Abstract
Background
Long-acting (LA) formulations of cabotegravir, an HIV integrase inhibitor, and rilpivirine, an NNRTI, are in development as monthly or 2 monthly intramuscular (IM) injections for maintenance of virological suppression.
Objectives
To evaluate cabotegravir and rilpivirine CSF distribution and HIV-1 RNA suppression in plasma and CSF in HIV-infected adults participating in a substudy of the Phase 2b LATTE-2 study (NCT02120352).
Methods
Eighteen participants receiving cabotegravir LA 400 mg + rilpivirine LA 600 mg IM [every 4 weeks (Q4W), n = 3] or cabotegravir LA 600 mg + rilpivirine LA 900 mg IM [every 8 weeks (Q8W), n = 15] with plasma HIV-1 RNA <50 copies/mL enrolled. Paired steady-state CSF and plasma concentrations were evaluable in 16 participants obtained 7 (±3) days after an injection visit. HIV-1 RNA in CSF and plasma were assessed contemporaneously using commercial assays.
Results
Median total CSF concentrations in Q4W and Q8W groups, respectively, were 0.011 μg/mL and 0.013 μg/mL for cabotegravir (0.30% and 0.34% of the paired plasma concentrations) and 1.84 ng/mL and 1.67 ng/mL for rilpivirine (1.07% and 1.32% of paired plasma concentrations). Cabotegravir and rilpivirine total CSF concentrations exceeded their respective in vitro EC50 for WT HIV-1 (0.10 ng/mL and 0.27 ng/mL, respectively). All 16 participants had HIV-1 RNA <50 copies/mL in plasma and CSF, and 15 of 16 participants had HIV-1 RNA <2 copies/mL in CSF.
Conclusions
A dual regimen of cabotegravir LA and rilpivirine LA achieved therapeutic concentrations in the CSF resulting in effective virological control in CSF.
Introduction
Improvements in the health and wellbeing of people living with HIV infection continue to be driven by on-going developments in ART; however, several challenges to the effective treatment of HIV-1 infection remain. Despite simplified regimens, including single-tablet formulations of two or three active drugs, suboptimal treatment adherence still occurs and is associated with an increased risk of virological failure and emergence of antiretroviral resistance.1–5 The complexities of treating an ageing population with accumulating comorbidities and polypharmacy mean that drug–drug interactions are important considerations, often limiting the choice of an optimal ART regimen.4,5 Long-acting (LA) injectable ART may help address these challenges by providing a convenient, discreet treatment option that avoids the need for daily oral dosing, thereby mitigating the potential impact of non-adherence on the emergence of viral resistance. With LA dosing, ART can be directly observed in a healthcare setting, which may augment the decline in non-adherence currently seen with oral regimens. Additionally, the need to keep, store and transport medications and the possible impact these have on patient-reported stigma could be lessened.6–9 The removal of food requirements and interactions occurring at the level of the gastrointestinal tract (e.g. requirement for maintenance of gastric pH, metal cation chelation potential) are potential further advantages with parenteral administration.
Cabotegravir, an analogue of the integrase strand transfer inhibitor dolutegravir, exhibits subnanomolar potency (EC50 for WT HIV-1 clade B = 0.25 nM) and antiviral activity against a broad range of HIV-1 strains.10,11 Cabotegravir primarily undergoes glucuronidation via UGT1A1, and to a minor extent via UGT1A9, and does not induce or inhibit UGT- or CYP-mediated metabolism.12 Following oral administration, cabotegravir is rapidly absorbed and is >99.8% bound to plasma proteins with limited distribution outside the plasma and extracellular compartment. Cabotegravir is a low-clearance compound as evidenced by a terminal elimination half-life of 40 h following once-daily dosing.13–15 Rilpivirine is an NNRTI approved as a 25 mg once-daily oral tablet indicated, in combination with other antiretrovirals, for the treatment of HIV-1 infection in treatment-naive individuals with HIV-1 RNA ≤100 000 copies/mL.16,17 Oral rilpivirine is readily absorbed, highly bound to plasma proteins (99.7%), is primarily metabolized by CYP3A and does not induce or inhibit metabolic enzymes.16 Injectable LA suspension formulations of cabotegravir and rilpivirine are in Phase 3 clinical development for the maintenance of HIV virological suppression in adults.18,19 Both cabotegravir LA and rilpivirine LA are slowly absorbed into the systemic circulation following intramuscular (IM) administration reflected by apparent absorption rate-limited half-lives of approximately 40 days and 90–200 days, respectively, allowing for administration on a monthly or less frequent schedule.15,19–22 In a Phase 1 study, monthly IM cabotegravir LA (200 or 400 mg) and rilpivirine LA (600 or 900 mg) achieved therapeutically relevant plasma concentrations (>4-fold the protein binding-adjusted EC90 for WT HIV) of each drug within 3 days of administration; after the fourth dose of cabotegravir and the second dose of rilpivirine, maximum plasma concentrations (geometric mean 2.2–4.4 μg/mL and 126–168 ng/mL, respectively) were achieved after approximately 1 week, with prolonged exposure over the dosing interval.22
The LATTE-2 Phase 2b study showed that cabotegravir LA 400 mg + rilpivirine LA 600 mg every 4 weeks (Q4W) or cabotegravir LA 600 mg + rilpivirine LA 900 mg, every 8 weeks (Q8W), was as effective as daily oral three-drug therapy (oral cabotegravir + abacavir/lamivudine) at maintaining HIV-1 viral suppression through 160 weeks of treatment.18,23 IM injections were well tolerated and well accepted, with 97% of participants reporting high levels of treatment satisfaction at Week 9618 and approximately 70% of participants reporting that it was never inconvenient to receive their medication (HIV Medication Questionnaire).24 Week 48 results from two pivotal Phase 3 studies assessing monthly maintenance therapy in adults with prior virological suppression (HIV-1 RNA <50 copies/mL) on oral ART demonstrated the non-inferiority of IM cabotegravir LA + rilpivirine LA compared with oral dolutegravir/abacavir/lamivudine (FLAIR study, NCT02938520) or with oral PI-, integrase inhibitor- or NNRTI-based triple therapy (ATLAS study, NCT02951052).25,26 Cabotegravir LA and rilpivirine LA were well tolerated with few (<1.5%) confirmed virological failures across both studies.25,26
A challenge to the effective treatment of HIV-1 infection is the persistence of HIV replication in ‘sanctuary’ sites where drug penetration and the host’s immune response may be suboptimal. The CNS is one of these important sanctuary sites. Drug-resistant viruses distinct from those in the plasma have been identified in CSF.27 Latent and productive HIV infection in the brain, even at levels below the quantification limit of commercial assays, may be implicated in inflammation associated with neurocognitive impairment, which is still prevalent among people living with HIV.28–30 ART is effective at reducing HIV-1 RNA to undetectable levels in the plasma, but virus may still be found in CSF, particularly when antiretroviral penetration into the CSF results in levels below effective therapeutic concentrations.30–32 Discordance between plasma and CSF HIV-1 RNA levels has been associated with worse neurocognitive performance in treated individuals.27,30,33,34 Therefore, it is important to elucidate the distribution and efficacy of new antiretrovirals in the CSF.
This investigation aimed to determine plasma and CSF cabotegravir and rilpivirine concentrations; to measure HIV-1 RNA in plasma and CSF following IM administration of cabotegravir LA and rilpivirine LA; and to assess the relationships between these parameters. Safety and adverse effects for participants in the substudy were also assessed.
Patients and methods
Ethics
The study was performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants. The protocol was approved by the institutional review board of each study site.
Design and study population
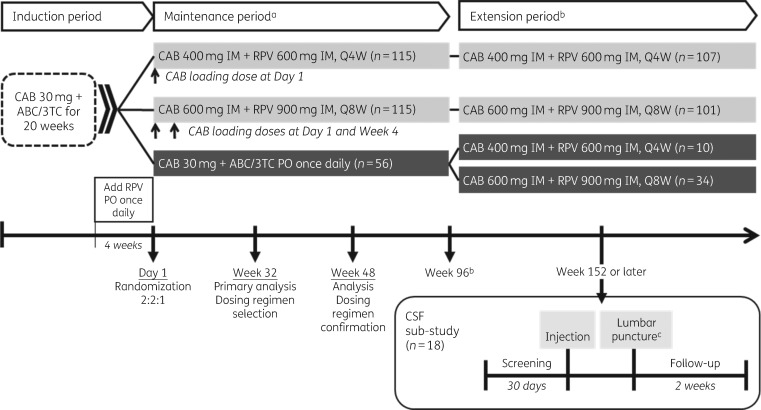
The CSF pharmacokinetic substudy was a USA-specific protocol amendment conducted at six study sites between 31 October 2017 and 21 February 2018 during the extension period of the LATTE-2 Phase 2b study (NCT02120352): a randomized, multicentre, open-label, non-inferiority, parallel-group, dose-ranging trial evaluating monthly or 2 monthly IM cabotegravir LA + rilpivirine LA versus oral comparator regimen (Figure 1).18,23 All study participants entering the extension period at Week 96 either continued to receive IM cabotegravir LA 400 mg + rilpivirine LA 600 mg Q4W or cabotegravir LA 600 mg + rilpivirine LA 900 mg Q8W, as originally randomized, or switched to either of the IM dosing regimens from the oral comparator regimen (oral cabotegravir + abacavir/lamivudine). The initial LATTE-2 study population included adults (aged 18 years or older) with HIV-1 infection who had no more than 10 days of previous ART, screening HIV-1 RNA of at least 1000 copies/mL and CD4+ T cell counts of at least 200 cells/mm³. Key exclusion criteria included: the presence of any major antiretroviral resistance-associated mutation; pregnancy; moderate or severe hepatic impairment; clinically relevant hepatitis C infection requiring treatment; serological evidence of chronic hepatitis B infection; laboratory values of clinical concern, including creatinine clearance less than 50 mL/min; or a need for chronic anticoagulants. The CSF pharmacokinetic substudy permitted enrolment of participants with HIV-1 RNA less than 50 copies/mL prior to and during the substudy screening period, who were willing to undergo lumbar puncture for collection of CSF and had no contraindications to lumbar puncture. Screening for the substudy was permitted at any study visit during the extension period and occurred within 30 days prior to the next scheduled study injection visit. For individuals in the Q8W group, an extra study visit was required to obtain screening HIV-1 RNA levels within this 30 day period. For those in the Q4W group, the screening visit coincided with the previous LATTE-2 injection visit.
Figure 1.
LATTE-2 Phase 2b study design and CSF substudy schedule. aSubjects who withdrew after at least one IM dose entered the long-term follow-up period (not shown). bSubjects could elect to enter Q4W and Q8W LA extension phase beyond Week 96. cLumbar puncture was carried out 1 week post-injection ± 3 days. ABC/3TC, abacavir/lamivudine; CAB, cabotegravir; PO, orally; Q4W, every 4 weeks; Q8W, every 8 weeks; RPV, rilpivirine.
Study endpoints
Endpoints for the CSF pharmacokinetic substudy were: total CSF concentrations of cabotegravir and rilpivirine 7 (±3) days after injection; plasma concentrations of cabotegravir (total and unbound) and rilpivirine (total) at the same timepoints as CSF measurements; and percentage of participants with plasma HIV RNA <50 copies/mL and <2 copies/mL in CSF and plasma. Cabotegravir and rilpivirine concentrations in plasma that were collected 7 (±3) days post-injection were considered Cmax values. Relationships between total cabotegravir and rilpivirine concentrations in plasma and CSF and between plasma HIV-1 RNA and CSF HIV-1 RNA were also evaluated. Safety endpoints included the incidence and severity of adverse events and laboratory abnormalities associated with the substudy.
Procedures and assessments
The Abbott RealTime HIV-1 assay, with a lower limit of quantification of 40 copies/mL, was used to measure plasma HIV RNA at screening, at the injection visit and at the lumbar puncture visit (7 ± 3 days after injection). The HIV-1 SuperLow assay [a proprietary modification of the NucliSENS EasyQ assay (bioMérieux), performed by bioMONTR Labs as previously described]35 and with a limit of quantification of 2 copies/mL, was used to measure HIV-1 RNA in the CSF at the lumbar puncture visit and in the plasma at injection and lumbar puncture visits.
Plasma and CSF samples were processed and frozen within 1 h of collection. Drug concentrations in plasma were determined using validated bioanalytical methods based on protein precipitation followed by HPLC-MS/MS analysis. Drug concentrations in CSF were determined using validated bioanalytical methods based on dilution followed by HPLC-MS/MS analysis. Cabotegravir concentrations in the plasma and CSF were analysed by Covance Inc. (Madison, WI, USA) as previously described.36 The lower and upper limits of quantification for cabotegravir were 25 ng/mL and 25 000 ng/mL, respectively, for total plasma concentration; 1 ng/mL and 2500 ng/mL for plasma unbound concentration; and 1 ng/mL and 1000 ng/mL for CSF concentration. Within-run precision (% CV) for the determination of cabotegravir in plasma was ≤6.8% and between-run precision (% CV) was ≤9.1%; accuracy (% bias) ranged from −8.8% ≤to ≤8.0%. Within-run precision (% CV) for the determination of cabotegravir in CSF was ≤9.1% and between-run precision (% CV) was ≤9.8%; accuracy (% bias) ranged from −4.5% ≤to ≤6.0%. Rilpivirine concentrations in plasma and CSF were analysed by PRA Health Sciences (Assen, the Netherlands) using a validated HPLC-MS/MS assay. After sample clean-up using protein precipitation, aliquots were spiked with a stable isotope-labelled internal standard (TMC278-13C-d4), which was also added to calibration standard and quality control samples. Chromatographic separation was achieved on an Acquity BEH C18 (50 × 2.1 mm, 1.7 μm) at 55°C. Gradient elution using ammonium formate and methanol was applied with a flow rate of 0.75 mL/min. A triple quadrupole mass spectrometer (API 4000) with turbo-ion spray was used in positive ion mode. Quantification was based on multiple reaction monitoring of the transitions of m/z 367.2–224.0 for rilpivirine and 372.2–225.0 for the internal standard. The lower limit of quantification for rilpivirine was 1 ng/mL. The effective linear range of quantification was 1–2000 ng/mL. The accuracy for rilpivirine quality control samples in plasma and in CSF met the predefined criterion of <−15% for the low, medium and high concentration quality control samples in at least 66.7% of all quality control results and in at least 50% of the results at each concentration level. The precision did not exceed 15% at each concentration level.
Safety was assessed by monitoring adverse events, clinical laboratory tests, vital signs and physical examinations at screening and during the injection and lumbar puncture visits. After the lumbar puncture visit, a 2 week safety follow-up assessment was carried out by phone for all substudy participants. Adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA).
Statistical analysis
As this CSF substudy aimed to describe cabotegravir and rilpivirine concentrations in plasma and CSF, no formal hypotheses were tested. A sample size of 20 evaluable participants was targeted.
The proportions of participants with HIV-1 RNA <50 copies/mL and <2 copies/mL in plasma and in CSF were calculated and summarized by treatment and visit. Safety data were also summarized by treatment for each visit. Plasma cabotegravir (total and unbound) and rilpivirine (total) concentration, CSF cabotegravir and rilpivirine total concentration was summarized by treatment.
Correlations were calculated between: total plasma cabotegravir versus total CSF cabotegravir; total plasma rilpivirine versus total CSF rilpivirine; and total plasma cabotegravir versus unbound plasma cabotegravir. The relationship between plasma pharmacokinetics and CSF samples (HIV-RNA) was assessed using plots or Pearson correlation coefficients if both CSF and plasma had actual measurable HIV-1 RNA data. SAS PROC CORR (Cary, NC, USA) was used to calculate correlation coefficients.
Results
A total of 18 participants in the extension period through to at least Week 152 of the LATTE-2 parent study were screened for the substudy. All 18 participants were receiving IM cabotegravir LA and rilpivirine LA Q4W (n = 3) or Q8W (n = 15); six participants had switched from oral dosing after the Week 96 visit and 12 participants had received IM dosing from the beginning of LATTE-2. At the time of substudy enrolment, all participants had been receiving cabotegravir LA and rilpivirine LA for at least 1 year. All 18 screened participants were enrolled and completed the substudy. The mean age of substudy participants was 38.3 years (range 19–64 years). Most participants were men and of white race (Table 1). No participants had clinical evidence of neurological disease at substudy entry, although no formal neuropsychological testing was performed. All plasma and CSF samples were collected between 5 and 9 days after dosing and were within the protocol’s specification of 7 (±3) days after injection. Lumbar puncture for CSF collection was not successful in two participants in the Q8W group.
Table 1.
CSF substudy population baseline characteristics
| Characteristicsa | Q8W IM (N = 15) | Q4W IM (N = 3) | Total (N = 18) |
|---|---|---|---|
| Age, years, mean (SD) | 37.2 (11.12) | 44.0 (13.08) | 38.3 (11.35) |
| Gender, n (%) | |||
| male | 12 (80) | 3 (100) | 15 (83) |
| female | 3 (20) | 0 | 3 (17) |
| BMI, kg/m2, mean (SD) | 26.7 (5.28) | 26.0 (1.51) | 26.5 (4.83) |
| Weight, kg, mean (SD) | 82.2 (15.27) | 87.0 (18.81) | 83.0 (15.40) |
| Ethnicity, n (%) | |||
| Hispanic/Latino | 4 (27) | 0 | 4 (22) |
| not Hispanic/Latino | 11 (73) | 3 (100) | 14 (78) |
| Race, n (%) | |||
| Asian | 1 (7) | 1 (33) | 2 (11) |
| black/African American | 5 (33) | 0 | 5 (28) |
| white | 9 (60) | 2 (67) | 11 (61) |
| Plasma HIV-1 RNA <50 copies/mL, n (%) | 15 (100) | 3 (100) | 18 (100) |
| CD4+ T cells, cells/mm3, mean (SD) | 827 (280) | 685 (164) | 803 (266) |
Q4W, cabotegravir LA 400 mg + rilpivirine LA 600 mg IM every 4 weeks; Q8W, cabotegravir LA 600 mg + rilpivirine LA 900 mg IM every 8 weeks.
All numbers are from the LATTE-2 parent study baseline except age, plasma HIV-1 RNA and CD4+ T cell count, which are from screening into the substudy.
Table 2 summarizes cabotegravir and rilpivirine concentrations in plasma and CSF. For both cabotegravir and rilpivirine, total concentrations in CSF were lower than total concentrations in plasma. Both cabotegravir and rilpivirine drug concentrations exceeded the in vitro EC50 for WT HIV-1 (cabotegravir, 0.10 ng/mL;10 rilpivirine, 0.27 ng/mL37) in all CSF specimens except one, in which the rilpivirine concentration was under 1 ng/mL (not quantifiable). This participant had a plasma rilpivirine concentration of 117 ng/mL with corresponding HIV-1 RNA of 5 copies/mL in the plasma and less than 2 copies/mL in the CSF. Total plasma cabotegravir concentrations significantly correlated with paired total CSF concentrations [Pearson correlation coefficient (r) = 0.899, P < 0.001]. Statistically significant correlations were not present between total and unbound cabotegravir concentrations in plasma (r = 0.229, P = 0.451) nor between unbound plasma cabotegravir and CSF cabotegravir concentrations (r = 0.156, P = 0.612). Total rilpivirine concentrations in plasma did not statistically significantly correlate with rilpivirine concentrations in CSF in this small sample (r = 0.432, P = 0.14). At the substudy lumbar puncture visit, all participants had HIV-1 RNA less than 50 copies/mL in both plasma and CSF (Table 3). In the Q8W arm, plasma HIV-1 RNA was less than 2 copies/mL in nine participants (60%) and above 2 copies/mL in six participants (with 3, 5, 5, 10, 15 and 42 copies/mL). One participant in the Q8W arm had a CSF viral load of above 2 copies/mL and plasma HIV-1 RNA of less than 2 copies/mL. Six participants (46%) in the Q8W group and all three participants in the Q4W group had both plasma SuperLow HIV-1 RNA and CSF SuperLow HIV-1 RNA below 2 copies/mL. As only one participant had HIV-1 RNA greater than 2 copies/mL in the CSF, assessment of the correlation between CSF drug concentrations and HIV-1 RNA was essentially meaningless: as expected, no significant correlation could be demonstrated for cabotegravir (r = 0.325, P = 0.278); the correlation was not tested for rilpivirine.
Table 2.
Summary of cabotegravir and rilpivirine concentrations in plasma and CSF 1 week (7 ± 3 days) after injection
| Pharmacokinetic parametera | Cabotegravir (μg/mL) |
Rilpivirine (ng/mL) |
||
|---|---|---|---|---|
| Q8W (n = 15) | Q4W (n = 3) | Q8W (n = 15) | Q4W (n = 3) | |
| Total plasma drug | 3.92 (1.30–6.41) | 3.02 (2.37–5.10) | 192 (91.7–378) | 134 (83.0–187) |
| Unbound plasma drug | 0.0047 (0.0007–0.0220) | 0.0019 (0.0014–0.0698) | NP | NP |
| Unbound fraction (%) at Cmax in plasmab | 0.103 (0.056–0.912) | 0.075 (0.062–1.45) | NP | NP |
| Total CSF drug | 0.0106 (0.0053–0.0245)c | 0.0127 (0.0082–0.0159) | 1.84 (NQ to 2.90)c, d | 1.67 (1.40–2.47) |
| Total CSF/total plasma (%) | 0.304 (0.218–0.449)c | 0.344 (0.312–0.421) | 1.07 (NQ to 1.52)c, d | 1.32 (1.25–1.69) |
All values shown are median (minimum–maximum). NP, not performed; NQ, not quantifiable; Q4W, cabotegravir LA 400 mg + rilpivirine LA 600 mg IM every 4 weeks; Q8W, cabotegravir LA 600 mg + rilpivirine LA 900 mg IM every 8 weeks.
All plasma samples and CSF samples were collected between 5 and 9 days after dosing and were within the protocol’s specification of 7 (±3) days after injection.
Cabotegravir and rilpivirine concentrations in plasma that were collected 7 (±3) days post-injection were considered Cmax values.
n = 13; failed to collect CSF for two participants.
One participant had rilpivirine CSF total as NQ (<1 ng/mL) and was imputed with a value of 0.
Table 3.
Summary of plasma and CSF HIV-1 RNA levels 1 week (7 ± 3 days) after injection
| Antiviral activity | HIV-1 RNA <50 copies/mL, n/N (%)a |
HIV-1 RNA <2 copies/mL, n/N (%)b |
||
|---|---|---|---|---|
| Q8W (N = 15) | Q4W (N = 3) | Q8W (N = 15) | Q4W (N = 3) | |
| Plasma HIV-1 RNA alone | 15/15 (100) | 3/3 (100) | 9/15c (60) | 3/3 (100) |
| CSF HIV-1 RNA alone | 13/13d (100) | 3/3 (100) | 12/13d,e (92) | 3/3 (100) |
| Both CSF HIV-1 RNA and plasma HIV-1 RNA | 13/13 (100) | 3/3 (100) | 6/13 (46) | 3/3 (100) |
Q4W, cabotegravir LA 400 mg + rilpivirine LA 600 mg IM every 4 weeks; Q8W, cabotegravir LA 600 mg + rilpivirine LA 900 mg IM every 8 weeks.
Abbott RealTime assay.
SuperLow assay.
Actual HIV-1 RNA levels in plasma for the 6 out of 15 participants who did not have <2 copies/mL: 3, 5, 5, 10, 15 and 42 copies/mL.
N = 13, failed to collect CSF for two participants.
All except one participant in the Q8W arm had CSF viral load <2 copies/mL. This participant had CSF HIV-1 RNA of 2 copies/mL and plasma HIV-1 RNA of <2 copies/mL.
Nineteen adverse events (1 during screening, 18 during treatment) were reported in eight participants during the substudy (Table S1, available as Supplementary data at JAC Online). Most adverse events were classified as Grade 1 (12 of 19) or Grade 2 (6 of 19) in intensity. Other than injection-site reactions, headache and back pain were the most common adverse events and generally resolved within 2 days. Two participants had an adverse event of post-lumbar puncture syndrome (MedDRA-coded): one developed a Grade 2 headache on Day 11, a day after lumbar puncture, which resolved on Day 19; the other developed a Grade 3 headache post-lumbar puncture on Day 8, which resolved on Day 10. Four participants reported a total of 10 injection-site reaction events: the most commonly reported was injection-site pain [in 3/18 (17%) participants] and all were Grade 1 or Grade 2 and resolved on study. No clinically significant laboratory values were observed during the substudy. There were no severe adverse events, adverse events leading to discontinuation, or deaths during the substudy.
Discussion
The CNS can be an important sanctuary site for persistent HIV replication, even in individuals with undetectable viral loads in the plasma.30,38–40 Distribution of antiretroviral drugs into the CNS and the subsequent impact on viral replication is of important clinical interest. In this substudy of HIV-1-infected adults receiving long-term LA dual therapy in the Phase 2b LATTE-2 clinical trial, cabotegravir and rilpivirine were measurable in the CSF approximately 1 week after scheduled LA IM injections in all but one participant (in whom rilpivirine was below the limit of detection in CSF). A timepoint of 1 week was chosen because this timepoint approximates the median time to reach peak plasma concentrations for both LA formulations.20,21 The dual regimen of cabotegravir LA + rilpivirine LA maintained CSF HIV-1 RNA below 50 copies/mL in all participants with nearly all participants having CSF HIV-1 RNA less than 2 copies/mL. Although drug concentrations in CSF were considerably lower than in plasma, they exceeded the in vitro EC50 for WT HIV-1. Median cabotegravir CSF levels were 106- and 127-fold above EC50 in the Q8W and Q4W groups, respectively, and median rilpivirine CSF levels were 7- and 6-fold above EC50 in the Q8W and Q4W groups, respectively. Taken together, these results support the conclusions that therapeutic concentrations of cabotegravir and rilpivirine are achieved in the CSF at steady-state following IM LA administration and demonstrate effective virological control in CSF for participants receiving the dual regimen of cabotegravir LA and rilpivirine LA.
The median cabotegravir total CSF-to-plasma ratio (0.30% to 0.34%) was comparable to that observed following oral administration of dolutegravir at 50 mg once daily (0.41%).35 These CSF-to-plasma ratios are lower than the ratio observed with raltegravir (approximately 6%); however, for both dolutegravir and cabotegravir, greater potency results in a higher CSF inhibitory quotient (ratio of drug concentration to EC50) than raltegravir (median of 66–90-fold for dolutegravir and 106- to 127-fold for cabotegravir compared with 4.5-fold for raltegravir).35,41 Unlike dolutegravir, median cabotegravir total CSF concentrations and CSF-to-plasma ratios appeared to be higher than median unbound plasma concentrations and unbound fraction in plasma. A possible explanation for this could be the involvement of active-transporter-mediated uptake along with passive diffusion in the CSF distribution of cabotegravir. Cabotegravir is a substrate for P-glycoprotein and breast cancer resistance protein (BCRP), two transporters reported to influence the CNS penetration of HIV drugs,42 whereas rilpivirine is not a substrate in vitro.43 Previous studies have shown that antiretrovirals in the CSF are usually mostly unbound; however, both etravirine and bictegravir have been shown to be predominantly protein-bound even in CSF.44,45 In the Q8W group, there was a statistically significant correlation between cabotegravir total concentrations in CSF and plasma; however, there was no significant correlation between cabotegravir CSF and unbound plasma concentrations, likely due to small sample size and large variability in the unbound plasma concentrations.
The median CSF-to-plasma ratio of rilpivirine measured in this study (Q8W: 1.07%; Q4W: 1.32%) was comparable to the CSF-to-plasma ratio of 1.4% reported earlier in a study where rilpivirine (25 mg) was administered orally for 60 days.46 The reported CSF distribution of other NNRTIs reflects the heterogeneity of this antiretroviral class in terms of size and protein-binding capacity: 0.5% for efavirenz, 1% for etravirine and 63% for nevirapine.27,34,44,47,48 In the present study, no significant correlation was found between total plasma and total CSF rilpivirine concentrations, which may be related to the small sample size and variability. This finding is consistent with previously published findings.46
In this substudy, the dual regimen of cabotegravir LA + rilpivirine LA maintained plasma and CSF HIV-1 RNA below 50 copies/mL in all participants. In all but one participant, CSF HIV-1 RNA was less than 2 copies/mL. Because of the high levels of suppression, no correlation could be identified between cabotegravir concentration in CSF and HIV-1 RNA in CSF, and the correlation between rilpivirine in CSF and HIV-1 RNA in CSF was not tested. In plasma, virological suppression to below 2 copies/mL was observed in all three individuals in the Q4W group and in 60% of participants in the Q8W group. All six individuals who had detectable HIV-1 RNA in the plasma had undetectable HIV-1 RNA in the CSF. Although previous reports have demonstrated an association between persistent low-level HIV-1 RNA in CSF during ART and worse neurocognitive performance, it is not clear what impact driving down HIV-1 RNA in CSF to very low levels (e.g. <2 copies/mL) has on neurocognitive performance.28,29 As we did not measure neurocognitive performance in this study, we cannot draw conclusions about the potential neurocognitive benefits of maintaining HIV-1 RNA in the CSF below 2 copies/mL.
Overall, non-injection-site adverse events were in line with those observed in previous studies.18,21,22 Injection-site reactions in the CSF substudy were less frequent than in the overall LATTE-2 population through to Week 96.18 This is consistent with recently reported observations in LATTE-2 through to Week 160 observations for the parent population23 and may be attributed to tolerance to IM therapy in these individuals who had been participating in the LATTE-2 study for more than 152 weeks. Following lumbar puncture, two participants reported an adverse event of headache. This was not unexpected and was considered to represent a typical post-lumbar puncture adverse event. No dose-related trends or differences between the Q8W and Q4W groups were observed with respect to non-injection-site reaction adverse events or clinical laboratory abnormalities. No new safety signals were detected during the study.
This study has several limitations. The small number of participants in the Q4W treatment group limits the power to test differences between dosing schedules; however, the narrow range of concentrations 1 week post-injection following a maintenance injection dose of 400 mg or 600 mg cabotegravir and 600 mg or 900 mg rilpivirine suggests the ability to detect a statistical difference between dosing arms would be limited even with a larger sample size. The substudy population enrolled predominantly white men, consistent with the demographics of the parent LATTE-2 study. Given that the participants were on drugs for many months, drug concentrations were assessed at a timepoint chosen to approximate peak plasma concentration; assessing pre-injection specimens (trough concentrations) would likely also be of interest; however, the dynamic range between peak and trough concentrations is limited due to blunting of the pharmacokinetic profile following LA administration by design. As only a single timepoint was measured for HIV-1 RNA in the CSF after a long duration of treatment, no information on the kinetics of HIV-1 RNA decline or time to reach undetectable concentrations in CSF is possible. Finally, neurocognitive performance was not assessed, precluding any comparisons of drug concentrations, HIV-1 RNA concentrations and neurocognitive performance.
In conclusion, at steady-state following Q8W and Q4W LA IM administration, cabotegravir and rilpivirine concentrations in CSF exceeded WT inhibitory concentrations for all individuals who had measurable levels obtained (with one possible exception in the participant who had a non-quantifiable rilpivirine concentration). All participants had HIV-1 RNA in CSF below 50 copies/mL with all but one achieving CSF HIV-1 RNA below 2 copies/mL. These results support the conclusion that a dual regimen of cabotegravir LA and rilpivirine LA can achieve therapeutic concentrations in the CSF and may provide effective control of HIV replication within this important sanctuary site.
Supplementary Material
Acknowledgements
We thank all the participants of the LATTE-2 CSF pharmacokinetic substudy and their families; the LATTE-2 CSF pharmacokinetic substudy clinical investigators and their staff; and the ViiV Healthcare, GlaxoSmithKline (in particular, Annie Cameron, previously of GlaxoSmithKline), PAREXEL and Janssen study team members.
Funding
This work was supported by ViiV Healthcare and Janssen Pharmaceuticals. Editorial assistance was provided by Esther Race of Articulate Science, UK, with funding provided by ViiV Healthcare.
Transparency declarations
S.L.L. has received payment from ViiV Healthcare for travel to HIV Glasgow 2018 for presentation of the findings of this project. A.M. has received honoraria for consulting for Merck, ViiV Healthcare, Janssen, and Gilead Sciences and the Men’s Health Foundation receives research support for clinical trials for which A.M. is an investigator. D.H. sits on the ViiV speaker’s bureau and advisory board. S.S. has received research grants from ViiV Healthcare. F.F. has received research support from Janssen, Merck and ViiV Healthcare and is a speaker for Gilead, Merck and ViiV Healthcare. S.F. and J.S.S. are employees and stockholders of GlaxoSmithKline. K.S., R.D’A. and P.P. are employees of ViiV Healthcare and stockholders of GlaxoSmithKline. H.C. is an employee and stockholder of Johnson and Johnson. J.D., C.B. and Y.L., have nothing to declare.
Author contributions
All authors made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. All authors were involved in drafting the manuscript and/or revising it critically for important intellectual content, approved the final version for submission and agree to be accountable for all aspects of the manuscript.
References
- 1. Nachega JB, Parienti JJ, Uthman OA. et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: a meta-analysis of randomized controlled trials. Clin Infect Dis 2014; 58: 1297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bangsberg DR, Perry S, Charlebois ED. et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS 2001; 15: 1181–3. [DOI] [PubMed] [Google Scholar]
- 3. Gardner EM, Sharma S, Peng G. et al. Differential adherence to combination antiretroviral therapy is associated with virological failure with resistance. AIDS 2008; 22: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV (version October 25, 2018). https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/0.
- 5.European AIDS Clinical Society. EACS Guidelines Version 9.1. http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html.
- 6. Omosanya OE, Elegbede OT, Agboola SM. et al. Effects of stigmatization/discrimination on antiretroviral therapy adherence among HIV-infected patients in a rural tertiary medical center in Nigeria. J Int Assoc Provid AIDS Care 2014; 13: 260–3. [DOI] [PubMed] [Google Scholar]
- 7. Genberg BL, Lee Y, Rogers WH. et al. Four types of barriers to adherence of antiretroviral therapy are associated with decreased adherence over time. AIDS Behav 2015; 19: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sweeney SM, Vanable PA.. The association of HIV-related stigma to HIV medication adherence: a systematic review and synthesis of the literature. AIDS Behav 2016; 20: 29–50. [DOI] [PubMed] [Google Scholar]
- 9. Kalichman SC, Mathews C, Banas E. et al. Treatment adherence in HIV stigmatized environments in South Africa: stigma avoidance and medication management. Int J STD AIDS 2019; 30: 362–70. [DOI] [PubMed] [Google Scholar]
- 10. Yoshinaga T, Kobayashi M, Seki T. et al. Antiviral characteristics of GSK1265744, an HIV integrase inhibitor dosed orally or by long-acting injection. Antimicrob Agents Chemother 2015; 59: 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karmon SL, Mohri H, Spreen W. et al. GSK1265744 demonstrates robust in vitro activity against various clades of HIV-1. J Acquir Immune Defic Syndr 2015; 68: e39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reese MJ, Bowers GD, Humphreys JE. et al. Drug interaction profile of the HIV integrase inhibitor cabotegravir: assessment from in vitro studies and a clinical investigation with midazolam. Xenobiotica 2016; 46: 445–56. [DOI] [PubMed] [Google Scholar]
- 13. Spreen W, Min S, Ford SL. et al. Pharmacokinetics, safety, and monotherapy antiviral activity of GSK1265744, an HIV integrase strand transfer inhibitor. HIV Clin Trials 2013; 14: 192–203. [DOI] [PubMed] [Google Scholar]
- 14. Ford SL, Gould E, Chen S. et al. Effects of etravirine on the pharmacokinetics of the integrase inhibitor S/GSK1265744. Antimicrob Agents Chemother 2013; 57: 277–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trezza C, Ford SL, Spreen W. et al. Formulation and pharmacology of long-acting cabotegravir. Curr Opin HIV AIDS 2015; 10: 239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janssen Therapeutics. EDURANT® (rilpivirine) prescribing information. Revised February 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/202022s011lbl.pdf.
- 17.Gilead Sciences. COMPLERA® (emtricitabine/rilpivirine/tenofovir disoproxil fumarate) tablets, prescribing information. Revised October 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/202123s029lbl.pdf.
- 18. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ. et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017; 390: 1499–510. [DOI] [PubMed] [Google Scholar]
- 19. Williams PE, Crauwels HM, Basstanie ED.. Formulation and pharmacology of long-acting rilpivirine. Curr Opin HIV AIDS 2015; 10: 233–8. [DOI] [PubMed] [Google Scholar]
- 20. Spreen W, Ford SL, Chen S. et al. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr 2014; 67: 481–6. [DOI] [PubMed] [Google Scholar]
- 21. Verloes R, Deleu S, Niemeijer N. et al. Safety, tolerability and pharmacokinetics of rilpivirine following administration of a long-acting formulation in healthy volunteers. HIV Med 2015; 16: 477–84. [DOI] [PubMed] [Google Scholar]
- 22. Spreen W, Williams P, Margolis D. et al. Pharmacokinetics, safety, and tolerability with repeat doses of GSK1265744 and rilpivirine (TMC278) long-acting nanosuspensions in healthy adults. J Acquir Immune Defic Syndr 2014; 67: 487–92. [DOI] [PubMed] [Google Scholar]
- 23. Margolis DA, Gonzalez Garcia J, Stellbrink HJ. et al. Safety, efficacy and durability of long-acting cabotegravir (CAB) and rilpivirine (rilpivirine) as two-drug IM maintenance therapy for HIV-1 infection: LATTE-2 week 160 results. J Int AIDS Soc 2018; 21 Suppl 8: 87. [Google Scholar]
- 24. Murray M, Pulido F, Mills A. et al. Patient satisfaction, tolerability and acceptability of cabotegravir (CAB) and rilpivirine (RPV) long-acting therapy in HIV-1-infected adults: LATTE-2 week 96 results. 22nd International AIDS Conference, Amsterdam, The Netherlands, 2018. Poster THPEB042. [Google Scholar]
- 25. Swindells S, Andrade-Villanueva J, Richmond GJ. et al. Long-acting cabotegravir + rilpivirine as maintenance therapy: ATLAS week 48 results Conference on Retroviruses and Opportunistic Infections , Seattle, Washington, 2019. Abstract 139. [Google Scholar]
- 26. Orkin C, Arastéh K, Hernandez-Mora MG. et al. Long-acting cabotegravir + rilpivirine for HIV maintenance: FLAIR week 48 results Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 2019. Abstract 140. [Google Scholar]
- 27. Antinori A, Perno CF, Giancola ML. et al. Efficacy of cerebrospinal fluid (CSF)-penetrating antiretroviral drugs against HIV in the neurological compartment: different patterns of phenotypic resistance in CSF and plasma. Clin Infect Dis 2005; 41: 1787–93. [DOI] [PubMed] [Google Scholar]
- 28. Carroll A, Brew B.. HIV-associated neurocognitive disorders: recent advances in pathogenesis, biomarkers, and treatment. F1000Res 2017; 6: 312.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olivier IS, Cacabelos R, Naidoo V.. Risk factors and pathogenesis of HIV-associated neurocognitive disorder: the role of host genetics. Int J Mol Sci 2018; 19: pii: E3594.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anderson AM, Muñoz-Moreno JA, McClernon DR. et al. Prevalence and correlates of persistent HIV-1 RNA in cerebrospinal fluid during antiretroviral therapy. J Infect Dis 2017; 215: 105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Letendre S, Marquie-Beck J, Capparelli E. et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 2008; 65: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nightingale S, Geretti AM, Beloukas A. et al. Discordant CSF/plasma HIV-1 RNA in patients with unexplained low-level viraemia. J Neurovirol 2016; 22: 852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yilmaz A, Izadkhashti A, Price RW. et al. Darunavir concentrations in cerebrospinal fluid and blood in HIV-1-infected individuals. AIDS Res Hum Retroviruses 2009; 25: 457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yilmaz A, Price RW, Gisslén M.. Antiretroviral drug treatment of CNS HIV-1 infection. J Antimicrob Chemother 2012; 67: 299–311. [DOI] [PubMed] [Google Scholar]
- 35. Letendre SL, Mills AM, Tashima KT. et al. ING116070: a study of the pharmacokinetics and antiviral activity of dolutegravir in cerebrospinal fluid in HIV-1-infected, antiretroviral therapy-naive subjects. Clin Infect Dis 2014; 59: 1032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shaik JSB, Ford SL, Lou Y. et al. A phase 1 study to evaluate the pharmacokinetics and safety of cabotegravir in patients with hepatic impairment and healthy matched controls. Clin Pharmacol Drug Dev 2019; 8: 664–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Center for Drug Evaluation and Research. Application number: 202022orig1s000. Microbiology Review. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202022Orig1MicroR.pdf.
- 38. Rose R, Nolan DJ, Maidji E. et al. Eradication of HIV from tissue reservoirs: challenges for the cure. AIDS Res Hum Retroviruses 2018; 34: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oliveira MF, Chaillon A, Nakazawa M. et al. Early antiretroviral therapy is associated with lower HIV DNA molecular diversity and lower inflammation in cerebrospinal fluid but does not prevent the establishment of compartmentalized HIV DNA populations. PLoS Pathog 2017; 13: e1006112.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lamers SL, Rose R, Maidji E. et al. HIV DNA is frequently present within pathologic tissues evaluated at autopsy from combined antiretroviral therapy-treated patients with undetectable viral loads. J Virol 2016; 90: 8968–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Croteau D, Letendre S, Best BM. et al. Total raltegravir concentrations in cerebrospinal fluid exceed the 50-percent inhibitory concentration for wild-type HIV-1. Antimicrob Agents Chemother 2010; 54: 5156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reese MJ, Savina PM, Generaux GT. et al. In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. Drug Metab Dispos 2013; 41: 353–61. [DOI] [PubMed] [Google Scholar]
- 43. Weiss J, Haefeli WE.. Potential of the novel antiretroviral drug rilpivirine to modulate the expression and function of drug transporters and drug-metabolising enzymes in vitro. Int J Antimicrob Agents 2013; 41: 484–7. [DOI] [PubMed] [Google Scholar]
- 44. Nguyen A, Rossi S, Croteau D. et al. Etravirine in CSF is highly protein bound. J Antimicrob Chemother 2013; 68: 1161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tiraboschi J, Imaz A, Khoo S. et al. Bictegravir concentrations and viral suppression in CSF of HIV-infected patients Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 2019. Abstract 474. [Google Scholar]
- 46. Mora-Peris B, Watson V, Vera JH. et al. Rilpivirine exposure in plasma and sanctuary site compartments after switching from nevirapine-containing combined antiretroviral therapy. J Antimicrob Chemother 2014; 69: 1642–7. [DOI] [PubMed] [Google Scholar]
- 47. Best BM, Koopmans PP, Letendre SL. et al. Efavirenz concentrations in CSF exceed IC50 for wild-type HIV. J Antimicrob Chemother 2011; 66: 354–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tiraboschi JM, Niubo J, Vila A. et al. Etravirine concentrations in CSF in HIV-infected patients. J Antimicrob Chemother 2012; 67: 1446–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.