Abstract
Fractures in the elderly represent a significant and rising socioeconomic problem. Although aging has been associated with delays in healing, there is little direct clinical data isolating the effects of aging on bone healing from the associated comorbidities that are frequently present in elderly populations. Basic research has demonstrated that all of the components of fracture repair -cells, extracellular matrix, blood supply, and molecules and their receptors— are negatively impacted by the aging process, which likely explains poorer clinical outcomes. Improved understanding of age-related fracture healing should aid in the development of novel treatment strategies, technologies, and therapies to improve bone repair in elderly patients.
Keywords: Fracture, Repair, Elderly, Clinical, Healing
Introduction:
The burden of musculoskeletal disorders is enormous. It is estimated that approximately 35 million Americans—fully 1 in 7—are affected at some time in their life. In addition to chronic, degenerative conditions such as osteoarthritis, back pain, and osteoarthritis, many lives are affected by trauma. Approximately 6.3 million fractures occur and are treated in the United States per year. While most of these heal uneventfully, approximately 10-15% result in nonunion, which creates tremendous disability and increases the cost of care and treatment of patients with such a complication.
The impact of impaired fracture union in the elderly population is even greater. Due to a decreased physiologic reserve, pre-existing co-morbidities, and greater perioperative complications of care, elderly patients are often unable to return to their pre-injury level of activity. Because of widespread osteoporosis in this population, the incidence of many fractures is much greater than in younger cohorts. The impact of this high incidence and propensity for complications of treatment is compounded by population trends: the 65+ cohort is the fastest growing segment of the population and is estimated to reach 20% by the year 2040 (1). Clearly, a greater understanding of the clinical trends and the underlying physiologic effects of aging on fracture repair are necessary to better understand and manage this staggering problem.
Factors that Affect Repair and Conditions that Contribute to Impaired Healing:
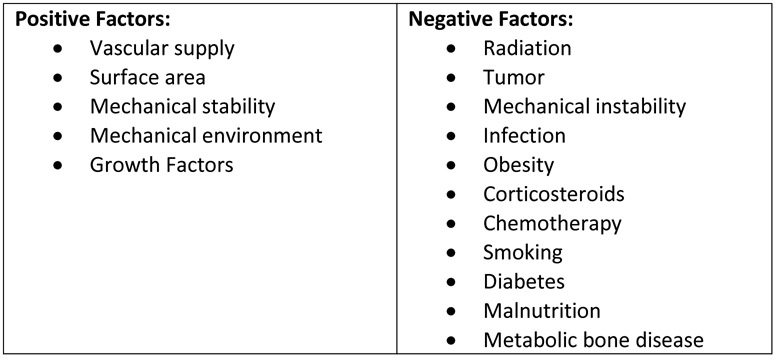
Numerous clinical factors have been demonstrated to impact normal bone healing (Figure 1). The natural aging process, age-related chronic disease, and, on occasion, treatment interventions can positively or negatively affect the healing process. Of note, positive factors can be influenced by appropriate treatment choices and surgical technique to ameliorate the negative impact of surgery. Minimally invasive techniques such as minimally invasive plate osteosynthesis (MIPO) and intramedullary stabilization have been demonstrated to preserve the local periosteal vascular supply while maintaining the soft tissue envelope and growth factor-containing hematoma that is compromised with traditional open methods of treatment. Load-sharing implants, locking screws, and improved design and testing of plating constructs provide adequate mechanical stability of a surgical repair to maintain fracture reduction and provide a favorable mechanical environment for direct or indirect fracture repair. Additionally, robust stable fixation allows for early mobilization and rehabilitation, reducing the long-term clinical impacts of a fracture.
Fig. 1.
Clinical Factors Affecting Bone Healing
Many negative factors that affect the healing process are inherently associated with aging, and each of the positive factors discussed above can be negatively impacted. Microvascular and large vessel occlusive disease can impair the local blood supply at a fracture site. The stability and durability of fracture fixation is greatly reduced in osteoporotic bone, and, as discussed below, the presence and effectiveness of growth factors in the local fracture environment are reduced in elderly patients.
Comorbid conditions and systemic diseases have been demonstrated to negatively affect fracture healing, and many of these are much more common in the elderly population. Obesity, diabetes mellitus, malnutrition, and malabsorption of necessary nutrients are prevalent in older patients. The burden of solid and hematogenous tumors and infection directly affect a patient’s metabolism, immune response, and healing. Medical interventions such as radiation treatment, chemotherapy, and corticosteroids damage the local tissue bed and blunt the normal inflammatory response to injury. The complex interrelationship and cumulative impact of all of these factors is difficult to quantify, but certainly negatively affect an older person’s ability to recover from a fracture.
Clinical Studies Addressing the Effects of Aging on Fracture Repair:
Despite the high incidence and impact of fractures in the elderly, there are very few large studies that adequately assess the impact of age on fracture healing. As there is no standard delineation of the geriatric age group or accurate method of determining physiologic age, most studies rely on a broad and somewhat arbitrary description when grouping patients. Additionally, few studies exist that address aging as an independent risk factor for nonunion. Rather, age must be correlated with other key risk factors, as discussed above.
Xu, et al., performed a systematic review of non-displaced femoral neck fractures in patients over the age of 65 (2). They identified 29 studies involving 5071 patients, and grouped 1120 patients into the nonoperative cohort, and 3951 into the operative group. They found poorer outcomes in the nonoperatively treated group, including union rates of 68.8% vs. 92.6% and osteonecrosis rates of 10.3% vs. 7.7%. The group concluded that surgery to treat non-displaced femoral neck fractures was associated with a higher union rate and a trend toward reduced osteonecrosis compared to nonoperative management.
Parker, et al., examined the radiographic outcomes of patients with femoral neck fractures treated with internal fixation in a prospective observational study of 1133 patients. They found an overall nonunion rate of 19.3% and a progressive increase by age group from 1 of 17 (5.9%) in patients less than 40 to 84 of 337 (24.9%) in the over 70 age group. When corrected for mortality, the incidence of nonunion in the 80+ age group continued to rise (3).
Co-morbidities, which may be considered a proxy for aging, similarly have been associated with an increased complication rate in the hip fracture population. In a prospective observational study of 122 patients under age 60 treated with internal fixation, Duckworth, et al. demonstrated an overall union rate of 68% and an overall complication rate of 32%. Failures were more common in patients over age 40, and importantly, pre-existing liver, renal, or respiratory disease were predictive of failure. They concluded that younger patients should be carefully screened for comorbidities, and those with significant concomitant diseases should be considered for alternate forms of treatment such as prosthetic joint replacement (4).
Finally, a large review of 4190 patients treated with low-intensity pulsed ultrasound was performed by Zura, et al. They found an overall healing rate of 96.2%, and found that comorbidities such as obesity, smoking, diabetes, vascular insufficiency, osteoporosis, cancer, and rheumatoid arthritis reduced healing rates. Patients over age 60 demonstrated similar healing rates to younger patients. However, the presence of comorbid conditions in this age group reduced healing more than in younger patients (5).
Clearly, more work is needed in large clinical studies to account for the impact of aging and its associated comorbid conditions to help delineate the causes of nonunion and improve the outcomes of older patients.
Physiology of fracture repair
Fracture repair follows a strict temporal sequence of events (6, 7). Key to each step are four major biological factors; cells, vascularization, extracellular matrix, and signaling molecules. The disruption of any one of these factors can result in fracture healing complications. Immediately following a fracture, a hematoma forms and the inflammatory response is initiated. Cells of the innate and adaptive immune system function to propagate the successive healing steps through secretions of cytokines and chemokines (8,9). Vascularization is promoted early in fracture healing and provides delivery of necessary nutrients, cells, and oxygen to further promote callus formation and reorganization (10,11,12). As the inflammatory stage proceeds towards resolution, osteochondral stem cells of mesenchymal origin migrate to the callus and differentiate into chondrocytes and osteoblasts. Stem cells contributing to fracture repair arise largely from the periosteum and have been reported to originate from the bone marrow and surrounding muscle (6, 13,14). In endochondral ossification, bone formation is preceded by the formation of a cartilage provisional matrix (15,16,17). The matrix is largely composed of type II collagen and proteoglycans that act as a scaffold to direct subsequent mineralization and bone formation (18). The extracellular matrix in fracture healing provides a storage site for cytokines, growth factors, and cell adhesion molecules to direct appropriate cell trafficking and differentiation (18). Throughout all healing phases, the coordination of the cellular processes is controlled by signaling molecules. The involved inflammatory, progenitor, osteoblasts, chondrocytes, and vascular endothelial cells produce cytokines, chemokines, and growth factors to regulate inflammation and promote healing (19).
Increased age negatively affects the physiology of fracture healing
Cells
Poor fracture healing outcomes in the elderly may be attributable to age-related changes to the osteochondral stem cell population. In both human and animal studies, osteochondral stem cells generally demonstrate decreased quantity and capacity for proliferation and differentiation in aged compared to young groups (20,21,22). Lopas et al. demonstrated that the significantly decreased bone and cartilage volume within a fracture callus of aged compared to young mice was directly associated with a decrease in osteochondral stem cell proliferation in the aged group (23). Specifically, periosteal osteochondral stem cells also showed decreased quantity in aged compared to young humans (24). The stem cells that were isolated from the periosteum demonstrated greater oxidative damage and increased senescence markers in older adults compared to younger individuals (25). In animal studies, chondrocyte and osteoblast differentiation was delayed in stem cells isolated from the periosteum of aged compared to young mice (26). Other sources of osteochondral stem cells that contribute to fracture healing are the muscle and bone marrow (27,28). Stem cells from both tissues also demonstrate decrease quantity and function in aged animals and humans (29,30,31).
Vascularization
Adequate vascularization is required for successful fracture healing; however, age-related changes to the vascular system may perturb fracture healing in elderly populations. In general, there is decreased perfusion to the skeletal system, with decreased osseous blood flow in aged compared to young humans (32,33). The decreased vascularization at the time of fracture in an aged population may delay angiogenesis within the fracture callus, resulting in delayed or inadequate delivery of nutrients, cells, and vascular endothelial signaling molecules (32,34). Additionally, a diminished vasodilation response of the vasculature in aged compared to young may humans may further reduce the blood profusion at the site of fracture (35). In animal models, a higher density of blood vessels within the fracture callus of young compared to old mice was associated with earlier detection and increased quantity of key biochemical regulators of angiogenesis, including vascular endothelial growth factor (VEGF), hypoxia inducible factor 1α, and matrix metallopeptidase 9 and 13 (11,36,37).
Extracellular Matrix
Age-related changes to the proteins and cells of the extracellular matrix may have a detrimental impact on fracture healing. With increased age, there is a reported decrease in mature enzymatic collagen cross-linking within bone, owing to a decrease in bone strength and toughness (38). Additionally, bone matrix proteins responsible for regulating mineralization of the matrix are decreased with age within the extracellular matrix (39). Cells of the extracellular matrix demonstrate an age-related perturbation of their response to mechanical stimuli resulting in impaired intracellular signaling and gene regulation with negative effects on tissue reorganization and maintenance (40). A provisional cartilage matrix is required for endochondral ossification. Timely formation, remodeling, and removal of the matrix is essential for subsequent bone formation. In aged animal studies, there is delayed formation of cartilage and a decrease in type II collagen within the callus (26). The extracellular matrix of the callus is a source of VEGF expression, thus delays in cartilage formation may limit the necessary signaling molecule within the callus (41).
Signaling molecules
The coordinated processes of fracture healing rely on the signaling molecules expressed from the involved cells and tissues. Bone morphogenetic proteins (BMPs) and VEGF are well understood to be critical for fracture healing (42,43). Reduced VEGF production during fracture healing has been demonstrated in aged compared to young animals (43,44). Additionally, the genetic regulation of these molecules is altered in aged compared young animals. Meyer, et al. demonstrated decreased transcriptional expression of BMP-2 within the callus of aged compared to young rats (45). Defective growth factor production may also arise from the intrinsic age-related changes to the cellular source of BMPs and VEGF. As described above, endothelial and osteoblast cells demonstrate decreased differentiation and function in aged humans and animals, a possible cause for the decreased production of BMP and VEGF.
Inflammation and fracture healing
Fracture healing is initiated by a robust inflammatory response. Following the initial response, resolution of inflammation must occur in a temporally controlled processes to allow for the subsequent anabolic stages of healing. An excessive or prolonged inflammatory phase could perturb the subsequent healing stages (46). Age-related changes to the inflammatory response have been attributed as an underlying cause of the myriad of age-related conditions and disease that affect elderly populations (47). Similarly, such age-related changes to the inflammatory response may be responsible for the poorer fracture healing outcomes in the elderly.
Macrophages are powerful immune regulators locally within a fracture callus (48). They are present early in fracture healing as an M1 phenotype expressing pro-inflammatory cytokines, and macrophages are also present later in healing as an M2 phenotype expressing anti-inflammatory cytokines and angiogenic and osteogenic growth factors (49,50). However, intrinsic age-related changes to macrophages, including decreased proliferation and chronic activation, may perturb inflammatory regulation within the callus (51,52). A detrimental effect of aged macrophages on fracture healing has been demonstrated in animal models. By blocking macrophage recruitment, fracture healing was improved in old mice compared to old mice with normal macrophage activity (53). Conversely, in the same experiment, blocking macrophage recruitment in young mice had a negative effect on fracture healing, suggesting their necessary role in fracture healing becomes perturbed with age (53). To investigate the direct contribution of the aged inflammatory response to fracture healing independently of other age-related changes to the organism, chimeric animal studies have been utilized. Xing et al. transplanted young mouse bone marrow into an old mouse before creating a tibia fracture (54). In this design, the osteochondral stem cells were derived from the old host and the inflammatory cells were derived from the young donor. The study demonstrated improved fracture healing in old mice with a young bone marrow transplant (54). As the inflammatory response initiates fracture healing, dysregulation of the aged-inflammatory response may have detrimental consequences on all proceeding stages of healing. Therapeutic targeting of the dysregulated inflammatory response may prove effective for management of fractures in elderly populations (55).
Conclusion:
Fractures in the elderly represent a significant socioeconomic problem that will continue to increase in scope in the coming decades. There is a paucity of clinical data that isolates the effects of aging on bone healing from those of associated comorbidities that are frequently present in this age group. Basic research has demonstrated that all of the components of fracture repair--cells, extracellular matrix, blood supply, and molecules and their receptors—are impacted by the aging process, which likely contributes to poorer clinical outcomes. Treatment strategies and new technological and therapeutic developments should address the multiple specific processes seen in aging tissues.
Footnotes
COI: The authors declares that they have no conflicts of interest.
BIBLIOGRAPHY
- 1.U.S. Census Bureau, Population Division, Population Projections Branch, Projected Population of the United States, by Age and Sex: 2000–2050. [Google Scholar]
- 2.Xu D, Bi FG, Ma CY, Wen ZF, Cai XZ. A systematic review of undisplaced femoral neck fracture treatments for patients over 65 years of age, with a focus on union rates and avascular necrosis. J Orthop Surg Res. 2017; 12: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker MJ, Raghavan R, Gurusamy K. Incidence of fracture-healing complications after femoral neck fractures. Clin Orthop Relat Res. 2007. May;458:175–9. [DOI] [PubMed] [Google Scholar]
- 4.Duckworth AD, Bennet SJ, Aderinto J, Keating JF. Fixation of intracapsular fractures of the femoral neck in young patients: risk factors for failure. J Bone Joint Surg Br. 2011. June;93(6):811–6. [DOI] [PubMed] [Google Scholar]
- 5.Zura R, Mehta S, Della Rocca GJ, Jones J, Steen RG. A cohort study of 4,190 patients treated with low-intensity pulsed ultrasound (LIPUS): findings in the elderly versus all patients. BMC Musculoskelet Disord. 2015. March 1;16:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hankenson KD, Zmmerman G, Marcucio R. Biological perspectives of delayed fracture healing Injury 2014;45:S8–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips AM. Overview of the fracture healing cascade. Injury 2005;36:55–57. [DOI] [PubMed] [Google Scholar]
- 8.Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Cruceta J, Graves BD, Einhorn TA. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor- alpha signaling. Cells Tissues Organs 2001;169:285–94. [DOI] [PubMed] [Google Scholar]
- 9.Einhorn TA., Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nature reviews. Rheumatology 2014;11:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colnot C, Lu C, Hu D, Helms JA. Distinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development. Dev Biol 2004;269:55–69. [DOI] [PubMed] [Google Scholar]
- 11.Lu C, Hansen E, Sapozhnikova A, Hu D, Miclau T, Marcucio RS. Effect of age on vascularization during fracture repair. J Orthop Res 2008;26:1384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobsen KA, et al. Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J Bone Miner Res 2008;23:596–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakahara H, Goldberg VM, Caplan AI. Culture-expanded human periosteal-derived cells exhibit osteochondral potential in vivo. J Orthop Res 1991;9:465–76. [DOI] [PubMed] [Google Scholar]
- 14.Abou-Khalil R et al. Role of Muscle Stem Cells During Skeletal Regeneration. Stem Cells 2015;33:1501–1511. [DOI] [PubMed] [Google Scholar]
- 15.Bahney C, Hu D. Miclau T, Marcucio R, The multifaceted role of the vasculature in endochondral fracture repair. Frontiers in endocrinology 2015;6:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colnot C Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res 2009;24:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le AX, Miclau T, Hu D, Helms JA. Molecular aspects of healing in stabilized and nonstabilized fractures. J Ortho Res 2001;19:78–84. [DOI] [PubMed] [Google Scholar]
- 18.Zuscik MJ, Hilton MJ, Zhang X, Chen D, O’Keefe RJ. Regulation of chondrogenesis and chondrocyte differentiation by stress. J Clin Invest 2008;118:429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury 2005;36:1392–404. [DOI] [PubMed] [Google Scholar]
- 20.Bergman R, et al. Age-related changes in osteogenic stem cells in mice. J Bone Miner Res 1996;11:568–577. [DOI] [PubMed] [Google Scholar]
- 21.Gruber R, Koch H, Doll BA, Tegtmeier F, Einhorn TA, Hollinger JO. Fracture healing in the elderly patient. Exp Gerontol 2006;41:1080–1093. [DOI] [PubMed] [Google Scholar]
- 22.Baxter M, et al. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells.2004;22:675–682. [DOI] [PubMed] [Google Scholar]
- 23.Lopas LA, Belkin NS, Mutyaba PL, Gray CF, Hankenson KD, Ahn J. Fracture in geriatric mice show decreased callus expansion and bone volume. Clin Orthop Relat Res 2014;472:3523–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakahara H, Goldberg VM, Caplan AI. Culture-expanded human periosteal-derived cells exhibit osteochondral potential in vivo. J Orthop Res 1991;9:465–76. [DOI] [PubMed] [Google Scholar]
- 25.Ferretti C, Lucarini G, Andreoni C, Salvolini E, Bianchi N, Vozzi G, et al. Human periosteal derived stem cell potential: the Impact of age. Stem Cell Rev 2015;11:487–500. [DOI] [PubMed] [Google Scholar]
- 26.Lu C, Miclau T, Hu D, Hansen E, Tsui K, Puttlitz C, Marcucio RS. Cellular basis for age- related changes in fracture repair. J Orthop Res 2005;23:1300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abou-Khalil R et al. Role of Muscle Stem Cells During Skeletal Regeneration. Stem Cells 2015;33:1501–1511. [DOI] [PubMed] [Google Scholar]
- 28.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 2003;33:919–26. [DOI] [PubMed] [Google Scholar]
- 29.Brack AS, Muñoz-Cánoves P. The ins and outs of muscle stem cell aging. Skeletal Muscle 2016;6:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sebastian S, Andrew S, Alexandra S. Aging of mesenchymal stem cells. Ageing Res Rev 2006;5:91–116. [DOI] [PubMed] [Google Scholar]
- 31.Lim JE, Son Y. Endogenous Stem Cells in Homeostasis and Aging. Tissue Eng Regen Med 2017; 14:679–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prisby RD, et al. Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO bioavailability in rats. J Bone Miner Res 2007;22:1280–8. [DOI] [PubMed] [Google Scholar]
- 33.Lahtinen T, Alhava EM, Karjalainen P, Romppanen T. The effect of age on blood flow in the proximal femur in man J Nucl Med 1981;22:966–972. [PubMed] [Google Scholar]
- 34.Collin-Osdoby P Role of vascular endothelial cells in bone biology. J Cell Biochem 199455:304–309. [DOI] [PubMed] [Google Scholar]
- 35.Spier S, Delp M, Meininger C, Donato A, Ramsey M, Muller-Delp J. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol 2004;1:947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frenkel-Denkberg G, Gershon D, Levy AP. The function of hypoxia-inducible factor 1 (HIF-1) is impaired in senescent mice. FEBS Lett 1999;462:341–344. [DOI] [PubMed] [Google Scholar]
- 37.Wagatsuma A Effect of aging on expression of angiogenesis-related factors in mouse skeletal muscle. Exp Gerontol 2006;41:49–54. [DOI] [PubMed] [Google Scholar]
- 38.Nyman JS, et al. Age-related effect on the concentration of collagen crosslinks in human osteonal and interstitial bone tissue. Bone 2006; 39:1210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu W, Robey PG, Boskey AL. (2008). The regulatory role of matrix proteins in mineralization of bone In: Osteoporosis. 3rd ed. Marcus R, Feldman D, Nelson D, Rosen CJ, editors. , editors. New York, NY: Academic Press, Chapter 9, pp. 191–240 [Google Scholar]
- 40.Wu M, Fannin J, Rice KM, Wang B, Blough ER. Effect of aging on cellular mechanotransduction. Ageing research reviews 2011;10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferguson C, Alpern E,Miclau T, Helms J. Does adult fracture repair recapitulate embryonic skeletal formation? Mechanisms of Development 1999;87:57–66. [DOI] [PubMed] [Google Scholar]
- 42.Bostrom MP. Expression of bone morphogenetic proteins in fracture healing. Clin Orthop Relat Res 1998;355:S116–23. [DOI] [PubMed] [Google Scholar]
- 43.Rivard A, et al. Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J Biol Chem 2000;275:29643–29647. [DOI] [PubMed] [Google Scholar]
- 44.Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J Histochem Cytochem 2003;51:1119–1130. [DOI] [PubMed] [Google Scholar]
- 45.Meyer RA, et al. Gene expression in older rats with delayed union of femoral fractures. J Bone Joint Surg Am 2003;85:1243–1254. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt-Bleek K, et al. Inflammatory phase of bone healing initiates the regenerative healing cascade. Cell Tissue Res 2012;347:567–573. [DOI] [PubMed] [Google Scholar]
- 47.Franceschi C, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000;908:244–254. [DOI] [PubMed] [Google Scholar]
- 48.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 2013;25:445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrante CJ, Leibovich SJ. Regulation of Macrophage Polarization and Wound Healing. Adv Wound Care 2012;1:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 2010;32:593–597 [DOI] [PubMed] [Google Scholar]
- 51.Sebastian C, Herrero C, Serra M, Lloberas J, Blasco MA, Celada A. Telomere shortening and oxidative stress in aged macrophages results in impaired STAT5a phosphorylation. J Immunol 2009;183:2356–64. [DOI] [PubMed] [Google Scholar]
- 52.Ramanathan R, et al. Serum chitotriosidase, a putative marker of chronically activated macrophages, increases with normal aging. J Gerontol A Biol Sci Med Sci 2013;68:1303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slade Shantz JA, Yu YY, Andres W, Miclau T, Marcucio R. Modulation of macrophage activity during fracture repair has differential effects in young adult and elderly mice. J Orthop Trauma 2014;28:S10–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xing Z, Lu C, Hu D, Miclau T 3rd, Marcucio RS. Rejuvenation of the inflammatory system stimulates fracture repair in aged mice. J Orthop Res 2010;28:1000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark D, Nakamura M, Miclau T, Marcucio R. Effects of Aging on Fracture Healing. Current Osteoporos Rep 2017;15:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]