Abstract
Colistin-induced nephrotoxicity is commonly associated with elevation of serum creatinine level or a reduction of urine output. Uncommonly, tubulopathy associated with colistin has been reported. Here we present a unique case of a 46-year-old man who developed polyuria, hypokalaemia, hypocalcaemia, hypomagnesemia and metabolic alkalosis after 3 days of therapy with intravenous colistimethate sodium. After ruling out other causes, a diagnosis of colistin-induced acquired Bartter syndrome was made. The patient required daily aggressive intravenous repletion of fluids and electrolytes. However, polyuria and metabolic abnormalities abated only after drug discontinuation.
Keywords: infections, drugs: infectious diseases, fluid electrolyte and acid-base disturbances
Background
Colistin is one of the few available agents to treat multidrug-resistant gram-negative infections which is often used as a last available resort.1 Nephrotoxicity has been the most worrying side effect of the medication which was why colistin was discontinued in the 1970s, shortly after being introduced.2 With limited alternatives and recent studies showing lower incidence of nephrotoxicity associated with use of more purified colistin (colistimethate instead of colistin sulfate), it has re-emerged as an important antimicrobial agent in a select group of patient.2–4 Most studies have demonstrated colistin-induced nephrotoxicity as acute kidney injury or acute tubular necrosis and have described it in the form of increasing creatinine, decreasing glomerular filtration rate or need to undergo hemodialysis.2 5 6 The mechanism of nephrotoxicity is via an increase in tubular epithelial cell permeability, which results in cation–anion and water influx leading to cell swelling and cell lysis. Risk factors of colistin nephrotoxicity include duration and dose of the antibiotic, co-administration of other nephrotoxic drugs, and the patient-related factors such as age, hypoalbuminemia, hyperbilirubinemia, underlying disease and severity of illness.1 We report a unique case of an acquired Bartter-like syndrome (BLS) after 3 days of intravenous colistin therapy in an adult. To the best of our knowledge, only three other similar cases (two in adults, one in a preterm infant) have been described in world literature—making this case rather extraordinary.
Case presentation
A 46-year-old man with a history of paraparesis secondary to TB myeloarachnoiditis (diagnosed outside based on imaging features and cerebrospinal fluid report), stage IV ischial and sacral decubitus ulcer. Presented to our hospital with scrotal ulcer and fever for 7 days with Foley’s catheter in situ. He was taking four-drug antitubercular therapy (ATT) comprising isoniazid, rifampicin, pyrazinamide and ethambutol for the last 45 days.
At the time of admission, the patient’s blood pressure was 120/70 and heart rate was 114 beats per minute; respiratory rate was 24 per minute. The patient had a temperature of 38.2°C and was found to be hyporesponsive (Glasgow Coma Scale score 10/15). A physical examination also identified 2×2.6 cm scrotal ulcer with abscess formation, 5.2×4.8 cm sacral ulcer with foul-smelling drainage and a 3.4×2.8 cm left ischial ulcer. A diagnosis of Fournier’s gangrene and infected bedsore was made. After sending initial cultures, the patient was started on injection teicoplanin and piperacillin–tazobactam. The patient underwent surgical debridement of bedsore and Fournier’s gangrene under general anaesthesia the next day. The postoperative phase was uneventful, and the patient was extubated after 24 hours of surgery. The patient improved consistently, and his surgical sites were healthy. However, 3 days after extubating, the patient developed progressively increasing fever and respiratory distress. His chest X-ray showed new-onset infiltrates in the lower lobe of the left lung. Because of worsening type 1 respiratory failure patient was reintubated, and the antibiotic was upgraded to imipenem. Piperacillin–tazobactam was discontinued. His endotracheal aspirate grew Acinetobacter baumannii complex sensitive only to colistin following which the patient was started on 5 mg/kg daily dose of intravenous colistin, divided into three doses throughout the day and ATT was continued. The patient’s fever resolved, ventilatory parameters improved significantly and the patient was planned for extubation. However, the patient had new-onset hypotension responsive to fluid.
Investigations
His blood investigations revealed serum sodium 128 mEq/L, serum potassium 3.0 mEq/L, serum magnesium 0.74 mEq/L, serum bicarbonate 28 mEq/L, serum calcium 3.89 mEq/L and serum creatinine 0.30 mg/dL. On subsequent days despite daily aggressive electrolyte repletion, the patient was noted to have persistent hypokalaemia, with levels between 2.5 and 3.4 mEq/L, metabolic alkalosis with serum bicarbonate level between 26 and 30 mEq/L, and polyuria with urine output around 4.0–5.0 L daily. Serum creatinine remains within the normal range of 0.25–0.60 mg/dL. The patient did not have any diarrhoea or vomiting. Urine studies showed urine spot potassium 107 mEq/L, urine sodium 91 mEq/L and urine calcium 25 mg/dL, confirming renal wasting of these electrolytes. Urine potassium:creatinine ratio was 6.6 mEq/g. Serum and urine osmolality was 270 mOsm/kg and 360 mOsm/kg, respectively. Urinary transtubular potassium gradient was elevated (value being 27). The urinary calcium:creatinine ratio was 0.83 mg/g. Urine magnesium and chloride concentrations were not done.
Differential diagnosis
The causes of fluid responsive hypotension in a hospitalised critically ill patients are new-onset sepsis, fluid depletion in the form of diarrhoea or blood loss, and right ventricular myocardial infarction.
The differential diagnosis of polyuria is drug-induced diuresis, central or nephrogenic diabetes insipidus, and solute diuresis. Dyselectrolytemia can be caused by drugs like diuretics or other causes of acquired tubulopathy
Treatment
The patient was put on potassium and calcium supplementation, along with antibiotics and ATT.
Outcome and follow-up
In the absence of any other medications, including diuretics, unexplained electrolyte disturbances and polyuria were most likely suggestive of tubulopathy. It appeared to be associated with colistin therapy with resultant acquired BLS. Colistin was discontinued and cefoperazone sulbactam with teicoplanin was continued. After 2 days of stopping colistin, polyuria improved dramatically. His metabolic alkalosis and hypomagnesemia also resolved with partial improvement in hypokalaemia and hypocalcaemia, for which supplementation was continued. His pneumonia resolved; he was tracheotomised and weaned off from a ventilator. After around 2 weeks of resolution of BLS, the patient had new-onset sepsis due to urinary tract infection with disseminated intravascular coagulopathy. Despite an escalation of antibiotics, the patient progressed to refractory septic shock. Despite appropriate treatment for the same, the patient steadily deteriorated and he expired on the 48th day of hospitalisation.
Discussion
Bartter syndrome is a hereditary, renal tubular salt-wasting disorder resulting from defective sodium chloride reabsorption in the medullary thick ascending loop of the loop of Henle and is characterised by metabolic alkalosis, hypokalaemia, hypochloremia, hyper-reninemia with normal blood pressure. Based on various genetic defects causing defective sodium chloride reabsorption, Bartter syndrome has been classified to types I, II, III, IV, IVb and V.7 Acquired BLS has also been described, especially following the use of aminoglycoside antibiotics.8 Certain diuretics and other medications including amphotericin B, ciclosporin and cisplatin have also been associated with BLS.
In vitro electrophysiological studies demonstrated that prolonged exposure to colistin could directly damage mammalian urothelium by interfering with transepithelial conduction. Similarly to aminoglycosides, colistin may directly activate the calcium-sensing receptor in the medullary thick ascending loop of Henle, resulting in hypokalaemia, metabolic alkalosis and hypomagnesemia. This may also result in hypercalciuria and lower-than-normal serum calcium levels.9 Two other drugs (piperacillin–tazobactam and rifampicin) used in our patient have been anecdotally reported to lead to electrolyte disturbances like hypokalemia, hypocalcaemia and so on.10 11 In this case, piperacillin–tazobactam was discontinued before the onset of BLS—making it an unlikely causative factor. Also, the patient was on rifampicin for more than 45 days and had received it throughout the hospital stay. However, the manifestations and abatement of BLS coincided with the introduction and withdrawal of colistin, respectively. This led us to strongly believe that colistin was the culprit drug in this case.
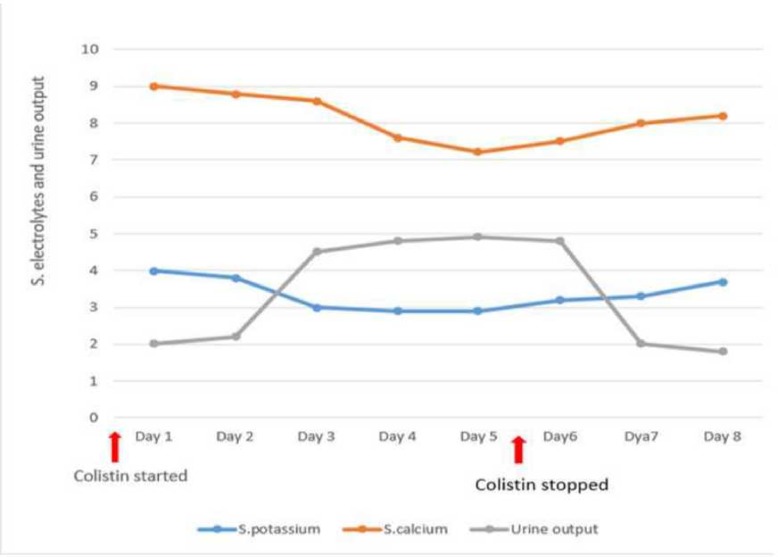
Our patient received therapy with colistimethate and developed unexplainable hypokalaemia, metabolic alkalosis, hypocalcaemia, hypomagnesemia and other electrolyte abnormality of renal origin. The metabolic alterations associated with hypotension were all suggestive of Bartter syndrome. The temporal relationship of the development of electrolyte abnormalities and its prompt abatement after discontinuing colistin therapy was indicative of colistin being the causative agent. We felt it inappropriate to re-challenge the patient with colistin again to prove our hypothesis as the patient had already received 6 days of colistin therapy and showed a remarkable improvement of markers of lung function and other markers of sepsis. As per the causality assessment of suspected adverse drug reactions, the association of BLS with colistin in our case could be labelled as ‘Probable’.12 The temporal relationship of the development of electrolyte abnormalities, polyuria, and its prompt abatement after discontinuing colistin is depicted in figure 1.
Figure 1.
Graphical representation of electrolyte and 24-hour urinary output trend.
An extensive review of the literature revealed only three other cases (two in adults and one in a preterm infant) of BLS after colistin use. In two of these cases, BLS occurred after 2 weeks of therapy while in the third case, it happened after 4 days of colistin therapy. Description of similar previous case reports including our case report is given in table 1.
Table 1.
Description of previous similar case reports
| Sample number | Author name | Clinical profile of the patient | Onset of BLS after starting colistin | Outcome after stopping colistin |
| 1. | Choksi and Shah13 | A 32-year-old man with ischial and sacral decubitus ulcer | 4 weeks | BLS resolved after 6 days |
| 2. | Cakir et al 14 | 28 week 740 g preterm infant receiving intravenous venous colistin for necrotising enterocolitis | 4 days | Polyuria and dyselectrolytemia resolved after 2 days and 7 days, respectively |
| 3. | Kamal Eldin et al 15 | 58-year-old man receiving intravenous colistin for wound infection | 2 weeks | BLS resolved after 6 days of cessation of colistin |
| 4. | Our case | 46-year-old man with sacral decubitus ulcer with ventilator-associated pneumonia | 3 days | BLS resolved after 2 days |
BLS, Bartter-like syndrome.
Learning points.
A patient receiving colistimethate sodium may present with polyuria, hypokalaemia and other electrolyte abnormalities suggestive of Bartter syndrome. One should be aware of this rare entity.
Electrolyte abnormalities (Bartter-like syndrome, BLS) during therapy with colistimethate may occur with normal renal function.
BLS associated with colistin, though life-threatening, is usually reversible on discontinuing the culprit drug.
Footnotes
Contributors: Manuscript writing and literature review was done by MT and MM. Case editing was done by AR and NKV. All four authors were part of treating team of patient.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Seyberth HW, Weber S, Kömhoff M, et al. Bartter’s and Gitelman’s syndrome. Curr Opin Pediatr 2017;29:179–86. [DOI] [PubMed] [Google Scholar]
- 2. Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect Dis 2006;6:589–601. 10.1016/S1473-3099(06)70580-1 [DOI] [PubMed] [Google Scholar]
- 3. Falagas M, Kasiakou S. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Critical Care 2006;10:R27 10.1186/cc3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spapen H, Jacobs R, Van Gorp V, et al. Renal and neurological side effects of colistin in critically ill patients. Ann Intensive Care 2011;1:14 10.1186/2110-5820-1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falagas ME, Fragoulis KN, Kasiakou SK, et al. Nephrotoxicity of intravenous colistin: a prospective evaluation. Int J Antimicrob Agents 2005;26:504–7. 10.1016/j.ijantimicag.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 6. Hartzell JD, Neff R, Ake J, et al. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis 2009;48:1724–8. 10.1086/599225 [DOI] [PubMed] [Google Scholar]
- 7. Lee BH, Cho HY, Lee H, et al. Genetic basis of Bartter syndrome in Korea. Nephrol Dial Transplant 2012;27:1516–21. 10.1093/ndt/gfr475 [DOI] [PubMed] [Google Scholar]
- 8. Chou C-L, Chau T, Lin S-H, et al. Acquired Bartter-like syndrome associated with gentamicin administration. Am J Med Sci 2005;329:144–9. 10.1097/00000441-200503000-00007 [DOI] [PubMed] [Google Scholar]
- 9. Kleta R, Bockenhauer D. Bartter syndromes and other salt-losing tubulopathies. Nephron Physiol 2006;104:p73–80. 10.1159/000094001 [DOI] [PubMed] [Google Scholar]
- 10. Torun AN, Eren MA, Demir M, et al. Recurrent symptomatic hypocalcemia during rifampicin therapy for brucellosis. Wien Klin Wochenschr 2011;123:566–8. 10.1007/s00508-011-0011-2 [DOI] [PubMed] [Google Scholar]
- 11. Kutluturk F, et al. A rare complication of antibiotic (piperacillin/tazobactam) therapy: resistant hypokalemia. J Med Cases 2012;3:355–7. [Google Scholar]
- 12. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000;356:1255–9. 10.1016/S0140-6736(00)02799-9 [DOI] [PubMed] [Google Scholar]
- 13. Choksi T, Shah S. Prolonged intravenous colistin use associated with acquired Bartter-like syndrome in an adult patient: a case report. EMJ Nephrol 2018;6:102–5. [Google Scholar]
- 14. Cakir U, Alan S, Zeybek C, et al. Acquired Bartter-like syndrome associated with colistin use in a preterm infant. Ren Fail 2013;35:411–3. 10.3109/0886022X.2012.761084 [DOI] [PubMed] [Google Scholar]
- 15. Kamal Eldin T, Tosone G, Capuano A, et al. Reversible hypokalemia and Bartter-like syndrome during prolonged systemic therapy with colistimethate sodium in an adult patient. Drug Saf Case Rep 2017;4:10 10.1007/s40800-017-0052-1 [DOI] [PMC free article] [PubMed] [Google Scholar]