Abstract
Our goal was to develop a behavioral measure of sensation seeking (SS). The Aroma Choice Task (ACT) assesses preference for an intense, novel, varied, and risky (exciting) option versus a mild, safe (boring) option using real-time odorant delivery. A total of 147 healthy young adults completed 40 binary choice trials. We examined (1) intensity and pleasantness of odorants, (2) stability of responding, (3) association with SS self-report, and (4) association with self-reported illicit drug use. Participants’ preference for the “exciting” option versus the safe option was significantly associated with self-reported SS (p < .001) and illicit drug use (p = .041). Odorant ratings comported with their intended intensity. The ACT showed good internal, convergent, and criterion validity. We propose that the ACT might permit more objective SS assessment for investigating the biological bases of psychiatric conditions marked by high SS, particularly addiction. The ACT measures SS behaviorally, mitigating some self-report challenges and enabling real-time assessment, for example, for functional magnetic resonance imaging (fMRI).
Keywords: substance use disorder, drug abuse, alcoholism, novelty seeking, endophenotype, addiction, risk taking
Sensation seeking (SS) has been conceptualized as a personality trait defined as desiring intense, exciting, varied, and novel stimulation with a willingness to take risks for those experiences (Arnett, 1994; Whiteside & Lynam, 2001; Zuckerman, 1990; Zuckerman, Eysenck, & Eysenck, 1978). SS is elevated in participants engaging in risky behaviors such as illicit drug, alcohol, and tobacco use; drunk driving; risky sexual behavior; criminality; and addictions (Arnett, 1990, 1994; Donohew et al., 2000; Zuckerman & Neeb, 1979, 1980). SS, or the related construct of novelty seeking, correlates with alcohol and drug use/abuse in a large number of cross-sectional studies (e.g., Ersche, Turton, Pradhan, Bullmore, & Robbins, 2010; Finn, Sharkansky, Brandt, & Turcotte, 2000; Hittner & Swickert, 2006; Jaffe & Archer, 1987; Khavari, Mabry, & Humes, 1977; Kosten, Ball, & Rounsaville, 1994; McGlothlin & Arnold, 1971; Segal & Singer, 1976; Wills, Vaccaro, & McNamara, 1994; Zhornitsky et al., 2012). Stable from early childhood (Masse & Tremblay, 1997), SS predicts later alcohol and drug use/abuse in longitudinal studies of children (Cloninger, Sigvardsson, & Bohman, 1988; Masse & Tremblay, 1997) and adolescents (MacPherson, Magidson, Reynolds, Kahler, & Lejuez, 2010; Pedersen, 1991). SS is thus an alcohol/substance use disorder risk factor with clinical and theoretical importance for early detection and intervention. Behavioral phenotyping could facilitate a “personalized medicine” approach to both childhood risk and/or treatment interventions. The goal of this study was to develop a behavioral laboratory measure of SS.
Self-Report Definitions and Measurement of SS
SS and its components have been described by the personality research literature in various ways, including thrill/adventure seeking, experience seeking, disinhibition, and boredom susceptibility (Sensation Seeking Scale–Form V [SSS-V]; Zuckerman et al., 1978), novelty and intensity of experience (Arnett Inventory of Sensation Seeking [AISS]; Arnett, 1994), novelty seeking (Tridimensional Personality Questionnaire; Cloninger, 1987), and venturesomeness (I7 Impulsiveness Questionnaire; Eysenck & Eysenck, 1978). SS is often studied within the larger context of “impulsivity”—broadly defined as lacking behavioral control—and variously described as risk taking, bold, adventurous, unreliable, and disordered (Depue & Collins, 1999). The various descriptions, inventories, and tasks for assessing impulsivity have led to some lexical disagreement regarding this collection of phenotypes (Meda et al., 2009). Categorically, these may be best described as, “discrete psychological processes that lead to impulsive-like behaviors” (Whiteside & Lynam, 2001, p. 685). To clarify the broad “impulsivity” concept, Whiteside and Lynam (2001) tested a number of extant impulsivity measures within the Five-Factor Model of personality (Revised NEO Personality Inventory [NEO-PI-R]; Costa & McCrae, 1992). A large sample (N = 437) completed 16 impulsivity scales/subscales (plus additional items), along with selected elements from the NEO-PI-R: Impulsiveness, Excitement seeking, Self-discipline, and Deliberation. From these, exploratory factor analysis led to item selection and the development of a new inventory: the UPPS Impulsive Behavior scale. The UPPS describes four unique facets: Urgency, (lack of) Premeditation, (lack of) Perseverance, and Sensation seeking, to describe impulsive-like traits (note the later addition of Positive Urgency for the current UPPS-P; Lynam, Smith, Whiteside, & Cyders, 2006). The authors’ description of the SS facet (seeking excitement, new experiences, and danger) aligns with previous conceptualizations from other researchers. Like the other personality facets identified under the umbrella of impulsivity, SS remains an independent and unique trait in spite of efforts to identify a single impulsivity factor (Cyders & Smith, 2007).
Although self-reported SS has generated a large and important literature, these measures do have some limitations. Two commonly used SS measures are the SSS-V (Zuckerman et al., 1978) and the AISS (Arnett, 1994). Like other self-report instruments, these excel in examining the chief expert on one’s behavior (one’s self), they are easily administered, and they capture a lifetime of behavior. However, they are limited in some important ways, including criteria contamination (e.g., items inquiring about alcohol/drug use when these are the outcomes of interest, Darkes, Greenbaum, & Goldman, 1998; and updated measure, Zuckerman, 2002), culturally dated items (e.g., “I stay away from anyone I suspect of being ‘gay’ or ‘lesbian’”; see Haynes, Miles, & Clements, 2000), and items confounded by age/physical abilities (e.g., mountain climbing or water skiing). Additionally, both the SSS-V and AISS may show restricted cross-cultural reliability (e.g., Berkowitz, 1967; Magaro, Smith, Cionini, & Velicogna, 1979)—for example, “A person should have considerable sexual experience before marriage” (SSS-V) in countries where premarital sex/adultery is a capital offense, or “When I listen to music, I like it to be loud” (AISS) for Amish participants. Thus, behavioral measurement of SS may overcome these limitations or provide complementary information regarding one’s level of SS.
Behavioral Measurement of SS
Although many behavioral laboratory tasks for generalized impulsivity exist, few behavioral measures correlate strongly with SS, and even fewer attempt to quantify SS specifically. Even with tasks designed explicitly to measure SS, previous work suggests that these likely would not correlate strongly with extant SS self-report measures. With impulsivity, for example, there is little overlap between behavioral tasks and self-report measures, examined across a range of measures (28-study meta-analysis; Cyders & Coskunpinar, 2011). Self-reported SS showed just one significant correlation (with delay response tasks) of r = .13, suggesting that these assessments largely measure separate tendencies. Thus, although well-validated monetary risk-taking tasks tap into related constructs, none overlap highly enough with SS to be considered a strong candidate for a behavioral measure of SS.
To our knowledge, “Stoplight” is the only published laboratory task explicitly designed to measure SS (as opposed to other related impulsive personality traits). Modified from the “Chicken” paradigm (Gardner & Steinberg, 2005), it simulates time-pressured driving (to a party) featuring risky decisions at yellow traffic lights (Steinberg et al., 2008). Stopping at the yellow light involves no risk but lost time; the “Go” response yields faster times to the party but risks collision. The sounds of a ticking clock and distant party noise are heard during the task, with collisions accompanied by squealing tires and crash noises, and a shattered windshield image. While the sights and sounds of the punishers (collision) are present, the positive hedonic sensory elements are missing; that is, there is no actual party or other sensory stimulation. As well-designed as the Stoplight task is, it does not exactly measure the aspect of SS we intend to capture—that is, sensory stimuli varying in intensity and novelty.
SS as a Biological Trait
Prior work indicates that the SS trait is governed by heritable neurobiological and genetic factors. Genetic effects explain 34% to 63% of variance in SS (Fulker, Eysenck, & Zuckerman, 1980; Hur & Bouchard, 1997; Koopmans, Boomsma, Heath, & van Doornen, 1995) and predict SS more strongly than environment (29% to 60% and 13% to 21% of variance explained, respectively; Stoel, De Geus, & Boomsma, 2006), making it highly heritable for a personality trait. Neuroimaging studies indicate that neural correlates of SS center on striatal and limbic systems, demonstrated with a variety of methods using fMRI (Abler, Walter, Erk, Kammerer, & Spitzer, 2006; Cservenka, Herting, Seghete, Hudson, & Nagel, 2013; Hawes et al., 2017; Joseph, Liu, Jiang, Lynam, & Kelly, 2009; Kruschwitz, Simmons, Flagan, & Paulus, 2012). SS has a clear biological basis with important behavioral consequences, but biological studies of personality have presented unique challenges. For example, the landmark personality genetics report of the dopamine D4 receptor 7-repeat allele association with novelty seeking (Ebstein et al., 1996) pointed toward heritable variants in the dopamine system, but a number of later studies found no such association (meta-analysis; Kluger, Siegfried, & Ebstein, 2002). Biological studies of personality attempt to detect relationships with small effect sizes, and this challenge is magnified by the variability introduced by differing questionnaires (Sen, Burmeister, & Ghosh, 2004). This led Ebstein (2006) to conclude that personality “might best be studied by laboratory-based paradigms” (p. 437) for phenotyping, arguing for behavioral approaches to augment self-report for better characterizing biological underpinnings of personality traits. Although compelling, the body of work describing the biological bases of SS has almost exclusively utilized self-report instruments; extension of this work toward elucidating the neurobiology of addiction disorders may require more objective behavioral methods.
Development of a New Behavioral SS Task: The Aroma Choice Task
Our motivation in developing the current behavioral SS assessment tool was primarily to facilitate identification of behavioral and biological correlates of SS in the context of addiction risk factors. Extant behavioral laboratory tasks of impulsivity-like traits generally lack sensory elements and show poor correspondence with self-reported SS—potentially limiting detection of biological markers. We believe that a behavioral measure of SS could reduce some of the challenges observed within this important literature, provide complimentary information to self-report SS assessment, and allow more objective investigations of biological correlates of SS, such as in neuroimaging research. Our task design was guided by trait descriptions from prior conceptualizations.
As the SS trait is largely defined by the preference for intense sensory experience (Andrew & Cronin, 1997; Arnett, 1994; Byrnes & Hayes, 2013; Kampov-Polevoy, Garbutt, Davis, & Janowsky, 1998; Mattes, 1994), we hypothesized that a choice task focused on intense sensory stimuli may yield a behavioral measure closer to real-world behavior. While impossible to simulate the range of real-world SS behaviors in the laboratory, we reasoned that an ideal behavioral test of SS would assess preference between contingencies of stimuli varying in intensity and valence and producing actual sensory consequences in multiple modalities. For simplicity, we settled on the single modality of olfaction.
We thus developed the Aroma Choice Task (ACT) to mirror prevailing descriptions of the SS trait: “the seeking of varied, novel, complex, and intense sensations and experiences, and the willingness to take physical, social, legal, and financial risks for the sake of such experience” (Zuckerman, 1994, p. 27); “a quality of seeking intensity and novelty in sensory experience” (Arnett, 1994, p. 290); and “1) a tendency to enjoy and pursue activities that are exciting and 2) an openness to trying new experiences that may or may not be dangerous” (Whiteside & Lynam, 2001, p. 686). The ACT models preferences for stimuli varying in intensity, novelty, and riskiness using actual sensory experiences presented in real time. The ACT was developed to (1) capture the essential elements of the SS trait as defined, (2) avoid criteria contamination, and (3) produce an objective behavioral metric. The task presents participants with the choice between two odorant options: boring (mild and vaguely pleasant) or more exciting (intense, novel, and varied, with a risk of unpleasantness). We focused on a single sensory modality, olfaction, to maximize simplicity and experimental control. Olfaction is ideal in several ways: Odorants are uniquely salient (Herz, 2004), possess discrete valence (Schiffman, 1974), can be precisely controlled, and deliver an immediate chemical sensory experience.
Our study aimed to establish face, convergent, and criterion-related validity of the ACT in a young adult sample. Our hypotheses are (1) odorant ratings would match our intended intensity and valence—that is, that weak odorants would be weaker than strong odorants, and the aversive odorant would be unpleasant; (2) the ACT would show reliable responding; (3) preference for the more exciting odorant option (choice ratio) would positively correlate with self-reported SS; and (4) choice ratio would positively correlate with self-reported drug taking.
Method
Participants
Students attending a large, urban Midwestern university were recruited through the human participants pool or directly via classroom announcements. Procedures were conducted on campus and approved by the university institutional review board. Participants were included only if they self-reported having a normal sense of smell and were proficient in English. Participants were excluded if they reported having a poor sense of smell, being overly sensitive to odors, having asthma, or being sensitive to volatile chemicals. Participants received course credit for their participation.
Measures and Experimental Stimuli
Odorant and Concentration Determination (Task Development).
Odorants were selected to represent categories of pleasant food (vanillin, orange, strawberry), nonfood (rose, linalyl acetate), and pungent aversive (propionic acid). Our pilot work targeted the threshold of detection (weak) and strong/very strong (strong) odors by iteratively adjusting concentrations based on ratings. Pleasantness was rated on a visual analog scale ranging from 1 to 9 in 0.5 increments anchored by very unpleasant to very pleasant, based on a previously used scale (Oberlin et al., 2013). While some prior work suggests that higher resolution (more response increments than the classic 5-item Likert-type scale) increases reliability (e.g., Weng, 2004), other studies call this into question (Simms, Zelazny, Williams, & Bernstein, 2019). Intensity ratings ranged from barely detectable to strongest ever on a quasilogarithmic scale specifically designed for chemical sense ratings (Green et al., 1996), with responses coded from 2 to 100. For continuity between the weak and the strong odorant classes, orange and rose (one food, and one nonfood) appeared in both sets, but in differing concentrations. Odorants were always called “smells” or “aromas” in the advertisements and participant instructions to avoid the negative associations with the word “odor.”
Olfactometer Odorant Delivery.
Participants were verbally instructed as follows:
For the next 12 minutes, you will make choices about some smells. The choice labeled “Standard” will likely be mild and pleasant. The choice labeled “Varied” will likely be stronger and pleasant, but there is a chance that it will be unpleasant. Upon making a choice, please inhale deeply through your nose to receive the aroma.
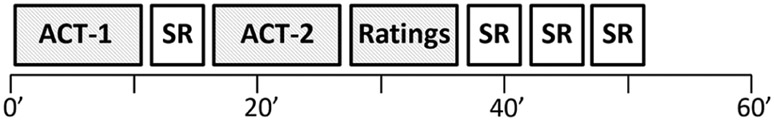
Participants faced a computer monitor in a chinrest/head frame with a 1.5-mm ID PTFE (polytetrafluoroethylene) air line angled at 45°, 2 cm from the center of their nostrils, delivering constant air flow at 2 L/min. Air flow and odorant delivery were governed by an air dilution olfactometer (Kareken et al., 2004; Lorig, Elmes, Zald, & Pardo, 1999) controlled by E-Prime® 2.0 (Psychology Software Tools, Pittsburg, PA) on a desktop personal computer. The olfactometer used here was originally designed for magnetic resonance imaging (MRI) applications, although its semiportable form permits testing in various settings (see Figure 1). Trials appeared as, “Which aroma would you prefer? Standard OR Varied” with a 5-second timeout and side randomization. Participants indicated their preference with a left or right mouse click. Immediately following selection, the auditory and visual prompt of “Ready?” preceded a 2-second burst of odorant in the air stream with an auditory prompt to “sniff.” A nasal cannula/pressure transducer confirmed inspiration to the “sniff” command. Intertrial intervals were jittered between 8 and 14 seconds. The specific odorant administered on a given trial was drawn from pseudorandomized arrays with presentation frequencies illustrated in Table 1. Two blocks of 20 trials each were separated by one self-report inventory to minimize sensory habituation/sensitization. Among the seven task order positions, the first ACT block was always in Position 1, 2, 3, or 4, with the position of the second ACT block and the ratings fixed relative to Block 1. The other tasks were assigned to the other task slots; see Figure 2 (note the ACT in Position 1). The dependent variable of interest, choice ratio, was the mean percent preference for “Varied.”
Figure 1.
Olfactometer cart.
Note. (Left) Participants’ heads were positioned on the chinrest/head frame (left) for alignment with the odorant delivery line and computer monitor. (Center foreground) odorant pellets in glass tubes (four shown here) diffused odorants into the air stream, produced by an air compressor (gray box mounted under cart), with rate indicated by ball float flowmeters (center background), and fine-adjusted with air metering valves. The Aroma Choice Task paradigm controlled real-time odorant delivery via an input/output interface (Personal Daq; Measurement Computing™) governing air flow solenoids (not visible).
Table 1.
Odorant Delivery Frequency by Choice Type.
| Odorant | Standard | Varied |
|---|---|---|
| Vanillin | 33.3% | 7.5% |
| Weak Orange | 33.3% | 7.5% |
| Weak Rose | 33.3% | 7.5% |
| Strong Orange | 17.5% | |
| Strong Rose | 17.5% | |
| Linalyl acetate | 17.5% | |
| Strawberry | 17.5% | |
| Propionic acid | 7.5% |
Note. Only weak odorants were presented for “Standard” choices; all odorants were presented for “Varied” choices, according to the percentages shown.
Figure 2.
Study day timeline.
Note. Odorants were presented (shaded) in the Aroma Choice Task (ACT) and the Ratings session. The two 20-trial ACT blocks were interrupted by one self-report (SR) inventory, and the order of self-report inventories was counterbalanced across participants.
Odorant Ratings.
Following the 40 choice trials (immediately after the second block of ACT choice trials), odorants were randomly presented one at a time, and participants rated them on intensity and pleasantness. The intensity and pleasantness rating scales described in the task development section were employed for all participants. Odorants were delivered in the same manner for ratings as during the choice task, with a plain air control rated as an odorant for comparison.
Odorants.
Weak odorants (Vanillin 5% wt/vol, Weak Orange 0.1% vol/vol, and Weak Rose 0.01% vol/vol) and Strong odorants (Strong Orange 100% vol/vol, Strong Rose 2% vol/vol, Linalyl acetate 20% vol/vol [floral, woody], Strawberry 40% vol/vol, and Propionic acid 15% vol/vol [pungent, rancid]) were diluted in 1,2-propanediol as needed; all odorant concentrates (except vanillin) were liquid. Orange, Rose, and Strawberry were kindly donated by International Flavors and Fragrances (Union Beach, New Jersey); Vanillin, Linalyl acetate, Propionic acid, and 1,2-propandiol were obtained from Sigma-Aldrich (St. Louis, Missouri). Absorbent polymer pellets were stored immersed in refrigerated odorant solutions and placed into the olfactometer just prior to the study.
Measures
Sensation-Seeking Scale–Form V.
This 40-item instrument was originally conceived to assess the optimal level of stimulation/arousal, predicated on the hypothesis that the tendency to seek increased levels of stimulation is an important marker for other behaviors and pathologies and is influenced by biological factors. This scale (Zuckerman, 2007) is widely used to examine risky behaviors and biological correlates of SS (for review, see Roberti, 2004). The SSS-V uses a forced-choice format in four 10-item subscales to assess different SS dimensions: Thrill and Adventure Seeking, Experience Seeking, Disinhibition, and Boredom Susceptibility (Zuckerman et al., 1978); see Zuckerman (1994) for updated item wording. Reliability for the total scale in the current sample (n = 143) was acceptable (α = .82).
Arnett Inventory of Sensation Seeking.
An attempt to improve SS assessment over the near-ubiquitous SSS-V inspired the development of the 20-item AISS, with two 10-item subscales targeting Novelty and Intensity. The improvements focused on removing criterion-contaminated items, incorporating a Likert-type format, using items not inherently limited by age/physical ability, and modernizing item wording (Arnett, 1994). Of note, the scale’s development was guided by the “biological predispositions in interaction with the social environment” (Arnett, 1994, p. 290), increasing the emphasis on the role of environment in the expression of SS. Although the AISS was not developed using psychometric methods, its total scale reliability was acceptable (α = .70), and both the total scale and subscales correlated with illicit drug use and other risky behaviors, suggesting criterion-related validity (Arnett, 1994). Reliability for the total scale in the current sample (n = 144) was relatively weak (α = .67).
Risky Behaviors Scale–Shortened.
With the goal of deriving a list of activities representing SS, Fischer and Smith (2004) created a questionnaire comprising a range of possible activities. The authors used trained raters to classify items as negative or nonnegative by their perception of a negative life outcome. The Likert-type scoring was based on never, 1 to 5 times, 6 to 10 times, 11 to 15 times, and 16 or more times over the past year. The derived subscales were Negative and Nonnegative, with 19 and 21 items and Cronbach’s alphas .81 and .84, respectively (Fischer & Smith, 2004). Our chief interest in the current study was in addiction risk, and so we employed the questionnaire as a checklist (removing four largely redundant athletic-related items) and increased the time frame to “lifetime” to more fully characterize behavior. We captured self-reported drug-taking behavior by summing the scores from the five items specific to illicit drugs: “Driven a car after using an illegal drug,” “Used cocaine,” “Used LSD,” “Used heroin,” and “Used ecstasy.” Reliability for the total scale in the current sample was high (α = .83), with n = 145.
Alcohol Use Disorders Identification Test.
The Alcohol Use Disorders Identification Test (AUDIT) was designed as a cross-national rapid screening tool for identifying hazardous drinking patterns (Saunders, Aasland, Babor, de la Fuente, & Grant, 1993). The 10-item scale yields a possible score of 40, with scores of 8 or more regarded as likely problem drinking, with Cronbach’s alpha of .67 for the five “alcohol problems ever” items in the U.S. sample (Saunders et al., 1993). Reliability for the total scale in the current sample was high (α = .82), with n = 144.
Mean self-report scores are reported (Table 2), along with normative data for comparison.
Table 2.
Self-Report Scores.
| M | SD | α | Comparisona M | |
|---|---|---|---|---|
| Sensation-Seeking Scale | ||||
| Thrill/Adventure | 6.2 | 3.0 | .83 | 7.8b |
| Experience Seeking | 5.4 | 2.2 | .62 | 4.8b |
| Disinhibition | 4.6 | 2.4 | .67 | 5.9b |
| Boredom Susceptibility | 2.7 | 1.9 | .57 | 3.2b |
| Arnett Inventory of Sensation Seeking | ||||
| Intensity | 26.2 | 4.3 | .53 | 26.86c |
| Novelty | 27.1 | 4.1 | .53 | 27.66c |
| Risky Behaviors Scale | ||||
| Negative | 31.5 | 7.7 | .79 | — |
| Nonnegative | 46.3 | 7.4 | .73 | — |
| Alcohol Use Disorders Identification Test | 4.3 | 4.9 | .82 | 4.06d |
Note. Numbers range from 142 to 145, due to missing data in some scales.
Subscale means for normative comparison; precision reproduced here as published.
n = 97 college-age males (Zuckerman et al., 1978).
n = 139 late adolescents (Arnett, 1994). Normative data not available for the modified Risky Behaviors Scale.
n = 226 nondepressed college athletes (Miller, Miller, Verhegge, Linville, & Pumariega, 2002).
Procedure
Participants completed informed consent, verified a normal sense of smell by self-report, then completed the ACT and self-report measures in approximately 50 minutes, in counterbalanced order.
Data Analysis Plan
SPSS 24 (IBM Corp., Armonk, New York) was used for analyses; alpha was set to .05; means and standard deviations are reported. Difference tests report effect sizes as Cohen’s d and its 95% confidence interval. Greenhouse–Geisser corrections were employed for tests of nonspherical data.
Primary Analyses.
The four primary analyses were as follows: (1) To confirm that perceptions of odorant classes matched instructions, intensity differences were assessed by paired t test (mean ratings of air control vs. weak vs. strong); pleasantness was determined by one-sample t tests against neutral. (2) ACT choice behavior was assessed for stability (paired t test between Blocks 1 and 2, i.e., trials 1-20 vs. 21-40) and post hoc one-sample t tests of mean choice ratio within five-trial bins against the overall mean, reliability (Cronbach’s alpha with five-trial bin mean choice ratio treated as items), in addition to normality (Shapiro–Wilk), and deviation from chance (one-sample t test against 50%). (3) To test the association between ACT behavior and self-reported SS, choice ratio was entered into a linear regression model of self-reported SS. To reduce dimensionality of the six SS subscales, composite scores were generated by principal components analysis (PCA; Varimax rotation, Kaiser criterion) on self-reported SS. Components were characterized based on loadings of >.6 (e.g., Matsunaga, 2010). (4) Criterion-related validity was assessed in another linear model testing ACT choice ratio and self-reported drug taking. Both linear regression models included covariates known to influence SS (Blaszczynski, Wilson, & McConaghy, 1986; Zuckerman et al., 1978), that is, age, sex, and caregiver income in childhood (as a proxy for socioeconomic status), with standardized beta coefficients reported. Regression models of our primary analyses are presented showing hierarchical addition of important covariates.
Additional Analyses.
We performed additional analyses to better understand the measured behavior. To examine the possibility that sex moderated the association between behavioral SS and self-reported drug taking, we added an ACT choice ratio × sex interaction term to the same linear model. Overall sex differences in choice ratio or drug taking were also evaluated by t test. To test whether the ACT showed incremental validity over self-reported SS in predicting self-reported drug taking, we used a linear model and the self-reported sensation-seeking component from PCA (covarying for age, sex, and childhood income), with the subsequent addition of ACT choice ratio to the model. Unique explained variance (significantly increased R2) would indicate incremental validity. To determine if sensory perception influenced our primary findings, additional post hoc analyses of choice ratio and drug taking included intensity and pleasantness ratings. Zero-order correlations between choice ratio and self-report subscales are reported to clarify the relationships between measures. To test the possibility that aversion sensitivity may influence choice behavior, the behavioral effect of the malodorant (propionic acid) on subsequent choice ratio was assessed by comparing the choice ratio of the five trials following propionic acid delivery with participants’ mean choice ratio (paired t test).
Results
Sample Characteristics
One hundred and forty-seven participants were enrolled, but two participants were completely excluded from analyses—one for language reasons (nonnative speaker with comprehension problems) and the other for technical equipment issues. The remaining participants (n = 145) were largely young adults (M = 21.0 years, SD = 4.5 years; range = 18-49 years), 58% male, 73% Caucasian (plus 9% African American, 6% Asian, 5% biracial, and 6% Hispanic), with an average caregiver income in childhood of $99,210 (SD = $60,529; range $10,000 to $200,000+). Of these, five participants were missing data—either a self-report inventory (two lacked SSS-V, one lacked AISS, one lacked AUDIT) or childhood income—but were used where possible, and were removed listwise from analyses requiring those data points.
ACT Odorant Perceptions.
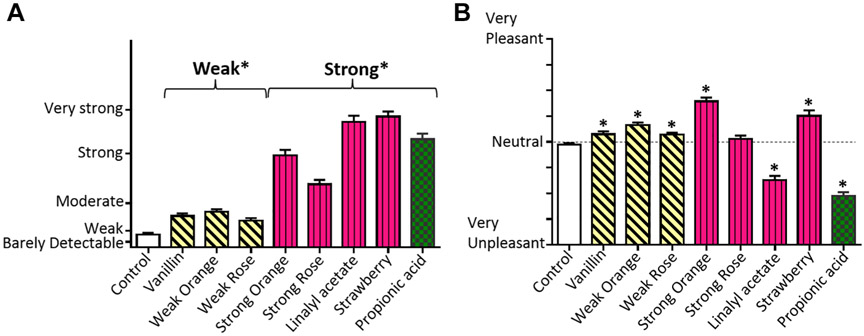
Strong odorants were rated as more intense than weak odorants, which were more intense than the odorless control (Figure 3A and Supplemental Table 1; supplementary material is available online). The odorless control and Strong Rose were rated neutral, the weak odorants and Strong Orange and Strawberry were pleasant, and linalyl acetate and propionic acid were unpleasant (Figure 3B and Supplemental Table 1).
Figure 3.
Ratings. (A) Intensity. (B) Pleasantness.
Note. Intensity: Strong odorants (maroon/vertical hatching or green/checkered) were more intense than Weak odorants (yellow/diagonal hatching), which were more intense than the odorless control. Pleasantness: The Weak odorants and Strong Orange and Strawberry were perceived as pleasant, and linalyl acetate and propionic acid were unpleasant (dotted line indicating neutrality). The y-axis labels are spatially proportional to the rating scales.
*ps < .05.
ACT Choice Behavior.
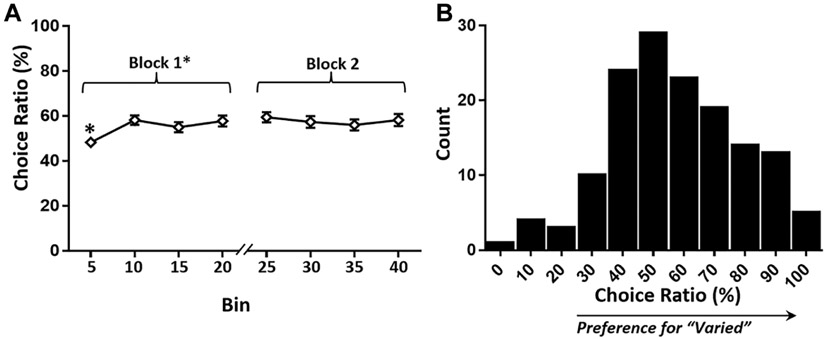
Choice ratio was lower in Block 1 versus Block 2, t(144) = −2.23, p = .027, d = −0.13 [−0.24, −0.015], driven by lower choice ratio in the first bin (five trials), which was lower than the mean; other bins did not differ from the mean (Figure 4A and Supplemental Table 2). The malodorant did not alter subsequent choices among participants who experienced it, t(126) = −1.17, p = .24, d = −0.081 [−0.22, 0.055]. Overall reliability was good, Cronbach’s α = .89. Relative preference for “Standard” versus “Varied” did not violate normality (p = .13) (Figure 4B), and revealed a slight preference for “Varied,” t(144) = 3.59, p < .001, d = 0.30 [0.13, 0.46], one-sample t test against 50% (M = 56.3%, SD = 21.1%).
Figure 4.
(A) Choice ratio by five-trial bin. (B) Choice ratio histogram.
Note. Choice ratio was lower in the first block (Trials 1-20) than the second due to “safe” choice behavior in the first five trials. Bins are five-trial M ± SEM, named by the last trial in the bin. Choice ratio was normally distributed in n = 145.
*p < .05.
PCA Component Extraction
The combined self-reported SS subscales (SSS-V and AISS) were reduced to two components with eigenvalues of 2.79 and 1.04, explaining 46.5% and 17.3% of variance, respectively. These were labeled Sensation Seeking and Disinhibited based on loadings (see Table 3.
Table 3.
Principal Component Analysis of Self-Reported Sensation Seeking; n = 145.
| Component |
||
|---|---|---|
| Sensation seeking | Disinhibited | |
| Sensation-Seeking Scale | ||
| Thrill/Adventure | .86a | .077 |
| Experience Seeking | .68a | .24 |
| Disinhibition | .29 | .74a |
| Boredom Susceptibility | .033 | .84a |
| Arnett Inventory of Sensation Seeking | ||
| Intensity | .71a | .26 |
| Novelty | .82a | .046 |
Note. Principal components analysis limited to n = 142 due to three participants’ missing data. Varimax-rotated solutions shown.
High factor loadings (>0.6).
ACT Behavior and Self-Reported Sensation Seeking.
The SS component, derived from PCA (detailed below), reflected our best estimate of self-reported SS. The regression model with choice ratio and demographic covariates significantly predicted the SS component, R2 = .18, F(4, 136) = 7.33, p < .001. Choice ratio was a significant predictor (β = .37, p < .001), but age, sex, and childhood income were nonsignificant (Table 4, Model 4). A regression model with choice ratio and demographic covariates significantly predicted the Disinhibited component, R2 = .11, F(4, 136) = 4.11, p = .004, but choice ratio was not significant (β = .015, p = .86), nor was age (β = .086, p = .29); sex and childhood income were significant (β = −.28, p = .001; β = .17, p = .040, respectively). Age deviated from normality (Supplemental Table 4), owing to its concentration around the typical college age, so regression was performed again using a natural-log transformation of age (e.g., Tabachnick & Fidell, 1989); results were qualitatively similar (Table 4, Model 5).
Table 4.
Aroma Choice Task Association With Self-Reported Sensation Seeking; n = 141.
| Predictor | R2 | F | β | t | p | |
|---|---|---|---|---|---|---|
| Model 1 | .16 | 25.87 | <.001 | |||
| Choice ratio | 0.40 | 5.09 | <.001 | |||
| Model 2 | .16 | 12.86 | <.001 | |||
| Choice ratio | 0.40 | 5.07 | <.001 | |||
| Age | 0.01 | 0.18 | .858 | |||
| Model 3 | .18 | 9.67 | <.001 | |||
| Choice ratio | 0.37 | 4.72 | <.001 | |||
| Age | 0.02 | 0.23 | .82 | |||
| Sex | −0.14 | −1.71 | .089 | |||
| Model 4 | .18 | 7.33 | <.001 | |||
| Choice ratio | 0.37 | 4.67 | <.001 | |||
| Age | 0.01 | 0.15 | .88 | |||
| Sex | −0.13 | −1.70 | .092 | |||
| Childhood income | −0.05 | −0.66 | .51 | |||
| Model 5 | .18 | 7.33 | <.001 | |||
| Choice ratio | 0.37 | 4.68 | <.001 | |||
| ln Age | 0.02 | 0.21 | .83 | |||
| Sex | −0.13 | −1.70 | .092 | |||
| Childhood income | −0.05 | −0.65 | .52 |
Associations With Self-Reported Drug Taking.
The regression model with choice ratio and demographic covariates significantly predicted self-reported drug taking, R2 = .090, F(4, 139) = 3.45, p < .010, with choice ratio significant (β = .17, p = .041) and childhood income (β = .23, p = .006), but neither age nor sex (Table 5, Model 4). Transformed age yielded similar results, with age reaching significance (Table 5, Model 5).
Table 5.
Aroma Choice Task Association With Drug Taking (n = 144).
| Model | Predictor | R2 | F | β | t | p |
|---|---|---|---|---|---|---|
| Model 1 | .02 | 2.83 | .095 | |||
| Choice ratio | 0.14 | 1.68 | .095 | |||
| Model 2 | .04 | 2.58 | .079 | |||
| Choice ratio | 0.15 | 1.82 | .071 | |||
| Age | 0.13 | 1.52 | .13 | |||
| Model 3 | .04 | 1.88 | .14 | |||
| Choice ratio | 0.16 | 1.90 | .059 | |||
| Age | 0.13 | 1.50 | .14 | |||
| Sex | 0.06 | 0.69 | .49 | |||
| Model 4 | .09 | 3.45 | .010 | |||
| Choice ratio | 0.17 | 2.06 | .041 | |||
| Age | 0.15 | 1.83 | .070 | |||
| Sex | 0.06 | 0.67 | .51 | |||
| Childhood income | 0.23 | 2.81 | .006 | |||
| Model 5 | .10 | 3.75 | .006 | |||
| Choice ratio | 0.16 | 1.99 | .049 | |||
| ln Age | 0.17 | 2.11 | .036 | |||
| Sex | 0.05 | 0.66 | .51 | |||
| Childhood income | 0.23 | 2.86 | .005 |
Additional Analyses.
Sex did not moderate the association between choice ratio and drug taking (β = −.35, p = .26). Although males’ choice ratios were higher than those of females, t(143) = 2.04, p = .043 (M = 59.3%, SD = 20.0%; M = 52.1%, SD = 22.0%, respectively), drug taking did not differ between sexes (p = .65). ACT choice ratio did not show incremental validity when added to a model using self-reported SS as the sensation-seeking predictor of drug taking, R2 = .17, F(4, 136) = 7.09, p < .001; and R2 = .18, F(5, 135) = 5.72, p < .001, respectively, with nonsignificant change in variance explained, F(1, 135) = 0.37, p = .54. To assess the possibility that odorant sensitivity was related to self-reported drug taking, we added mean perception ratings (weak and strong) to the linear regression model with choice ratio and demographics; this analysis remained significant, R2 = .15, F(8, 135) = 2.89, p = .005, and revealed a positive association between the intensity perception of strong odorants and drug taking (β = .22, p = .017), with choice ratio and childhood income remaining significant, but other factors nonsignificant. Continuous predictors used in regression models are summarized in Supplemental Table 4.
Discussion
The ACT shows face and criterion-related validity (task design and ratings, and association with drug use, respectively) with good internal consistency. We believe that it captures central elements of SS—as described by others— with the current study providing the first steps toward establishing construct validity. The ACT permits the empirical measurement of behavioral SS by providing actual sensory experiences and consequences in real time. Choice behavior in the ACT is associated with self-reported SS (R = .42), suggesting that they both tap into similar constructs but that each provides unique, complementary information. Consistent with prior work indicating that self-reported SS predicts later drug use (Cloninger et al., 1988; MacPherson et al., 2010; Masse & Tremblay, 1997; Pedersen, 1991), the ACT detects the same risk factor using behavioral measurement. While choice ratio was associated with self-reported illicit drug taking, it did not show incremental validity over the existing self-report measures we used.
We demonstrated that behavioral SS is not strongly biased in most participants, that is, toward “Standard” or “Varied,” thus maximizing the range of detection with this odorant series. Behavioral SS is normally distributed in our sample, making it amenable to parametric testing, with behavior remaining stable throughout the 40-trial session— suggesting that additive changes in sensory sensitivity, if present, did not affect choice behavior. Interestingly, choice ratio was lower in the first five trials, suggesting an initially conservative strategy at least until some stimuli were sampled. Odorant intensity and pleasantness were consistent with our intent, and self-reported SS positively correlated with choice ratio. Unexpectedly, higher perceived intensity was a significant predictor of self-reported drug taking, suggesting the possibility that greater sensory sensitivity confers a risk for drug taking. Importantly, high-intensity ratings did not confound the relationship between choice ratio and self-reported drug taking. Apart from the first five trials, choice ratio remained stable throughout the 40-trial session, even after a fresh air break or malodorant, suggesting trait-like stability.
Within the personality literature, SS has largely been grouped within the broader domain of impulsivity. Measuring impulsivity is challenging, as noted by Depue and Collins (1999), “because the content of the measures of impulsivity is heterogeneous, ranging from purely motor and cognitive impulsivity to novelty and SS, boldness, thrill and adventure seeking, and risk-taking” (p. 495). Whiteside and Lynam (2001) and Cyders and Smith (2007) found that the multifactorial concept of impulsivity could be described by five distinct and unique facets: sensation seeking (preference for excitement), positive and negative urgency (emotional rash action), lack of perseverance (performing difficult tasks), and lack of premeditation (acting without considering future consequences). The latter two facets, aversion to performing undesirable present tasks and disregarding future outcomes, arguably represent impulsivity descriptions from the behavioral literature—that is, the conflict between present gratification and future gain (Ainslie, 1975; Kirby, 1997; Petry, Bickel, & Arnett, 1998; Rachlin & Green, 1972). Behavioral studies of this particular type of impulsivity (small immediate vs. larger delayed; i.e., delay discounting) suggest that it is largely orthogonal to self-reported SS (e.g., Mitchell, 1999). It remains yet unknown how behavioral SS, as measured in ACT or similar tasks, will relate to behavioral impulsivity or other forms of self-reported impulsivity. Searching for consilience between behavioral and self-reported impulsivity scores, Cyders and Coskunpinar (2011) performed a meta-analysis of 28 studies containing both tasks and inventories designed to assess impulsivity. They found little overlap (rs < .14) between behavioral tasks and self-report inventories ostensibly measuring analogous constructs. The authors note that the differing theoretical constructs informing the various measures may explain much of this divergence. “Siloization” has long been identified as an important cause:
Unfortunately, with a few exceptions, researchers interested in the personality trait of impulsivity, in the experimental analysis of impulsive behaviour, in psychiatric studies of impulsivity or in the neurobiology of impulsivity form largely independent schools, who rarely cite one another’s work, and consequently rarely gain any insight into their own work from the progress made by others. (Evenden, 1999, p. 348)
In spite of the unimpressive correspondence of self-reported and behavioral impulsivity (Cyders & Coskunpinar, 2011), our task performed well, with rs > .27 on four subscales specifically measuring the constructs informing this task (subscales of SSS-V TAS [Thrill and Adventure Seeking] and ES [Experience Seeking], and AISS Intensity and Novelty; Supplemental Table 3). Interestingly, ACT did not correlate with AUDIT (problematic drinking), although AUDIT correlated with TAS and ES. Given that alcohol drinking is legal behavior and drug taking is illegal, this dissociation suggests the intriguing possibility that ACT behavior reflects more antisocial tendencies, whereas self-reported SS reflects more socially normalized behaviors. Explicitly designed future studies would be required to speculate further on this possibility. Overall, we believe that our present effort succeeded in bridging a gap between self-reported SS and behavioral measurement of a specific construct of SS.
As researchers focused on addiction, we are especially interested in precise measurement of SS and other impulsivity-like traits, given their importance in addiction risk and pathology. We believe that comprehensive trait characterization is critical to understanding addiction phenotypes and etiology. Disambiguating these traits might best be accomplished by using behavioral and self-report methods in parallel (Sharma, Markon, & Clark, 2014), with nonoverlapping variance captured by divergent methods (Cyders & Coskunpinar, 2011; Lane, Cherek, Rhoades, Pietras, & Tcheremissine, 2003), and both showing valid prediction of later addiction behavior (Cloninger et al., 1988; Fernie et al., 2013). As previously noted (e.g., Steinberg et al., 2008; Whiteside & Lynam, 2001), SS is distinct from other impulsivity-like traits. For example, seeking the intense and novel sensations of scuba diving in a carefully planned excursion is highly future-conscious behavior and not necessarily an impulsive choice (defined as nonplanning or disinhibition), whereas a classically impulsive choice such as ignoring retirement planning is presumably not motivated by desiring intense and novel sensations per se. Clarifying these traits through more precise and comprehensive measurement should capture more variance, better describe behavioral phenotypes, and ultimately lead to models offering greater predictive utility (Ebstein, 2006; Steinberg et al., 2008).
The difficulty in collecting accurate self-report data in drug users and heavy drinkers (Magura & Kang, 1996; Northcote & Livingston, 2011) highlights an important strength of the ACT, namely, that its true purpose is unclear to most participants. Anecdotally, many participants assume that the task’s purpose is to assess olfaction. With the task’s true purpose obfuscated, demand characteristics are presumably limited and therefore affect measurement less than a more obvious method might. In spite of any clear connection or reference to drug use—which participants might infer from self-report inventories of SS—the ACT still detected an association with illicit drug taking, highlighting a key strength of the method. Additionally, laboratory behavioral tasks, such as the ACT, excel at capturing behavior at a defined point in time, thus enabling detection of shifts in behavior, such as with manipulations designed to inhibit SS. Another potential application of this task is investigating brain activation at the time of choice; the olfactometer’s portability and MRI-compatibility permit use with the prevalent neuroimaging modalities—fMRI, EEG/ERP (electroencephalography/event-related potential), PET (positron emission tomography). The ACT could find utility in translational studies of SS traits. For example, a slightly modified ACT task could be easily used with animal models or children at the preverbal stage for assessing correlated traits or prediction of clinical outcomes. While our present interest in behavioral SS is rooted in its association with addiction risk, broader applications in personality research are possible. Our findings suggest that ACT choice ratio corresponds with the SSS-V subscales TAS and ES, which are highly related to the Extraversion and Openness domains, respectively (Aluja, García, & García, 2003). Additional studies designed to characterize ACT choice ratio in terms of personality will be required to better understand which facets of Extraversion and Openness most closely align with this behavior.
Some limitations warrant discussion. The use of a relatively high-functioning college sample for initial validation comes at the risk of limiting generalizability—although with the strength of being a nonclinical sample to compare future work against. Another concern with college samples is limited variability, which may limit the range over which comparisons might be made. We inferred behavioral SS from behavior involving only one sensory modality, olfaction, meaning that extrapolation to other sensory modalities should be made with caution until further testing is performed. The illicit drug taking reported here was self-reported, therefore making it subject to some of the same limitations outlined for other self-report metrics. As the Zuckerman and Arnett trait descriptions largely inspired the task design, we focused on those SS inventories, at the cost of using arguably better-designed/validated instruments, such as the UPPS (Whiteside & Lynam, 2001). Future studies will focus on discriminant validity, with additional measures tested, both self-report and behavioral. We note that the covariates of age, sex, and childhood income influenced the association between ACT and drug taking, such that the association was only trend level with no covariates (Table 5). We find the influence of these covariates unsurprising, as these are known to strongly influence SS (Steinberg et al., 2008; Zuckerman et al., 1978)—although note that they were largely nonsignificant predictors of drug taking. While subjects rated pleasantness and intensity of odorants, a more comprehensive evaluation of olfactory sensitivity may be required to quantify expectations governing choice behavior in odorant-based choice tasks such as the ACT. More generally, the ACT requires relatively intact olfaction, which could restrict samples to participants without allergies, congestion, or extreme sensitivity to odorants (although we did not encounter this problem in n = 145).
Conclusions
We endeavored to use a specific SS conceptualization (risk-taking to experience intense, novel, and varied stimuli) in order to create a behavioral task that reflects self-reported SS and drug use. We believe that this study offers promising preliminary data supporting the use of the ACT in expanded work. The ACT offers objective behavioral measurement of SS that is easy to analyze, compliments self-report, limits demand characteristics, is presumably less constrained by language barriers, and provides a metric corresponding with real-world risky behavior. Addiction-relevant traits will require precise phenotyping to advance biological studies of addiction, whether behavioral, genetic, or neurobiological. We believe that behavioral tasks can augment self-report inventories by increasing phenotypic precision, in service of advancing biological studies of personality. A dearth of behavioral models in SS research leaves a gap that we hope the ACT can begin to fill.
Supplementary Material
Acknowledgments
We are grateful to Melissa Kerfoot, Drew E. Winters, and Jean D. De Jesus for excellent technical assistance; Taylor Hunton for editing assistance; and Dr. Jaroslaw Harezlak for statistical advice. We also thank Drs. Stephen Warrenburg and Aleksey Dumer of International Flavors and Fragrances for generously providing odorants and Dwight Hector for design and construction of the olfactometer.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (R00AA023296) and (K01AA020102), and National Institutes of Health (R25GM109432).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental material for this article is available online.
References
- Abler B, Walter H, Erk S, Kammerer H, & Spitzer M (2006). Prediction error as a linear function of reward probability is coded in human nucleus accumbens. Neuroimage, 31, 790–795. doi: 10.1016/j.neuroimage.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Ainslie G (1975). Specious reward: A behavioral theory of impulsiveness and impulse control. Psychological Bulletin, 82, 463–496. [DOI] [PubMed] [Google Scholar]
- Aluja A, García O, & García LF (2003). Relationships among extraversion, openness to experience, and sensation seeking. Personality Individual Differences, 35, 671–680. [Google Scholar]
- Andrew M, & Cronin C (1997). Two measures of sensation seeking as predictors of alcohol use among high school males. Personality and Individual Differences, 22, 393–401. [Google Scholar]
- Arnett J (1990). Drunk driving, sensation seeking, and egocentrism among adolescents. Personality and Individual Differences, 11, 541–546. [Google Scholar]
- Arnett J (1994). Sensation seeking: A new conceptualization and a new scale. Personality and Individual Differences, 16, 289–296. [Google Scholar]
- Berkowitz WR (1967). Use of the sensation-seeking scale with Thai subjects. Psychological Reports, 20, 635–641. [Google Scholar]
- Blaszczynski AP, Wilson AC, & McConaghy N (1986). Sensation seeking and pathological gambling. British Journal of Addiction, 81, 113–117. [DOI] [PubMed] [Google Scholar]
- Byrnes NK, & Hayes JE (2013). Personality factors predict spicy food liking and intake. Food Quality and Preference, 28, 213–221. doi: 10.1016/j.foodqual.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR (1987). A systematic method for clinical description and classification of personality variants: A proposal. Archives of General Psychiatry, 44, 573–588. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Sigvardsson S, & Bohman M (1988). Childhood personality predicts alcohol abuse in young adults. Alcoholism, Clinical and Experimental Research, 12, 494–505. [DOI] [PubMed] [Google Scholar]
- Costa PTJ, & McCrae RR (1992). Revised NEO Personality Inventory manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Cservenka A, Herting MM, Seghete KL, Hudson KA, & Nagel BJ (2013). High and low sensation seeking adolescents show distinct patterns of brain activity during reward processing. Neuroimage, 66, 184–193. doi: 10.1016/j.neuroimage.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, & Coskunpinar A (2011). Measurement of constructs using self-report and behavioral lab tasks: Is there overlap in nomothetic span and construct representation for impulsivity? Clinical Psychology Review, 31, 965–982. [DOI] [PubMed] [Google Scholar]
- Cyders MA, & Smith GT (2007). Mood-based rash action and its components: Positive and negative urgency. Personality and Individual Differences, 43, 839–850. [Google Scholar]
- Darkes J, Greenbaum PE, & Goldman MS (1998). Sensation seeking–disinhibition and alcohol use: Exploring issues of criterion contamination. Psychological Assessment, 10, 71–76. [Google Scholar]
- Depue RA, & Collins PF (1999). Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences, 22, 491–517; discussion 518-569. [DOI] [PubMed] [Google Scholar]
- Donohew L, Zimmerman R, Cupp PS, Novak S, Colon S, & Abell R (2000). Sensation seeking, impulsive decision-making, and risky sex: Implications for risk-taking and design of interventions. Personality and Individual Differences, 28, 1079–1091. [Google Scholar]
- Ebstein RP (2006). The molecular genetic architecture of human personality: Beyond self-report questionnaires. Molecular Psychiatry, 11, 427–445. doi: 10.1038/sj.mp.4001814 [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, Blaine D, … Belmaker RH (1996). Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait of novelty seeking. Nature Genetics, 12, 78–80. doi: 10.1038/ng0196-78 [DOI] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Pradhan S, Bullmore ET, & Robbins TW (2010). Drug addiction endophenotypes: Impulsive versus sensation-seeking personality traits. Biological Psychiatry, 68, 770–773. doi: 10.1016/j.biopsych.2010.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL (1999). Varieties of impulsivity. Psychopharmacology, 146, 348–361. [DOI] [PubMed] [Google Scholar]
- Eysenck SB, & Eysenck HJ (1978). Impulsiveness and venturesomeness: Their position in a dimensional system of personality description. Psychological Reports, 43, 1247–1255. [DOI] [PubMed] [Google Scholar]
- Fernie G, Peeters M, Gullo MJ, Christiansen P, Cole JC, Sumnall H, & Field M (2013). Multiple behavioural impulsivity tasks predict prospective alcohol involvement in adolescents. Addiction, 108, 1916–1923. doi: 10.1111/add.12283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Sharkansky EJ, Brandt KM, & Turcotte N (2000). The effects of familial risk, personality, and expectancies on alcohol use and abuse. Journal of Abnormal Psychology, 109, 122–133. [DOI] [PubMed] [Google Scholar]
- Fischer S, & Smith GT (2004). Deliberation affects risk taking beyond sensation seeking. Personality and Individual Differences, 36, 527–537. [Google Scholar]
- Fulker DW, Eysenck SBG, & Zuckerman M (1980). A genetic and environmental analysis of sensation seeking. Journal of Research in Personality, 14, 261–281. [Google Scholar]
- Gardner M, & Steinberg L (2005). Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Developmental Psychology, 41, 625–635. doi: 10.1037/0012-1649.41.4.625 [DOI] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, & Higgins J (1996). Evaluating the “Labeled Magnitude Scale” for measuring sensations of taste and smell. Chemical Senses, 21, 323–334. [DOI] [PubMed] [Google Scholar]
- Hawes SW, Chahal R, Hallquist MN, Paulsen DJ, Geier CF, & Luna B (2017). Modulation of reward-related neural activation on sensation seeking across development. Neuroimage, 147, 763–771. doi: 10.1016/j.neuroimage.2016.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes CA, Miles JNV, & Clements K (2000). A confirmatory factor analysis of two models of sensation seeking. Personality and Individual Differences, 29, 823–839. [Google Scholar]
- Herz RS (2004). A naturalistic analysis of autobiographical memories triggered by olfactory visual and auditory stimuli. Chemical Senses, 29, 217–224. [DOI] [PubMed] [Google Scholar]
- Hittner JB, & Swickert R (2006). Sensation seeking and alcohol use: A meta-analytic review. Addictive Behaviors, 31, 1383–1401. doi: 10.1016/j.addbeh.2005.11.004 [DOI] [PubMed] [Google Scholar]
- Hur YM, & Bouchard TJ Jr. (1997). The genetic correlation between impulsivity and sensation seeking traits. Behavior Genetics, 27, 455–463. [DOI] [PubMed] [Google Scholar]
- Jaffe LT, & Archer RP (1987). The prediction of drug use among college students from MMPI, MCMI, and sensation seeking scales. Journal of Personality Assessment, 51, 243–253. doi: 10.1207/s15327752jpa5102_8 [DOI] [PubMed] [Google Scholar]
- Joseph JE, Liu X, Jiang Y, Lynam D, & Kelly TH (2009). Neural correlates of emotional reactivity in sensation seeking. Psychological Science, 20, 215–223. doi: 10.1111/j.1467-9280.2009.02283.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Garbutt JC, Davis CE, & Janowsky DS (1998). Preference for higher sugar concentrations and Tridimensional Personality Questionnaire scores in alcoholic and nonalcoholic men. Alcoholism, Clinical and Experimental Research, 22, 610–614. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Claus ED, Sabri M, Dzemidzic M, Kosobud AE, Radnovich AJ, … Li TK (2004). Alcohol-related olfactory cues activate the nucleus accumbens and ventral tegmental area in high-risk drinkers: Preliminary findings. Alcoholism, Clinical and Experimental Research, 28, 550–557. [DOI] [PubMed] [Google Scholar]
- Khavari KA, Mabry E, & Humes M (1977). Personality correlates of hallucinogen use. Journal of Abnormal Psychology, 86, 172–178. [DOI] [PubMed] [Google Scholar]
- Kirby KN (1997). Bidding on the future: Evidence against normative discounting of delayed rewards. Journal of Experimental Psychology: General, 126, 54–70. [Google Scholar]
- Kluger AN, Siegfried Z, & Ebstein RP (2002). A meta-analysis of the association between DRD4 polymorphism and novelty seeking. Molecular Psychiatry, 7, 712–717. doi: 10.1038/sj.mp.4001082 [DOI] [PubMed] [Google Scholar]
- Koopmans JR, Boomsma DI, Heath AC, & van Doornen LJ (1995). A multivariate genetic analysis of sensation seeking. Behavior Genetics, 25, 349–356. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Ball SA, & Rounsaville BJ (1994). A sibling study of sensation seeking and opiate addiction. Journal of Nervous and Mental Disease, 182, 284–289. [DOI] [PubMed] [Google Scholar]
- Kruschwitz JD, Simmons AN, Flagan T, & Paulus MP (2012). Nothing to lose: Processing blindness to potential losses drives thrill and adventure seekers. Neuroimage, 59, 2850–2859. doi: 10.1016/j.neuroimage.2011.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Rhoades HM, Pietras CJ, & Tcheremissine OV (2003). Relationships among laboratory and psychometric measures of impulsivity: Implications in substance abuse and dependence. Addictive Disorders & Their Treatment, 2, 33–40. [Google Scholar]
- Lorig TS, Elmes DG, Zald DH, & Pardo JV (1999). A computer-controlled olfactometer for fMRI and electrophysiological studies of olfaction. Behavior Research Methods, Instruments & Computers, 31, 370–375. [DOI] [PubMed] [Google Scholar]
- Lynam DR, Smith GT, Whiteside SP, & Cyders MA (2006). The UPPS-P: Assessing five personality pathways to impulsive behavior. West Lafayette, IN: Purdue University. [Google Scholar]
- MacPherson L, Magidson JF, Reynolds EK, Kahler CW, & Lejuez CW (2010). Changes in sensation seeking and risk-taking propensity predict increases in alcohol use among early adolescents. Alcoholism, Clinical and Experimental Research, 34, 1400–1408. doi: 10.1111/j.1530-0277.2010.01223.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaro P, Smith P, Cionini L, & Velicogna F (1979). Sensation-seeking in Italy and the United States. Journal of Social Psychology, 109, 159–165. doi: 10.1080/00224545.1979.9924191 [DOI] [PubMed] [Google Scholar]
- Magura S, & Kang SY (1996). Validity of self-reported drug use in high risk populations: A meta-analytical review. Substance Use & Misuse, 31, 1131–1153. [DOI] [PubMed] [Google Scholar]
- Masse LC, & Tremblay RE (1997). Behavior of boys in kindergarten and the onset of substance use during adolescence. Archives of General Psychiatry, 54, 62–68. [DOI] [PubMed] [Google Scholar]
- Matsunaga M (2010). How to factor-analyze your data right: Do’s, don’ts, and how-to’s. International Journal of Psychological Research, 3(1), 97–110. [Google Scholar]
- Mattes RD (1994). Influences on acceptance of bitter foods and beverages. Physiology & Behavior, 56, 1229–1236. [DOI] [PubMed] [Google Scholar]
- McGlothlin WH, & Arnold DO (1971). LSD revisited. A ten-year follow-up of medical LSD use. Archives of General Psychiatry, 24, 35–49. [DOI] [PubMed] [Google Scholar]
- Meda SA, Stevens MC, Potenza MN, Pittman B, Gueorguieva R, Andrews MM, … Pearlson GD (2009). Investigating the behavioral and self-report constructs of impulsivity domains using principal component analysis. Behavioural Pharmacology, 20, 390–399. doi: 10.1097/FBP.0b013e32833113a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BE, Miller MN, Verhegge R, Linville HH, & Pumariega AJ (2002). Alcohol misuse among college athletes: Self-medication for psychiatric symptoms? Journal of Drug Education, 32, 41–52. doi: 10.2190/JDFM-AVAK-G9FV-0MYY [DOI] [PubMed] [Google Scholar]
- Mitchell SH (1999). Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology, 146, 455–464. [DOI] [PubMed] [Google Scholar]
- Northcote J, & Livingston M (2011). Accuracy of self-reported drinking: observational verification of “last occasion” drink estimates of young adults. Alcohol and Alcoholism, 46, 709–713. doi: 10.1093/alcalc/agr138 [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, Albrecht DS, Yoder KK, & Kareken DA (2013). Beer flavor provokes striatal dopamine release in male drinkers: Mediation by family history of alcoholism. Neuropsychopharmacology, 38, 1617–1624. doi: 10.1038/npp.2013.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen W (1991). Mental health, sensation seeking and drug use patterns: A longitudinal study. British Journal of Addiction, 86, 195–204. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, & Arnett M (1998). Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction, 93, 729–738. [DOI] [PubMed] [Google Scholar]
- Rachlin H, & Green L (1972). Commitment, choice and self-control. Journal of the Experimental Analysis of Behavior, 17, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberti JW (2004). A review of behavioral and biological correlates of sensation seeking. Journal of Research in Personality, 38, 256–279. [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, & Grant M (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction, 88, 791–804. [DOI] [PubMed] [Google Scholar]
- Schiffman SS (1974). Physicochemical correlates of olfactory quality. Science, 185(4146), 112–117. [DOI] [PubMed] [Google Scholar]
- Segal B, & Singer JL (1976). Daydreaming, drug and alcohol use in college students: A factor analytic study. Addictive Behaviors, 1, 227–235. [Google Scholar]
- Sen S, Burmeister M, & Ghosh D (2004). Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. American Journal of Medical Genetics B Neuropsychiatric Genetics, 127B, 85–89. doi: 10.1002/ajmg.b.20158 [DOI] [PubMed] [Google Scholar]
- Sharma L, Markon KE, & Clark LA (2014). Toward a theory of distinct types of “impulsive” behaviors: A meta-analysis of self-report and behavioral measures. Psychological Bulletin, 140, 374–408. [DOI] [PubMed] [Google Scholar]
- Simms LJ, Zelazny K, Williams TF, & Bernstein L (2019). Does the number of response options matter? Psychometric perspectives using personality questionnaire data. Psychological Assessment, 31, 557–566. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, & Woolard J (2008). Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Developmental Psychology, 44, 1764–1778. doi: 10.1037/a0012955 [DOI] [PubMed] [Google Scholar]
- Stoel RD, De Geus EJ, & Boomsma DI (2006). Genetic analysis of sensation seeking with an extended twin design. Behavior Genetics, 36, 229–237. doi: 10.1007/s10519-005-9028-5 [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, & Fidell LS (1989). Using multivariate statistics (2nd ed.). New York, NY: HarperCollins. [Google Scholar]
- Weng L (2004). Impact of the number of response categories and anchor labels on coefficient alpha and test-retest reliability. Educational Psychological Measurement, 64, 956–972. [Google Scholar]
- Whiteside SP, & Lynam DR (2001). The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences, 30, 669–689. [Google Scholar]
- Wills TA, Vaccaro D, & McNamara G (1994). Novelty seeking, risk taking, and related constructs as predictors of adolescent substance use: An application of Cloninger’s theory. Journal of Substance Abuse, 6, 1–20. [DOI] [PubMed] [Google Scholar]
- Zhornitsky S, Rizkallah E, Pampoulova T, Chiasson JP, Lipp O, Stip E, & Potvin S (2012). Sensation-seeking, social anhedonia, and impulsivity in substance use disorder patients with and without schizophrenia and in non-abusing schizophrenia patients. Psychiatry Research, 200, 237–241. doi: 10.1016/j.psychres.2012.07.046 [DOI] [PubMed] [Google Scholar]
- Zuckerman M (1990). The psychophysiology of sensation seeking. Journal of Personality, 58, 313–345. [DOI] [PubMed] [Google Scholar]
- Zuckerman M (1994). Behavioral expressions and biosocial bases of sensation seeking (1st ed.). New York, NY: Cambridge University Press. [Google Scholar]
- Zuckerman M (2002). Zuckerman-Kuhlman Personality Questionnaire (ZKPQ): An alternative five-factorial model In de Raad B & Perugini M (Eds.), Big Five assessment (pp. 377–395). Seatte, WA: Hogrefe & Huber. [Google Scholar]
- Zuckerman M (2007). The Sensation Seeking Scale V (SSS-V): Still reliable and valid. Personality Individual Differences, 43, 1303–1305. [Google Scholar]
- Zuckerman M, Eysenck S, & Eysenck HJ (1978). Sensation seeking in England and America: Cross-cultural, age, and sex comparisons. Journal of Consulting and Clinical Psychology, 46, 139–149. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, & Neeb M (1979). Sensation seeking and psychopathology. Psychiatry Research, 1, 255–264. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, & Neeb M (1980). Demographic influences in sensation seeking and expressions of sensation seeking in religion, smoking and driving habits. Personality and Individual Differences, 1, 197–206. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.