Abstract
Mediastinal malignant melanoma is rare as both primary and metastatic lesions. We present the case of a 50-year-old man with diagnosis of recurrent melanoma of the mediastinum. Our patient was previously treated for cutaneous melanoma in 2001 with surgical excision. He presented with symptoms of exertional dyspnoea, dull chest pain and non-productive cough for 12 weeks. CT revealed a large heterogeneously enhancing mass, measuring 10.7×7.6 cm, centred within the aortopulmonary window which abutted the adjacent pericardium. Open biopsy of the epicardial mass was performed via left anterior thoracotomy. Immunohistochemical stains performed on the mass were positive for CD99, focally positive for CD56, SOX10, S100 and WT-1. A diagnosis of metastatic melanoma was established. The patient was started on pembrolizumab with pending BRAF testing. V600E and V600K mutations in exon 15 of the BRAF gene were codetected, and the patient was treated with dabrafenib and trametinib.
Keywords: oncology, skin cancer
Background
According to the most recent data from Surveillance Epidemiology and End Results, melanoma accounts for about 1.2% of all cancer deaths although it is responsible for the most skin cancer deaths in the USA.1 The skin remains the most common location for primary malignant melanoma (MM) with the liver, lung, brain and bone as the most common sites for metastatic disease. Metastasis to the mediastinum is extremely rare as a primary or metastatic deposit, with only a handful of cases citing mediastinal involvement.2–7 Mediastinal masses have been described as having broad histopathological features, which can be classified as anterior, middle or posterior mediastinal masses with anterior masses commonly comprising teratomas, thymomas, lymphomas and other rare malignancies such as melanoma and Pancoast tumours.8 Based on a study of 100 cases of metastasis to lung or mediastinum, about 5% of cases were found to be due to melanoma.9 We present a rare case of recurrent metastatic melanoma in a patient with a large mediastinal mass.
Case presentation
A 50-year-old man presented with dull chest pain, worsening dyspnoea with exertion and a non-productive cough over the last 12 weeks. The patient’s medical history was significant for melanoma diagnosed in 2001. The melanoma was localised to the upper right side of his back and was treated with surgical resection only. Otherwise, the patient’s surgical history and medical history were unremarkable. His family medical history was significant for coronary artery disease in his father but no familial cancers. The patient was a non-smoker. Two months prior to presentation, the patient was seen at an outside hospital with similar complaints. At that time, he was found to have a pericardial effusion on echocardiogram. He underwent pericardiocentesis with removal of 1 L of haemorrhagic fluid. Serological evaluation and fluid cultures remained negative, and the aetiology of his effusion was not determined. CT of the chest at the time was reportedly normal.
At the time of presentation, the patient was afebrile and in no apparent distress. He had no reported cervical or axillary lymphadenopathy on physical examination. His oxygen saturation was 95% on room air. ECG showed normal sinus rhythm and an incomplete right bundle branch block. Troponin levels were negative but brain natriuretic peptide was 187.0 (0–100 pg/mL) and D-dimer was 2349 (<500 ng/mL). With the patient’s recent history of pericardial effusion, an echocardiogram was obtained, which showed no effusion, normal systolic and diastolic function, and a normal ejection fraction.
Investigations
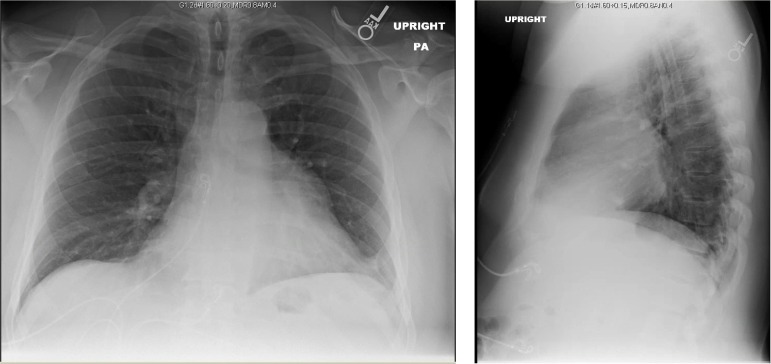
Conventional chest radiography demonstrated prominent soft tissue at the aortopulmonary window that surrounded the left hilar bronchovasculature with associated air bronchograms (figure 1).
Figure 1.
Chest radiography with prominent soft tissue at the aortopulmonary window.
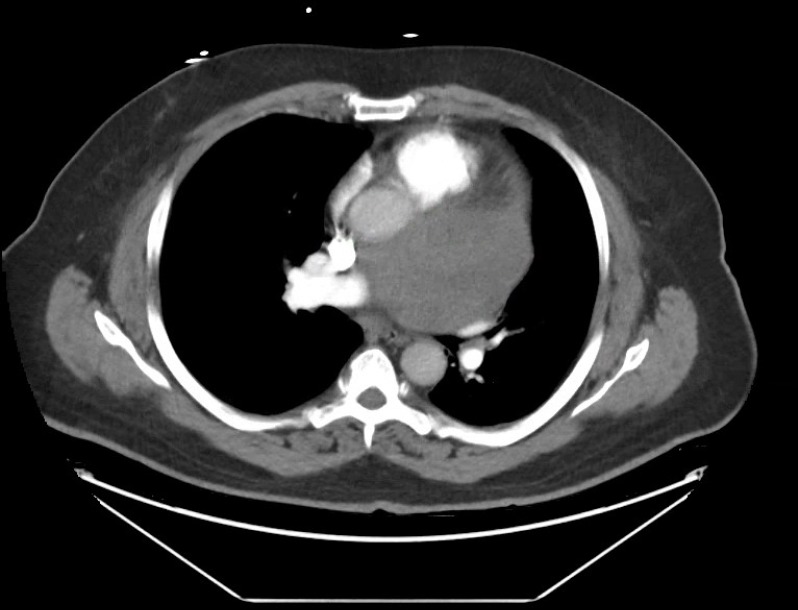
CT angiogram (CTA) was done to evaluate for pulmonary embolism given the patient’s presentation and elevated D-dimer. Multiple contiguous axial CT images were obtained through the chest following the administration of intravenous contrast. CTA revealed a large heterogeneously enhancing mass, measuring 10.7×7.6 cm, centred within the aortopulmonary window which abutted the adjacent pericardium as seen in figure 2. This appeared to be extraluminal to adjacent cardiac chambers and resulted in a mild mass effect on the adjacent vascular structures and airways. The differential diagnosis for this mass included: paraganglioma, sarcoma, inflammatory pseudotumour and metastatic disease.
Figure 2.
Mediastinal mass measuring 10 cm.
In addition, there was mild narrowing of the left mainstem bronchus by extrinsic compression from the mediastinal mass. However, no discrete enlarged mediastinal or hilar lymph nodes were identified. Further imaging was recommended.
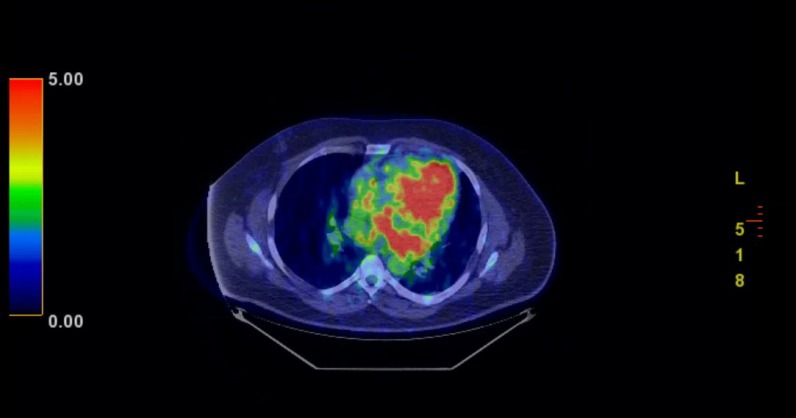
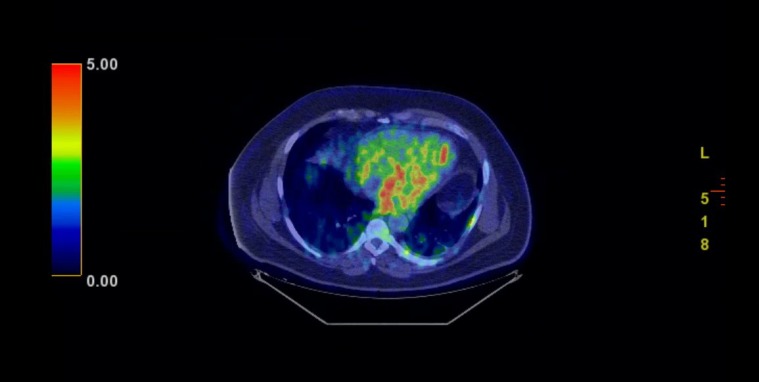
Positron emission tomography (PET) scan redemonstrated the large mediastinal, heterogeneously attenuating mass, centred in the aortopulmonary window that exhibited peripherally increased fludeoxyglucose (FDG) uptake, most pronounced posteriorly (figure 3). Differential considerations included infected haematoma, large necrotic lymph node and haemorrhagic metastasis. There was also mild FDG uptake within ill-defined opacities in the bilateral lung bases, likely representative of patchy pneumonitis (figure 4).
Figure 3.
Significant uptake in the periphery of the mass.
Figure 4.
Scattered uptake in bilateral lung bases, likely pneumonitis.
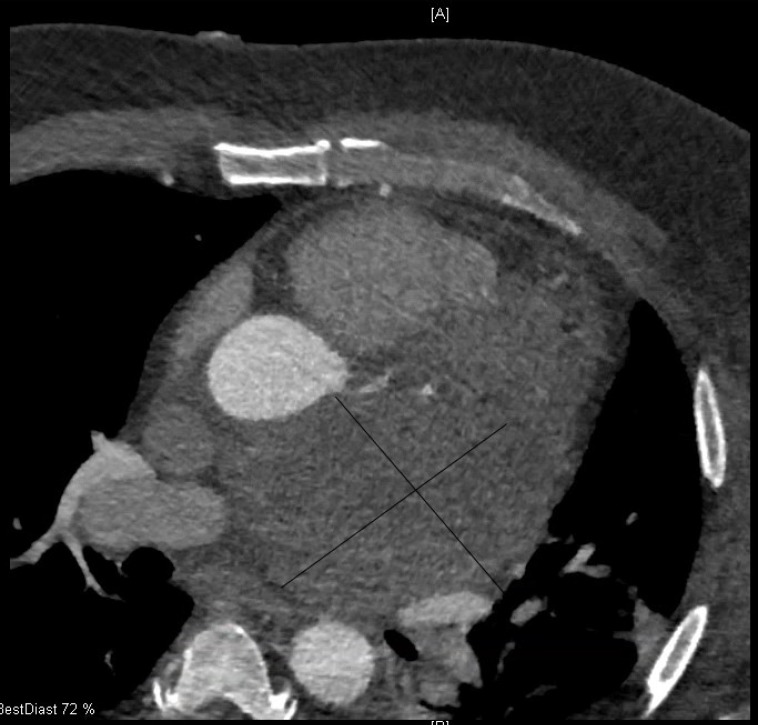
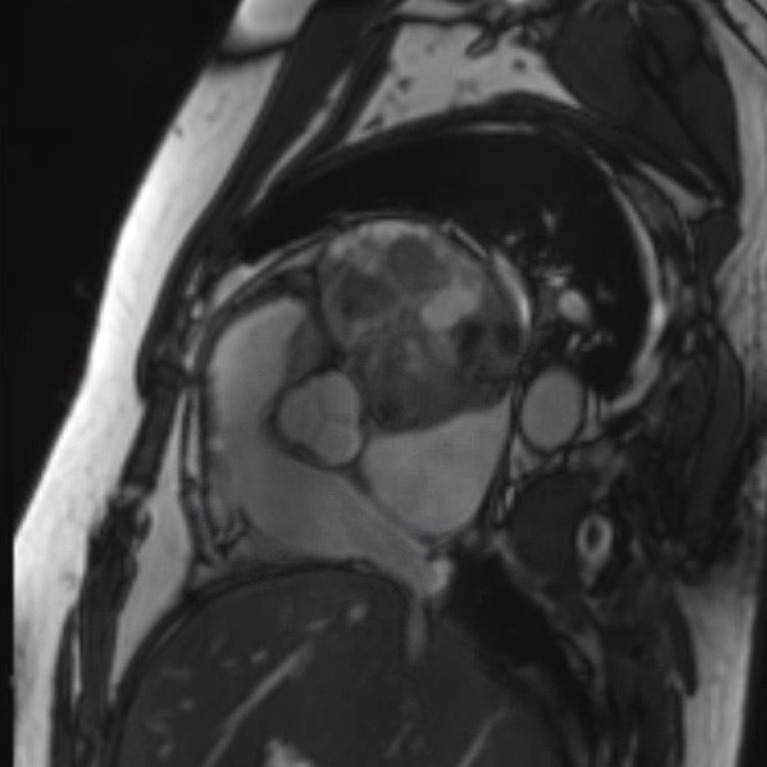
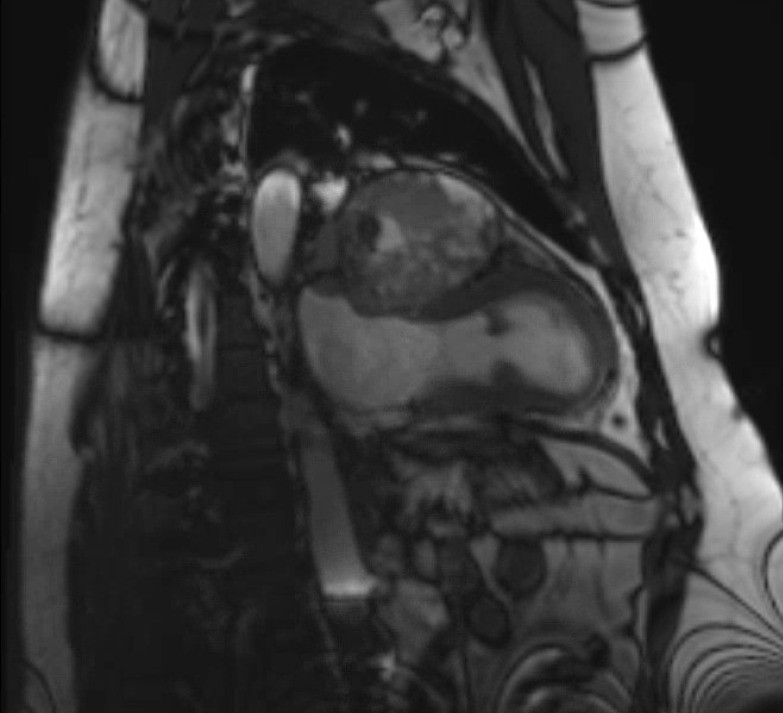
Cardiac MRI was performed to further characterise the mediastinal mass, as well as to assess pericardial and cardiac involvement. The large mediastinal mass was again noted and measured 9.9×6.9 cm. It was located posterior to the aortic root and inferior to the pulmonary vein. It appeared to be adherent to several structures, specifically the more basal portion of the anterior and anterolateral wall, the left atrial roof and the left atrial free wall. Furthermore, the mass was in contact with the left superior pulmonary vein causing a mild degree of mass effect and compression. There was minimal enhancement with contrast imaging, likely related to the presence of necrotic tissue (figures 5–7).
Figure 5.
Computed tomography scan long-axis, two-chamber view demonstrating 11.1 x 7.0cm mass.
Figure 6.
Cardiac magnetic resonance image, aortic valve view. This demonstrates 11.1cm x 7.0cm mass.
Figure 7.
Cardiac magnetic resonance image demonstrating mass posterior to the aortic root and inferior to the pulmonary vein, measuring 9.9 ×6.9 cm.
On hospital day 3, the patient was taken to the operating room for an open biopsy of the epicardial mass via left anterior thoracotomy. An intraoperative frozen section showed high-grade malignancy of unknown aetiology which was deferred to permanent and additional immunohistochemical study. Immunohistochemical stains performed on the tumour were positive for CD99, focally positive for CD56, SOX10, S100 and WT-1. Given the patient’s history, a diagnosis of metastatic melanoma was made.
Outcome and follow-up
Ten days after discharging from the hospital, the patient was seen by oncology in outpatient follow-up. During this initial visit, BRAF testing of the tumour was ordered. The V600E and V600K mutations in exon 15 of the BRAF gene were codetected in the tumour specimen. Initially, the patient received one dose of pembrolizumab while awaiting results of the BRAF testing. The patient was switched to dabrafenib and trametinib when the genetic analysis confirmed the tumour was BRAF positive. He remains on these oral agents with close follow-up scheduled.
Discussion
In cases previously described, mediastinal involvement of melanoma was typically reported as primary in origin. However, melanoma may sometimes present metastatically in the absence of a primary lesion. For example, among 1600 patients treated for malignant melanomas at Duke University Medical Center between 1970 and 1980, 16.3% developed thoracic metastasis with patterns including multiple pulmonary nodules, solitary nodule, miliary pattern, mediastinal and/or hilar adenopathy, pleural effusion, lytic bone lesions, extrapleural mass and combined lesions.10 Further, in a retrospective analysis of consecutive patients with melanoma at the University of Texas MD Anderson Cancer Center from 1990 to 2001, Cormier et al 11 reported patients with metastatic mediastinal melanoma in mediastinal lymph nodes arising from an unknown primary site had favourable long-term survival. Melanoma of unknown primary lesions has been reported to account for 1%–8% of melanoma.12–14 Although there are numerous reports of primary malignant melanoma, there is a paucity of literature citing metastasis in the setting of recurrent cutaneous melanoma.
Melanotic tumours of the mediastinum have a broad differential diagnosis. This includes, but is not limited to, pigmented extra-adrenal paraganglioma, pigmented carcinoid tumour of the thymus, melanotic schwannoma, melanotic neuroectodermal neoplasm and primary malignant melanoma.15 Theories for primary metastatic melanoma of the mediastinum include in utero migration of melanocytes with rest of embryonic respiratory tract from primitive foregut.7 However, cutaneous melanoma metastasis has been shown to arise via three main metastatic pathways: (1) as satellite or in‐transit metastasis, (2) as regional lymph node metastasis and (3) as distant metastasis at the time of primary recurrence.16
Li et al summarised the sequelae of mediastinal melanoma’s local compression symptoms.3 They reported cardiac tissue compression by a mediastinal tumour could bring about persistent precordial pain2; and if the tumour compresses an adjacent bronchus or the oesophagus, symptoms of progressive dyspnoea and dysphagia can be observed.7 17 These findings are consistent with the fact that most mediastinal masses are found incidentally through imaging or local compressive symptoms. In our patient, 2 months prior to presentation, a large pericardial effusion was drained with negative cytological and fluid culture evaluation. The chest CT was reported as normal, but further review of images obtained from that hospital demonstrated the presence of a mediastinal mass. At the time of presentation, our patient had dull chest pain, worsening dyspnoea with exertion and a non-productive cough. Local compressive symptoms were present in our patient’s disease course and prompted further evaluation.
A pulmonary aetiology of our patient’s condition was initially evaluated due to our patient’s presentation and elevated D-dimer. A CTA was performed with the suspicion of a pulmonary embolus. Although a pulmonary embolus was not found, it led to the discovery of our patient’s mediastinal mass. Subsequent imaging further characterised the mass and shed light on a rare disease process. Although there have been numerous cases of primary metastatic melanoma of the mediastinum reported, our patient’s presentation with recurrent metastatic melanoma was quite unusual, and to our knowledge, less than a handful of cases have been reported.
In conclusion, this case report adds to our collective knowledge of the unusual way in which metastatic melanoma can present. The prognosis and management of both patients with primary malignant melanoma or metastatic disease to the mediastinum have not been well defined due to the rarity of these cases. However, physicians must remain vigilant in diagnosing recurrent metastatic melanoma in patients with a known history of cutaneous melanoma. Through the use of chest X-rays and CT scans, we can help characterise unknown mediastinal masses. PET scans and MRI’s can also be useful for evaluating mediastinal masses; however, diagnosis can only be achieved through biopsy or surgery. When evaluating such masses, both primary and metastatic melanoma should be considered even in the absence of mediastinal or hilar adenopathy.
Learning points.
Physicians must be vigilant when evaluating new symptoms and medical illnesses in patients with history of cutaneous melanoma.
Despite its rarity, metastatic mediastinal melanoma should be considered when evaluating new mediastinal masses, particularly those with local compressive symptoms.
Further evaluation of patients with compressive symptoms should include advanced radiographic imaging if suspicion for primary or metastatic disease is suspected.
Footnotes
Contributors: JWK is the responsible consultant for the patient and was involved in the management of the patient all throughout and also provided guidance in preparing the article, and revising and editing the article as per BMJ format. JWK directly contacted the patient to obtain written consent. EK contributed to manuscript writing, review of literature and editing. CJ contributed to manuscript writing, editing, collection of data, radiological review and summary of the patient case.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Seer cancer STAT facts: melanoma of the skin. Bethesda, MD. National cancer Institute, 2019. Available: https://seer.cancer.gov/statfacts/html/melan.html
- 2. Park S-Y, Kim MY, Chae EJ. Primary malignant melanoma of the mediastinum: radiologic and pathologic correlation in two case. Korean J Radiol 2012;13:823–6. 10.3348/kjr.2012.13.6.823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Z, Jia H, Zhang B, et al. The clinical features, treatment, and prognosis of primary mediastinal malignant melanoma: a case report. Medicine 2017;96:e6436 10.1097/MD.0000000000006436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lau CL, Bentley RC, Gockerman JP, et al. Malignant melanoma presenting as a mediastinal mass. Ann Thorac Surg 1999;67:851–2. 10.1016/S0003-4975(99)00075-2 [DOI] [PubMed] [Google Scholar]
- 5. Karuppiah SV, Buchan KG. Primary malignant melanoma: a rare cause of mediastinal mass. Jpn J Thorac Cardiovasc Surg 2006;54:396–8. 10.1007/s11748-006-0004-7 [DOI] [PubMed] [Google Scholar]
- 6. Takao H, Shimizu S, Doi I, et al. Primary malignant melanoma of the anterior mediastinum: CT and Mr findings. Clin Imaging 2008;32:58–60. 10.1016/j.clinimag.2007.04.025 [DOI] [PubMed] [Google Scholar]
- 7. Kalra A, Kalra A, Palaniswamy C, et al. Primary malignant melanoma presenting as superior mediastinal mass. Int J Surg Case Rep 2011;2:239–40. 10.1016/j.ijscr.2011.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Youn HC. A primary malignant melanoma of the mediastinum with gross surgical view. J Thorac Dis 2016;8:E133–6. 10.3978/j.issn.2072-1439.2016.01.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson RM, Lindskog GE. 100 cases of tumor metastatic to lung and mediastinum. treatment and results. JAMA 1967;202:94–8. [PubMed] [Google Scholar]
- 10. Chen JT, Dahmash NS, Ravin CE, et al. Metastatic melanoma in the thorax: report of 130 patients. AJR Am J Roentgenol 1981;137:293–8. 10.2214/ajr.137.2.293 [DOI] [PubMed] [Google Scholar]
- 11. Cormier JN, Xing Y, Feng L, et al. Metastatic melanoma to lymph nodes in patients with unknown primary sites. Cancer 2006;106:2012–20. 10.1002/cncr.21835 [DOI] [PubMed] [Google Scholar]
- 12. Katz KA, Jonasch E, Hodi FS, et al. Melanoma of unknown primary: experience at Massachusetts General Hospital and Dana-Farber cancer Institute. Melanoma Res 2005;15:77–82. 10.1097/00008390-200502000-00013 [DOI] [PubMed] [Google Scholar]
- 13. Sutherland CM, Chmiel JS, Bieligk S, et al. Patient characteristics, treatment, and outcome of unknown primary melanoma in the United States for the years 1981 and 1987. Am Surg 1996;62:400–6. [PubMed] [Google Scholar]
- 14. Chang P, Knapper WH. Metastatic melanoma of unknown primary. Cancer 1982;49:1106–11. [DOI] [PubMed] [Google Scholar]
- 15. Pujani M, Hassan MJ, Jetley S, et al. Malignant melanoma presenting as a mediastinal malignant melanoma presenting as a mediastinal unknown primary origin? Turk Patoloji Derg 2017;33:168–70. 10.5146/tjpath.2014.01290 [DOI] [PubMed] [Google Scholar]
- 16. Leiter U, Meier F, Schittek B, et al. The natural course of cutaneous melanoma. J Surg Oncol 2004;86:172–8. 10.1002/jso.20079 [DOI] [PubMed] [Google Scholar]
- 17. Meacci E, Mulè A, Cesario A, et al. Posterior mediastinal melanoma causing severe dysphagia: a case report. J Med Case Rep 2008;2:316 10.1186/1752-1947-2-316 [DOI] [PMC free article] [PubMed] [Google Scholar]