Sir,
Human cytomegalovirus (CMV) remains a serious complication of HSCT. In 2017, letermovir was approved for prophylaxis of CMV infection for high-risk patients following allogeneic HSCT.1,2 Letermovir is an inhibitor of CYP3A4 and inducer of CYP2C19/2C9, which are common enzymatic pathways for many medications used in HSCT, including voriconazole.2–4 Voriconazole is metabolized by CYP2C9 and CYP2C19, and co-administration with letermovir may lead to reduced voriconazole exposure through induction of these pathways.3,4 In a study of healthy subjects who received letermovir 480 mg daily with voriconazole, voriconazole AUC and maximum serum concentration were reduced by 44% and 39%, respectively.4 In addition, interpatient variability can be significant, with plasma concentrations of voriconazole varying up to 100-fold between patients.5 Although letermovir is known to reduce voriconazole exposure, there are limited published data describing the implications of this interaction in clinical practice. Here, we report two cases of a clinically significant drug interaction between voriconazole and letermovir.
Case 1
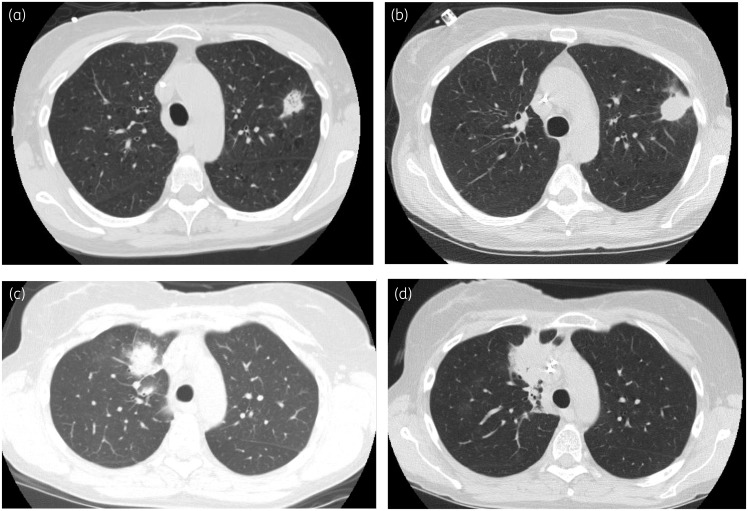
A 58-year-old woman received a reduced-intensity allogeneic HSCT for acute myeloid leukaemia conditioned with cyclophosphamide, fludarabine, thiotepa and total body irradiation. Ten days after transplant, she was started on voriconazole 300 mg twice daily, after an initial loading dose, for invasive pulmonary aspergillosis diagnosed based on a pulmonary nodule seen on chest CT scan and positive galactomannan of 1.088 from bronchoalveolar lavage (BAL) (Figure 1a). Two days earlier, she began letermovir 480 mg daily for CMV prophylaxis. The voriconazole trough concentration obtained 5 days after voriconazole initiation was 1.1 mg/L. Neutrophil engraftment occurred on day +13. On day +35 post-HSCT, a chest CT scan demonstrated interval enlargement of the pulmonary nodule (Figure 1b). The voriconazole trough measurement on day +44 showed a subtherapeutic concentration of 0.6 mg/L. The patient reported full adherence to her regimen. Her voriconazole dose was increased to 350 mg twice daily. The trough obtained on day +83 was subtherapeutic at 0.5 mg/L, and her voriconazole dose was increased to 400 mg twice daily with a measured concentration of 0.8 mg/L on day +95. Because follow-up imaging demonstrated a slow but interval decrease in nodule size and discontinuation of letermovir was planned for the next day per protocol, no further changes were made. The voriconazole trough measurement obtained 1 week after discontinuation of letermovir was 1.4 mg/L. The patient had some nausea due to mild gastrointestinal graft-versus-host disease (GVHD), which did not require systemic steroids and was managed with oral beclomethasone, which was unlikely to influence absorption of oral voriconazole. No other interacting medications were identified, and liver function tests remained within normal limits throughout treatment.
Figure 1.
(a) CT scan of Patient 1 obtained on day +10 after transplant immediately prior to initiation of voriconazole, demonstrating left upper lobe pulmonary nodule. (b) CT scan of Patient 1, obtained on day +35 after transplant, demonstrating interval enlargement and increase in density of the left upper lobe nodule. (c) CT scan of Patient 2, obtained on day +11 after transplant, immediately prior to initiation of voriconazole. (d) CT scan of Patient 2, obtained on day +27, demonstrating worsening right upper lobe consolidation.
Case 2
A 54-year-old woman received a matched unrelated allogeneic HSCT for myelodysplastic syndrome conditioned with busulfan and cyclophosphamide. Eleven days following transplant, the patient was diagnosed with invasive pulmonary aspergillosis based on a chest CT with new pulmonary nodules (Figure 1c) and serum and BAL galactomannan of 0.788 and 5.029, respectively. She was started on voriconazole 200 mg twice daily after a loading dose with a 2 week course of micafungin 100 mg daily. Neutrophil engraftment occurred on day +12. On day +19, she began letermovir 480 mg daily for CMV prophylaxis. The following day, her voriconazole trough was 1.5 mg/L. On day +20, 4 days after initiation of letermovir, voriconazole trough concentration was subtherapeutic at 0.6 mg/L. Voriconazole was increased to 300 mg twice daily. On day +27, imaging demonstrated worsening right upper lobe (RUL) consolidation (Figure 1d). The voriconazole trough drawn at this time was 1.1 mg/L and on day +33 voriconazole was increased to 350 mg twice daily. The voriconazole trough on day +41 was 0.9 mg/L. Voriconazole was increased to 400 mg twice daily and a trough level on day +62 was 1.1 mg/L. The patient reported full adherence, had no reported symptoms of GVHD, and no other interacting medications were identified. Repeat chest CT scan on day +69 demonstrated some improvement in her RUL pulmonary consolidation. Eventually, voriconazole was switched to isavuconazole due to fluoride level elevation associated with bone pain.
These cases highlight the need for awareness of the interaction between letermovir and voriconazole. As institutions increasingly adopt letermovir for CMV prophylaxis, the potential for clinically meaningful drug interactions as evidenced by these two cases will also rise. Notably, no significant effects of letermovir on posaconazole pharmacokinetics have been observed as posaconazole is not metabolized by the cytochrome P450 pathway.4 No drug interaction is expected with fluconazole; as isavuconazole is metabolized by CYP3A4, letermovir may increase isavuconazole exposure.3 If patients must receive voriconazole and letermovir concomitantly, voriconazole levels should be monitored with increased frequency. We suggest that the voriconazole level should be checked at least 1 week after letermovir discontinuation to guide voriconazole dose reduction as the cytochrome P450 induction effect wanes; this should be done in conjunction with close monitoring for signs and symptoms of voriconazole neurotoxicity.6,7
Funding
This work was supported by a grant from the National Cancer Institute at the National Institutes of Health (P30 CA15704).
Transparency declarations
M.B. has served as a consultant for and received research support from Merck, Astellas, Chimerix, GSK, Takeda (formerly Shire) and has served as a consultant for Helocyte. S.A.P. participates in clinical trials with Chimerix, and has received research support from Global Life Technologies, Inc. All other authors: none to declare.
References
- 1. Marty FM, Ljungman P, Chemaly RF. et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med 2017; 377: 2433–44. [DOI] [PubMed] [Google Scholar]
- 2.PrevymisTM Package Insert. Merck & Co. Inc, 2017. [Google Scholar]
- 3.PrevymisTM Assessment Report. European Medicines Agency, 2017. [Google Scholar]
- 4. Marshall WL, McCrea JB, Macha S. et al. Pharmacokinetics and tolerability of letermovir coadministered with azole antifungals (posaconazole or voriconazole) in healthy subjects. J Clin Pharmacol 2018; 58: 897–904. [DOI] [PubMed] [Google Scholar]
- 5. Boyd AE, Modi S, Howard SJ. et al. Adverse reactions to voriconazole. Clin Infect Dis 2004; 39: 1241–4. [DOI] [PubMed] [Google Scholar]
- 6. Dolton MJ, Ray JE, Chen SC. et al. Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring. Antimicrob Agents Chemother 2012; 56: 4793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pascual A, Calandra T, Bolay S. et al. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis 2008; 46: 201–11. [DOI] [PubMed] [Google Scholar]