Abstract
Bronchopulmonary dysplasia (BPD) is occasionally associated with tracheobronchomalacia, and it is this combination that can lead to serious outcomes. The most severe cases require tracheostomies, ventilatory support and eventually even tracheal stents or surgery. Ventilation in patients with tracheomalacia is complicated without a good patient-ventilator synchrony; the neurally adjusted ventilatory assist (NAVA) mode is potentially beneficial in these cases. This case report presents a patient affected by BPD and severe tracheobronchomalacia who was tracheostomised and ventilated 24 hours a day and who suffered from episodes of airway collapse despite using the NAVA mode. It was necessary to increase the positive end-expiratory pressure to 20 cmH2O (the PEEP-20 manoeuvre) for several minutes during an episode; this allowed the trachea to remain open and allowed us to optimise the patient’s ventilation. This strategy has previously been described in a patient with tracheomalacia, reducing the frequency and need for sedation in the following episodes.
Keywords: mechanical ventilation, paediatric intensive care, paediatrics
Background
Patient-ventilator asynchrony, where there is suboptimal interaction between the patient and the ventilator, in paediatrics is more frequent in infants. Unfortunately, this usually means increased use of sedatives and subsequently the use of controlled modes of ventilation. This strategy is associated with lengthening of time on the ventilator and therefore an increased length of stay in the paediatric intensive care unit (PICU). Additionally, sedative drugs have been associated, especially in preterm babies, with neurological side effects.
Although it is not a very common problem, asynchrony secondary to airway collapse due to tracheobronchomalacia is associated with a considerable increase in morbidity and mortality.1 Procedures such as surgical tracheoplasty or stenting of the airways are not always available.2 3
The use of modes such as neurally adjusted ventilatory assist (NAVA) offering better synchrony and newer strategies to transitorily stent the airway, such as the PEEP-20 manoeuvre (increasing the positive end-expiratory pressure to 20 cmH2O), could be an alternative strategy leading to better outcomes.
Case presentation
A 7-month-old patient who was born prematurely at 24 weeks with bronchodysplasia and severe tracheobronchomalacia was transferred from the neonatal intensive care unit. The patient had a tracheostomy (cannula number 4.5 with a deflated cuff) and required round-the-clock mechanical ventilation: assisted/controlled pressure mode, inspiratory pressure of 19 cmH2O, PEEP of 9 cmH2O and fractional inspired oxygen (FiO2) of 0.6. In the PICU, the ventilation settings were gradually adjusted to an inspiratory pressure of 22 cmH2O, PEEP of 12 cmH2O and FiO2 of 0.6 to achieve acceptable volumes for age and weight.
Since birth the patient has experienced intermittent episodes of desaturation and cyanosis, with respiratory difficulty and diminished air entry in both lung fields, predominantly worse in the right lung, and expiratory stridor. Consequently, flexible bronchoscopy was performed in the PICU which led to a diagnosis of severe tracheobronchomalacia of the pars membranacea (figure 1).
Figure 1.
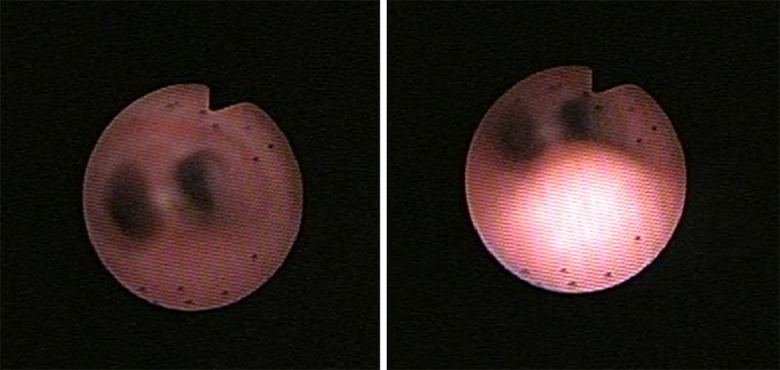
Images from the fibrobronchoscopy performed at basal condition (left) and at the time of crisis (right), with collapse of the membranous part of the trachea.
During these episodes of asynchrony (figure 2), even when using the most sensitive inspiratory trigger, deep sedation was eventually needed (figure 3), along with an increase in ventilation parameters or ventilation with a resuscitation bag.
Figure 2.
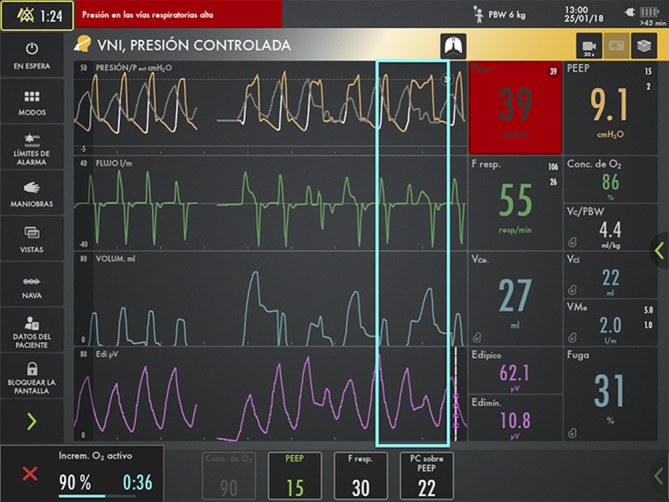
Severe patient-ventilator asynchrony in the assisted/controlled pressure mode. The grey line represents the pressure curve which the patient would perform through activation of the NAVA mode and its neural trigger, while the yellow curve corresponds to the pressure controlled by the ventilator. The light bluebox highlights a period of extreme asynchrony between the theoretical NAVA mode neurally triggered (grey line) and the current Pressure control mode (yellow line), pressure triggered (white line). NAVA, neurally adjusted ventilatory assist.
Figure 3.
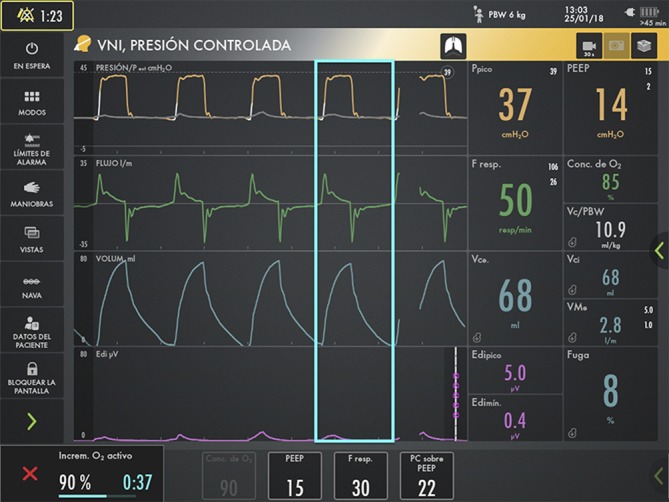
The patient improved once his spontaneous respirations were suppressed (Edi peak diminished from 62 to 5 microvolts) after receiving a bolus of sedation. The light bluebox shows a complete respiratory cycle delivering perfectly the whole tidal volume to the patient. Edi, electrical activity of the diaphragm.
To optimise patient-ventilator synchrony, the NAVA mode was initiated. NAVA is a mechanical ventilation mode which uses the electrical activity of the diaphragm (Edi) as the trigger, measured through electrodes placed at the distal oesophagus of a nasogastric tube (NAVA catheter). Once the patient starts a respiratory impulse with its corresponding electrical activity, the electrical gradient between the Edi maximum and Edi minimum is multiplied by the set NAVA level, resulting in the pressure support over PEEP the patient will receive (figure 4).4 5
Figure 4.
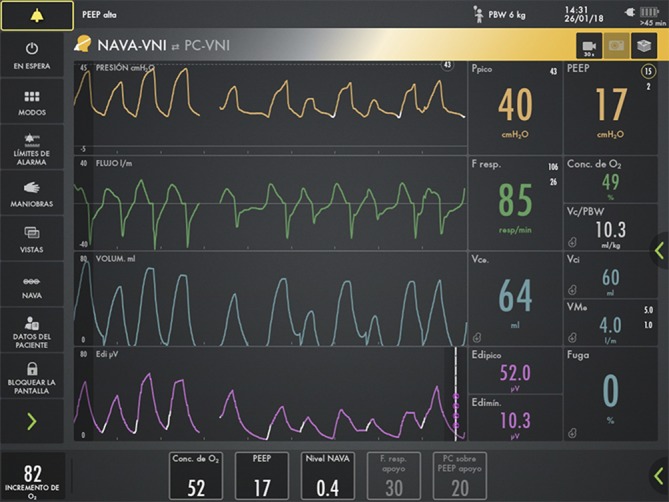
The patient, previously on a pressure controlled with a volume guarantee mode, is adapted to the NAVA mode, with NAVA level of 0.4 and PEEP of 17 cmH2O, achieving a good patient-ventilator synchrony. NAVA, neurally adjusted ventilatory assist; PEEP, positive end-expiratory pressure.
The patient was stabilised using the following ventilation parameters: PEEP 11 cmH2O, NAVA level 0.7, FiO2 0.4–0.5, achieving adequate synchrony and tidal volumes between 6 and 8 mL/kg.
In spite of better synchrony with this mode, the episodes of airway collapse still persisted. Therefore the PEEP-20 manoeuvre was added to the management of the airway obstruction crises, where the PEEP level was increased up to 20 cmH2O, the FiO2 was increased to 1, the NAVA level was increased according to needs, and the pressure limit alarm was maintained at 40 cmH2O (figure 5).
Figure 5.
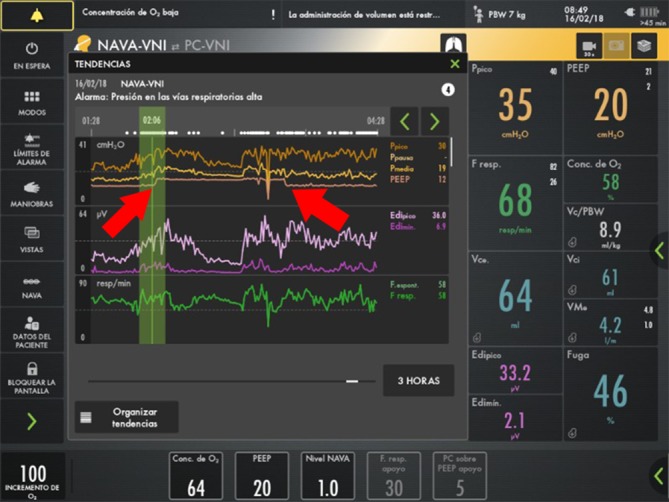
During an episode of airway collapse, increased airway pressure, diminished tidal volume, and increased Edi maximum and Edi minimum are observed. When the increased PEEP manoeuvre was applied with increased FiO2, the values returned to normal. Red arrows show the beginning and the end of the PEEP-20 manoeuvre. Edi, electrical activity of the diaphragm; FiO2, fractional inspired oxygen; PEEP, positive end-expiratory pressure.
In the graph of trends it can be observed that one of the patient’s episodes of airway collapse was resolved with the PEEP-20 manoeuvre and with the increase of FiO2 to 1, while airway pressure was maintained at 35 cmH2O, tidal volume was maintained at an acceptable value for the patient, and no deep sedation was required during the episode.
From that moment on, sedation was not required in the majority of occasions as the episodes were resolved with the increase of expiratory pressure and the self-adjustable support for the patient’s respiratory efforts. For the episodes where sedation was needed, intranasal midazolam was used.
The frequency of PEEP-20 manoeuvres per month progressively reduced: 10 in February 2018, 4 in March, 4 in April, 2 in May and 1 in June. No haemodynamic alterations or air leaks were observed in any of the episodes, similar to the case described in Pons-Odena et al.4
Although a pH study did not suggest acid gastro-oesophageal reflux, antireflux surgery was performed (on May 14) due to a strong clinical association between food intake and abdominal pain causing airway collapse. Finally a procedure to insert a customised tracheostomy cannula (length close to the carina) with a water-filled low-pressure balloon (July 3) was performed.
Outcome and follow-up
Once the episodes stopped, the patient was ventilated with a home ventilator and was discharged after 1 month (August 8), without needing hospital readmission.
Since then, the patient has not needed tracheal opening manoeuvres and was able to be partially weaned off the ventilator. Currently (July 2019) the patient enjoyed 12-hour periods of disconnect from mechanical ventilator and was ventilated with Pressure Control (PC) mode, peak inspiratory pressure (PIP) of 16 cmH2O and PEEP of 6 cmH2O at night. On November 2019, patient was adapted to nocturnal non-invasive support and was succesfully decannulated.
Discussion
Ventilatory management of tracheostomised patients with severe tracheomalacia is not adequately described in the medical literature. Using continuous positive airway pressure (CPAP) through tracheostomy is recommended in patients for long periods of time or when associated bronchomalacia is not suitable for surgery or stenting of the airway.6 Using the NAVA ventilation mode allowed us to achieve a better adaptation of our patient to the ventilator. This is a very significant achievement as an adequate adaptation is difficult in small patients who require sedation and high ventilation parameters in the majority of cases. Being able to monitor the activity of the diaphragm with the NAVA mode was also a great help to determine the patient’s work of breathing. Recently, Edi maximum and associated derived variables have been correlated with oesophageal pressure time product, so a decrease in Edi maximum could be considered an indirect measurement of the patient’s response to the manoeuvre.7
Quantitative assessment of the collapsibility of the trachea has been described.8 This methodology could be used to titrate statically the required PEEP to prevent tracheal collapse. Unfortunately it is not clear if the obtained values could be clinically useful in a dynamic scenario, such as a crying baby.
It is understandable that PEEP-20 manoeuvre may not be suitable for all patients, even in the same patient during repeated episodes. Nevertheless, using our previous experience4 and this particular case of patient, lower levels of PEEP could not reverse the cyanosis and led to sedation of the patient and bag resuscitation. Additionally, trying to avoid peak pressure over 35 cmH2O (pressure alarm limited to 40) but maintaining a sufficient pressure gradient to deliver proper tidal volume was our main reason to stop at a maximum PEEP level of 20 cmH2O.
The use of the PEEP-20 manoeuvre during episodes of severe airway collapse could be an alternative therapeutic procedure to avoid deep sedation and ventilation with a resuscitation bag.
Learning points.
The use of the PEEP-20 manoeuvre (increasing positive end-expiratory pressure to 20 cmH2O) in this patient permitted a reduction in the need for sedation during episodes of severe airway obstruction, which suggests that it could be a potential therapeutic option in the treatment of patients with airway obstruction crises secondary to tracheobronchomalacia.
The patient-ventilator synchrony, which was achieved with the neurally adjusted ventilatory assist mode, suggests that it is a therapeutic option to take into account for patients who go through a difficult weaning process caused by an inability to activate the trigger in conventional modes due to age or pathology.
Continuous level of PEEP around 10 cmH2O should be considered in selected patients with severe tracheobronchomalacia.
Footnotes
Twitter: @Martí
Contributors: MP-O and MOC conceived the case report, acquired the data and contributed to writing the manuscript. FJC contributed to writing the manuscript. All authors read, reviewed and approved the final version of this case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Okata Y, Hasegawa T, Bitoh Y, et al. . Bronchoscopic assessments and clinical outcomes in pediatric patients with tracheomalacia and bronchomalacia. Pediatr Surg Int 2018;34:55–61. 10.1007/s00383-017-4209-x [DOI] [PubMed] [Google Scholar]
- 2. van der Zee DC. New developments towards the management of severe cases of tracheobronchomalacia. J Thorac Dis 2016;8:3484–5. 10.21037/jtd.2016.12.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fayoux P, Sfeir R. Management of severe tracheomalacia. J Pediatr Gastroenterol Nutr 2011;52 Suppl 1:S33–4. 10.1097/MPG.0b013e3182132d76 [DOI] [PubMed] [Google Scholar]
- 4. Pons-Odena M, Verges A, Arza N, et al. . Combined use of neurally adjusted ventilatory assist (NAVA) and vertical expandable Prostethic titanium rib (VEPTR) in a patient with spondylocostal dysostosis and associated bronchomalacia. BMJ Case Rep 2017;2017. doi: 10.1136/bcr-2016-217027. [Epub ahead of print: 14 Feb 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ducharme-Crevier L, Beck J, Essouri S, et al. . Neurally adjusted ventilatory assist (NAVA) allows patient-ventilator synchrony during pediatric noninvasive ventilation: a crossover physiological study. Crit Care 2015;19:44 10.1186/s13054-015-0770-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mok Q. Airway problems in Neonates-A review of the current investigation and management strategies. Front Pediatr 2017;5:60 10.3389/fped.2017.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Essouri S, Baudin F, Mortamet G, et al. . Relationship between diaphragmatic electrical activity and esophageal pressure monitoring in children. Pediatr Crit Care Med 2019;20:e319–25. 10.1097/PCC.0000000000001981 [DOI] [PubMed] [Google Scholar]
- 8. Okazaki J, Isono S, Hasegawa H, et al. . Quantitative assessment of tracheal collapsibility in infants with tracheomalacia. Am J Respir Crit Care Med 2004;170:780–5. 10.1164/rccm.200312-1691OC [DOI] [PubMed] [Google Scholar]


