Abstract
Hereditary transthyretin amyloidosis is a rare progressive systemic disease. We describe a physically active 46-year-old man who presented with dyspnoea on exertion. An echocardiogram showed increased left ventricular wall thickness and diastolic dysfunction, but normal systolic function. The QRS voltage on ECG was normal. The patient was diagnosed with hypertrophic cardiomyopathy, and several years passed before establishment of the accurate diagnosis of hereditary transthyretin amyloidosis caused by the rare mutation ATTR Phe33Leu, previously described in only five case reports. Further investigation revealed neuropathy and nephropathy, and the patient developed severe heart failure. The patient is treated with tafamidis, has undergone heart transplantation and is currently planned for liver transplant. Hereditary transthyretin amyloidosis is likely underdiagnosed, especially in patients presenting with cardiomyopathy. A discrepancy between the left ventricular mass indicated by echocardiogram and that on ECG is an important indicator of amyloidosis, as is involvement of multiple organs.
Keywords: heart failure, renal system, neurology (drugs and medicines)
Background
Amyloidosis is a rare progressive and eventually fatal disease in which extracellular deposition of amyloid fibrils leads to characteristic histological changes in organs including the heart and those of the gastrointestinal tract and nervous system. The type of precursor protein, organs involved and quantity of deposition determine clinical manifestations.1 2 When the precursor protein is transthyretin (TTR), two forms of disease can arise: hereditary ATTR amyloidosis, caused by mutated TTR, or wild-type (non-mutant) ATTR.2 Hereditary ATTR amyloidosis primarily causes neuropathy and may also be associated with cardiomyopathy, but the phenotype varies with specific mutation as well as among patients with the same mutation.3 The diagnosis of ATTR amyloidosis is challenging and it is likely underdiagnosed.4 Here, we describe a case of a patient presenting with cardiomyopathy who was initially diagnosed with hypertrophic cardiomyopathy but later accurately diagnosed with hereditary ATTR amyloidosis caused by a rare mutation.
Case presentation
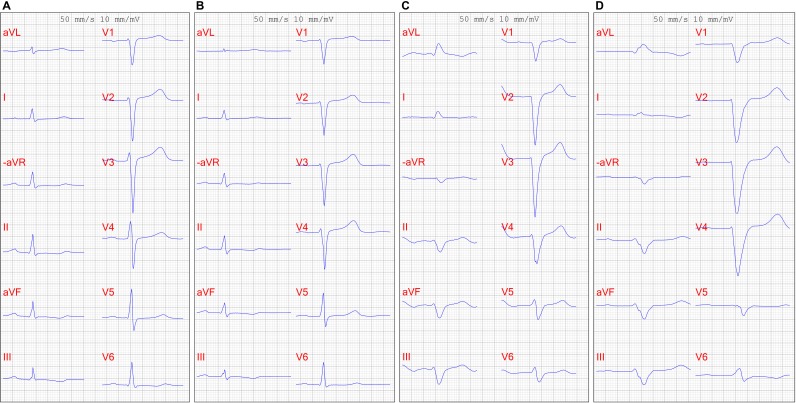
A previously healthy 46-year-old man presented to his primary care physician in November 2012 with dyspnoea on exertion. The patient had a high activity level and exercised daily, alternating running, cycling, karate and weight training. The patient was born in Poland but has lived in Sweden since age 20. The patient’s father died of unspecified heart disease in his 50s, and the patient had two healthy siblings and one child. A 12-lead ECG showed sinus rhythm, negative T waves in inferior leads and normal QRS voltage (figure 1A). Laboratory testing revealed a creatinine level of 156 µmol/L. A transthoracic echocardiogram revealed a mildly enlarged left atrium, normal-sized ventricles, increased wall thickness of all left ventricular segments with a maximum thickness of 17 mm and evidence of diastolic dysfunction. The left ventricular systolic function was normal (video 1). A stress echocardiogram showed no signs of left ventricular outflow obstruction. The patient was referred to the cardiology department in Örebro University Hospital and was diagnosed with hypertrophic cardiomyopathy. Genetic testing was not performed. The patient was followed with annual transthoracic echocardiograms and clinical visits and had a slight decline in left ventricular ejection fraction from 60% in 2012 to 2014, to 50% in 2015 and 2016. Cardiac troponin and N-terminal pro-brain natriuretic peptide levels were not measured.
Figure 1.
ECG in (A) 2012, showing sinus rhythm and normal QRS voltage; (B) 2013, showing sinus rhythm and increasing QRS width and decreasing QRS voltage; (C) 2017, showing atrial flutter and low QRS voltage and (D) 2019, showing atrial flutter, left bundle branch block, and low QRS voltage.
Video 1.
Investigations
In June 2017, the patient presented to the emergency department with a 4-week history of progressive dyspnoea. Physical examination revealed signs of heart failure, and an ECG showed atrial flutter with a ventricular rate of 118 bpm. Laboratory testing revealed a creatinine level of 162 µmol/L, an estimated glomerular filtration rate of 39 mL/min/1.73 m2, troponin I of 118 ng/L and an N-terminal pro-brain natriuretic peptide level of 3115 ng/L. The patient was admitted, and a transthoracic echocardiogram showed impaired left ventricular ejection fraction of 25% and diastolic dysfunction (video 2). The patient underwent electrical cardioversion that restored sinus rhythm and was administered apixaban, beta blockers, ACE inhibitors, and spironolactone. Imaging of the urinary tract and serum and urine protein electrophoresis showed no pathology and the patient was discharged after 3 days.
Video 2.
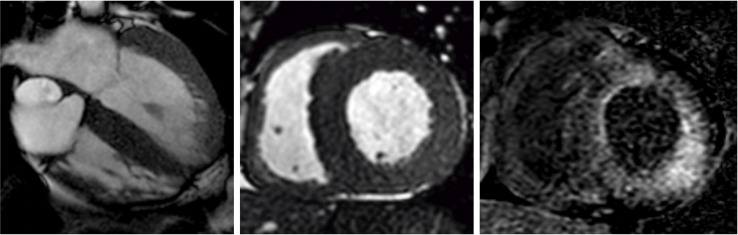
Due to recurrent atrial flutter, two radiofrequency catheter ablations of the cavotricuspid isthmus were conducted, but the atrial arrhythmia recurred and was considered permanent. During the invasive electrophysiology study, several areas of low voltage were observed, which, together with frequent episodes of verified orthostatic hypotension, raised the suspicion of amyloidosis. Cardiac MRI revealed severe global hypertrophy of both ventricles and delayed subendocardial gadolinium enhancement consistent with amyloidosis (figure 2A–2C). An abdominal fat pad biopsy examined with Congo red staining and polarised light revealed no amyloid deposits. The patient was also diagnosed with stage three chronic kidney disease. A renal biopsy showed amyloid deposits in extraglomerular vessels along with moderate parenchymal changes. Characterisation of amyloid type was not possible. To rule out light-chain amyloidosis, a bone marrow biopsy was conducted that showed hypocellular fully mature bone marrow without plasmacytosis of clonal plasma cell populations. In March 2018, genetic testing of peripheral blood showed that the patient was heterozygotic for the pathogenic variant c.157T>C (ATTR Phe33Leu), consistent with the diagnosis of hereditary ATTR amyloidosis.
Figure 2.
Cardiac MRI. (A) Apical four-chamber view and (B) short axis view showing severe global hypertrophy of both ventricles; (C) short axis view showing diffuse delayed subendocardial gadolinium enhancement, all findings characteristic of amyloidosis.
The patient was referred to the Swedish Centre for Familial Amyloidosis at Umeå University Hospital. Neurological examination showed moderate peripheral axonal polyneuropathy in both upper and lower extremities and bilateral carpal tunnel syndrome. 99mTechnetium-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy revealed massive cardiac uptake of technetium in both ventricles. A further abdominal fat pad biopsy revealed large quantities of amyloid composed of a mixture of full-length TTR and TTR fragments. The centre of excellence recommended treatment with tafamidis and lifesaving heart transplantation, followed by liver transplant and subsequent consideration of kidney transplant. The patient was evaluated for heart transplant and placed on a waiting list in December 2018, but a week later presented to the emergency department with sudden onset of syncope. In-hospital ECG monitoring revealed ventricular tachycardia followed by syncope that required prompt treatment with cardioversion and infusions of amiodarone. The patient received an implantable cardioverter-defibrillator, was treated with oral amiodarone and experienced no further arrhythmias. From 2012 to 2019, there has been considerable progression of ECG findings, with prolonged PR interval and QRS width increasing to left bundle branch block and, eventually, atrial flutter, along with QRS voltage decreasing from normal to low (figure 1A–1D).
Differential diagnosis
Left ventricular thickening due to amyloid infiltration can be misdiagnosed on echocardiography as left ventricular hypertrophy as a consequence of sarcomeric hypertrophic cardiomyopathy, hypertensive heart disease, Fabry disease or mitochondrial cardiomyopathy.5 6 However, our patient initially showed normal QRS voltage, which was inconsistent with the substantial left ventricular hypertrophy seen on repeated echocardiograms. Furthermore, the QRS voltage decreased over time and the patient ultimately exhibited low voltage complexes, which is suggestive of infiltrative disease but limited by low sensitivity.7 In contrast, most patients with sarcomeric hypertrophic cardiomyopathy and hypertensive heart disease demonstrate increased QRS voltage.6 The uptake of technetium in both ventricles further excluded sarcomeric hypertrophic cardiomyopathy, since only myocardium infiltrated by TTR takes up technetium and has both high sensitivity for ATTR cardiomyopathy.8 The absence of hypertension precluded hypertensive heart disease.6 Our patient was diagnosed with renal failure, which may also be present in Fabry disease and mitochondrial cardiomyopathy.6 However, cardiac MRI showed severe global hypertrophy of both ventricles, and late gadolinium enhancement pattern spoke strongly for amyloid cardiomyopathy. The patient was also found to exhibit systemic disease, further substantiating the diagnosis of amyloidosis.2 The initial abdominal fat pad biopsy showed no amyloid deposits, but, due to the irregular distribution of amyloid deposits, a negative biopsy does not exclude amyloidosis.2 A second fat pad biopsy and genetic analysis of peripheral blood led to the definitive diagnosis of hereditary ATTR amyloidosis and excluded Fabry disease and mitochondrial cardiomyopathy. In general, a fat pad biopsy has relatively poor sensitivity for ATTR cardiomyopathy, whereas endomyocardial biopsy has very high sensitivity and specificity.7 Light-chain amyloidosis could have been a differential diagnosis, but serum and urine protein electrophoresis was normal, and a bone marrow biopsy showed no monoclonal population of plasma cells, excluding that diagnosis.5
Treatment
The patient has been treated with tafamidis since May 2018 and underwent heart transplant in March 2019.
Outcome and follow-up
The patient recovered well after the heart transplant and was last seen in the cardiology outpatient clinic in November 2019. He is currently working 50% time and doing weight training five to six times a week. The patient has some trouble with walking due to polyneuropathy, but can ride a bicycle without difficulty. The patient is currently on a waiting list for domino liver transplantation, and treatment with tafamidis is to continue until the procedure. Laboratory tests reveal a creatinine level of 120 µmol/L and a glomerular filtration rate of 57 mL/min/1.73 m2, and kidney transplant is currently not considered necessary. At 3-month follow-up at the transplant centre, an endomyocardial biopsy showed grade 2 rejection, and the patient is being treated with corticosteroids with positive results.
Discussion
We describe a case of hereditary ATTR amyloidosis due to the rare ATTR Phe33Leu mutation in which the patient was initially diagnosed with hypertrophic cardiomyopathy and was later also diagnosed with nephropathy and neuropathy. Several years passed before the accurate diagnosis, during which the patient developed severe heart failure requiring a heart transplant. The patient is currently treated with tafamidis and planned for liver transplant.
Hereditary ATTR amyloidosis is a dominantly inherited heterozygous disorder. The most common mutation, endemic in Portugal, Japan and northern Sweden, is ATTR Val30Met. The substitution of leucine for phenylalanine at position 33 (ATTR Phe33Leu) is a rare mutation first identified in 1990 and since described in four additional case reports. It was initially seen in an American male of Polish-Lithuanian decent who presented at age 53 with rapidly progressing peripheral polyneuropathy and orthostatic hypotension, later developing cardiomyopathy.9 10 The second was a 65-year-old Polish-American female who presented with ascites but was diagnosed with asymptomatic cardiomyopathy and later died of unknown cause.11 The third case was a man from northern Sweden who had previously undergone surgery for bilateral carpal tunnel syndrome and presented with painful peripheral polyneuropathy at age 46. He also had asymptomatic cardiomyopathy and underwent a liver transplant. The patient’s great-grandfather originated from Germany.12 The fourth case, a Taiwanese man, presented with diarrhoea at age 47 and later developed orthostatic hypotension, polyneuropathy and cardiomyopathy.13 The fifth is a Hungarian male who was misdiagnosed with hypertrophic cardiomyopathy at age 51, but who also described symptoms of polyneuropathy at presentation. This patient was treated with tafamidis and was on a waiting list for heart transplant when the case report was published.14 All described patients, as well as our patient, showed varying degrees of cardiomyopathy and polyneuropathy, which seem to be the predominant symptoms in patients with the ATTR Phe33Leu mutation. Bilateral carpal tunnel syndrome is also commonly reported with this mutation. The Hungarian patient presented with symptoms similar to those of the patient in the current case. The patient who presented with ascites stands out from the others and illustrates how the phenotype can vary even among patients with the same ATTR genotype. The patient described in this report is of Polish ethnicity, and several of the patients with the ATTR Phe33Leu mutation may share ancestry from Eastern Europe, although new mutations can arise, as in the Asian patient. A family history may not be apparent in hereditary ATTR amyloidosis due to variation in penetrance.2 However, our patient’s father died of unspecified heart disease, which may have been related to cardiomyopathy resulting from hereditary ATTR amyloidosis.
Diagnosing hereditary ATTR amyloidosis can be challenging, especially in patients who present with symptoms associated with cardiomyopathy, as did our patient. Accurately diagnosing patients with amyloidosis requires, first and foremost, a high degree of clinical suspicion, as stated in recently published expert consensus recommendations.7 Identification of cardiac involvement is critical, as this determines prognosis.7 An important clue to amyloidosis is a discrepancy between the assessment of left ventricular mass on echocardiogram and that on ECG, as seen in our patient. Interestingly, our patient showed multiple progressive ECG changes with QRS voltage decrease over time and advancing conduction system disease. The patient also had evidence of systemic disease with nephropathy and neuropathy, further indicative of ATTR amyloidosis.2
Symptomatic treatment is important in patients with hereditary ATTR amyloidosis, but disease-modifying therapies are rapidly expanding. Since the mutant TTR is mainly produced by the liver, liver transplant has been the gold standard to reduce production and slow disease progression.2 However, continued wild-type TTR deposition on established amyloid deposits, especially in the heart, can limit the effectiveness of liver transplantation, especially in patients with non-Val30Met mutations.15 Patients in advanced stages of cardiomyopathy may therefore benefit from combined heart and liver transplant.16 Since 2018, three novel disease-modifying pharmaceutical treatments have emerged. Patisiran and inotersen, gene modifiers that reduce hepatic TTR synthesis, have shown efficacy in slowing progression and even improving polyneuropathy in randomised, double-blind, placebo-controlled trials.17 18 Tafamidis, a small molecule stabiliser of the TTR tetramer, was demonstrated in a randomised trial to be superior to placebo in extending survival among patients with hereditary ATTR cardiomyopathy.19 Therapeutic options for ATTR amyloidosis are increasing, but require accurate and timely diagnosis of the disease.
Patient’s perspective.
I have always been a physically active person and have competed in several sports at the elite level. In October, 8 years ago, I suffered a herniated disc that hampered exercise. I recovered and began exercising again in March of the following year. To my surprise, I experienced unusual breathlessness on exertion, which had never been the case after previous injuries. After exercising regularly for 1 month, I could run no further than 200 m. I eventually sought my primary care physician and underwent a number of investigative procedures. A cardiologist told me that I had hypertrophic cardiomyopathy. I accepted my impaired physical state and continued to do weight training. I fathered a second son, and annual check-ups showed no progression. I was not prescribed medication.
In June 2017, I had a cold and did not exercise for a few days. The weakness and breathlessness worsened, and I eventually sought the emergency department. I was admitted to a cardiology ward, diagnosed with heart failure and started medication. The physician also observed impaired renal function, and I was told that further investigation was needed. My physical state was poor. I could walk no more than 10 m and up only a single flight of stairs without resting. I tried to bicycle to work every day, but what had previously been a 15 min trip now took 30–40 min, as I needed to rest often.
Meanwhile, other symptoms began, including tenderness in my feet and easy bruising of the skin, especially around the eyes. At one point, my girlfriend undertook an internet search of my symptoms and found them to be suggestive of amyloidosis. After further investigation and biopsies, I was diagnosed with hereditary amyloidosis. I was referred to a centre of excellence where I received further information about the disease. Except for the physical impairment, I felt well and was unaware of the seriousness of disease. I was regularly followed up by a cardiologist and a nephrologist and eventually informed that I would not survive more than a few months to a year without a new heart. I would also need a liver transplant to stop progression of other symptoms and to prevent amyloid deposition in the new heart. I worsened physically and, in December 2018, was put on the waiting list for heart transplant. Soon afterwards I fainted at home. In the hospital, my heart stopped for 3–4 min, but my life was saved and I had a pacemaker implanted. After a week, I could go back to work but, unfortunately, exercise was very limited. I tried to bike to work, but on multiple occasions had to take the bus. Thanks to the medications and the pacemaker/defibrillator, I felt confident that I would not die suddenly. On March 12, it was time for the heart transplant. After being sedated for 5 days, I regained consciousness with a new heart but feeling very weak and tired. The rehabilitation went well, and I returned home after 4 weeks. Even while in the hospital, I gained strength beyond what it had been for many years. My main symptom was gout, but after receiving a new medication, this was nearly eliminated.
Six months after the transplant, I feel very well. I have some trouble with polyneuropathy, manifested as numbness in my feet and weakness in my legs. These problems do not prevent me from having a good life. I cannot do everything I did 10 years ago, but I consider my quality of life to be high under the circumstances. I am now awaiting liver transplantation. Meanwhile, I try to exercise as much as I can to be as healthy as possible before the new transplant. This time I know what awaits me, and I want to improve my odds. I experienced rejection 3 months after the heart transplant and will need to medicate for the rest of my life, but it feels like a low price to pay. During my illness, I have felt mentally well, and I think that this has been an advantage. I look forward to the liver transplant, as I know that my condition will then stabilise. Of course, many negative things can happen, but that is nothing that concerns me or over which I can have control. I am endlessly grateful for the help and the resources that have been provided to me. All physicians and other healthcare personnel have done their absolute best to help me, and I feel very confident for the future.
Learning points.
Amyloidosis is likely underdiagnosed.
Misdiagnosis of ATTR amyloidosis is common, especially in patients presenting with cardiomyopathy.
A discrepancy between the assessment of left ventricular mass on echocardiogram and that on ECG, along with evidence of systemic disease, are important indicators of amyloidosis.
Acknowledgments
We thank Dr Mats Lidén, Department of Radiology, Örebro University Hospital, for providing the magnetic resonance images.
Footnotes
Contributors: The patient is currently under the care of BS. AJS performed the ablations and AB the cardioverter defibrillator implant. AB proposed the case report. All authors discussed the results and contributed substantially to the manuscript and approved the final version. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy of any part of this report are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Planté-Bordeneuve V, Said G. Familial amyloid polyneuropathy. Lancet Neurol 2011;10:1086–97. 10.1016/S1474-4422(11)70246-0 [DOI] [PubMed] [Google Scholar]
- 2. Ando Y, Coelho T, Berk JL, et al. Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J Rare Dis 2013;8:31 10.1186/1750-1172-8-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rapezzi C, Quarta CC, Riva L, et al. Transthyretin-Related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol 2010;7:398–408. 10.1038/nrcardio.2010.67 [DOI] [PubMed] [Google Scholar]
- 4. Damy T, Costes B, Hagège AA, et al. Prevalence and clinical phenotype of hereditary transthyretin amyloid cardiomyopathy in patients with increased left ventricular wall thickness. Eur Heart J 2016;37:1826–34. 10.1093/eurheartj/ehv583 [DOI] [PubMed] [Google Scholar]
- 5. Dubrey SW, Hawkins PN, Falk RH. Amyloid diseases of the heart: assessment, diagnosis, and referral. Heart 2011;97:75–84. 10.1136/hrt.2009.190405 [DOI] [PubMed] [Google Scholar]
- 6. Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of cardiology (ESC). Eur Heart J 2014;35:2733–79. 10.1093/eurheartj/ehu284 [DOI] [PubMed] [Google Scholar]
- 7. Maurer MS, Bokhari S, Damy T, et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail 2019;12:e006075 10.1161/CIRCHEARTFAILURE.119.006075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rapezzi C, Quarta CC, Guidalotti PL, et al. Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur J Nucl Med Mol Imaging 2011;38:470–8. 10.1007/s00259-010-1642-7 [DOI] [PubMed] [Google Scholar]
- 9. Ii S, Minnerath S, Ii K, et al. Two-Tiered DNA-based diagnosis of transthyretin amyloidosis reveals two novel point mutations. Neurology 1991;41:893–8. 10.1212/WNL.41.6.893 [DOI] [PubMed] [Google Scholar]
- 10. Harding J, Skare J, Skinner M. A second transthyretin mutation at position 33 (Leu/Phe) associated with familial amyloidotic polyneuropathy. Biochim Biophys Acta 1991;1097:183–6. 10.1016/0925-4439(91)90033-6 [DOI] [PubMed] [Google Scholar]
- 11. Myers TJ, Kyle RA, Jacobson DR. Familial amyloid with a transthyretin leucine 33 mutation presenting with ascites. Am J Hematol 1998;59:249–51. [DOI] [PubMed] [Google Scholar]
- 12. Holmgren G, Hellman U, Jonasson J, et al. A Swedish family with the rare Phe33Leu transthyretin mutation. Amyloid 2005;12:189–92. 10.1080/13506120500221989 [DOI] [PubMed] [Google Scholar]
- 13. Chen C-H, Huang C-W, Lee M-J. A case of familial amyloidotic polyneuropathy with a rare Phe33Leu mutation in the TTR gene. J Formos Med Assoc 2014;113:575–6. 10.1016/j.jfma.2012.07.026 [DOI] [PubMed] [Google Scholar]
- 14. Csillik A, Pozsonyi Z, Soós K, et al. [Transthyretin familial amyloid polyneuropathy - three Hungarian cases with rare mutations (His88Arg and Phe33Leu)]. Ideggyogy Sz 2016;69:245–53. 10.18071/isz.69.0245 [DOI] [PubMed] [Google Scholar]
- 15. Dubrey SW, Davidoff R, Skinner M, et al. Progression of ventricular wall thickening after liver transplantation for familial amyloidosis. Transplantation 1997;64:74–80. 10.1097/00007890-199707150-00014 [DOI] [PubMed] [Google Scholar]
- 16. Raichlin E, Daly RC, Rosen CB, et al. Combined heart and liver transplantation: a single-center experience. Transplantation 2009;88:219–25. 10.1097/TP.0b013e3181ac60db [DOI] [PubMed] [Google Scholar]
- 17. Adams D, Gonzalez-Duarte A, O'Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med 2018;379:11–21. 10.1056/NEJMoa1716153 [DOI] [PubMed] [Google Scholar]
- 18. Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med 2018;379:22–31. 10.1056/NEJMoa1716793 [DOI] [PubMed] [Google Scholar]
- 19. Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018;379:1007–16. 10.1056/NEJMoa1805689 [DOI] [PubMed] [Google Scholar]