increased’, one with an SAE of postoperative ileus, one with SAE of arterial injury and one with SAEs of acute pancreatitis and intra-abdominal haematoma and an AE of hypertransaminasaemia)]. The majority of increases resolved rapidly following discontinuation of treatment. However, for the patient with postoperative ileus, ALT levels remained 2 × ULN on Day 43 at an unscheduled visit after the LFU visit. The subject remained asymptomatic of hepatic injury throughout and the event did not require treatment. Two of the six patients with PCS increases in transaminases also experienced PCS increases in total bilirubin (>1.5 × ULN and 100% change from baseline). In both cases, alternative aetiologies were evident (thrombosis of left suprahepatic vein/hepatic artery injury and acute pancreatitis). There were no confirmed Hy’s law cases (definition of Hy’s criteria provided in Text S1).
No deaths occurred during the AE collection period defined in the protocol. Two fatal events were reported, one prior to the patient receiving study treatment and one which occurred 1 week after the LFU visit, when the patient was no longer taking part in the study. Both cases were assessed as not related to study treatment.
Assessment and medical monitoring/review of vital sign and ECG data did not reveal any clinically relevant abnormalities attributed to aztreonam/avibactam.
Efficacy
Investigator assessment of clinical response at EOT and TOC is summarized in Table 5. Overall, 67.6% and 73.9% of patients experienced clinical cure at EOT in the MITT and mMITT populations, respectively. Approximately 60% of patients overall were assessed as being cured at TOC (58.8% in the MITT population and 60.9% in the mMITT population).
Table 5.
Investigator assessment of clinical responses at the EOT and TOC visits (MITT and mMITT populations)
| Timepoint/response | Cohort 1 | Cohorts 2 + 3 | Total |
|---|---|---|---|
| EOT | |||
| MITT population | n = 16 | n = 18 | N = 34 |
| cure | 10 (62.5) | 13 (72.2) | 23 (67.6) |
| failure | 3 (18.8) | 4 (22.2) | 7 (20.6) |
| indeterminate | 2 (12.5) | 0 | 2 (5.9) |
| mMITT population | n = 12 | n = 11 | N = 23 |
| cure | 8 (66.7) | 9 (81.8) | 17 (73.9) |
| failure | 2 (16.7) | 1 (9.1) | 3 (13.0) |
| indeterminate | 1 (8.3) | 0 | 1 (4.3) |
| TOC | |||
| MITT population | n = 16 | n = 18 | N = 34 |
| cure | 10 (62.5), 95% CI 35.4–84.8 | 10 (55.6), 95% CI 30.8–78.5 | 20 (58.8) |
| failure | 3 (18.8) | 5 (27.8) | 8 (23.5) |
| indeterminate | 3 (18.8) | 3 (16.7) | 6 (17.6) |
| mMITT population | n = 12 | n = 11 | N = 23 |
| cure | 8 (66.7), 95% CI 34.9–90.1 | 6 (54.5), 95% CI 23.4–83.3 | 14 (60.9) |
| failure | 2 (16.7) | 2 (18.2) | 4 (17.4) |
| indeterminate | 2 (16.7) | 3 (27.3) | 5 (21.7) |
Some patients did not have a clinical response assessment at one or more visits. Values shown are n (%).
Baseline pathogens
In total, 23/34 (67.6%) patients had a pathogen identified at baseline and comprised the mMITT population. E. coli, Klebsiella pneumoniae and Klebsiella oxytoca were the most commonly isolated Enterobacteriaceae species (Table S3). Nine of these 23 (39.1%) patients had a single pathogen isolated and 14/23 (60.9%) had more than one pathogen isolated. The baseline pathogen profile was balanced across cohorts and consistent with the cIAI population. The individual aztreonam/avibactam MIC distributions for all baseline pathogens (aerobes) in the mMITT population are displayed in Table S4.
Discussion
REJUVENATE is the first study of aztreonam/avibactam in patients with a representative burden of infection and, as such, represents an important clinical milestone. PK and safety of aztreonam/avibactam were evaluated in patients with cIAI using dosing regimens selected to achieve PK/PD targets based on preclinical and healthy volunteer PK data. Following review of safety/tolerability and PK assessments in Cohort 1, the avibactam component of the combined dose was increased to optimize target attainment based on an updated population PK/PD model and target attainment rate estimations.23
Aztreonam/avibactam is one of the few treatments in clinical development specifically designed to target serious infections caused by MBL-producing Enterobacteriaceae, particularly those harbouring other β-lactamases, such as ESBLs. This study forms part of a streamlined development programme being pursued for aztreonam/avibactam, driven by the lack of effective treatment options and the prior preclinical and clinical experience with both aztreonam and avibactam.12,16,17
The steady-state mean clearance of aztreonam in patients with cIAI was similar compared with healthy volunteers who received similar dosing regimens [6.4 L/h versus 5.3–7.4 L/h, respectively (NCT01689207)]. Clearance of avibactam was somewhat lower in cIAI, versus healthy volunteers (10.1–10.5 L/h versus 10.3–15.5 L/h, respectively). Mean aztreonam and avibactam apparent volume of distribution at steady-state after IV administration in patients with cIAI was modestly higher than the mean values reported in young adult healthy volunteers. This was not unexpected in patients with acute IAI and recent surgery, as distribution may be affected by changes in blood volume, circulation, extracellular fluid and circulating plasma protein levels.24 Exposures of aztreonam and avibactam achieved in patients with cIAI were consistent with the predicted exposures for both avibactam dose regimens based on the updated population PK models.23
Importantly, the PK results for aztreonam and avibactam reported here confirm that the dosing regimen studied in Cohorts 2 and 3 is appropriate for the Phase 3 programme.
Aztreonam/avibactam was generally well tolerated and the pattern of reported AEs resembles those observed in the ceftazidime/avibactam clinical development programme, which also used additional metronidazole in patients with cIAI.16,17,25
In line with the hepatic safety profile of aztreonam monotherapy,12 drug-induced liver injury effects were expected and liver parameters were therefore monitored. AEs of ‘hepatic enzyme increased’ were reported in nine (26.5%) patients overall. Most of these AEs concerned asymptomatic transaminase elevations in patients in Cohort 1, the majority of which did not meet potentially clinically significant criteria based on laboratory parameter assessment and none of which met Hy’s law criteria.
Procedural injuries to the liver, or medical history, such as diabetes, steatosis, cholelithiasis or chronic hepatitis C, may have contributed to the occurrence of transaminase elevations; severe cIAI, surgery and anaesthetics can also result in increases in liver enzymes.26–28 In addition, most patients received concomitant medications, such as paracetamol or additional antibiotics, which are known to have potential effects on the liver.29
Of note, elevations in liver transaminases associated with aztreonam are usually reversible with treatment discontinuation and typically occur without overt signs or symptoms of hepatobiliary dysfunction.12 This is reflected by the observations in the current study, in which most of the AEs relating to liver enzyme increases were asymptomatic and recovered during the study.
The incidence of diarrhoea was slightly higher than the safety profile presented in the aztreonam summary of product characteristics (SmPC);12 it is difficult to assess whether this is due to a true increase in incidence or underlying intra-abdominal pathology. The increase in occurrence of diarrhoea is not considered to be of clinical significance, as events were mild and mostly resolved before the LFU visit. None were related to C. difficile. Nevertheless, the finding should be investigated further in future studies.
In the current study, approximately 60% of patients with cIAI attained clinical cure at TOC. Although enrolment was opened to patients with CLCR of 31–50 mL/min in Cohorts 2 and 3, the assessment of aztreonam/avibactam in such patients was limited by the fact that only one patient with baseline CLCR <50 mL/min was enrolled. This patient discontinued on Day 2 and had no evaluable PK data available for analysis. MDR bacteria are emerging with increasing incidence worldwide and effective antibiotic medication is often limited or unavailable, resulting in increased morbidity and mortality.1 Although currently rare in most parts of Europe, outbreaks due to CRE, including MBL, have the potential to be severe and have been reported in various EU countries.30 Overall, the findings strongly support further development of aztreonam/avibactam; although, as no MBL- or ESBL-producing bacteria were detected in the isolates from the 34 patients in the present study, the contribution of avibactam in the aztreonam/avibactam combination to in vivo efficacy in this subgroup of species could not be evaluated and further studies are therefore needed to investigate this aspect of the study drug combination.
In summary, the PK data for aztreonam and avibactam in cIAI patients reported here confirm that the aztreonam/avibactam 500/167 mg (30 min infusion) loading dose and 1500/500 mg (3 h infusions) maintenance dose q6h dosing regimen, in patients with CLCR >50 mL/min, is appropriate for the Phase 3 development programme. The overall safety profile of aztreonam/avibactam is in line with that of aztreonam alone, with a favourable risk–benefit profile. Results from this study support the clinical development of aztreonam/avibactam.
Supplementary Material
Acknowledgements
We thank the patients and their families involved in this study and the REJUVENATE Study Group. We also thank Karen Cheng, Safety, Pfizer; Michele Wible, Statistics, Pfizer; Boudewijn de Jonge, Microbiology, Pfizer; and Joseph W. Chow, Clinical, Pfizer for their contributions to the preparation of the clinical study report for the REJUVENATE study. Medical writing support was provided by Melanie More of Prime, Knutsford, Cheshire, UK and was funded by Pfizer. The sponsor was involved in the study design, collection, analysis and interpretation of data, as well as data-checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions and data interpretation lies with the authors. Data from this study were presented in poster format at the 29th European Congress of Clinical Microbiology and Infectious Diseases, 13–16 April 2019, Amsterdam, The Netherlands. Results from this study have been posted on www.clinicaltrials.gov (NCT02655419) and www.clinicaltrialsregister.eu (2015-002726-39) databases.
Members of the REJUVENATE study group
Ana Cristina Padial Aguado, Reina Sofía University Hospital, Córdoba, Spain; Miguel Montejo Baranda, Hospital Universitario Cruces, Bizkaia, Spain; Carlos García Bernedo, Hospital del Mar, Barcelona, Spain; Marc Bludau, University Hospital Cologne, Cologne, Germany; Lucía Boix-Palop, Hospital Universitario Mútua de Tarrasa, Barcelona, Spain; Karen Cheng, Pfizer, Sandwich, Kent, UK; Boudewijn de Jonge, Pfizer, Cambridge, MA, USA; Francisco Javier González de Molina, Hospital Universitario Mútua de Tarrasa, Barcelona, Spain; Pilar Retamar Gentil, Hospital Universitario Virgen Macarena, Seville, Spain; Julia Guzmán-Puche, Reina Sofía University Hospital, Córdoba, Spain; Virginia Palomo Jiménez, Hospital Universitario Virgen Macarena, Seville, Spain; José A. López-Ruiz, Hospital Universitario Virgen Macarena, Seville, Spain; Enrique Montero Mateos, Hospital Universitario Virgen del Rocío, Seville, Spain; Cristina Roca Oporto, Hospital Universitario Virgen del Rocío, Seville, Spain; Guillaume Piessen, Claude Huriez University Hospital, Lille, France; Deborah Postil, Centre Hospitalier Universitaire de Limoges, Limoges, France; Rosa M. Jiménez Rodríguez, Hospital Universitario Virgen del Rocío, Seville, Spain; Javier Padillo Ruiz, Hospital Universitario Virgen del Rocío, Seville, Spain; Jan Rupp, Universitätsklinikum Schleswig-Holstein, Lübeck, Germany; Rafael Morales Soriano, Hospital Son Espases, Mallorca, Spain; Michele Wible, Pfizer, Collegeville, PA, USA; Ángela Cano Yuste, Reina Sofía University Hospital, Córdoba, Spain; Silvia Gómez-Zorrilla, Hospital del Mar, Barcelona, Spain.
Funding
This work was supported and sponsored by Pfizer; with funding from Innovative Medicines Initiative (IMI) Combatting Bacterial Resistance in Europe - Carbapenem-Resistance (COMBACTE-CARE) and Allergan.
Transparency declarations
This study was originally sponsored by AstraZeneca. AstraZeneca’s rights to aztreonam/avibactam were acquired by Pfizer in December 2016. The study is now sponsored by Pfizer. This research project receives support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115523 | 115620 | 115737 resources of which are composed of financial contribution from the European Union Seventh Framework Programme (FP7/2007–2013) and EFPIA companies in-kind contribution. The research leading to these results was conducted as part of the COMBACTE-CARE consortium. For further information refer to www.COMBACTE.com. This aztreonam/avibactam development programme also receives funding from Allergan.
O.A.C., J.M.C., J.T.C., M.J.R.H., L.T.A., E.C., J.P.H., C.Q., U.Z., C.M.R.F., D.A. and S.J-J. received institutional funding for the conduct of the study from the Innovative Medicines Initiative under an overarching grant agreement. Spanish sites were partly selected within the REIPI (Spanish Network for Research in Infectious Disease) network, collaborating within COMBACTE. C.M.R.F. and S.J-J. are representatives of the Spanish research Network (SCReNPT13/0002/0010-PT17/0017/0012). O.A.C. is supported by the German Federal Ministry of Research and Education, has received research grants from Actelion, Amplyx, Astellas, Basilea, Cidara, Da Volterra, F2G, Gilead, Janssen Pharmaceuticals, Medicines Company, MedPace, Melinta Therapeutics, Merck/MSD, Pfizer, Scynexis, is a consultant to Actelion, Allecra Therapeutics, Amplyx, Astellas, Basilea, Biosys UK Limited, Cidara, Da Volterra, Entasis, F2G, Gilead, IQVIA, Matinas, MedPace, Menarini Ricerche, Merck/MSD, Octapharma, Paratek Pharmaceuticals, Pfizer, PSI, Rempex, Scynexis, Seres Therapeutics, Tetraphase and Vical, and received lecture honoraria from Astellas, Basilea, Gilead, Merck/MSD and Pfizer. E.C. has accepted grants, speaking engagements and conference invitations from: Astellas, AstraZeneca, Novartis, Pfizer and MSD. S.R. is an employee of and shareholder in Pfizer. G.T. is a former employee of AstraZeneca and current contractor to Pfizer. A.L. is a former contractor to AstraZeneca and current employee of Pfizer. S.O’B. is a former employee of AstraZeneca and of Pfizer.
Author contributions
O.A.C., J.M.C., A.L., C.Q., U.Z., D.A., S.O’B. designed and conceived the study. All authors had roles in acquisition, analysis and/or interpretation of the data. U.Z., D.A., G.T. and S.J-J. provided administrative, technical or material support. O.A.C., J.M.C., C.Q. and A.L. supervised the study. All authors were involved in drafting and/or critically revising the Article. All authors approved the final version of the article.
Data sharing
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual deidentified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (i) for indications that have been approved in the USA and/or EU or (ii) in programmes that have been terminated (i.e. development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Contributor Information
COMBACTE-CARE consortium/REJUVENATE Study Group:
Ana Cristina Padial Aguado, Miguel Montejo Baranda, Carlos García Bernedo, Marc Bludau, Lucía Boix-Palop, Karen Cheng, Boudewijn de Jonge, Francisco Javier González de Molina, Pilar Retamar Gentil, Julia Guzmán-Puche, Virginia Palomo Jiménez, José A López-Ruiz, Enrique Montero Mateos, Cristina Roca Oporto, Guillaume Piessen, Deborah Postil, Rosa M Jiménez Rodríguez, Javier Padillo Ruiz, Jan Rupp, Rafael Morales Soriano, Michele Wible, Ángela Cano Yuste, and Silvia Gómez-Zorrilla
References
- 1. Carlet J, Jarlier V, Harbarth S. et al. Ready for a world without antibiotics? The Pensieres Antibiotic Resistance Call to Action. Antimicrob Resist Infect Control 2012; 1: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Theuretzbacher U. Global antimicrobial resistance in Gram-negative pathogens and clinical need. Curr Opin Microbiol 2017; 39: 106–12. [DOI] [PubMed] [Google Scholar]
- 3. Tangden T, Giske CG.. Global dissemination of extensively drug-resistant carbapenemase-producing Enterobacteriaceae: clinical perspectives on detection, treatment and infection control. J Intern Med 2015; 277: 501–12. [DOI] [PubMed] [Google Scholar]
- 4. Walsh TR, Weeks J, Livermore DM. et al. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis 2011; 11: 355–62. [DOI] [PubMed] [Google Scholar]
- 5. Mojica MF, Bonomo RA, Fast W.. B1-metallo-β-lactamases: where do we stand? Curr Drug Targets 2016; 17: 1029–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rossi F, Baquero F, Hsueh PR. et al. In vitro susceptibilities of aerobic and facultatively anaerobic Gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: 2004 results from SMART (Study for Monitoring Antimicrobial Resistance Trends). J Antimicrob Chemother 2006; 58: 205–10. [DOI] [PubMed] [Google Scholar]
- 7. Chen YH, Hsueh PR, Badal RE. et al. Antimicrobial susceptibility profiles of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections in the Asia-Pacific region according to currently established susceptibility interpretive criteria. J Infect 2011; 62: 280–91. [DOI] [PubMed] [Google Scholar]
- 8. Kurup A, Liau KH, Ren J. et al. Antibiotic management of complicated intra-abdominal infections in adults: the Asian perspective. Ann Med Surg (Lond) 2014; 3: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldstein EJ, Snydman DR.. Intra-abdominal infections: review of the bacteriology, antimicrobial susceptibility and the role of ertapenem in their therapy. J Antimicrob Chemother 2004; 53 Suppl 2: ii29–36. [DOI] [PubMed] [Google Scholar]
- 10. Sartelli M, Chichom-Mefire A, Labricciosa FM. et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg 2017; 12: 29.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cano A, Gutierrez-Gutierrez B, Machuca I. et al. Risks of infection and mortality among patients colonized with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: validation of scores and proposal for management. Clin Infect Dis 2018; 66: 1204–10. [DOI] [PubMed] [Google Scholar]
- 12. Squibb B-M. Azactam 1g powder for solution for injection or infusion, vial. Summary of product characteristics 2017. https://www.medicines.org.uk/emc/product/3773/smpc.
- 13. Biedenbach DJ, Kazmierczak K, Bouchillon SK. et al. In vitro activity of aztreonam-avibactam against a global collection of Gram-negative pathogens from 2012 and 2013. Antimicrob Agents Chemother 2015; 59: 4239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sader HS, Mendes RE, Pfaller MA. et al. Antimicrobial activities of aztreonam-avibactam and comparator agents against contemporary (2016) clinical Enterobacteriaceae isolates. Antimicrob Agents Chemother 2017; 62: e01856-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Docquier JD, Mangani S.. An update on β-lactamase inhibitor discovery and development. Drug Resist Updat 2018; 36: 13–29. [DOI] [PubMed] [Google Scholar]
- 16.Allergan. AVYCAZ (ceftazidime and avibactam) for injection, for intravenous use.2018. https://www.allergan.com/assets/pdf/avycaz_pi.
- 17.Pfizer. Zavicefta 2g/0.5g powder for concentration for solution for infusion. Summary of product characteristics 2018. https://www.medicines.org.uk/emc/medicine/33061.
- 18. Livermore DM, Mushtaq S, Warner M. et al. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 2011; 55: 390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crandon JL, Nicolau DP.. Human simulated studies of aztreonam and aztreonam-avibactam to evaluate activity against challenging gram-negative organisms, including metallo-β-lactamase producers. Antimicrob Agents Chemother 2013; 57: 3299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh R, Kim A, Tanudra MA. et al. Pharmacokinetics/pharmacodynamics of a β-lactam and β-lactamase inhibitor combination: a novel approach for aztreonam/avibactam. J Antimicrob Chemother 2015; 70: 2618–26. [DOI] [PubMed] [Google Scholar]
- 21. Karlowsky JA, Kazmierczak KM, de Jonge BLM. et al. In vitro activity of aztreonam-avibactam against Enterobacteriaceae and Pseudomonas aeruginosa isolated by clinical laboratories in 40 countries from 2012 to 2015. Antimicrob Agents Chemother 2017; 61: doi:10.1128/AAC.00472-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edeki T, Zhou D, van den Berg F. et al. A phase I, 3-part placebo-controlled randomised trial to evaluate the safety, tolerability and pharmacokinetics of aztreonam-avibactam in healthy subjects. 26th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Amsterdam, The Netherlands, 2016. Poster EV0643.
- 23. Das S, Riccobene T, Carrothers TJ. et al. Population pharmacokinetic/pharmacodynamic (PK/PD) modelling to optimise aztreonam-avibactam dose selection. 29th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Amsterdam, The Netherlands, 2019. Poster P3719.
- 24. Kennedy JM, Riji AM.. Effects of surgery on the pharmacokinetic parameters of drugs. Clin Pharmacokinet 1998; 35: 293–312. [DOI] [PubMed] [Google Scholar]
- 25. Mazuski JE, Gasink LB, Armstrong J. et al. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis 2016; 62: 1380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andrei VE, Schein M, Margolis M. et al. Liver enzymes are commonly elevated following laparoscopic cholecystectomy: is elevated intra-abdominal pressure the cause? Dig Surg 1998; 15: 256–9. [DOI] [PubMed] [Google Scholar]
- 27. Guo K, Ren J, Wang G. et al. Early liver dysfunction in patients with intra-abdominal infections. Medicine (Baltimore) 2015; 94: e1782.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nishiyama T, Yokoyama T, Hanaoka K.. Liver function after sevoflurane or isoflurane anaesthesia in neurosurgical patients. Can J Anaesth 1998; 45: 753–6. [DOI] [PubMed] [Google Scholar]
- 29. Chalasani N, Fontana RJ, Bonkovsky HL. et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924–34, 34.e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Centre for Disease Prevention and Control. Carbapenem-resistant Enterobacteriaceae - first update. 2018. https://ecdc.europa.eu/sites/portal/files/documents/RRA-Enterobacteriaceae-Carbapenems-European-Union-countries.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.