Abstract
This 49-year-old woman was referred to ear, nose and throat (ENT) with primary hyperparathyroidism. Imaging studies failed to localise the adenoma so she required four-gland parathyroid exploration. She also required diagnostic left hemithyroidectomy as she had a U3 nodule with multiple insufficient fine needle aspirations (FNAs). Intraoperatively, the left thyroidectomy proceeded uneventfully. No convincing left inferior parathyroid gland was identified however palpation revealed a 1 cm mass just medial to carotid artery. This was excised as probable ectopic parathyroid gland. She was discharged two days later. Thirteen days postoperatively she attended Eye Casualty with a left-sided Horner’s syndrome. A CT angio of aortic arch was normal. She was reviewed at ENT outpatients. Histopathology report of the expected ectopic parathyroid gland returned as benign ganglioneuroma, likely arising from her left sympathetic chain. Horner’s syndrome is a common side effect from excision of ganglioneuromas, but an incredibly rare side effect from thyroid or parathyroid surgery.
Keywords: ear, nose and throat/otolaryngology; thyroid disease; otolaryngology/ENT; head and neck surgery
Background
The cervical sympathetic chain is not a structure that is routinely encountered or damaged during thyroid and parathyroid surgery however it is in close proximity. In this case a rare benign tumour arising from the sympathetic chain was excised, resulting in a postoperative Horner’s syndrome. Informed consent is a very important part of each patient’s surgical journey. While Horner’s syndrome is an incredibly rare side effect following thyroid or parathyroid surgery, this case report poses a question as to whether we should be consenting for this complication preoperatively.
Case presentation
This 49-year-old woman was referred by the endocrinology team with primary hyperparathyroidism (pHPT). She had a serum adjusted calcium of 2.8 mmol/L with a parathyroid hormone level of 80 pg/mL and an estimated glomerular filtration rate (eGFR) of 52 mL/min. Sestamibi, Single-Photon Emission Computed Tomography (SPECT-CT) and ultrasound imaging could not localise a specific parathyroid adenoma. Ultrasound revealed a U3 thyroid nodule with three non-diagnostic fine needle aspiration attempts so she required diagnostic left hemithyroidectomy and four-gland parathyroid exploration. Intraoperatively, the left hemithyroidectomy proceeded uneventfully. A left superior parathyroid gland was located and identified as normal. A right inferior parathyroid adenoma was excised and a normal right superior parathyroid gland was identified. No convincing left inferior parathyroid gland was identified so we searched in ectopic locations. Palpation revealed a 1 cm mass just medial to the carotid artery. This was excised as probable ectopic parathyroid gland. A suspected lymph node was also excised from the left side of the neck during the procedure.
In the immediate postoperative period she recovered well and the surgical drain was removed. The wound was healthy with no haematoma. She was noted to have minor left-sided peri-orbital oedema. This seemed to be resolving and she was clinically well so she was discharged home 2 days later. Thirteen days postoperatively she attended Eye Casualty with a left-sided ptosis. History revealed that when exercising the left side of her face was not sweating (anhydrosis) and examination demonstrated a left-sided miosis. This is clinically known as Horner’s syndrome.
Investigations
Preoperative imaging
US neck
No identifiable parathyroid gland. Generally U2 nodules within left thyroid gland and a U3 marginally exophytic nodule of left thyroid lobe with single calcification and small hypoechoic probable cyst in direct juxtaposition.
SPECT-CT and sestamibi scans
No evidence of functioning parathyroid adenoma on either the washout or SPECT studies.
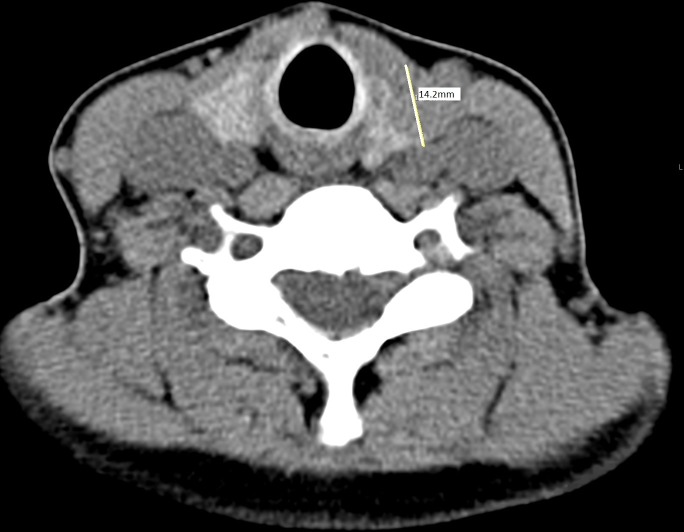
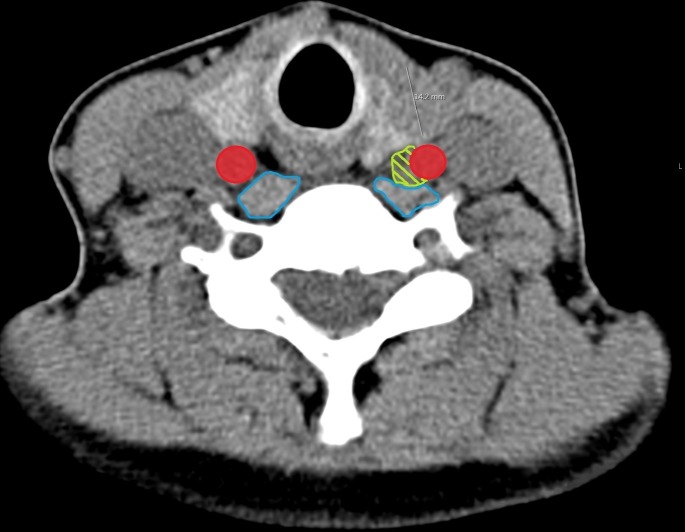
The ganglioneuroma was not initially seen on SPECT-CT; however, in retrospect it can be visualised (figures 1 and 2).
Figure 1.
Nuclear Medicine (NM) parathyroid SPECT-CT scan. Line measuring 14.2 mm left thyroid nodule.
Figure 2.
Annotated NM parathyroid SPECT-CT. Carotid artery in red, ganglioneuroma in green, musculature in blue. Note ganglioneuroma indenting the musculature.
Postoperative investigations
Horner’s syndrome was confirmed pharmacologically in eye casualty.
A CT angio from aortic arch to circle of Willis was performed to rule out any carotid artery dissection, aneurysm or any intracranial cause of Horner’s syndrome. It found no abnormalities.
Histopathology report from surgery found
-
Left level IV suspected ectopic parathyroid gland: single piece of tissue, 13×8×3 mm, weighing 1 g:
Benign ganglioneuroma. No evidence of malignancy.
-
Left thyroid lobe, 41×22×20 mm, weighing 9 g:
Multinodular goitre. Some nodules show partial Hurthle cell change. No malignancy.
-
Left neck node, single piece of tissue, 9×8×4 mm, 1 g:
Thymic tissue with unremarkable parathyroid tissue at the periphery. No lymph node.
-
Right inferior parathyroid adenoma, two pieces of tissue: first piece 18×9×5 mm, 0.67 g and second piece 8×6×5 mm:
Features keeping with a parathyroid adenoma. No malignancy. The smaller piece shows features in keeping with thymic tissue.
Postoperative calcium: 2.17 mmol/L.
Postoprative parathormone: 25 pg/mL.
Postoprative eGFR: 51 mL/min.
Differential diagnosis
Prior to excision of the left level IV mass, our main differential diagnoses were either ectopic parathyroid gland or lymph node (with potential metastatic disease). Neurogenic tumours are a rare but important differential diagnosis of a mass in the neck.
The underlying cause of the left-sided Horner’s syndrome became apparent after the histopathology was reported as a left-sided ganglioneuroma. Other important iatrogenic/postsurgical differential diagnoses were excluded by CT angio, including carotid artery aneurysm or dissection. Other causes of neuropraxia include compression from haematoma or stretching of the nerves during intraoperative retraction. It was unlikely to be of any intracranial origin as symptoms commenced after surgery and exploration of her neck.
Treatment
Ganglioneuromas are benign and do not require any further treatment or follow-up after excision.
Horner’s syndrome requires no specific treatment. We explained to her the cause of her symptoms and that it is usually transient.
Outcome and follow-up
The patient was followed up for 8 months postoperatively. Her Horner’s syndrome persists but is less pronounced than immediately postoperatively. Her parathyroid hormone is within the normal limits at 17 pg/mL and so is her serum-adjusted calcium at 2.38 mmol/L. Her renal function has improved and now she now has an eGFR of >60 mL/min. She was discharged from the ear, nose and throat clinic after a successful surgery with an unfortunate postoperative complication.
Discussion
Ganglioneuromas are rare, benign, non-invasive tumours derived from neural crest cells that usually migrate into the sympathetic ganglia or adrenal medulla. They are at the mature, well differentiated end of a spectrum of peripheral neuroblastic tumours, without malignant potential. Neuroblastomas and ganglioneuroblastomas are at the other less differentiated end of the spectrum, with malignant potential. There is no easy way to discriminate between the different types of peripheral neuroblastic tumours so tissue diagnosis is required.1 FNA results can be misleading because if aspirate fails to target the mature ganglion (characteristic of gnaglioneuroma), it may suggest other malignant neurogenic tumours with malignant potential. Surgical excision is therefore the treatment of choice. There have been rare case reports of malignancy from ganglioneuromas, however it is believed that these tumours are actually metastases of neuroblastomas or ganglioneuroblastomas that have subsequently matured into ganglioneuromas. They are only found in the cervical neck region in 8% of cases.2
Ganglioneuromas usually present in patients under 20 years old and with a slight female predominance. An American case report of an incidental cervical ganglioneruma published in the Journal of Louisiana State Medical Society in 2010 stated that to the best of their knowledge their 41-year-old patient was the oldest documented patient with a cervical ganglioneuroma.3 Our patient is 49, and after performing a literature review we have not found any evidence suggesting this is not now the oldest age at diagnosis.
Clinically cervical ganglioneuromas are usually asymptomatic but can present with compressive symptoms due to mass effect on adjacent structures, for example, nerves and vascular structures.4 Catecholamines can be released in 37%–39% of cases so patients may present with diarrhoea, sweating, hypertension, flushing or renal acidosis.1 5
A literature review over a 10-year period from all English language case reports performed in 2012 found a total of only 26 cases of ganglioneuroma located in the head and neck. They are usually solitary (88.5%). Following total excision they have an excellent prognosis, with no recurrence in the literature review cases. Horner’s syndrome was a common postoperative finding in 38.5% of cases, with symptoms usually resolving fully within a few months.2 Horner’s syndrome is characterised by the classical signs of unilateral miosis, ptosis and anhydrosis, all of which our patient had, and apparent enophthalmus. Rarer signs include facial flushing, retinal vascular dilatation, transient decreased intraocular pressure and hemiatrophy of the face. It is caused by disruption of the sympathetic pathway to the eye.6 Retrospectively, the periorbital oedema noted postoperatively may have actually been lid ptosis and apparent enophthalmus. A full eye examination could have led to a quicker diagnosis but would not have changed the outcome.
In patients with pHPT, multiglandular disease has an incidence of between 8% and 33%. There is no level I or II evidence yet to answer which patients with pHPT should undergo bilateral neck exploration as opposed to minimally invasive parathyroid gland surgery; however, this European consensus report advises it should be considered with negative preoperative imaging, in Multiple Endocrine Neoplasia syndrome(MEN) or if inadequate decrease of intraoperative parathyroid hormone (iOPTH) following excision of the image located adenoma.7 In our case, preoperative investigations failed to localise a specific parathyroid adenoma and we do not have iOPTH monitoring available, so a bilateral four-gland neck exploration was necessary.
With regards to iOPTH monitoring, some may argue that it may add additional cost and time to surgery, however there is evidence suggesting its value in both positive and negative localisation imaging pHPT cases. A study carried out by Bergenfelz et al showed that using iOPTH monitoring in patients with negative sestamibi and ultrasound imaging decreased the rate of persistent pHPT from 27% to 9% (p=0.03).8
A study by Calò et al has also demonstrated the merit of iOPTH monitoring in those with positive localisation imaging. Out of 50 patients that were sestamibi positive but ultrasound negative, they found seven patients (14%) did not show a sufficient drop in iOPTH so required bilateral neck exploration, in which five additional pathological glands were found. One case was later found to have an ectopic mediastinal gland on further sestamibi imaging. Additionally they found that 2.63% (3/114) of patients with both positive sestamibi and ultrasound did not have a sufficient drop in iOPTH so went on to have bilateral neck exploration, all of which had an additional pathological gland identified.9
The use of iOPTH has also been shown to prevent the need for bilateral neck exploration in sestamibi negative imaging cases. A study published in Langenbeck’s Archives of Surgery showed they could achieve a 94% cure rate of pHPT with a unilateral approach in 46% of cases. This resulted in shorter operative time and less incidence of postoperative hypocalcaemia.10 Unfortunately we do not have the use of iOPTH monitoring in our department yet but it is something we are aiming to introduce in the near future. Current National Institute for Health and Care Excellence (NICE) guidelines recommend not using iOPTH monitoring in first time parathyroid gland surgery.11
During our bilateral neck exploration, we could not locate a left inferior parathyroid gland, so we searched in ectopic locations. An article published in the American Journal of Surgery found that 16% of patients operated on for pHPT had ectopic parathyroid glands. 62% of these were inferior glands, of which 30% were intrathymic.12 2.7% of all ectopic parathyroid glands found were located in the carotid sheath. Our histopathology reports (see Investigations section) demonstrate a right parathyroid adenoma associated with some thymic tissue and also left-sided thymic tissue with some unremarkable parathyroid tissue. We excised the ganglioneuroma from just medial to the carotid artery within the carotid sheath. We could not identify any obvious entry or exit nerve to the ganglioneuroma. We assumed it was either ectopic parathyroid gland or potentially a malignant lymph node. Macroscopically, we were not able to distinguish between what could be potentially be thymic tissue, ectopic parathyroid, lymph node or in this case ganglioneuroma, so all had to be excised.
Horner’s syndrome has also been found to be a rare complication following thyroidectomy. Theories of pathophysiology include ischaemia induced nerve damage after ligating the inferior thyroid vessels, and from local trauma to the sympathetic chain during lateral retraction or identification of the recurrent laryngeal nerve.13 It is important to be aware of all anatomical structures around you when operating to minimise the risk iatrogenic injury.
Retrospectively, without the use of iOPTH monitoring, we believe that this patient’s Horner’s syndrome was unavoidable. With iOPTH monitoring, if we had achieved a satisfactory decrease in parathyroid hormone levels we may not have had to explore ectopic locations and it could have potentially been avoided. On retrospective review of the CT images (figures 1 and 2), we can see a small mass adjacent to the carotid artery but it was not originally reported. Given the rarity of ganglioneuroma and her known hyperparathyroidism, we would have probably have assumed it to be a parathyroid adenoma and excised it. If the reporting radiologist had suggested potential ganglioneuroma, it would still have to be excised for histological confirmation, but we would have been better equipped to consent for Horner’s syndrome.
The question we pose is: given the close proximity of the sympathetic chain to the thyroid and parathyroid glands, and given that we know the carotid sheath is a known site for ectopic parathyroid gland, when performing thyroidectomy or parathyroid gland exploration should we routinely consent the patient’s for damage to the sympathetic chain and Horner’ syndrome? Given the rarity of ganglioneuroma, it is not something we are likely to encounter again, however there is always the small risk of iatrogenic damage to the sympathetic chain so it is certainly something that we will consider in the future.
Learning points.
Ganglioneuroma is an extremely rare but recognised cause of a neck mass so must be considered in the differential diagnoses.
Four gland parathyroid exploration is required when localisation imaging fails to adequately demonstrate a singular parathyroid adenoma and if no intraoperative parathyroid hormone (iOPTH) monitoring is available.
iOPTH monitoring can reduce the need for bilateral neck dissection and therefore save time and morbidity.
Surgeons must have an awareness of all nearby important structures when operating to minimise risk of iatrogenic injury.
Informed consent is an integral part of surgery.
Acknowledgments
Dr Barry James, Consultant Radiologist in Craigavon Area Hospital who kindly reviewed and annotated SPECT-CT images.
Footnotes
Contributors: DMcC: Primary author invovled in case concept, write up and literature review. AK: ENT registrar invovled with case concept, write-up and review. MK: Consultant ENT surgeon looking after the patient. Involved in case concept and review.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Katılmış H, Öztürkcan S, Adadan I, et al. Cervical ganglioneuroma. Int J Pediatr Otorhinolaryngol Extra 2006;1:157–9. 10.1016/j.pedex.2006.04.006 [DOI] [Google Scholar]
- 2. Ma J, Liang L, Liu H. Multiple cervical ganglioneuroma: a case report and review of the literature. Oncol Lett 2012;4:509–12. 10.3892/ol.2012.767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cavanaugh DA, Jawahar A, Harper J, et al. Cervical ganglioneuroma in an adult man: case report and literature review of a rare occurrence. J La State Med Soc 2010;162:218–21. [PubMed] [Google Scholar]
- 4. Califano L, Zupi A, Mangone GM, et al. Cervical ganglioneuroma: report of a case. Otolarynology Head Neck Surg 2001;124:115–6. [DOI] [PubMed] [Google Scholar]
- 5. Geoerger B, Hero B, Harms D, et al. Metabolic activity and clinical features of primary ganglioneuromas. Cancer 2001;91:1905–13. [DOI] [PubMed] [Google Scholar]
- 6. Kong YX, Wright G, Pesudovs K, et al. Horner syndrome. Clin Exp Optom 2007;90:336–44. 10.1111/j.1444-0938.2007.00177.x [DOI] [PubMed] [Google Scholar]
- 7. Barczyński M, Bränström R, Dionigi G, et al. Sporadic multiple parathyroid gland disease—a consensus report of the European Society of endocrine surgeons (ESES). Langenbecks Arch Surg 2015;400:887–905. 10.1007/s00423-015-1348-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergenfelz AOJ, Wallin G, Jansson S, et al. Results of surgery for sporadic primary hyperparathyroidism in patients with preoperatively negative sestamibi scintigraphy and ultrasound. Langenbecks Arch Surg 2011;396:83–90. 10.1007/s00423-010-0724-0 [DOI] [PubMed] [Google Scholar]
- 9. Caló PG, Pisano G, Loi G, et al. Surgery for primary hyperparathyroidism in patients with preoperatively negative sestamibi scan and discordant imaging studies: the usefulness of intraoperative parathyroid hormone monitoring. Clin Med Insights Endocrinol Diabetes 2013;6:CMED.S13114–7. 10.4137/CMED.S13114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thier M, Nordenström E, Bergenfelz A, et al. Surgery for patients with primary hyperparathyroidism and negative sestamibi scintigraphy—a feasibility study. Langenbecks Arch Surg 2009;394:881–4. 10.1007/s00423-009-0524-6 [DOI] [PubMed] [Google Scholar]
- 11. National Institute for Health and Care Excellence Hyperparathyroidism (primary): diagnosis, assessment and initial management. NICE guideline May 2019 [NG132, 1.4.2]. Available: https://www.nice.org.uk/guidance/ng132/chapter/Recommendations#surgical-management [Accessed 21 Oct 2019]. [PubMed]
- 12. Phitayakorn R, McHenry CR. Incidence and location of ectopic abnormal parathyroid glands. Am J Surg 2006;191:418–23. 10.1016/j.amjsurg.2005.10.049 [DOI] [PubMed] [Google Scholar]
- 13. Seneviratne SA, Kumara DS, Drahaman AMP. Horner’s syndrome: an unusual complication of thyroidectomy: a case report. J Med Case Rep 2016;10:300 10.1186/s13256-016-1072-7 [DOI] [PMC free article] [PubMed] [Google Scholar]