Abstract
Objective:
HIV prevention and treatment studies demonstrate that pharmacologic adherence metrics are more accurate than self-report. Currently-available metrics use liquid-chromatography/tandem-mass-spectrometry (LC-MS/MS), which is expensive and laboratory-based. We developed a specific and sensitive antibody against tenofovir, the backbone of treatment and prevention, but conversion to a lateral flow assay (LFA) –analogous to a urine pregnancy test- is required for point-of-care testing. We describe the development of the first LFA to measure antiretroviral adherence in real-time.
Methods:
Previous work in a directly-observed therapy study of providing tenofovir-disoproxil fumarate (TDF) to HIV-noninfected volunteers at various simulated adherence patterns defined the appropriate cut-off for the LFA (1500ng tenofovir/ml urine). We developed the LFA using a sample pad for urine; a conjugate pad coated with TFV-specific antibodies conjugated to colloidal gold nanoparticles; a nitrocellulose membrane striped with tenofovir-antigen (test line) and a control line; with an absorbent pad to draw urine across the reaction membrane.
Results:
We tested 300 urine samples collected from the directly-observed therapy study by this LFA and the gold-standard method of LC-MS/MS. The LFA demonstrated 97% specificity (95% CI: 93% to 99%) and 99% sensitivity (94% to 100%) compared to LC-MS/MS. The LFA accurately classified 98% of patients who took a dose within 24 hours as adherent.
Conclusion:
We describe the development and validation of the first point-of-care assay to measure short-term adherence to HIV prevention and treatment in routine settings. The assay is low-cost, easy-to-perform and measures the breakdown product (tenofovir) of both TDF and tenofovir alafenamide (TAF). This assay has the potential to improve HIV and PrEP outcomes worldwide by triggering differentiated service delivery with further study merited.
Keywords: Antiretroviral treatment, PrEP, adherence, tenofovir, lateral flow assay, point-of-care, urine
INTRODUCTION
The pre-exposure prophylaxis (PrEP) trials revealed the profound limitations of self-reported adherence for PrEP.1,2 Indeed, pharmacologic adherence metrics (where drug levels were measured in a biomatrix such as plasma) were critical to the interpretation of all the major PrEP trials.1–3 PrEP implementation and roll-out programs, when possible, are now incorporating objective metrics of adherence.4 In the context of antiretroviral treatment (ART), there is increasing interest in objective adherence monitoring using drug levels to avert both virologic resistance and the need for second or third-line regimens.5 For instance, virologic failure with a low drug level would be indicative of adherence challenges, whereas virologic failure with adequate adherence could trigger resistance testing.6 However, for objective adherence monitoring to be scaled up for both HIV prevention and treatment, a real-time, inexpensive, easy-to-perform adherence monitoring tool will be needed.
The currently-available pharmacologic adherence metrics, where antiretroviral drug levels are measured in a biomatrix such as plasma,7 urine,8 dried blood spots (DBS),9 or hair10, require expensive spectrometry-based technology and skilled laboratory-based personnel. For pragmatic reasons, therefore, these metrics have been restricted mainly to the research setting. Antibody-based tests, if converted into a point-of-care assay platform, allow for immediate and low-cost detection of the compound of interest in the clinical setting, similar to the technology used for the urine-based pregnancy test.11
Tenofovir-based compounds - tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide (TAF) - are the backbone of most ART regimens worldwide. TDF/emtricitabine (FTC) is the only approved agent for PrEP, with TAF/FTC under consideration at the Food and Drug Administration as another option for oral PrEP.12 Both TDF and TAF are broken down into tenofovir, the metabolite found in both plasma and urine. Urine collection is noninvasive compared to blood sampling. Therefore, a urine-based point-of-care assay to objectively confirm tenofovir drug-taking in routine clinical settings could have broad applications.
Our group has recently developed an antibody-based immunoassay to measure tenofovir in urine.8,13,14 Tenofovir levels by the ELISA-immunoassay showed high sensitivity, specificity and accuracy when compared to concentrations measured by the gold-standard method of liquid chromatography tandem mass spectrometry (LC-MS/MS) among participants provided TDF/FTC in a large directly-observed therapy (DOT) study.13 Since ELISA-immunoassays are cumbersome, the translation of an antibody-based test to a lateral flow assay (LFA) format,15 similar to an over-the-counter urine pregnancy test, will allow for implementation at the clinical point-of-care. This paper describes the development of an LFA from our tenofovir-based immunoassay and its validation by comparing the LFA’s performance characteristics to the gold standard of LC-MS/MS.
METHODS:
Development of the lateral flow assay:
Using urine samples collected from a DOT study (TARGET, ) in which 30 HIV-noninfected volunteers from Thailand received directly-observed TDF 300mg/FTC 200mg at 7, 4 and 2 doses per week (to simulate high, moderate and low adherence levels, respectively), we modeled an appropriate adherence benchmark for the lateral flow assay. As previously described, we found that a urine concentration of 1500 nanograms (ng)/milliliter (mL) of tenofovir in the urine balanced high specificity for adherence with good sensitivity for non-adherence.13
We then developed the lateral flow assay (LFA) for tenofovir using previously-described methods.15 The LFA test strip components include a sample pad onto which the test sample (e.g. urine) is applied; a conjugate pad coated with tenofovir specific antibodies conjugated to colloidal gold nanoparticles; a nitrocellulose membrane striped with a test line consisting of a tenofovir antigen and a control line consisting of anti-rabbit antibody; and an absorbent pad designed to draw the sample across the reaction membrane by capillary action. These components are all affixed to inert backing material and packaged within a plastic casing.
Validating the LFA results against LC-MS/MS-determined concentrations:
Laboratory methods:
To evaluate the performance of the LFA, urine samples are aliquoted for measurement by both LC-MS/MS and the LFA. For the LC-MS/MS-based method, as previously described,8 tenofovir is separated from one thousand-times diluted urine via reverse-phase high-performance LC and quantified by MS/MS using electrospray positive ionization in multiple reaction monitoring mode (TFV, 287.9/175.9 m/z (Q1/Q3)). The lower limit of quantification (LLOQ) of the LC-MS/MS-based assay is 500 ng/mL. For the LFA, 2–3 drops of urine are applied from the urine sample on to the LFA and, after approximately two minutes, the lines on the LFA window are read.
Statistical analysis:
We calculated the sensitivity, specificity and accuracy of the LFA compared to LC-MS/MS by cross-tabulating values above/below the 1500ng/mL threshold by the two different assays. Because misclassification was very rare, we present confidence intervals based on exact calculations using the binomial distribution.16
RESULTS:
LFA development
A photograph of the fully-developed LFA is shown in Figure 1. The urine first encounters the colored particles labeled with tenofovir antibody, so free tenofovir in the urine sample will bind the antibodies, preventing them from binding to the antigen line (test line) containing the tenofovir antigen. The presence of tenofovir in the urine, therefore, results in no color signal on the test line. Absence of tenofovir in the urine causes the antibodies to migrate to the antigen pad and bind strongly to the tenofovir antigen, resulting in a dark test line. This competitive assay, therefore, demonstrates a colored line in samples negative for tenofovir and no line in samples positive for tenofovir.
Figure 1:
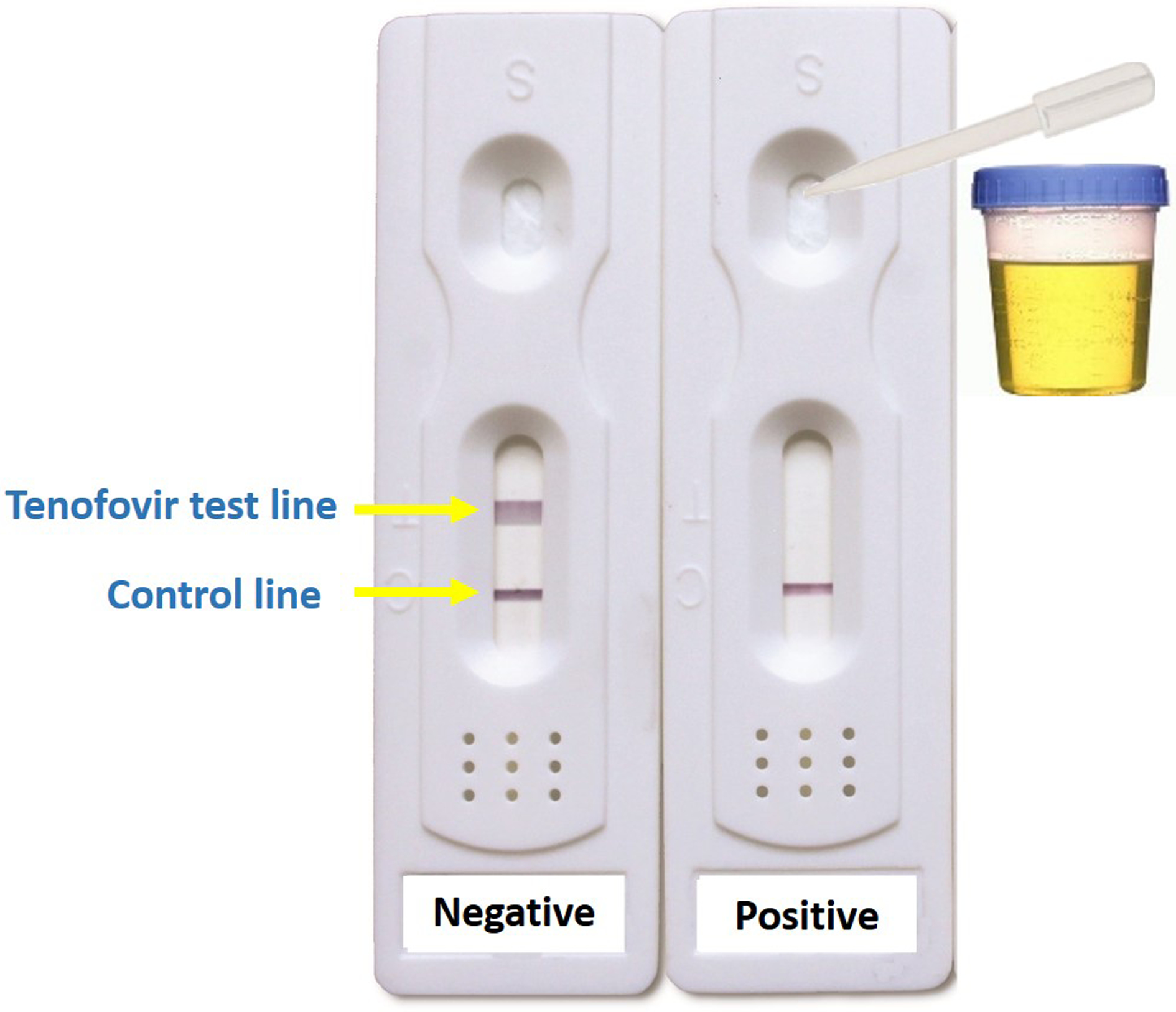
Prototype for first lateral flow assay for tenofovir detection in urine
This LFA requires 2–3 drops of urine to be dropped into the divot indicated on Figure 1 and takes <2 minutes to yield results. The test on the left shows a urine sample without tenofovir present. The test on the right shows a urine sample taken 8 hours after an HIV-noninfected volunteer was administered TDF/FTC 300mg/200mg.
Accuracy, sensitivity and specificity of the LFA:
Of 637 urine samples collected among the participants in the TARGET DOT study,13 300 were randomly selected to be tested by both the LFA and the gold standard method of LC-MS/MS for validation. Figure 2 shows the 2 × 2 table of tenofovir in the urine via the LFA versus LC-MS/MS with the established cut-off of 1500ng/mL. The accuracy of the LFA compared to LC-MS/MS was 97% (95% confidence interval (CI): 95% to 99%). Among the 213 tenofovir-negative samples by LC-MS/MS (≤1500ng/mL), 206 were also negative by the LFA, indicating 97% specificity (95% CI: 93% to 99%). Of the 87 tenofovir-positive samples by LC-MS/MS (>1500ng/ml), 86 were also positive by the LFA, indicating 99% sensitivity (95% CI: 94% to 100%).
Figure 2:
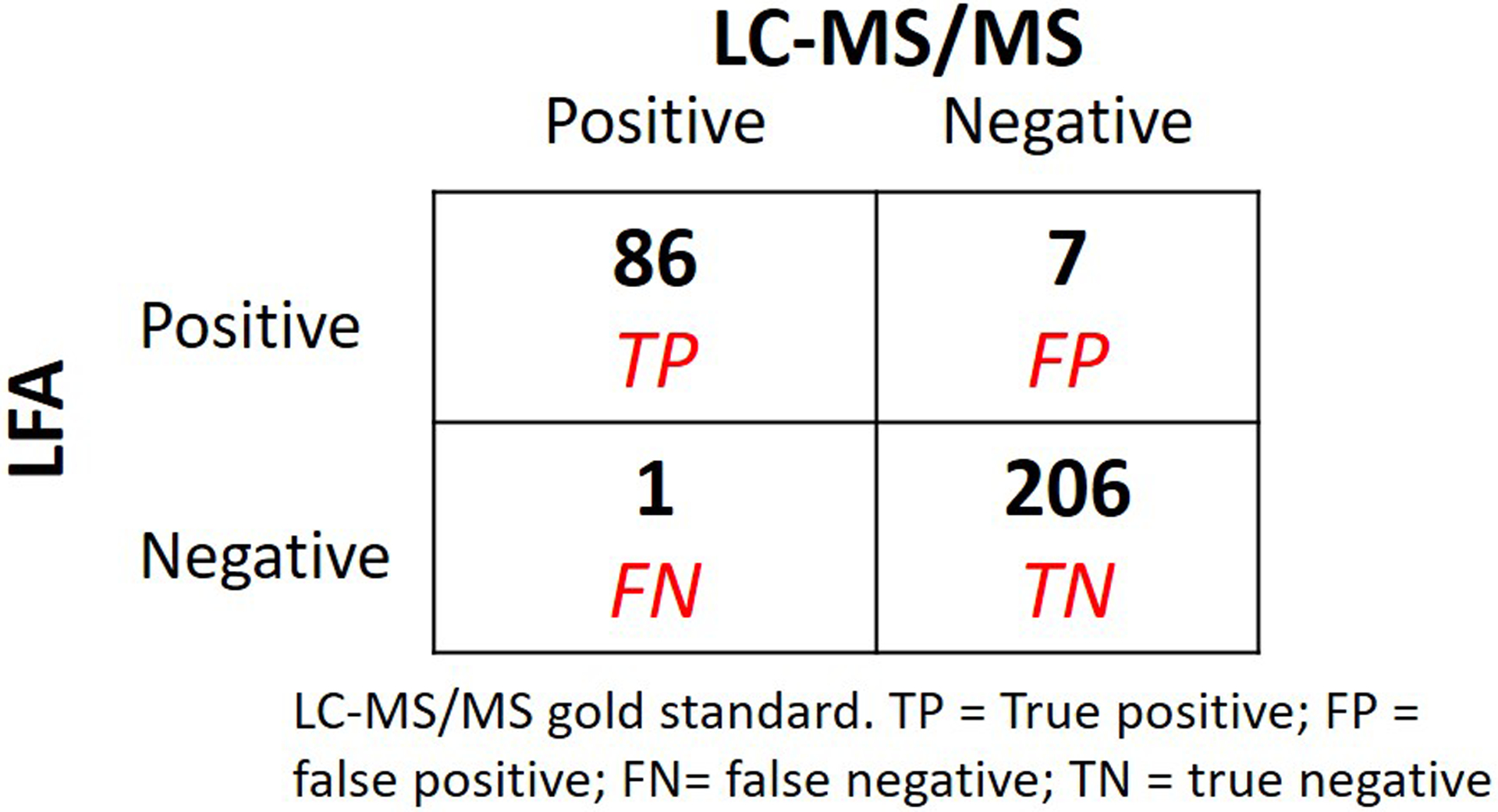
Test characteristics of lateral flow assay
DISCUSSION:
This study describes development and validation against the gold-standard method of LC-MS/MS of a novel urine based lateral flow assay to detect adherence to tenofovir-based regimens. This LFA will allow for point-of-care testing for tenofovir in routine clinic-based settings. Given that TDF or TAF (both metabolized into tenofovir) are the backbone of most ART regimens and of oral PrEP worldwide, this test should have wide-reaching implications for both treatment and prevention. LFA technology allows for real-time testing and this particular product takes <2 minutes to interpret results, allowing adherence information to be objectively measured during a routine clinical visit. Urine collection has acceptability and feasibility advantages to blood collection.17,18 Moreover, the test is inexpensive (<$2 per test), easy to use and can be readily performed and interpreted by non-trained personnel.
Our LFA, like the ELISA-based immunoassay test,13 is highly specific (97%) and sensitive (99%) when compared to the gold-standard method. These performance characteristics will minimize misclassification of adherence at a clinic visit. Based on qualitative data from the VOICE study from participants who were provided feedback on plasma tenofovir levels, we deliberately chose a cut-off for the urine assay that maximized specificity, i.e., minimizing the chance that an individual would be told they were non-adherent when actually taking the drug.19 We estimate that a patient who has taken a TDF/FTC dose 24 hours ago will have a 98% probability of the LFA being positive at this cut-off and the probability of the test remaining positive if the last dose was taken >4 days prior will be low (≤17%).13
In the context of PrEP, objective adherence monitoring is helpful since self-reported adherence has limitations, including social desirability bias,20 and there is no surrogate for adherence (such as HIV viral loads in individuals with HIV). Moreover, the ability to monitor adherence at the point-of-care will allow for real-time feedback on adherence, which itself can improve adherence.19 Timely information on how a patient is adhering to PrEP can trigger an immediate HIV test and real-time adherence interventions before prevention effectiveness is compromised.21 Finally, since this metric provides information on dosing within the prior 4–7 days, with minimal impact of cumulative adherence on its assessment of recent adherence, it has the potential to confirm self-reported PrEP dosing patterns on a weekly basis in the context of on-demand PrEP, meriting further study.
In the setting of HIV treatment, World Health Organization guidelines recommend that patients on ART with failure to achieve virologic suppression or with new virologic failure be enrolled into a protocol of enhanced adherence counseling with more frequent HIV viral load monitoring until suppression is achieved.22 Providing a low-cost tool to monitor drug-taking behavior at the clinical point-of-care can allow for enhanced adherence counseling even before a viral load is known or the rationing of viral load testing until the urine test improves. A low-cost point-of-care assay can also monitor for adherence between less frequent and more expensive viral load measurements on ART. Finally, in second-line ART failure, a point-of-care adherence metric in combination with viral load testing can determine when costly viral drug resistance testing may be required (e.g. a low adherence test with a high viral load suggest adherence challenges; a good adherence result with a high viral load suggests resistance).6 Of note, the adherence threshold to maintain virologic suppression may be different than what is required to maintain the effectiveness of PrEP, so future studies in treatment cohorts will determine if the assay cut-offs should differ by indication.
This assay will have limitations. Urine and plasma levels of tenofovir provide information on adherence over short periods of time (1–7 days) and a limitation of any short-term metric is the possibility of “white coat adherence,” where adherence improves transiently before a visit. This phenomenon has not yet been observed in PrEP, with plasma levels of tenofovir serving as the primary adherence metric in every one of the placebo-controlled trials of PrEP.23 However, only with deployment of a widely-used point-of-care assay for monitoring short-term adherence can the extent of this limitation be determined. Combining short and long-term metrics of adherence (such as tenofovir levels in urine and hair23) to define patterns of adherence can circumvent this limitation as the transfer of the technology of performing hair assays to laboratories in low-and-middle-income countries expands.24 Finally, although tenofovir is the metabolite of both TDF and TAF, the currently-developed assay should be used with TDF only. The appropriate cut-off for tenofovir in the urine assay with TAF needs to be determined and that work is underway. Regulatory approval and acceptability, feasibility and utility studies are also underway ().
In conclusion, we have developed and validated the first LFA for tenofovir in urine, allowing for point-of-care objective adherence monitoring for both ART and PrEP. This test is easy to perform, inexpensive, and rapid, allowing for immediate patient feedback, timely adherence support and the activation of relevant laboratory testing in real-time. Further study of the impact of this novel adherence tool on optimizing the effectiveness of HIV treatment and prevention in diverse populations is needed.
ACKNOWLEDGEMENTS:
Funding for this work and the development of the antibody at Abbott Rapid Diagnostics was provided by the National Institute of Allergy and Infectious Diseases/ National Institutes of Health (NIAID/NIH) R01AI143340 (P.I. Gandhi). Three authors (WCR, GW, MV) as indicated on the title page are from Abbott Rapid Diagnostics. Further funding from this work came from NIAID/NIH R21AI127200 and R01AI136648 (P.I. Drain). M.A.S. was supported by T32AI060530 (P.I. Havlir). We wish to thank the participants of the TARGET study and their families.
REFERENCES:
- 1.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Straten A, Brown ER, Marrazzo JM, et al. Divergent adherence estimates with pharmacokinetic and behavioural measures in the MTN-003 (VOICE) study. J Int AIDS Soc. 2016;19(1):20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celum CL, Mgodi N, Bekker LG, et al. Adherence 3 months after PrEP Initation among Young African Women in HPTN 082 Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, Washingon, March 4–7 2019. (abstract 995). [Google Scholar]
- 5.Kimulwo MJ, Okendo J, Aman RA, et al. Plasma nevirapine concentrations predict virological and adherence failure in Kenyan HIV-1 infected patients with extensive antiretroviral treatment exposure. PLoS One. 2017;12(2):e0172960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermans LE, Steegen K, ter Heine R, et al. PI Drug-Level Testing as a Screening Tool for Drug Resistance in 2nd-Line ART Failure Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, Washingon, March 4–7 2019. (abstract 461). [Google Scholar]
- 7.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi M, Bacchetti P, Rodrigues WC, et al. Development and Validation of an Immunoassay for Tenofovir in Urine as a Real-Time Metric of Antiretroviral Adherence. EClinical Medicine (Published by The Lancet); https://doiorg/101016/jeclinm201808004 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo-Mancilla JR, Zheng JH, Rower JE, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29(2):384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandhi M, Greenblatt RM. Hair it is: the long and short of monitoring antiretroviral treatment. Ann Intern Med. 2002;137(8):696–697. [DOI] [PubMed] [Google Scholar]
- 11.Chard T. Pregnancy tests: a review. Hum Reprod. 1992;7(5):701–710. [DOI] [PubMed] [Google Scholar]
- 12.Hare CB, Coll J, Ruane P, et al. The Phase 3 Discover STudy: Daily F/TAF or F/TDF for HIV Pre-Exposure Prophylaxis Conference on Retroviruses and Opportunistic Infections; March 4–7, 2019; Seattle: Abstract 104LB. [Google Scholar]
- 13.Gandhi M, Bacchetti P, Spinelli MA, et al. Brief Report: Validation of a Urine Tenofovir Immunoassay for Adherence Monitoring to PrEP and ART and Establishing the Cutoff for a Point-of-Care Test. J Acquir Immune Defic Syndr. 2019;81(1):72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spinelli MA, Glidden DV, Rodrigues WC, et al. Low tenofovir level in urine by a novel immunoassay is associated with seroconversion in a PrEP demonstration project. AIDS. 2019(5):867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koczula KM, Gallotta A. Lateral flow assays. Essays Biochem. 2016;60(1):111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agresti A., Kateri M. (2011) Categorical Data Analysis Lovric M. (eds) International Encyclopedia of Statistical Science. Springer, Berlin, Heidelberg [Google Scholar]
- 17.Marrazzo JM, Scholes D. Acceptability of urine-based screening for Chlamydia trachomatis in asymptomatic young men: a systematic review. Sex Transm Dis. 2008;35(11 Suppl):S28–33. [DOI] [PubMed] [Google Scholar]
- 18.Hadland SE, Levy S. Objective Testing: Urine and Other Drug Tests. Child Adolesc Psychiatr Clin N Am. 2016;25(3):549–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Straten A, Montgomery ET, Musara P, et al. Disclosure of pharmacokinetic drug results to understand nonadherence. AIDS. 2015;29(16):2161–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kagee A, Nel A. Assessing the association between self-report items for HIV pill adherence and biological measures. AIDS Care. 2012;24(11):1448–1452. [DOI] [PubMed] [Google Scholar]
- 21.Spinelli MA, Glidden DV, Anderson PL, et al. Brief Report: Short-Term Adherence Marker to PrEP Predicts Future Nonretention in a Large PrEP Demo Project: Implications for Point-of-Care Adherence Testing. J Acquir Immune Defic Syndr. 2019;81(2):158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization (WHO) Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidance; December 2018; http://www.who.int/hiv/pub/guidelines/ARV2018update/en/ (Accessed March 26, 2019). [Google Scholar]
- 23.Koss CA, Bacchetti P, Hillier SL, et al. Differences in Cumulative Exposure and Adherence to Tenofovir in the VOICE, iPrEx OLE, and PrEP Demo Studies as Determined via Hair Concentrations. AIDS Res Hum Retroviruses. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandhi M, Devi S, Bacchetti P, et al. Measuring Adherence to Antiretroviral Therapy via Hair Concentrations in India. J Acquir Immune Defic Syndr. 2019;81(2):202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]


