Abstract
Losartan was the 9th most prescribed drug in the US in 2016, and several other angiotensin-II receptor blockers (ARBs) are widely prescribed. Since July 2018, more than two dozen specific ARB products have been recalled owing to the presence of potentially carcinogenic nitrosamine impurities in selected lots. As is the case with all FDA drug recalls, the ARB recalls have been voluntary on the part of the companies involved. In April 2019, the FDA categorized marketed ARB products with respect to nitrosamine impurities: (1) not present, (2) to be determined with no prior lots removed from the market (“TBD”), or (3) to be determined in the context of prior lots having been removed from the market (“TBD*”). The data were structured as hundreds of rows of products. Owing to the complexity of these data, more than a year into the recalls, it remains difficult for clinicians to understand which ARB products are free of impurities.
Keywords: Angiotensin receptor blocker, nitrosamine, FDA, impurities, drug safety, generic drugs, hypertension
Introduction
Losartan was the 9th most prescribed drug in the US in 2016,1 and several other angiotensin-II receptor blockers (ARBs) are widely prescribed. Since July 2018, more than two dozen specific ARB products have been recalled in the US owing to the presence of potentially carcinogenic nitrosamine impurities in at least some lots of those products.2 As is the case with all US drug recalls,3,4 the ARB recalls have been voluntary on the part of the companies involved.
The recalls have also occurred on an international scale. The United Kingdom’s Medicines and Healthcare Products Regulatory Agency has issued a series of announcements of recalls of ARB products,5 and the German Federal Institute for Drugs and Medical Devices took an active role in investigating the ARB recalls.6 The European Medicines Agency has reacted to the problem of impurities in ARB products with a variety of actions,7 and the European Directorate for the Quality of Medicines suspended Certificates of Suitability for some ARB products.8 It has been reported that at least 24 countries have taken action regarding these impurities.9
The FDA has recently revealed additional information about the processes leading to the presence of these impurities. The FDA has noted that “nitrosamine impurities can form during API (active pharmaceutical ingredient) processing under certain processing conditions and in the presence of some types of raw materials and starting materials.” Additionally, another cause for such nitrosamine impurities is “from the use of contaminated raw materials used in the manufacturing process.” However, the most alarming observation made was that “some ARB producers have identified a nitrosamine in their finished API, even though they are using processes incapable of forming a nitrosamine impurity.”10 The FDA has stated a concern that a process known as solvent recovery may have played a role in at least some of these incidents.10 There is recent evidence that nitrosamine impurities are not restricted to the ARB class; some ranitidine products have been reported to contain nitrosamine.11
In April 2019, the FDA categorized marketed ARB products with respect to nitrosamine impurities: (1) not present, (2) to be determined with no prior lots removed from the market (“TBD”), or (3) to be determined in the context of prior lots having been removed from the market (“TBD*”).12 The data were structured as hundreds of rows of products. Owing to the complexity of these data, more than a year into the recalls, it remains difficult for clinicians to understand which ARB products are free of impurities.
Methods
To gain an understanding of the current status of the FDA investigation, two authors separately analyzed the data after having agreed to the figure format in advance. Since some entries in the FDA list were presented as specific dosage forms (e.g., a company’s 32 mg tablet listed separately from a 16 mg tablet) and since the amount of active pharmaceutical ingredient might influence the probability of detecting nitrosamines, companies’ ARB products were separated into dosage forms. Some products were combination tablets, and thus, we categorized data for single drugs and drug combinations by the ARB contained in the products.
For robustness, the analysts used independent methods to prepare the data for analysis and to construct the figure. PMG manually separated the entries into 1,109 dosage forms, creating a graph in Prism (GraphPad Software, Inc), and JBB scripted his analysis in R (version 3.5.2), separating the entries into 1,111 dosage forms. The difference of 2 rows (a 0.2% difference) was reconciled after identification of two omissions from the manually-curated database. The numerical analyses resulting from the two approaches were identical after rounding to whole numbers.
Results
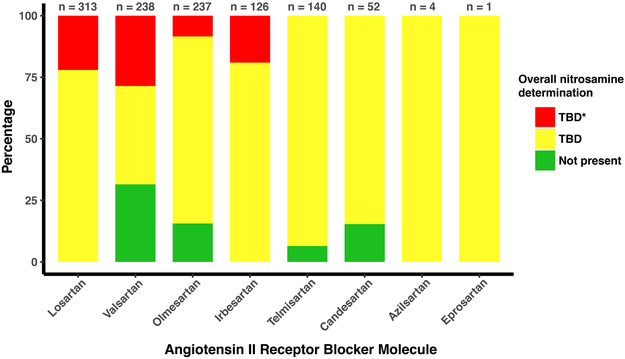
Figure 1 shows the principal findings. As of August, 2019—more than a year after the recalls began—the information currently available from FDA indicates the detectability of nitrosamines remains to be determined for all losartan, irbesartan, azilsartan, and eprosartan products currently marketed in the US.
Figure 1.
Percent of ARB-containing products (at the level analysis of each manufacturer’s dosage forms [dosage strengths], total n=1,111) in three categories assessed by the US Food and Drug Administration. Not present (green) indicates that the FDA has completed a comprehensive assessment. TBD (yellow) indicates that “one or more parts of FDA’s assessment remain incomplete.” TBD* (red) indicates that “certain lots of the product did have impurity levels above interim acceptable limits, however they have already been removed from the market.”
Some olmesartan products are listed as “to be determined” with a “*” indicating prior lots have been removed from the market; we have been unable locate any public statements regarding detection of nitrosamines in olmesartan products. We do not find olmesartan on the FDA’s list of recalled drug products,13 its separate list of recalled ARB products,14 its webpage summarizing ARB recalls,15 or the FDA’s searchable database of enforcement reports.16
The FDA data show that a fraction of available valsartan, olmesartan, candesartan, and telmisartan generic products have been placed by the FDA in the “not present” category.
Discussion
The principal new findings of our analysis are that no losartan, irbesartan, azilsartan, or eprosartan product on the US market is known to be free of nitrosamine impurities per current FDA data, and some olmesartan lots have been withdrawn from the market without being featured among the medications discussed in FDA press releases. Nitrosamines were not present in a small number of US-marketed generic ARB products. However, it is not clear how clinicians can direct a pharmacy to dispense only those manufacturers’ generic product since state-specific laws offer prescribers a means of directing pharmacies to dispense a brand name product, rather than one manufacturer’s generic product.
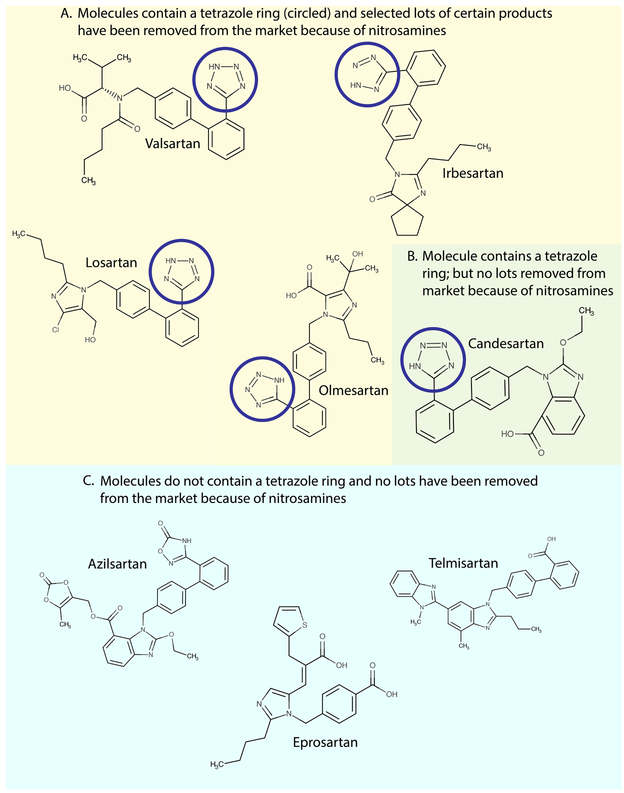
Although we have some information regarding potential sources of contamination, the origin of each impurity at each manufacturing site is not clear. As of now, nitrosamines have been reported in ARBs with a tetrazole ring (Figure 2). Evidence for how nitrosamines entered the supply chain has implicated the synthesis of the tetrazole ring.2 Whether this suspicion will lead to a shift in prescriptions to telmisartan, eprosartan, and azilsartan remains to be determined.
Figure 2.
The structures of the US-marketed ARB molecules, with tetrazole ring circled when present. Panel A shows molecules containing a tetrazole ring and for which selected lot of some products have been removed from the market via publicly announced recalls (valsartan, irbesartan, losartan) or through an unspecified mechanism (olmesartan). Panel B shows the structure of candesartan, an ARB molecule containing a tetrazole ring; no recalls related to nitrosamine impurities have affected candesartan products as of now. Panel C shows US-marketed ARB molecules that do not contain a tetrazole ring, none of which has been affected by recalls.
With respect to the different ARB products, FDA states that they are “prioritizing the assessments by those of highest patient need and in response to credible information about nitrosamine contamination.” Nonetheless, one of the most commonly prescribed drugs in the US—losartan—has no products for which a determination of “not present” has been made.
An up-to-date explanation of these recalls with attention to the above unexpected findings would benefit clinicians and their patients. Diligent clinicians aware of the which generic products have been fully vetted by FDA with respect to nitrosamine impurities are nonetheless unable to prescribe a specific manufacturer’s generic drug, suggesting policy changes might be needed.
Acknowledgement
The authors thank Andrea Berrido for helpful discussion of the ideas in the manuscript.
Sources of Funding
Funding: Dr. Byrd is funded by National Institutes of Health award K23HL128909.
Footnotes
Disclosures
None.
References
- 1.Losartan Potassium, ClinCalc DrugStats Database. Free U.S. outpatient drug usage statistics. https://clincalc.com/DrugStats/Drugs/LosartanPotassium. Accessed June 3, 2019.
- 2.Byrd JB, Chertow GM, Bhalla V. Hypertension Hot Potato - Anatomy of the Angiotensin-Receptor Blocker Recalls. N Engl J Med. 2019;380:1589–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulero A FDA Works to Improve Voluntary Drug Recall Process. https://www.raps.org/news-and-articles/news-articles/2019/4/fda-works-to-improve-voluntary-recall-processes. Accessed September 8, 2019.
- 4.Gibbons DC. Who can Recall what FDA’s Mandatory Recall Authority is? A U.S. District Court Could Not…. http://www.fdalawblog.net/2014/08/who-can-recall-what-fdas-mandatory-recall-authority-is-a-us-district-court-could-not/. Accessed September 8, 2019.
- 5.United Kingdom Medicines and Healthcare Products Regulatory Agency. Class 2 Medicines recall: Accord Healthcare Limited - Losartan Potassium 50mg Film-coated Tablets, PL 20075/0022 and Losartan Potassium 100mg Film-coated Tablets, PL 20075/0023. https://www.gov.uk/drug-device-alerts/class-2-medicines-recall-accord-healthcare-limited-losartan-potassium-50mg-film-coated-tablets-pl-20075-0022-and-losartan-potassium-100mg-film-coated-tablets-pl-20075-0023. Accessed September 7, 2019.
- 6.European Medicines Agency. Valsartan: Review of Impurities Extended to Other Sartan Medicines. https://www.ema.europa.eu/en/news/valsartan-review-impurities-extended-other-sartan-medicines. Accessed September 8, 2019.
- 7.European Medicine Agency. Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 28-31 January 2019. https://www.ema.europa.eu/en/news/meeting-highlights-committee-medicinal-products-human-use-chmp-28-31-january-2019. Accessed September 8, 2019.
- 8.European Directorate for the Quality of Medicines. Actions on CEPs. https://www.edqm.eu/en/actions-ceps#CEP%20suspension. Accessed September 8, 2019.
- 9.Farrukh MJ, Tariq MH, Malik O, Khan TM. Valsartan recall: global regulatory overview and future challenges. Ther Adv Drug Saf. 2019; 10: 2042098618823458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.United States Food and Drug Administration. Statement on the agency’s ongoing efforts to resolve safety issue with ARB medications. https://www.fda.gov/news-events/press-announcements/statement-agencys-ongoing-efforts-resolve-safety-issue-arb-medications. Accessed September 9, 2019.
- 11.United States Food and Drug Administration. Statement alerting patients and health care professionals of NDMA found in samples of ranitidine. https://www.fda.gov/news-events/press-announcements/statement-alerting-patients-and-health-care-professionals-ndma-found-samples-ranitidine. Accessed September 16, 2019.
- 12.United States Food and Drug Administration. FDA’s Assessment of Currently Marketed ARB Drug Products. https://www.fda.gov/drugs/drug-safety-and-availability/fdas-assessment-currently-marketed-arb-drug-products. Accessed June 7, 2019.
- 13.United States Food and Drug Administration. Drug Recalls. https://www.fda.gov/drugs/drug-safety-and-availability/drug-recalls. Accessed September 9, 2019.
- 14.United States Food and Drug Administration. Search List of Recalled Angiotensin II Receptor Blockers (ARBs) including Valsartan, Losartan and Irbesartan. https://www.fda.gov/drugs/drug-safety-and-availability/search-list-recalled-angiotensin-ii-receptor-blockers-arbs-including-valsartan-losartan-and. Accessed July 16, 2019.
- 15.United States Food and Drug Administration. FDA Updates and Press Announcements on Angiotensin II Receptor Blocker (ARB) Recalls (Valsartan, Losartan, and Irbesartan). https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-angiotensin-ii-receptor-blocker-arb-recalls-valsartan-losartan. Accessed July 16, 2019.
- 16.United States Food and Drug Administration. Enforcement Reports. https://www.accessdata.fda.gov/scripts/ires/index.cfm#tabNav_advancedSearch. Accessed August 30, 2019.