Abstract
A 25-year-old woman presented a challenging diagnosis of acute rheumatic fever (ARF). Initial symptoms included dry cough and three minor Jones criteria (unabating fever (38.4°C, 0d), elevated acute phase reactants (C-reactive protein, 13d) and joint pain (monoarthralgia) in her neck (0d)). ARF was diagnosed only after presentation of two major Jones criteria (polyarthritis/polyarthralgia (16d) and erythema marginatum (41d)) and positive antistreptolysin O titre (44d). Parotid swelling, peripheral oedema, elevated liver enzymes and diffuse lymphadenopathy complicated the diagnosis. Throat swab, chorea and carditis were negative or absent. Atypical ARF is challenging to recognise. There is no diagnostic test and its presentation is similar to that of other diseases. While the 2015 Jones criteria modification increased specificity of ARF diagnosis, atypical cases may still be missed, especially by physicians in developed countries. Suspicion of atypical ARF, especially after travel to high incidence regions, would allow for earlier treatment and prevention of rheumatic heart disease.
Keywords: general practice / family medicine, global Health, infectious diseases, medical management
Background
Diagnosing ARF can be a challenge as it is a rare disease with symptoms similar to those of other diseases. Furthermore, diagnosis is primarily clinical as there are no diagnostic biomarkers. Diagnosis is based on identification of sufficient features that meet the combination of major and minor Jones criteria.1 Major criteria and frequency of occurrence include polyarthritis and/or polyarthralgia in high-risk populations (75%), carditis (>50%), chorea (<30%), subcutaneous nodules (<10%) and erythema marginatum (<6%).1 2 Minor criteria for high-risk populations include fever (≥38.0°C; >90%), monoarthralgia, elevated erythrocyte sedimentation rate ((ESR) >30 mm in the first hour), elevated acute phase reactants such as C-reactive protein (CRP) (>3.0 mg/dL) and prolonged PR interval on ECG.1 2 The presence of two major criteria or one major and two minor Jones criteria are diagnostic for ARF. Another important consideration is the response of ARF to anti-inflammatory medications with 48 hours, and an ARF diagnosis should be reconsidered otherwise.2 Since ARF resembles other diseases, evidence of a preceding Group A streptococcal (GAS) infection is needed (eg, antistreptolysin O titre (ASO)/anti-DNase B, positive streptococcal test/throat culture or rapid group A streptococcal carbohydrate antigen test).1 We should stress that Jones himself indicated that these criteria offered the least likelihood of misdiagnosis.3
The diagnosis is even more difficult when ARF presents atypically as in our case where major Jones criteria only manifested 16 and 41 days after onset of symptoms, the fever was non-responsive to antipyretics and non-ARF features including peripheral oedema, parotid swelling and liver dysfunction were present. Furthermore, travel to a developing country further complicated the diagnosis as chikungunya, dengue fever and Zika virus are endemic and share many symptoms. Missed diagnosis of atypical ARF can result in serious cardiovascular morbidity and mortality.
The Jones criteria is not perfect. It was developed in 1944 and revised in 1992 and 2015. The 1992 revision resulted in low sensitivity in high prevalence regions, with missed diagnosis of cases presenting only with minor Jones criteria such as monoarthralgia and low-grade fever.4 Sensitivity of diagnosis was improved with separate guidelines for high-risk populations in the 2015 Jones revision.5 This is of importance as Grenada, the country of our patient’s travel, has a high incidence of ARF (52/100 000 Grenada vs 0.6/100 000 USA) with endemic rheumatic heart disease.6 7
The fact that ARF presents differently in high-risk populations was recognised in the 2015 Jones revision, with separate guidelines to increase sensitivity of diagnosis. ARF can also have presentations that do not follow the classical Jones criteria. Atypical ARF was documented over 70 years ago in published reports for ~1500 adult cases (eg, n=1000,8 367,4 243).9 It was speculated that the milder form of rheumatic fever which presents with arthralgia and low-grade fever results in increased misdiagnoses.8 However, this disease is now rare in developed countries and atypical presentation in adults is no longer as well recognised.
Using the 2015 modified Jones criteria for high-risk populations, our specific case initially met only minor Jones criteria (fever, joint pain (monoarthralgia) in the neck and elevated acute phase reactants/ESR). Interestingly, this case displayed two of the most common features of ARF (arthralgia and fever) early on in its course.2 8 However, diagnosis of ARF using minor criteria is not recommended and is of limited diagnostic value since these features occur in other diseases.2 10 While this case could be classified as poststreptococcal reactive arthritis (when Jones criteria are not satisfied and/or there is no response to anti-inflammatory/antipyretics),1 10–12 such cases have been noted to develop into ARF at times and may represent a mild or early form of the disease.11 Additionally, liver dysfunction (elevated liver enzymes) may have obscured the diagnosis.13 14
Jones criteria for ARF diagnosis in our patient were not met until presentation of a second major Jones criteria, and a positive ASO titre >1 month following initial presentation. The final diagnosis was made based on two major and three minor modified 2015 Jones criteria. The major Jones criteria were polyarthritis/polyarthralgia (16d) and erythema marginatum (41d), an extremely rare symptom. The three minor criteria were fever, monoarthralgia (joint pain in the neck) and elevated acute phase reactants/ESR. It was also important to note that our patient travelled to a country with high incidence of ARF.
ARF cases are expected to be more frequent with increasing rate of travel to regions with high incidence of disease, and appropriate management is required to prevent significant morbidity and mortality.15 Dr Jones on page 482 of his 1944 paper clearly summarises what should be done with findings such as those in our case: ‘In my experience transient mild polyarthritis, without other diagnostic features suggestive of rheumatic fever or some other medical condition, rarely proves to be a problem of serious import. One must accept, I feel, one exception to this. Knowledge of the epidemiologic disease pattern of the community in which the disease develops may present strong presumptive evidence of rheumatic fever. For instance, if the patient has been exposed to a known beta-hemolytic streptococcus or scarlet fever epidemic, any joint symptoms become more significant. If the patient has had tonsillitis, pharyngitis or even a cold in the past two or three weeks, and sérologie tests (such as anti-streptolysin determination) indicate a recent hemolytic streptococcus infection, the burden of proof rests with the physician who would not interpret such a syndrome as rheumatic fever, since this represents the usual epidemiologic pattern of-the disease.’3
We suggest that clinicians consider atypical ARF in adults from high-risk populations (history of travel to a developing country), with a fever of unexplained origin (≥38.0°C), arthralgia, elevated ESR or CRP and positive ASO titre or similar streptococcal diagnostic test.
Case presentation
On 10 December 2016 (set as 0d), a 25-year-old woman returned to North America from her school in the Caribbean. On the night of her return, she developed an unabating fever, dry cough and joint pain (monoarthralgia) in her neck, which persisted for nearly 2 weeks. This prompted her to seek consultation with her family physician 2 days later. A blood workup for Zika and Dengue serology (occasional viral epidemics in the Caribbean), blood culture and malaria parasite blood smear were ordered, all of which yielded negative results. Subsequently, she went to the emergency department on 23 December 2016 (13d). Her temperature was 38.4°C (101.1°F) with blood pressure, pulse, respiration and oxygen saturation within normal limits. Blood work showed an elevated white cell count (WCC), elevated ESR and acute phase reactants (CRP, ferritin) and evidence of liver dysfunction (elevated aspartate aminotransferase (AST), alanine transaminase (ALT), gamma-glutamyl transferase (GGT) and lactate acid dehydrogenase) (table 1).
Table 1.
Investigations performed in North America (2016–2017). Abnormal values are in bold. Days postinitial presentation (10 December 2016; set as 0d) are also indicated
| Investigation | Results | ||
| 23 Dec 2016 (13d) | 5 Jan 2017 (26d) |
Normal range | |
| AST (U/L) | 216 | 43 | <35 |
| ALT (U/L) | 262 | 34 | <35 |
| GGT (U/L) | 51 | 69 | <43 |
| LDH (U/L) | 466 | 115–230 | |
| Hb (g/L) | 118 | 107 | 115–155 |
| WCC (×109/μL) | 4.7 | 14.8 | 4–11 |
| Platelets (×109/L) | 159 | 273 | 150–400 |
| Ferritin (ng/mL) | 4144 | 5–148 | |
| CRP (mg/L) | 194.6 | 339.3 | 0–3 |
| ESR (mm/hour) | 0–20 | ||
| b-hCG (IU/L) | <1 | <2 | |
| Bilirubin (total) (μmol/L) | 7 | 7 | <17 |
| Alkaline phosphatase (U/L) | 75 | 86 | 35–120 |
| TSH (mU/L) | 1.16 | 1.63 | 0.32–5.04 |
| Glucose (random) (mmol/L) | 8.6 | 3.3–11 | |
| Sodium (mmol/L) | 139 | 134 | 135–148 |
| Potassium (mmol/L) | 4.3 | 3.8 | 3.6–4.7 |
| Creatinine (μmol/L) | 82 | 83 | 45–90 |
| eGFR (mL/min) | 86 | 84 | >60 |
| Hepatitis B (core Ab and surface Ag) | NEG | ||
| Hepatitis C (Ab) | NEG | ||
| Zika | NEG | ||
| Dengue | NEG | ||
| T reponema p allidum | NEG | ||
| Malaria | NEG | ||
| HIV 1 and 2 | NEG | ||
| Blood culture | NEG | ||
| Chest X-ray | NORM | ||
| Urinalysis | NORM | ||
| Urine culture/MSU (CFU/L) | <104 | ||
| Skin flora (CFU/L) | <10 M | ||
| ANA (U) | 0.2 | <1.0 | |
| C-citrullinated peptide (U) | 5.6 | <20 | |
Ab, antibody; Ag, antigen; ANA, antinuclear antibodies; CFU, colony-forming unit; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hb, haemoglobin; MSU, midstream specimen of urine; NEG, negative; NORM, normal; WCC, white cell count.
She was diagnosed with a viral infection and instructed to take Tylenol and Aleve. Three days later (26 December 2017; 16d), she developed upper and lower limb oedema, lethargy, anorexia and polyarthralgia especially at the end of range of motion in her shoulders, elbows, wrists, knees and ankles, metacarpophalangeal joints, metatarsophalangeal joints and proximal interphalangeal joints. Inflammatory polyarthritis affected her knees, metatarsophalangeal joints, wrists and metacarpophalangeal/proximal interphalangeal joints (figure 1). There was clear evidence of synovitis in her wrists and metacarpophalangeal/metatarsophalangeal joints (figure 2). The patient showed tenderness at the lateral margin of the right knee and her left knee was palpably hot with joint effusion. Sexual history and investigations for sexually transmitted infections were not significant.
Figure 1.
The patient presented with pitting, peripheral oedema in her feet at 6 days (26 December 2016) following initial symptoms (10 December 2016). This feature is typically not present in acute rheumatic fever.
Figure 2.
The patient presented with synovitis (metacarpophalangeal/proximal intertarsalphalangeal joints), a major Jones criteria, at 6 days (26 December 2016) following initial symptoms (10 December 2016).
The patient initially presented atypically with three minor Jones criteria for ARF (fever, joint pain (monoarthralgia) in her neck and elevated (CRP, 13d)). She was only diagnosed with ARF after delayed presentation of two major Jones criteria: polyarthritis/polyarthralgia (16d) and erythema marginatum (41d). The delayed ASO titre (44d) assisted in the final diagnosis, providing evidence of prior streptococcal infection.
Investigations
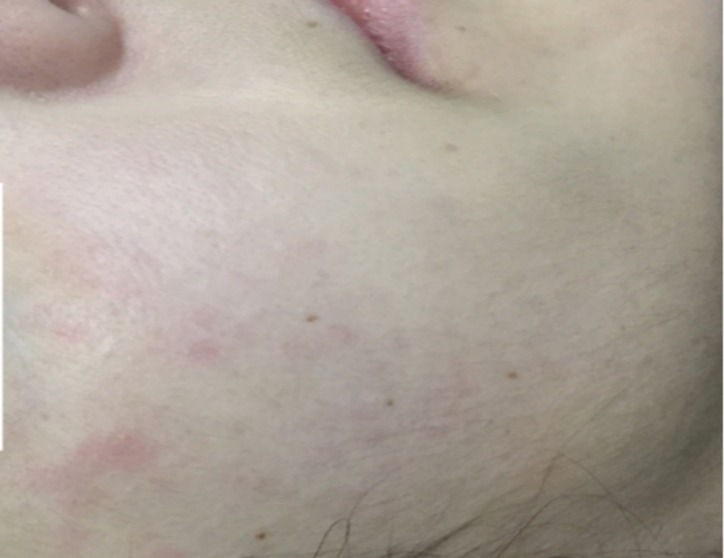
The patient returned to her Caribbean medical school 3 weeks after the onset of her symptoms with no significant improvement while taking prescribed Tylenol and Aleve. She then came to the University Health Centre with the same symptoms (13 January 2017; 34d). Laboratory tests were ordered (table 2), and a follow-up appointment was scheduled. On physical examination 41 days following the onset of symptoms (20 January 2017), the patient had pallor and peripheral oedema in upper and lower limbs up to the elbows and knees, respectively (figure 1). Peripheral lymphadenopathy, bilateral parotid swelling, joint swelling and joint tenderness were also noted in upper and lower limbs. An erythematous, macular, non-pruritic and blanching rash was seen on the left forearm, torso, legs and face (to a lesser extent) (figures 3 and 4). The rash was not raised, bumpy or episodic nor was it similar to a dermatitis or drug-induced reaction. It was accentuated with heat.
Table 2.
Investigations performed in the Caribbean (2017). Abnormal values are in bold. Days postinitial presentation (10 December 2016; set as 0d) are also indicated
| Investigation | Results | |||||||
| 13 January (34d) | 23 January (44d) | 9 February (61d) | 21 February (73d) | 8 March (89d) | 30 March (111d) | 2 May (114d) | Normal range | |
| AST (U/L) | 118 | 135.6 | 50 | 37.4 | 28.5 | 34.9 | 34.9 | <35 |
| ALT (U/L) | 78.1 | 158.5 | 118.7 | 51.5 | 28.2 | 32.4 | 23.8 | <35 |
| Hb (g/L) | 85 | 81 | 89 | 98 | 108 | 115 | 122 | 115–155 |
| WCC (×109/µL) | 18.4 | 15.3 | 19.8 | 8.9 | 8.2 | 4.8 | 4 .l | 4–11 |
| Platelets (×109/L) | 503 | 523 | 409 | 302 | 263 | 292 | 214 | 150–400 |
| Ferritin (ng/mL) | >1500 | >1500 | 751 | 400 | 13 | 5–148 | ||
| CRP (mg/L) | >100 | 49.8 | 4.85 | 0–3 | ||||
| ESR (mm/hour) | 84 | 47 | 52 | 35 | 0–20 | |||
| Bilirubin (total) (mg/dL) | 0.81 | 0.7 | 0.76 | 0.2–1.2 | ||||
| Alkaline phosphatase (U/L) | 245 | 193.1 | 192.5 | 35–120 | ||||
| Sodium (mmol/L) | 134.3 | 141.1 | 140.3 | 135–148 | ||||
| Potassium (mmol/L) | 4.22 | 5.18 | 3.65 | 3.6–4.7 | ||||
| Creatinine (mg/dL) | 0.7 | 0.9 | 0.6–1.1 | |||||
| ASO titre (IU/mL) | 800 | 400 | 400 | <200 | ||||
| Throat swab/culture | NEG | |||||||
| Urinalysis | NORM | |||||||
| TB PPD | NEG | |||||||
| Mononucleosis | NEG | |||||||
| 2D TT echocardiography | NORM | |||||||
| Rheumatoid factor | NEG | NEG | ||||||
| Abdominal and pelvic ultrasound | NORM | |||||||
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hb, haemoglobin; NEG, negative; NORM, normal; TB PPD, tuberculosis purified protein derivative test; WCC, white cell count.
Figure 3.
The patient presented with erythematous, macular, blanching, non-pruritic rash on her face, a major Jones criteria, at 41 days (20 January 2017) following initial symptoms (10 December 2016). (Parotid swelling is not observable in this image.)
Figure 4.
The patient presented with erythematous, macular, blanching, non-pruritic rash on her posterior torso, a major Jones criteria at 41 days (20 January 2017) following initial symptoms (10 December 2016).
Cardiovascular findings, specifically the other Jones criteria pertaining to carditis and prolonged PR interval, were unremarkable. ARF presents with rheumatic heart disease 50%–78% of the time1; however, electrocardiography and echocardiography showed no abnormality in our patient.
Based on the patient’s arthralgia, fever and oedema, further laboratory tests were performed. On her first three visits to the University Health Centre in the Caribbean, WCC and ESR were markedly elevated as were acute phase reactants (CRP and ferritin, table 2). The ESR and acute phase reactants indicated an inflammatory response, and along with the polyarthralgia and polyarthritis, suggested ARF. However, at this time throat swab culture was negative for streptococcal infection (table 2). This is not remarkable as only 25% of patients have a positive throat culture test at the time of diagnosis 10+ days later.10 The delayed presentation of the rare but specific major Jones criterion erythema marginatum on 20 January 2017 (41d),1 further confirmed the ARF diagnosis. At this point in time, the patient presented with two major and three minor Jones criteria.
The diagnosis was delayed by several weeks because of a lack of sufficient modified 2015 Jones criteria diagnostic evidence in the early stages of her illness. A confirmed diagnosis of ARF was made following significantly elevated ASO titres (23 January 2017; 44d; table 2), a serological marker of prior streptococcal infection; and the patient was prescribed penicillin and corticosteroids. The elevated laboratory values declined over subsequent weeks following the initiation of treatment on 26 January 2017 (47d) (table 2). Follow-up examinations showed remarkable improvements in clinical features. The patient’s peripheral oedema, joint swelling and pain, erythema marginatum and fever resolved while lab values such as ESR, CRP, ferritin, AST, ALT and WBC decreased within 3 months of treatment (table 2). Haemoglobin levels also increased (table 2). On an 8 February 2017 (60d) follow-up visit, the patient’s rash was no longer present. She was asymptomatic for ARF on repeat follow-up several months later.
Due to her atypical ARF presentation, the patient was confidently diagnosed with ARF. She finally met complete modified 2015 high-risk population Jones criteria with two major features (16 and 41d), three minor features (0 to 13d) and a positive ASO titre (44d) indicating prior streptococcal infection.
Differential diagnosis
The differential diagnoses considered in the history, physical examination and investigation of this patient included systemic viral infections, sexually transmitted infections, Reiter’s disease/reactive arthritis, systemic lupus erythematosus (SLE), rheumatoid arthritis, septic arthritis and Still’s disease. Note that blood cultures, rheumatoid factor, cyclic citrullinated peptide antibodies and antinuclear antibodies were negative in this case. This thus decreased the likelihood of diagnosis for diffuse septic arthritis, rheumatoid arthritis or SLE.
Unremarkable sexual history investigations and lack of bowel and urinary irregularities made reactive arthritis unlikely.
Still’s disease presents with a spiking fever (>39°C), polyarthralgia, sore throat, generalised lymphadenopathy, hepatosplenomegaly and serositis.16 17 Furthermore, a salmon-coloured, evanescent, shortlived papular rash is typically found on the chest and thighs during each febrile episode.17 Jones indicated that there may be confusion of ARF with Still’s disease.3 While interleukin 18 levels may be of value in differentiating Still’s disease from ARF,16 18 this differential diagnosis was ruled out based on the following:
The fever was low grade (38.4°C) and unabating, not spiking or episodic (quotidian or double quotidian).
There was a lack of organomegaly, pharyngitis and abdominal symptoms.
Erythema marginatum, as seen in this patient was macular (figure 3) and not raised, bumpy or episodic as in Still’s disease. Furthermore, this major Jones criterion is extremely rare and highly specific for rheumatic fever.1
Lastly and importantly, our patient met complete Jones criteria (two major features, three minor features and positive ASO titre) and thus satisfied all minimum ARF diagnostic requirements (1. evidence of prior streptococcal infection (ASO titre) and 2. Either two major features or one major and two minor features).
Usually, patients with arthritis of the small joints of the hand and fever non-responsive to anti-inflammatory drugs are diagnosed with poststreptococcal reactive arthritis.19 However, some patients do go on to develop ARF, suggesting that they originally had ARF rather than poststreptococcal reactive arthritis.19 This is likely the situation in our case as our patient eventually met complete Jones.
A more detailed list of differential diagnoses for ARF symptoms can be found in table 3 which has been modified from Gewitz et al 1 and Webb et al.20
Table 3.
Differential diagnoses of ARF1 20
| Arthritis | Carditis | Chorea |
| Septic arthritis | Mitral regurgitation | Drug intoxication |
| Connective tissue and other autoimmune diseases (eg, systemic lupus erythematosus, systemic vasculitis, psoriatic arthritis, juvenile idiopathic arthritis) | Mitral valve prolapse | Wilson disease |
| Viral arthropathy (eg, hepatitis, rubella, parvovirus, Epstein-Barr virus, cytomegalovirus, Lyme disease) | Myxomatous mitral valve | Tic disorder and Tourette syndrome |
| Reactive arthritis | Fibroelastoma | Choreoathetoid cerebral palsy |
| Lyme disease | Congenital mitral valve disease | Encephalitis |
| Sickle cell anaemia | Congenital aortic valve disease | Familial chorea |
| Infective endocarditis | Infective endocarditis | Intracranial tumour |
| Leukaemia or lymphoma | Cardiomyopathy | Lyme disease |
| Gout and pseudogout | Myocarditis | Hormonal |
| Poststreptococcal reactive arthritis | Kawasaki disease | Metabolic (eg, Lesch-Nyhan, hyperalaninemia, ataxia telangiectasia) |
| Henoch-Schoenlein purpura | Pericarditis | Antiphospholipid antibody syndrome |
| Still’s disease | Autoimmune (eg, systemic lupus erythematosus), vasculitis, NMDA receptor antibody encephalitis) | |
| Sarcoidosis | ||
| Hormonal (eg, hyperthyroidism, pregnancy, oral contraceptives) | ||
| Wilson disease | ||
| Drugs and toxins |
ARF, acute rheumatic fever; NMDA, N-methyl-D-aspartate receptor antibody.
Treatment
Initially, the patient used over-the-counter Tylenol and Aleve for pain and fever. However, based on the delayed diagnosis of ARF, the patient was later administered penicillin. On 26 January 2017, Penicillin G 1.6 million units intramuscular was administered and changed to Penicillin V 250 mg two times per day on 13 February 2017. Prednisone 60 mg/day orally was also prescribed and tapered by 5 mg every 3 days. Salicylates were not used throughout the course of treatment.
Outcome and follow-up
Two weeks following commencement of therapy on 26 January 2017 (47d) the patient’s symptoms, signs and acute phase reactants started to regress (table 2). By week 9 (30 March 2017; 111d), ESR decreased by 58%, CRP decreased by 95%, AST decreased by 74%, ALT decreased by 80%, ferritin decreased by 99% and WCC returned within normal limits (table 2). The patient recovered without any further complications over the course of several months.
Discussion
ARF is thought to arise due to a type II hypersensitivity cross-reaction between streptococcal and host antigens 2–3 weeks following a group A beta-haemolytic streptococcal (GAS) infection, and can affect the heart, joints and brain.4 It is clinically diagnosed via symptoms matching a combination of major and minor 2015 revised Jones criteria1 (table 4). Untreated ARF can develop into rheumatic heart disease and thus a treatable disease transforms into a disease with long-term morbidity and mortality.
Table 4.
Revised 2015 Jones Criteria for diagnosing ARF1 5 32
| A. For all patient populations with evidence of preceding group A streptococcal (GAS) infection | |
| Diagnosis: initial ARF | 2 Major manifestations; or 1 major plus 2 minor manifestations |
| Diagnosis: recurrent ARF | 2 Major; or 1 major and 2 minor; or 3 minor manifestations |
| B. Major criteria | |
| Low-risk populations* | Moderate-risk and high-risk populations |
| Carditis (clinical or subclinical†) | Carditis (clinical or subclinical†) |
| Arthritis—polyarthritis only (35%–88%) | Arthritis—monarthritis or polyarthritis, and/or polyarthralgia |
| Chorea (2%–30%) | Chorea |
| Erythema marginatum (<6%) | Erythema marginatum |
| Subcutaneous nodules (1%–13%) | Subcutaneous nodules |
| C. Minor criteria | |
| Low-risk population* | Moderate-risk and high-risk populations |
| Polyarthralgia (75%) | Monoarthralgia |
| Fever (≥38.5°C) (>90%) | Fever (≥38.0°C) |
| ESR ≥60 mm/hour and/or CRP ≥3.0 mg/dL | ESR ≥30 mm/hour and/or CRP ≥3.0 mg/dL |
| Carditis—prolonged PR interval after accounting for age variability (only if carditis is NOT considered a major criterion) | Carditis—prolonged PR interval after accounting for age variability (only if carditis is NOT considered a major criterion) |
*Low-risk population are those with incidence ≤2 per 100 000 school-aged children or all-age rheumatic heart disease prevalence ≤1 per 100 000 per year.
†Subclinical carditis: seen only on echocardiography without auscultatory findings.
ARF, acute rheumatic fever; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
Our patient was a challenging case which was initially thought to be poststreptococcal reactive arthritis due to polyarthralgia, fever and non-responsiveness to antipyretics. Initially, the patient did not meet the 2015 Jones criteria, even for high-risk populations, manifesting only three minor Jones features. ARF was only considered approximately 1 month after initial onset of fever, with manifestation of two major Jones criteria polyarthritis/polyarthralgia (16d) and erythema marginatum (41d) (figures 2–4). ARF is a clinical diagnosis which in this case was aided by knowledge of epidemiology. The patient had recently travelled to Grenada, a country with high incidence of ARF.6 Since ARF resembles other diseases, evidence of a preceding streptococcal (GAS) infection was needed.1 There was only late confirmation of recent streptococcal infection as a result of positive ASO titres (44d). There was an absence of active bacterial infection in the throat swab cultured at the same time, but an absence of positive throat swab culture has been previously documented.4 21 This is not remarkable as only 25% of patients have a positive throat culture test at the time of diagnosis 10+ days later.10 Indeed, the ASO titre may persist for 3 months after subsidence of the causal infection.22 The patient thus had an atypical ARF presentation and only satisfied the Jones criteria for ARF 44 days after initial onset of symptoms, with two major and three minor criteria in the presence of a positive ASO titere.
There were several factors which made our case atypical ARF. Latency and initial lack of some features made the diagnosis difficult. However, such variability in typical Jones criteria in both adult ARF and high-risk populations had previously been described. For over 70 years, ARF was well known to present atypically in adults based on several published studies of >1500 people including military personnel.4 8 9 23 However, and importantly for an atypical ARF presentation, arthralgia, carditis, elevated ESR and low-grade persistent fever were the most common symptoms.4 8 9 19 23–26 It has been suggested that arthralgia and low-grade fever may represent a milder form of rheumatic fever8; and it was further suggested to make exceptions for such presentation of minor Jones criteria, especially when rheumatic fever is endemic in the population.3 Indeed, ARF used to be associated with fevers of unknown origin in the past.13 Lastly, our patient did not present with carditis. Carditis has been suggested to result from overstimulation of the vagal nerve endings by the rheumatic toxin.27 However, the age of onset may affect ARF severity as fewer adults (vs children) have a first attack of polyarthritis or arthralgia that later develops into carditis.28 The latency of symptoms (1–4 weeks) and low-grade fever for weeks to months has been previously described.21
We should note that diagnosis of the initial ARF attack using minor criteria such as monoarthralgia and fever is not recommended and remains of limited diagnostic value since they occur in other diseases.10 It is interesting to note that confirmation of prior streptococcal infection was previously a minor Jones criterion but had been removed in later revisions, affecting diagnostic sensitivity.26 However, we suggest that ARF should be considered with minor Jones criteria (monoarthralgia, low-grade fever of unknown origin and elevated ESR/CRP/acute phase reactants), especially with a history of travel to a developing nation and confirmed pre-existing streptococcal infection.
Our specific case had other interesting non-ARF features that made for a challenging diagnosis: peripheral oedema, liver dysfunction, parotid swelling, lymphadenopathy and lack of response to antipyretics. At first, the case appears to be more similar to poststreptococcal reactive arthritis, as in contrast to ARF, joint pain is unresponsive to antipyretics.11 12 If arthralgia is non-responsive to anti-inflammatory medications within 48 hours, the ARF diagnosis should be questioned.2 However, such poor response to salicylates in some ARF cases has been previously described.21 Our patient showed additional features of upper and lower limb oedema (figure 1) and bilateral parotid swelling. These later symptoms are not typically associated with ARF.1 Indeed, we found one report in which oedema was present in an atypical ARF case (5 days subsequent to onset of symptoms).29 Additionally, there was associated liver dysfunction. Elevated liver enzymes, suggestive of liver damage, have been noted in other ARF cases and obscured initial diagnoses.14 15 30 It is also important to note that renal function appears to be intact as creatinine and eGFR were found to be normal on multiple occasions, ruling out renal disease including poststreptococcal glomerulonephritis. Prior studies have suggested the existence of an initial and undiagnosed infection that predisposed the patient to ARF.23 This may have additionally complicated the diagnosis of our patient and masked the Jones criteria. Thus, we affirm that this was a challenging case.
Improvements in surveillance are supported by discussions regarding prevention, control and elimination of ARF at the 2018 71st WHO assembly, which acknowledged case detection, among others, as a barrier to progress and an area of future research interest.31 Atypical ARF presentation in adults is of importance because missed early diagnosis results in an increased potential for recurrence and severe, chronic, debilitating cardiovascular complications. These complications can be easily prevented with treatment once early diagnosis is made. Luckily, our patient eventually presented with complete Jones criteria and was able to be treated.
While diagnosis of the initial ARF attack using minor criteria such as monoarthralgia, fever and elevated acute phase reactants is not recommended as they are of limited diagnostic value due to similarities in other diseases,10 specific factors including travel to high-risk countries and prior streptococcal infection should be considered. In 1944, Jones advised that caution should be used when dismissing an ARF diagnosis in cases with only minor criteria of arthralgia and fever in areas with high incidence of ARF.3 This was echoed in a later statement: “In populations with high rates of rheumatic fever, where the consequences of missed diagnosis may outweigh those of over diagnosis, the positive predictive value of less stringent criteria for rheumatic fever diagnosis may be acceptable”.4 Thus, ARF should be considered with minor Jones criteria (arthralgia, low-grade fever of unknown origin, elevated ESR/CRP), history of travel to a developing nation and confirmed pre-existing streptococcal infection.
Patient’s perspective.
When I first arrived back home for winter break and started experiencing nightly fevers, I assumed I had caught an infection that would pass in a few days. I was seemingly healthy, and apart from being exhausted from the preceding school term I had just finished, nothing else felt wrong with me. However, nightly fevers turned into violent temperature swings that presented each evening like clockwork: I would become freezing cold to the point where I was in immense pain, then a high fever would follow. I soon developed a forceful cough that left my chest and abdomen extremely sore and made it impossible to sleep through the night. When these symptoms quickly worsened, I started to worry that something was seriously wrong.
I began to notice that I had become very weak. I was not able to lift household items any longer, and a hot rash had developed on my trunk and limbs. I was devoid of energy, and my entire body ached. It progressed to the point that I couldn’t even lift my own arms to brush my hair, or turn my wrist to open door handles. Simply walking left me in pain. If I attempted to attend family outings, I was rushed home early in the evening as the (now routine) wave of feeling freezing cold would hit me, and my nightly routine would begin. When I looked down to my aching legs one night, I did not recognise my body. I had swelled up so much that I could no longer demarcate my feet from my legs. My socks couldn’t even feet over my feet, and I finally broke down. I felt helpless, as if my entire body had failed me. I tried everything to relieve the symptoms—topical hydrocortisol cream, Tylenol, ibuprofen, muscle relaxants—with no success. I was angry and frustrated that doctor’s in my hometown were unable to provide any explanation for what could possibly be happening. I had frequented the clinic, labs and hospital so much at this point that when I laid in the hospital bed waiting for answers, I felt like a lost cause.
When I arrived back to school in Grenada, against doctor’s recommendations, my condition had continued to diminish, and I felt afraid. I sought medical help at my school health clinic, while attempting to push through my studies and rigorous academic schedule. I was unable to focus or concentrate, nor attend the hours of lecture I had previously sat through with ease. When I was not at the clinic, my days were reduced to studying from my bed and sleeping majority of the day. I was completely dependent on others around me for the simplest tasks. I felt fragile and debilitated. I no longer had an appetite and felt as if I was fighting a constant uphill battle. I started to become doubtful that the cause of my condition would ever be found.
Being under the care of my doctor’s in Grenada brought me a level of comfort and reassurance through an otherwise tumultuous time. Despite being puzzled themselves on the cause of my condition, their willingness to search and exhaust every avenue gave me hope and made me more confident that I could get better. Despite feeling my absolute worst, I surged forward with my academic responsibilities while working with the doctors to find any possible solution. When my doctors reported that they had finally determined the cause of my illness, I felt a bittersweet sense of relief, as the previous months had left me very weak, both mentally and physically, and I knew the road to recovery would be long and arduous. Being compliant with daily medications became a priority that, admittedly, was not an easy process, but I was eager to start feeling better. The medications made me nauseous and irritable, and it became a part of my day that I dreaded. Although recovering was a very slow process (one that I would endure for many months afterwards), I gradually started to feel better as my symptoms subsided. I could move and walk with more ease, and I slowly started to regain the strength I had lost. Over the proceeding months I was able to look and feel more myself.
Suffering through a traumatic illness can be debilitating and effect an individual for the remainder of their lives. As chaotic, frightening and taxing as this experience was (both physically and mentally), I was able to endure the pain and suffering with an inner strength and confidence that I undoubtedly owe to my family, friends, and team of doctors that provided the utmost support and care throughout my experience.
Learning points.
The clinical picture of acute rheumatic fever (ARF) may be clouded by atypical features or underlying conditions in adults. Clinicians, especially family physicians and emergency physicians, should suspect ARF in cases of fever of unknown origin along with minor Jones criteria and a history of travel to a developing country (high-risk ARF population).
When presented with a case of persistent fever of unknown origin in an adult, especially if they have visited developing nations, clinicians should consider including screening for recent streptococcal infection (ASO titre and/or antideoxyribonuclease-B).
Though several attempts have been made to revise the Jones criteria for ARF diagnosis, ARF presentation is variable and there have been several cases of atypical or delayed presentation of Jones criteria. Thus, it should be acknowledged that the Jones criteria may not be sensitive enough to identify atypical cases. ‘In populations with high rates of rheumatic fever, where the consequences of missed diagnosis may outweigh those of over diagnosis, the positive predictive value of less stringent criteria for rheumatic fever diagnosis may be acceptable.’4
Footnotes
Contributors: JAS: identified the case, ordered tests, interpreted results, wrote and edited the paper; SJB: analyzed data, wrote and edited the paper, IVJM: initiated the project, analyzed data, wrote and edited the paper. SJB, JAS and IVJM composed, reviewed, researched, edited and approved this manuscript. JAS was the attending physician overseeing this case.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Gewitz MH, Baltimore RS, Tani LY, et al. Revision of the Jones criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography a scientific statement from the American heart association. Circulation 2015;131:1806–18. [DOI] [PubMed] [Google Scholar]
- 2. Sika-Paotonu D, Beaton A, Raghu A, et al. Acute Rheumatic Fever and Rheumatic Heart Disease In Streptococcus pyogenes: Basic Biology to Clinical Manifestations. Oklahoma City: University of Oklahoma Health Sciences Center, 2017. [Google Scholar]
- 3. Jones TD. The diagnosis of rheumatic fever. J Am Med Assoc 1944;126:481–4. 10.1001/jama.1944.02850430015005 [DOI] [Google Scholar]
- 4. Carapetis JR, Currie BJ. Rheumatic fever in a high incidence population: the importance of monoarthritis and low grade fever. Arch Dis Child 2001;85:223–7. 10.1136/adc.85.3.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beaton A, Carapetis J. The 2015 revision of the Jones criteria for the diagnosis of acute rheumatic fever: implications for practice in low-income and middle-income countries. Heart Asia 2015;7:7–11. 10.1136/heartasia-2015-010648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ayoub EM, Nelson B, Shulman ST, et al. Group A streptococcal antibodies in subjects with or without rheumatic fever in areas with high or low incidences of rheumatic fever. Clinical and Vaccine Immunology 2003;10:886–90. 10.1128/CDLI.10.5.886-890.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Watkins DA, Johnson CO, Colquhoun SM, et al. Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med 2017;377:713–22. 10.1056/NEJMoa1603693 [DOI] [PubMed] [Google Scholar]
- 8. Rosenberg DH. The clinical aspects of rheumatic fever in adults. N Engl J Med 1946;234:148–52. 10.1056/NEJM194601312340503 [DOI] [PubMed] [Google Scholar]
- 9. Ferguson C. Acute (febrile) polyarthritis in the army.. Can MAJ 1943;49:492–6. [PMC free article] [PubMed] [Google Scholar]
- 10. Dajani A, Ayoub E, Bierman F. Guidelines for the diagnosis of rheumatic fever: Jones criteria, updated 1992. JAMA 2011. [PubMed] [Google Scholar]
- 11. Albritton W, Boucher F, Delage G, et al. Poststreptococcal arthritis. Can J Infect Dis 1995;6:133–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maness DL, Martin M, Mitchell G. Poststreptococcal illness: recognition and management. Am Fam Physician 2018;97:517–22. [PubMed] [Google Scholar]
- 13. Roth AR, Basello GM. Approach to the adult patient with fever of unknown origin. Am Fam Physician 2003;68:2223–8. [PubMed] [Google Scholar]
- 14. Rasa M, Garcia A, Yamaga K, et al. Hematologic and hepatic anomalies in pediatric acute rheumatic fever. Ann Pediatr Res 2018;2:1016 http://www.remedypublications.com/annals-of-pediatric-research/articles/pdfs_folder/apr-v2-id1016.pdf [Google Scholar]
- 15. Catanchin A, O-Brien DP, Athan E. Acute rheumatic fever: an unusual cause of fever in a returned traveler. J Travel Med 2006;12:353–5. 10.2310/7060.2005.12611 [DOI] [PubMed] [Google Scholar]
- 16. Inoue N, Shimizu M, Tsunoda S, et al. Cytokine profile in adult-onset still's disease: comparison with systemic juvenile idiopathic arthritis. Clinical Immunology 2016;169:8–13. 10.1016/j.clim.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 17. Giacomelli R, Ruscitti P, Shoenfeld Y. A comprehensive review on adult onset still's disease. J Autoimmun 2018;93:24–36. 10.1016/j.jaut.2018.07.018 [DOI] [PubMed] [Google Scholar]
- 18. Wakiguchi H, Okazaki F, Suzuki Y, et al. Acute rheumatic fever associated with Tenosynovitis and a unique cytokine profile. Immunological Medicine 2018;41:43–5. 10.1080/09114300.2018.1451617 [DOI] [PubMed] [Google Scholar]
- 19. Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. The Lancet 2005;366:155–68. 10.1016/S0140-6736(05)66874-2 [DOI] [PubMed] [Google Scholar]
- 20. Webb RH, Grant C, Harnden A. Acute rheumatic fever. BMJ 2015;351:h3443–8. 10.1136/bmj.h3443 [DOI] [PubMed] [Google Scholar]
- 21. Griffith G. Rheumatic fever: its recognition and treatment. JAMA 1947. [DOI] [PubMed] [Google Scholar]
- 22. Stuart RD. Anti-Streptolysin titres of human sera in health and in various streptococcal infections. J Hyg 1936;36:26–36. 10.1017/S0022172400043394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Copeman WSC. Observations on the natural history of acute rheumatic fever. Ann Rheum Dis 1944;4:11–21. 10.1136/ard.4.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sokolow M, Snell AM. Atypical features of rheumatic fever in young adults. J Am Med Assoc 1947;133:981–9. 10.1001/jama.1947.02880140011003 [DOI] [PubMed] [Google Scholar]
- 25. Reynell PC. Atypical rheumatic fever in young adults. Ann Rheum Dis 1948;7:221–4. 10.1136/ard.7.4.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khriesat I, Najada AH. Acute rheumatic fever without early carditis: an atypical clinical presentation. Eur J Pediatr 2003;162:868–71. 10.1007/s00431-003-1320-x [DOI] [PubMed] [Google Scholar]
- 27. Perry CB. Review of the literature on acute rheumatism during the years 1939-1945. Ann Rheum Dis 1947;6:162–83. 10.1136/ard.6.3.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DeLiee E, Dodge K, McEwen C. The prognostic significance of age at onset in initial attacks of rheumatic fever. Am Heart J 1943. [Google Scholar]
- 29. Ilgenfritz S, Dowlatshahi C, Salkind A. Acute rheumatic fever: case report and review for emergency physicians. J Emerg Med 2013;45:e103–6. 10.1016/j.jemermed.2013.04.037 [DOI] [PubMed] [Google Scholar]
- 30. Nickeson RW, Brewer EJ, Ferry GD. Diagnosis of rheumatic fever obscured by liver disease. J Rheumatol 1981;8:138–40. [PubMed] [Google Scholar]
- 31. Organization WH Rheumatic fever and rheumatic heart disease: report by the director General. World Heal. Assem 2018;71 http://apps.who.int/gb/ebwha/pdf_files/WHA71/A71_25-en.pdf?ua=1 [Google Scholar]
- 32. Zühlke LJ, Beaton A, Engel ME, et al. Group A Streptococcus, acute rheumatic fever and rheumatic heart disease: epidemiology and clinical considerations. Curr Treat Options Cardiovasc Med 2017;19 10.1007/s11936-017-0513-y [DOI] [PMC free article] [PubMed] [Google Scholar]