Abstract
Background
Pulmonary infections caused by non-tuberculous mycobacteria (NTM) are hard to treat and have low cure rates despite intensive multidrug therapy.
Objectives
To assess the feasibility of tedizolid, a new oxazolidinone, for the treatment of Mycobacterium avium and Mycobacterium abscessus.
Methods
We determined MICs of tedizolid for 113 isolates of NTM. Synergy with key antimycobacterial drugs was assessed using the chequerboard method and calculation of the FIC index (FICI). We performed time–kill kinetics assays of tedizolid alone and combined with amikacin for M. abscessus and with ethambutol for M. avium. Human macrophages were infected with M. abscessus and M. avium and subsequently treated with tedizolid; intracellular and extracellular cfu were quantified over time.
Results
NTM isolates generally had a lower MIC of tedizolid than of linezolid. FICIs were lowest between tedizolid and amikacin for M. abscessus (FICI = 0.75) and between tedizolid and ethambutol for M. avium (FICI = 0.72). Clarithromycin and tedizolid showed initial synergy, which was abrogated by erm(41)-induced macrolide resistance (FICI = 0.53). Tedizolid had a weak bacteriostatic effect on M. abscessus and combination with amikacin slightly prolonged its effect. Tedizolid had concentration-dependent activity against M. avium and its efficacy was enhanced by ethambutol. Both combinations had a concentration-dependent synergistic effect. Tedizolid could inhibit the intracellular bacterial population of both M. avium and M. abscessus.
Conclusions
Tedizolid should be further investigated in pharmacodynamic studies and clinical trials for M. avium complex pulmonary disease. It is less active against M. abscessus, but still promising.
Introduction
Non-tuberculous mycobacteria (NTM) are causative agents of chronic, opportunistic pulmonary infections in susceptible patient populations.1 In NTM pulmonary disease (NTM-PD) Mycobacterium avium complex (MAC) and Mycobacterium abscessus are the most common causative pathogens.2,3 Current treatment regimens consist of at least three drugs for a duration of 18–24 months,1,4 but treatment outcome is still poor. In meta-analyses, 50% (M. abscessus) to 70% (MAC) of patients who tolerate recommended regimens achieve prolonged culture conversion.5 In addition, this multidrug treatment is associated with significant adverse effects.6
The oxazolidinone linezolid is recommended as part of first-line treatment of M. abscessus disease and as a second-line treatment of MAC pulmonary disease.1 Because of the necessity of prolonged treatment, the linezolid dose has to be lowered to avoid or delay adverse events.7 Given linezolid’s very high MICs (median 16 mg/L),8 these low doses are unlikely to be effective.
Tedizolid is an oxazolidinone that, like linezolid, targets the 23S ribosomal RNA of the 50S ribosomal subunit.9 Its favourable toxicity profile and superior penetration into epithelial lining fluid, compared with linezolid, has already led to sporadic use in NTM-PD treatment.10–12 A recent study using an intracellular hollow-fibre system infection model identified tedizolid as highly bactericidal against intracellular MAC,13 but lower in vitro activity of tedizolid alone against M. abscessus is reported.8,14,15
Even with promising in vitro performance and toxicity profile, any new drug to be incorporated into NTM disease treatment regimens should have a firm preclinical basis, including at least measurements of synergy with established antimycobacterial drugs and intracellular activity.
Here, we assess the in vitro and ex vivo activity of tedizolid as well as synergism between tedizolid and key antimycobacterial drugs using the chequerboard method and time–kill kinetics assays.
Methods
Bacterial strains
M. abscessus subspecies abscessus CIP 104536, Mycobacterium fortuitum ATCC 6841, M. avium ATCC 700898, Mycobacterium peregrinum ATCC 700686, Mycobacterium chimaera DSM 44623, Mycobacterium simiae ATCC 25275 and Mycobacterium intracellulare DSM 43223 reference strains were purchased from their relevant culture collections. Clinical isolates were acquired from the collection of the Department of Medical Microbiology at Radboud University Medical Center, Nijmegen, The Netherlands. Isolates were stored in trypticase soy broth with 40% glycerol at −80°C and freshly cultured before each experiment.
Susceptibility and stability testing
MICs of tedizolid phosphate (the active moiety of tedizolid; henceforth referred to as tedizolid; MSD) were determined by broth microdilution in CAMHB (BD Biosciences, Drachten, the Netherlands) according to CLSI guidelines.16 We tested concentrations ranging from 0.125 to 128 mg/L on a total of 113 NTM isolates (7 reference strains and 106 clinical isolates). MBCs were determined for reference strains of M. abscessus and M. avium by quantification of cfu for tedizolid concentrations that showed no visible growth in microdilution, on Middlebrook 7H10 agar plates (BD Biosciences). To determine whether tedizolid should be considered bacteriostatic or bactericidal, we calculated the MBC/MIC ratio and considered a ratio >8 to be bacteriostatic, as previously described.17 We tested tedizolid stability by pre-incubating broth microdilution assay plates for 7, 14 and 21 days at 30°C or 37°C before inoculating them with M. abscessus CIP 104536 or M. avium ATCC 700898, respectively. To assess a correlation between linezolid and tedizolid MIC, we ln(x + 1) transformed the MIC values and performed a Spearman’s ρ two-tailed correlation test using a Gaussian approximation.
Synergy testing
We assessed synergy between tedizolid and other antimycobacterial drugs using the broth microdilution chequerboard method.18 Tedizolid was evaluated against a total of 12 clinical and reference strains of NTM [tedizolid in combination with clarithromycin, cefoxitin, tigecycline or amikacin for rapidly growing mycobacteria (RGM; M. abscessus, M. fortuitum, M. peregrinum and M. chelonae) and tedizolid in combination with clarithromycin, rifampicin, ethambutol, amikacin or minocycline for M. avium] as previously described.19 Drug interactions were interpreted according to the FIC index (FICI) and were classified as synergistic (FICI ≤0.5), having no interaction (FICI >0.5–4.0) or antagonistic (FICI >4.0). We selected the most promising candidate antibiotics based on the FICI value for subsequent experiments in time–kill interaction assays.
Time–kill kinetics assays
Time–kill kinetics assays were performed with M. abscessus and M. avium reference strains in duplicate. In short, bacteria were cultured in individual bottles containing 10 mL of CAMHB with 0.05% Tween 80 and for M. avium 10% OADC growth supplement (BD Biosciences). We tested tedizolid concentrations ranging from 0.25× to 32× MIC and a growth control, all inoculated with a bacterial inoculum approximating 105–106 cfu/mL and incubated at 30°C (for M. abscessus) or 37°C (M. avium), shaken constantly and ventilated through a filter. We used the following predetermined timepoints: days 0, 1, 2, 3, 4, 5, 7, 14 and 21 for M. abscessus and an additional day 28 for M. avium. At these timepoints, the bacterial population of each bottle was quantified using a 10-fold serial dilution with triplicate spot-plating of each well on M7H10 agar plates. Plates were incubated at 30°C for 3 days for M. abscessus and at 37°C for 7 days for M. avium. Time–kill kinetics for tedizolid interactions were determined following the protocol described above. Single-drug tedizolid, companion and a tedizolid/companion combination were tested with three concentrations (0.5×, 1× and 2× MIC) in CAMHB. We defined a bactericidal effect as a >1 log cfu decrease in the course of the interaction compared with the start inoculum. To assess the resistant M. avium and M. abscessus populations in the combination time–kill kinetics assays, we prepared Middlebrook 7H11 (M7H11) agar plates containing 5× MIC of the companion drug and, to assess some form of oxazolidinone resistance, 5× MIC of linezolid as an indicator for measuring emerging tedizolid resistance. M7H11 plates were prepared from powder (Sigma–Aldrich) according to the manufacturer’s recommendation, with 10% OADC growth supplement and antibiotic added after autoclaving. Plates were inoculated as described before. Inoculated M7H11 plates were kept for 10 days at 30°C for M. abscessus and for 21 days at 37°C for M. avium.
Response surface analysis
Response surface analysis to assess interaction according to Bliss independence was performed as previously described.20 AUC was calculated from the log cfu versus time plots using the trapezoidal rule after averaging the result form the two replicates and normalizing to the baseline colony count. The effect was then calculated according to effectx = AUCgrowth control − AUCx, where x is any given curve other than the growth control.
To assess potential interactions in the time–kill kinetics experiments we calculated the expected effect for a combination under Bliss independence (Ecomb,BI) to be compared with the observed effect (Ecomb,obs). Since Bliss independence builds on probability theory, the maximum effect to be evaluated is limited to 1 and therefore all effects were normalized to the Emax of the most potent drug (tedizolid). After simplification this gives the following formula: Ecomb,BI = EA + EB − (EA × EB)/Emaxhigh, where EA and EB are the effect sizes of drug A or B separately and Emaxhigh is the highest maximum effect. The Emax of tedizolid was determined by fitting a sigmoidal Emax model to the concentration–response data using ordinary least squares.
The difference between observed and expected effect (ΔE) was quantified as a percentage difference relative to the expected: ΔE = (Ecomb,obs − Ecomb,BI)/Ecomb,BI. We defined a ΔE of 0 ± 10% as no interaction, anything less than −10% was defined as antagonistic and anything more than 10% was defined as synergistic.
Ex vivo intracellular assays
PBMCs were isolated from buffy coats obtained from three healthy volunteers (Sanquin Bloodbank, Nijmegen, The Netherlands). Informed consent from these volunteers was obtained for use of their blood for scientific purposes, as approved by the Ethics Committee of Radboud University Medical Center, Nijmegen, The Netherlands. Macrophage differentiation was performed as described previously.21 One day prior to the experiment, cells were dissociated and re-seeded in antibiotic-free medium in a 96-well plate (1 × 105 cells/well).
On the day of infection, the cell culture medium was removed and M. avium was added to the macrophages at an moi of 1:5 (cells:bacteria), and M. abscessus was added at an moi of 1:1. The macrophages were incubated with mycobacteria for 1 h at 37°C to allow phagocytosis before medium was changed to RPMI supplemented with 10% human pooled serum. Tedizolid was added at 2× MIC and was present for the remainder of the experiment. At the indicated timepoints, the number of extracellular bacteria was quantified through plating of the supernatant on Middlebrook 7H10 agar for cfu counts. To determine the number of intracellular bacteria, macrophages were washed with warm PBS and then subjected to hypotonic lysis in sterile water for 10 min. The intracellular fraction was then also quantified through serial dilutions and plating of the different dilutions on Middlebrook 7H10 plates. All samples were analysed in triplicate.
Statistics
Calculations were performed using GraphPad Prism version 5.03 (GraphPad Software Inc., La Jolla, CA, USA) or R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/). Error bars in the figures show SEMs.
Results
MICs, MBCs and stability
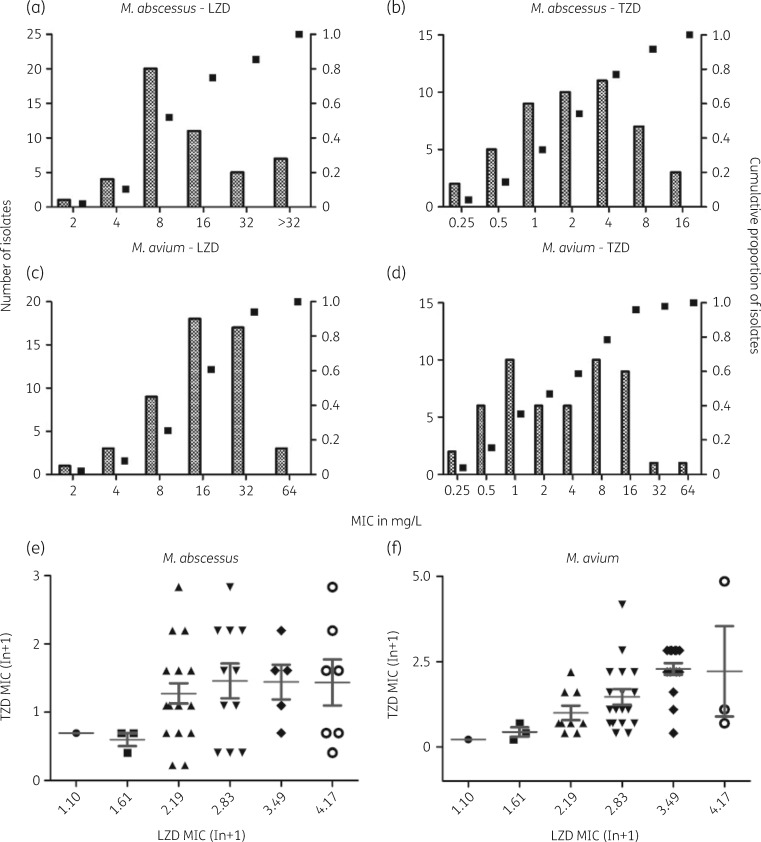
The MICs of tedizolid for clinically relevant NTM are given in Table 1. MICs were generally lower for RGM than for slowly growing mycobacteria (SGM). For both M. abscessus (Figure 1a and b) and M. avium (Figure 1c and d), there is a trend visible for more potent in vitro activity of tedizolid compared with linezolid against NTM. For M. abscessus, tedizolid and linezolid MICs are not correlated (Spearman’s ρ = 0.2304, P = 0.1192; Figure 1e), but they are correlated for M. avium (Spearman’s ρ = 0.5921, P < 0.0001; Figure 1f). Individual linezolid and tedizolid MICs per isolate are given in Table S1 (available as Supplementary data at JAC Online). All wells without visible growth still produced cfu after plating, regardless of organism, leading to an MBC of >128 mg/L for both M. avium and M. abscessus and MBC/MIC ratios of 16 for M. avium and 32–64 for M. abscessus, defining tedizolid as bacteriostatic. Tedizolid MICs remained stable for M. abscessus and M. avium after a pre-incubation period of 7 days, but, after 14 days of pre-incubation, we measured increasing tedizolid MICs as well as some sedimentation at higher concentrations (Table S1).
Table 1.
Tedizolid and linezolid MICs for clinical isolates of SGM and RGM, in CAMHB; for SGM, the broth was enriched with 10% OADC growth supplement
| Organism | n | MIC50/MIC90 or MIC (mg/L) | Tedizolid | Linezolid |
|---|---|---|---|---|
| RGM | ||||
| M. abscessus | 47 | MIC50/MIC90 | 2/8 | 8/>32 |
| M. abscessus CIP 104536 | MIC | 4 | >32 | |
| M. fortuitum | 2 | MIC | 0.25 | 2 |
| M. fortuitum ATCC 6841 | MIC | 2 | 8 | |
| M. peregrinum ATCC 700686 | MIC | 1 | NA | |
| Mycobacterium chelonae | 2 | MIC | 0.5–1 | 8–16 |
| SGM | ||||
| M. avium | 51 | MIC50/MIC90 | 2/16 | 16/32 |
| M. avium ATCC 700898 | MIC | 8 | 16 | |
| M. chimaera | 2 | MIC | 4 | 8–16 |
| M. chimaera DSM 44623 | MIC | 1 | 4 | |
| M. intracellulare | 2 | MIC | 4–8 | 16–32 |
| M. intracellulare DSM 43223 | MIC | 2 | <1 | |
| M. simiae ATCC 25275 | MIC | 2 | 16 |
NA, not applicable.
Figure 1.
MIC distributions of linezolid and tedizolid for clinical isolates of M. abscessus (n = 47) and M. avium (n = 51). (a) Linezolid MIC distribution for M. abscessus. (b) Tedizolid MIC distribution for M. abscessus. (c) Linezolid MIC distribution for M. avium. (d) Tedizolid MIC distribution for M. avium. (e) Correlation between linezolid MIC and tedizolid MIC for M. abscessus. MICs were not correlated (n = 47, Spearman’s ρ = 0.2304, two tailed, P = 0.1192). (f) Correlation between linezolid MIC and tedizolid MIC for M. avium. MICs are correlated (n = 51, Spearman’s ρ = 0.5921, two tailed, P < 0.0001). LZD, linezolid; TZD, tedizolid.
Synergism of tedizolid with other drugs
FICIs of tedizolid combinations are shown in Table 2. For M. abscessus, the tedizolid/amikacin combination yielded the lowest FICI (average = 0.75; range = 0.56–1). Notably, the lowest FICI for tedizolid/amikacin was observed for the M. abscessus reference strain (FICI = 0.56). Though the initial synergism between tedizolid and clarithromycin seemed promising, it was rapidly abrogated by emergence of induced clarithromycin resistance facilitated by the erm(41) gene. From this, we chose amikacin as a combination drug for subsequent time–kill assays against M. abscessus. A combination of tedizolid and cefoxitin was synergistic against M. fortuitum (FICI = 0.375) and a tedizolid/tigecycline combination was synergistic against M. peregrinum (FICI = 0.375).
Table 2.
FICIs of tedizolid in combination with clarithromycin, cefoxitin, tigecycline or amikacin for RGM, and tedizolid in combination with clarithromycin, rifampicin, ethambutol, amikacin or minocycline for M. avium
|
M. abscessus (n = 5) |
M. fortuitum (n = 1) | M. peregrinum (n = 1) | M. chelonae (n = 1) | ||
|---|---|---|---|---|---|
| Combination | average FICI | range (min–max) | FICI | FICI | FICI |
| Tedizolid/clarithromycin | 0.53 | 0.28–0.625 | 0.75 | 1 | 1 |
| Tedizolid/cefoxitin | 0.81 | 0.56–1 | 0.375 | 0.625 | 0.5 |
| Tedizolid/tigecycline | 1.01 | 0.56–1.25 | 1 | 0.375 | 0.625 |
| Tedizolid/amikacin | 0.75 | 0.56–1 | 0.75 | 0.5 | 0.75 |
|
| |||||
|
MAC (n = 4) |
|||||
| Combination | average FICI | range (min–max) | |||
|
| |||||
| Tedizolid/clarithromycin | 1 | 0.75–1.25 | |||
| Tedizolid/rifampicin | 1.25 | 0.5–2.5 | |||
| Tedizolid/ethambutol | 0.72 | 0.5–1 | |||
| Tedizolid/amikacin | 0.81 | 0.5–1 | |||
| Tedizolid/minocycline | 0.78 | 0.625–1 | |||
Tedizolid had no average synergistic interaction for MAC isolates, but had isolated FICIs of 0.5 (Table 2). The lowest average FICI was found in combination with ethambutol (average = 0.72; range = 0.5–1). From this, we chose ethambutol as a combination drug for subsequent time–kill assays against M. avium. We observed no antagonistic interactions.
Time–kill kinetics assays
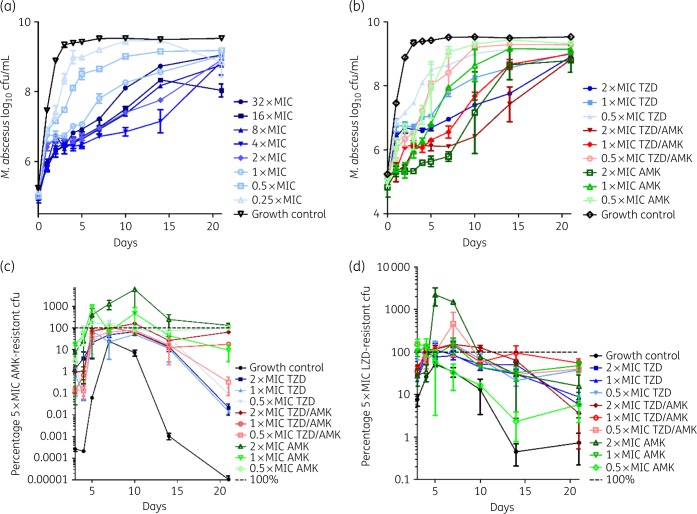
The time–kill kinetics for M. abscessus are shown in Figure 2(a) for the tedizolid dose–response assay and Figure 2(b) for the companion time–kill kinetics for the tedizolid/amikacin combination assay. All conditions of tedizolid show growth until day 2, after which the bacterial loads remain static until day 5 or continued a slightly inhibited growth following a concentration-dependent pattern. Notably, the 32× MIC combination shows the second highest bacterial load by day 21. The combination time–kill kinetics assays in Figure 2(b) show over the course of the experiment that the combination conditions have a consistently lower bacterial burden than the single-drug conditions and the 2× MIC combination condition has a static bacterial burden until day 10. An exception to this overall trend is the 0.5× MIC combination condition, which does not seem to prolong the bacteriostatic activity compared with the single-drug conditions. Compared with tedizolid, amikacin shows a more pronounced bacteriostatic effect, but all conditions show sustained growth by day 10. The percentage of the amikacin-resistant population is shown in Figure 2(c). All treatment conditions induced amikacin resistance, but conditions treated with amikacin showed the highest resistant subpopulation. The percentage of the linezolid-resistant subpopulation as a proxy for the tedizolid-resistant subpopulation is shown in Figure 2(d). No clear trends are visible and all conditions induce linezolid resistance similarly.
Figure 2.
Time–kill kinetics of tedizolid against M. abscessus CIP 104536. (a) Dose–response of tedizolid (MIC = 4 mg/L). (b) Combination time–kill kinetics of tedizolid and amikacin (MIC = 32 mg/L). (c) Percentage of the 5× MIC amikacin-resistant subpopulation of (b). (d) Percentage of the 5× MIC linezolid-resistant subpopulation of (b). AMK, amikacin; LZD, linezolid; TZD, tedizolid.
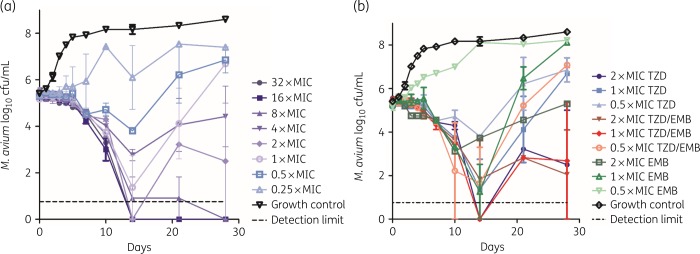
The time–kill kinetics for M. avium are shown in Figure 3(a) for the tedizolid dose–response assay and Figure 3(b) for the companion time–kill kinetics for the tedizolid/ethambutol combination assay. At concentrations higher than 1× MIC, tedizolid achieves at least >1 log kill by day 28. Notably, the 4× MIC condition does not follow the same dose–response trend and has a higher bacterial burden on day 28 than the 2× MIC condition. The combination time–kill kinetics assays in Figure 3(b) show that, overall, the combination conditions have a lower bacterial burden than the single-drug conditions and all conditions follow a dose–response distribution. In most combination conditions, we observe that the SEM exceeds 1 log at some point, indicating high variability between samples. Both the 1× MIC and 2× MIC combination conditions end on a par with the 2× MIC tedizolid condition on day 28. We did not detect a linezolid-resistant subpopulation in any of the conditions (data not shown).
Figure 3.
Time–kill kinetics of tedizolid against M. avium ATCC 700898. (a) Dose–response of tedizolid (MIC = 8 mg/L). (b) Combination time–kill kinetics of tedizolid and ethambutol (MIC = 4 mg/L). EMB, ethambutol; TZD, tedizolid.
Response surface analysis
The sigmoidal Emax curves for tedizolid on M. abscessus and M. avium are shown in Figure S1(a) and Figure S1(b), respectively. For M. abscessus, the fitted Emax was 34.1 log10 cfu/mL × day, the EC50 was 0.73× MIC and the estimated Hill slope was 2.63. For M. avium, the fitted Emax was 176 log10 cfu/mL × day, the EC50 was 0.94× MIC and the estimated Hill slope was 0.84. The calculated ΔE percentages as well as Ecomb,obs, Ecomb,BI and the effect sizes of the companion drugs in the combination time–kill kinetics assays are shown in Table 3.
Table 3.
Response surface analysis results, as well as interaction effect size, by deviation from expected combination effect under Bliss independence in percentage ΔE
| Combination (concentration) | Effect (log10 cfu/mL × day) |
||||
|---|---|---|---|---|---|
| tedizolid | amikacin or ethambutol | observed, combination | expected, combinationa | ΔE (%) | |
| M. abscessus | |||||
| tedizolid/amikacin (0.5× MIC) | 8.93 | −95.6 | 16.3 | 62.2 | −126 |
| tedizolid/amikacin (1× MIC) | 22.6 | 24.2 | 33.7 | 31.1 | 8.24 |
| tedizolid/amikacin (2× MIC) | 34.8 | 35.6 | 48.8 | 34.9 | 39.7 |
| M. avium | |||||
| tedizolid/ethambutol (0.5× MIC) | 65.8 | 9.39 | 102 | 71.6 | 42.3 |
| tedizolid/ethambutol (1× MIC) | 96.5 | 81.8 | 145 | 133 | 8.81 |
| tedizolid/ethambutol (2× MIC) | 137 | 95.1 | 137 | 159 | −13.4 |
Under Bliss independence.
The combination of tedizolid and amikacin against M. abscessus had an antagonistic interaction at low concentrations (ΔE = −126%) and was synergistic at higher concentrations (ΔE = 39.7%). This is mainly because of a prolonged lower cfu count from day 6 onwards, after which the 2× MIC tedizolid/amikacin condition performed better than the single-drug conditions as it prevented regrowth. For M. avium, the interaction between tedizolid and ethambutol is considered synergistic at lower concentrations (ΔE = 42.3%) and turns slightly antagonistic at higher concentrations (ΔE = −13.4%).
Ex vivo intracellular assays
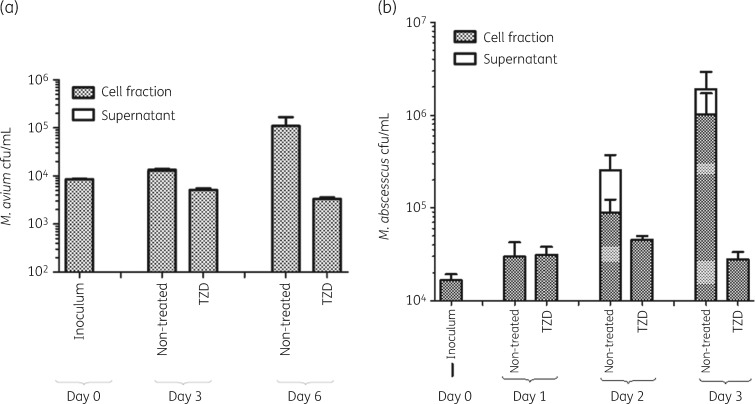
Intracellular and extracellular cfu/mL counts of M. avium are shown in Figure 4(a). Tedizolid reduced the intracellular bacterial load by 66% below stasis over the course of 6 days. The extracellular bacterial population was comparatively low (2% of total cfu counts). Intracellular and extracellular cfu/mL counts of M. abscessus are shown in Figure 4(b). Overall, tedizolid could hold the bacterial load static compared with the non-treated control over the course of 3 days. Especially on day 2, we observe a large extracellular M. abscessus population in the non-treated controls, but not in the treated conditions. By day 3, the intracellular bacterial load of the treated conditions is lower by a factor of 500 than the non-treated condition. Notably, little extracellular bacterial load is detected in the tedizolid-treated conditions.
Figure 4.
Ex vivo infection assays of mycobacteria-infected macrophages treated with and without tedizolid (2× MIC). (a) Intracellular and extracellular cfu counts of M. avium. (b) Intracellular and extracellular cfu counts of M. abscessus. TZD, tedizolid.
Discussion
Tedizolid holds promise as the successor to linezolid in the treatment of mycobacterial diseases, because of its increased in vitro activity8 and lower toxicity in treatment of other bacterial infections, compared with linezolid.12,22 Linezolid is recommended in M. abscessus therapy and is a second-line agent in MAC disease treatment,1 although there are no clinical data supporting its efficacy. A more active and better-tolerated alternative to linezolid is desirable. In this study, we confirm previous MIC distribution data for MAC and M. abscessus found by Brown-Elliott and Wallace8 and also find that tedizolid generally has a lower MIC than linezolid. Notably, the tedizolid MIC distribution for M. avium seems to be bimodal, emphasizing the need to perform in vitro drug susceptibility testing. The mechanistic background underlying this bimodal MIC distribution requires further study. Tedizolid showed concentration-dependent activity against M. avium and bacteriostatic activity against M. abscessus in time–kill experiments and promising intracellular activity against both M. avium and M. abscessus.
With our time–kill experiments with M. abscessus, we confirm a study performed by Compain et al.,14 who report modest exposure-dependent activity of tedizolid. Combination experiments with tedizolid and amikacin reveal modest, concentration-dependent synergy relative to Bliss independence. Tedizolid’s potential is further supported by its performance alone in human macrophages, where it holds the bacterial burden static over 3 days at 2× MIC. A previous study showed similar tedizolid activity against M. abscessus, in THP-1 cells.15
For M. avium, few in vitro studies beyond MIC determinations are available. The aforementioned hollow-fibre system study by Deshpande et al.13 shows intracellular killing of 2.0 log10 cfu/mL over 14 days, which translates well to our observed killing effect in time–kill assays. These researchers also assessed tedizolid together with a ceftazidime/avibactam + rifabutin + moxifloxacin combination in a hollow-fibre system infection model, reporting a stronger killing effect than an azithromycin/ethambutol/rifabutin regimen.23 Though these results are promising, adding tedizolid to the standard regimen or exploiting our observed synergism with ethambutol by replacing rifampicin might be an approach more translatable to clinical trials.
Translating in vitro findings to the clinical reality is challenging, especially when integrating tedizolid in a multidrug regimen exploiting its synergism with ethambutol. Deshpande et al.13 propose a dose of 200 mg daily if no interacting co-medication is used, treating M. avium-infected THP-1 cells in a hollow-fibre system. Based on this study, Deshpande et al.13 suggested a tedizolid breakpoint of 1 mg/L. Based on our MIC distribution data, a 1 mg/L breakpoint would define <40% of MAC isolates as susceptible.
A more favourable safety profile of tedizolid versus linezolid has only been established for a 200 mg/day dose for up to 6 days;24 the safety profile of prolonged administration of higher doses is uncertain. The efficacy and long-term safety of different tedizolid doses within multidrug regimens should be investigated in pharmacodynamic models and ultimately clinical trials.
Our study has several limitations to consider. Most importantly, all our assays are static assays where drug is added only at the beginning and not continuously, making human pharmacokinetics impossible to model. A hollow-fibre system experiment might be interesting, especially if assessing the tedizolid/ethambutol synergism as well as counteracting drug stability issues and medium exhaustion. The high proportion of resistant cfu in the resistance monitoring assays surpassing 100% remains unexplained. Regrettably, we could also not monitor the linezolid resistance in M. avium time–kill assays. For this, lower concentrations of linezolid (3× MIC) might have been helpful. Also, linezolid was merely used as a proxy for tedizolid resistance and it might not mirror the actual resistance to tedizolid in M. abscessus resistance monitoring, possibly leading to an overestimation of resistance in these assays. Lastly, translating MICs, synergy and time–kill kinetics to possible clinical efficacy is challenging, especially for M. fortuitum and M. peregrinum, where we only tested the reference strains. Ideally, this will be established in a stepwise process of hollow-fibre system studies and clinical trials.
Concluding, we confirmed tedizolid as a potent asset in MAC pulmonary disease treatment; it is less active against M. abscessus, but still interesting as a replacement for linezolid. With the observed synergistic interactions, especially with ethambutol, a tedizolid/ethambutol/azithromycin regimen might be an interesting candidate regimen for further pharmacodynamic studies and ultimately clinical trials in MAC pulmonary disease.
Supplementary Material
Acknowledgements
We thank MSD for kindly providing us with tedizolid phosphate and appreciate the collaboration.
Funding
Jakko van Ingen is supported by a personal grant from the Netherlands Organization for Scientific Research (NWO/ZonMW grant Veni 016.176.024). This grant funded laboratory equipment as well as consumables used in this study.
Transparency declarations
None to declare.
References
- 1. Griffith DE, Aksamit T, Brown-Elliott BA. et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175: 367–416. [DOI] [PubMed] [Google Scholar]
- 2. Hoefsloot W, van Ingen J, Andrejak C. et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J 2013; 42: 1604–13. [DOI] [PubMed] [Google Scholar]
- 3. Stout JE, Koh W-J, Yew WW.. Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis 2016; 45: 123–34. [DOI] [PubMed] [Google Scholar]
- 4. Floto RA, Olivier KN, Saiman L. et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis: executive summary. Thorax 2016; 71: 88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Ingen J, Ferro BE, Hoefsloot W. et al. Drug treatment of pulmonary nontuberculous mycobacterial disease in HIV-negative patients: the evidence. Expert Rev Anti Infect Ther 2013; 11: 1065–77. [DOI] [PubMed] [Google Scholar]
- 6. Philley JV, Griffith DE.. Management of nontuberculous mycobacterial (NTM) lung disease. Semin Respir Crit Care Med 2013; 34: 135–42. [DOI] [PubMed] [Google Scholar]
- 7. Winthrop KL, Ku JH, Marras TK. et al. The tolerability of linezolid in the treatment of nontuberculous mycobacterial disease. Eur Respir J 2015; 45: 1177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown-Elliott BA, Wallace RJ.. In vitro susceptibility testing of tedizolid against nontuberculous mycobacteria. J Clin Microbiol 2017; 55: 1747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burdette SD, Trotman R.. Tedizolid: the first once-daily oxazolidinone class antibiotic. Clin Infect Dis 2015; 61: 1315–21. [DOI] [PubMed] [Google Scholar]
- 10. Conte JE Jr, Golden JA, Kipps J. et al. Intrapulmonary pharmacokinetics of linezolid. Antimicrob Agents Chemother 2002; 46: 1475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Housman ST, Pope JS, Russomanno J. et al. Pulmonary disposition of tedizolid following administration of once-daily oral 200-milligram tedizolid phosphate in healthy adult volunteers. Antimicrob Agents Chemother 2012; 56: 2627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yuste JR, Bertó J, Del Pozo JL. et al. Prolonged use of tedizolid in a pulmonary non-tuberculous mycobacterial infection after linezolid-induced toxicity. J Antimicrob Chemother 2017; 72: 625–8. [DOI] [PubMed] [Google Scholar]
- 13. Deshpande D, Srivastava S, Pasipanodya JG. et al. Tedizolid is highly bactericidal in the treatment of pulmonary Mycobacterium avium complex disease. J Antimicrob Chemother 2017; 72 Suppl 2: ii30–5. [DOI] [PubMed] [Google Scholar]
- 14. Compain F, Soroka D, Heym B. et al. In vitro activity of tedizolid against the Mycobacterium abscessus complex. Diagn Microbiol Infect Dis 2018; 90: 186–9. [DOI] [PubMed] [Google Scholar]
- 15. Le Run E, Arthur M, Mainardi J-L.. In vitro and intracellular activity of imipenem combined with tedizolid, rifabutin, and avibactam against Mycobacterium abscessus. Antimicrob Agents Chemother 2019; 63: e01915-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CLSI. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes—Second Edition: M24 2011. [PubMed]
- 17. Ruth MM, Sangen JJN, Pennings LJ. et al. Minocycline has no clear role in the treatment of Mycobacterium abscessus disease. Antimicrob Agents Chemother 2018; 62: e01208-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 2003; 52: 1. [DOI] [PubMed] [Google Scholar]
- 19. Ruth MM, Sangen JJN, Remmers K. et al. A bedaquiline/clofazimine combination regimen might add activity to the treatment of clinically relevant non-tuberculous mycobacteria. J Antimicrob Chemother 2019; 74: 935–43. [DOI] [PubMed] [Google Scholar]
- 20. Wicha SG, Kees MG, Kuss J. et al. Pharmacodynamic and response surface analysis of linezolid or vancomycin combined with meropenem against Staphylococcus aureus. Pharm Res 2015; 32: 2410–8. [DOI] [PubMed] [Google Scholar]
- 21. Ruth MM, Magombedze G, Gumbo T. et al. Minocycline treatment for pulmonary Mycobacterium avium complex disease based on pharmacokinetics/pharmacodynamics and Bayesian framework mathematical models. J Antimicrob Chemother 2019; 74: 1952–61. [DOI] [PubMed] [Google Scholar]
- 22. Hall RG, Smith WJ, Putnam WC. et al. An evaluation of tedizolid for the treatment of MRSA infections. Expert Opin Pharmacother 2018; 19: 1489–94. [DOI] [PubMed] [Google Scholar]
- 23. Deshpande D, Srivastava S, Pasipanodya JG. et al. A novel ceftazidime/avibactam, rifabutin, tedizolid and moxifloxacin (CARTM) regimen for pulmonary Mycobacterium avium disease. J Antimicrob Chemother 2017; 72 Suppl 2: ii48–53. [DOI] [PubMed] [Google Scholar]
- 24. Lan S-H, Lin W-T, Chang S-P. et al. Tedizolid versus linezolid for the treatment of acute bacterial skin and skin structure infection: a systematic review and meta-analysis. Antibiotics (Basel) 2019; 8: E137.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.