Abstract
There is a need to quantify and reproduce the mechanical behavior of brain tissue for a variety of applications from designing proper training models for surgeons to enabling research on the effectiveness of personal protective gear, such as football helmets. The mechanical response of several candidate phantom materials, including hydrogels and emulsions, was characterized and compared to porcine brain tissue under similar strains and strain rates. Some candidate materials were selected since their compositions were similar to brain tissue, such as emulsions that mimic the high content of lipids. Others, like silicone, were included since these are currently used as phantom materials. The mechanical response of the emulsion was closer to that of the native porcine brain tissue than the other candidates. The emulsions, created by addition of oil to a hydrogel, were able to withstand compressive strain greater than 40%. The addition of lipids in the emulsions also prevented the syneresis typically seen with hydrogel materials. This allowed the emulsion material to undergo freeze-thaw cycles with no significant change in their mechanical properties.
Introduction
Research on the mechanical properties of soft tissues was initially motivated by the desire to improve the fundamental understanding of the relationship between the tissue’s mechanical properties to their biological processes (e.g., mechano-transduction) and to design biomimetic engineered tissues. Research has also resulted in the development/improvement of mathematical models applicable to in silico brain models used for surgery and injury simulation. Furthermore, the need to study brain mechanical properties has recently been motivated by the need to improve the training of medical professionals by developing more accurate haptic devices [1], simulating brain tissue for research studies tied to shock [2]–[5], and the need to test computer-integrated tools and robot-aided surgery and virtual reality techniques [6]–[8]. To improve physical brain phantoms, it is critical to create materials with the mechanical behavior similar to that of brain tissue that can be used in the training of surgeons, realistic haptic devices, and/or within research studies on personal protective gear, such as helmet testing.
The ideal phantom aims to reproduce the physical, chemical, and mechanical properties of brain tissue. The goal of this study was to either develop a new or identify an existing a brain phantom material with properties suitable for clinical training and surgical planning. We considered three design requirements for the material development: (1) similar compressive stiffness, (2) be structurally stable at room temperature and (3) easy to manipulate while maintaining its structure for manufacturing. The synthetic materials explored for this study included silicones of different hardness, gelatin with and without a chromium crosslinker, agarose, and two emulsions with varying composition.
Brain tissue mechanical properties have been extensively characterized in vitro under the modes of shear [9]–[13], compression [14]–[19] and tension [16], [20]–[24] as well as in situ and in vivo under the modes of magnetic resonance elastography (MRE) [25]–[30], surface suction [31] and indentation [24], [32]–[35]. The human brain is formed by gray matter that constitutes the brain cortex. White matter is composed of the myelinated nerve cell projections (axons) that connect the gray matter areas of the brain to each other. The concentration of lipids in gray matter, white matter and myelin of human brain for a child and an adult were initially established by O’ Brien et al. [36]. A normal human brain of an adult is mainly composed of water, comprising 78.8 wt%, lipids conform 11.5 wt% and non-lipid-residuals make up 9.7 wt%. However, the relationship between the tissue’s composition and mechanical property is not well understood. After years of research about brain tissue mechanical properties, some disagreement regarding differences in mechanical properties of white and gray matter tissues still remains. It has been suggested that gray matter does not have significant mechanical anisotropy (i.e. large differences in directional properties). In contrast, white matter tissue is composed of oriented nerve fibers and is stiffer than gray matter [23], [37], [38]. In general, white matter is considered to be anisotropic while gray matter is nearly isotropic [38]. Therefore, a more consistent response can be found for gray matter than for white matter, that is, less variability in the mechanical characterization [23]. According with Kaster et al. [39], the difference in elastic modulus between white and gray matter is statistically significant (p<0.01) and that seems to validate the assumption made in MRE studies [30][40] that conclude white matter is stiffer than gray matter. The human cerebral cortex (gray matter), which is also a folded sheet, has a depth ranging from 1.0-4.5 mm [41]. This small thickness, complex folding geometry, and extremely soft and delicate properties of brain tissue made it difficult to perform tests of the mechanical properties of gray matter separately from white matter under unconfined, in vitro compression tests.
Despite of more than 50 years of research on brain mechanics, it is still difficult to precisely report brain tissue’s mechanical properties due to the large variation of the results reported in literature. The variation in the reported viscoelastic properties might be attributed to several reasons such anisotropy, regional differences, white matter and gray matter heterogeneity and differences between species as well as specimen shape, specimen size, post-mortem time, in vivo in situ or in vitro experiments, definition of zero strain point, sample preparation and experimental methodology (tension, compression or indentation). Based on these difficulties, we characterized porcine brain tissue mechanical properties to define a protocol that would serve as a baseline in the development of brain tissue phantom materials. Porcine brain tissue was selected since prior work has shown that their mechanical properties are similar to that of human brain tissue [42].
Most prior studies on the development of phantoms that simulated brain mechanical properties have used silicones and/or hydrogels [43]. Silicones are considered relatively easy to manufacture and handle. However, they are typically stiffer than brain tissues. Hydrogels or hydrophilic gels are polymer networks that have the ability to swell by retaining significant amounts of water. Gelatin and agarose are hydrogels often used in the biomedical engineering field for tissue culture and are currently considered phantom materials for soft tissue when considering only mechanical properties. Gelatin is a product derived from collagen that is the principal component of skin, connective tissue, cartilage and bone. Whereas, agarose is a polymer generally extracted from seaweed and is frequently used in molecular biology for electrophoresis [44]–[47]. In this study, we tested silicone gels, agarose and collagen hydrogels, as well as two newly developed emulsions with compositions mimicking brain tissue.
Material and Methods
Porcine brain specimen preparation
Porcine brain tissue was selected as a substitute for human brain tissue due to their similar mechanical properties to human brain tissue, accessibility and possibility to reduce the post-mortem time testing. Two cylindrical samples from each hemisphere (anterior, medial and posterior regions) of each porcine brain were sectioned for compression testing. In our study of the porcine brain tissue mechanical properties, we use samples containing gray and white matter in order to ensure the integrity and stability of the samples during testing (the needed to be able to maintain a shape while performing mechanical tests). A total of 19 brains from six-month old pigs were collected within the Clemson University Godley Snell Research Center; these were a by-product of other terminal studies that followed the NIH guide for the care and use of laboratory animals. Porcine heads were collected immediately after the animals were sacrificed. The intact brain mean weight was 90.92 ± 5.16 g (n=19). Porcine heads were placed into ice to slow down degradation of the tissue and prevent dehydration immediately and transported to the dissection laboratory within 1 h post-mortem. The brains where then stored in phosphate buffered saline solution (PBS) and refrigerated at 4°C to avoid dehydration as studied in [13]. Prior to each experiment, the brain tissue was warmed to room temperature (~23°C). Before sample preparation, the dura-mater, midbrain, and thalamus were removed. Cylindrical specimens were obtained in the inferior-superior direction by utilizing a steel cutter with diameter of 20 mm. This method resulted in only a slight distortion of the pia mater and arachnoid membrane near the edge sliced with this steel cutter. It should be noted that the gyri remained part of the sample and each sampled contained both white and gray matter. Subsequently, specimens were transected with a surgical scalpel to make the samples ~10 mm thick and ensure that the faces were smooth. The diameter was recorded as an average between the diameter of the upper and bottom faces and the height was measure by the gap between testing machine platen when sample was loaded.
Silicone specimens
Silicone samples were obtained from Smooth-On Inc. (pat numbers: 000-35, 00-20, 00-10; Ecoflex-series; Macungie, PA). No modification to the recommended preparation of the mix was perform prior to testing. These samples were included since silicone is currently utilized as a phantom material for brain tissue by researchers [48]–[50] as well as commercial entities (e.g., Somso®, True Phantom Solutions, and MediVisuals Inc. models).
Gelatin specimen preparation
Gelatin was made by mixing 4 g, 6 g or 12 g of granulated gelatin (Carolina Biological Supply Company) into 100 ml of distilled water at 80 °C. Next, the solution was poured into 100 mm petri dishes to a depth of 10 mm and allowed to set at room temperature. These petri dishes were covered and stored up to 12 h at 4 °C to prevent dehydration and degradation prior to mechanical testing. Cylindrical samples (diameter 20 mm diameter, thickness 10 mm thickness) were cut using a steel cutter. Samples will be referred to as 4 g/g%, 6 g/g% or 12 g/g% gelatin.
Gelatin-chromium specimen preparation
To increase the cross-linking in gelatin, chromium was added to the gelatin samples since can act as a cross-linker agent. In our tests, we varied the chromium between 1.7 g/g%. Adding 1.7 g/g% of chromium to a 4 g/g% gelatin solution exhibited a similar behavior as brain tissue.
Gelatin-agarose hydrogels specimen preparation
A range of gelatin-agarose hydrogels were synthesized by varying the gelatin between 4-6 g/g% and the agarose between 0.4-0.6 g/g%. An example formulation for the 3 g/v% gelatin-1 g/g% agarose hydrogel included mixing 4 g of granulated gelatin (Carolina Biological Supply Company) with 0.4 g of agarose type I with low electroendesmosis (Sigma-Aldrich) into 100 ml of distilled water at 80 °C. Next, the solution was poured into petri dishes (diameter 100 mm) to a depth of 10 mm and allowed to set at room temperature. These petri dishes were covered and stored up to 12 h at 4 °C to prevent dehydration and degradation prior to mechanical testing. Cylindrical samples (20 mm diameter, 10 mm thickness) were obtain from each petri dish with the same steel cutter used to section the porcine brain tissue.
Emulsion specimen preparation
Two emulsions consisting of both a lipid and water phase were prepared are refer to as ‘Emulsion A’ and ‘Emulsion B’ and their compositions are reported in Table 1. Gelatin was the substitute for the non-lipid contentment of the brain with borax added to help crosslink. The water phase was prepared adding gelatin to distilled water at 50 °C. Once the solution became clear, borax was added. For the lipid phase, soybean lecithin and flax oil were mixed at 50 °C. In Emulsion A, 3% stearic acid was also added to the lipid mixture. The lipid phase for Emulsion A included organic, pure and unrefined flax oil (Barlean’s), soybean lecithin (VWR, L0023) and stearic acid (Merk, 1.43905). Flax oil contains many of the polyunsaturated lipids found in brain tissue [51], [52]. The soybean lecithin was used as an emulsifier. Stearic acid resembles properties similar to cholesterol in the brain. For Emulsion B, the lipid phase did not include stearic acid.
Table 1:
The composition of the two emulsions tested within this study.
| Emulsion composition (g) | ||
|---|---|---|
| Emulsion A | Emulsion B | |
| Gelatin | 4.0 | 4.0 |
| Lecithin | 5.0 | 5.0 |
| Stearic acid | 3.0 | 0.0 |
| Borax | 1.5 | 1.5 |
| Flax oil | 9.0 | 9.0 |
| Water | 80.0 | 80.0 |
Once both phases were ready, the lipid phase was added to the water phase and emulsified by mixing vigorously with an electric blender for 2 min. The resulting mixture was poured into 100 mm diameter petri dishes filled up to 10 mm. The samples were then allowed to set at room temperature and then covered and stored for 12 h at 4 °C to prevent dehydration until the experiment was performed. Right before mechanical testing, seven cylindrical specimens (20 mm diameter, 10 mm thickness) were cut using a round steel cutter.
Mechanical setup
Uniaxial unconfined compression testing was conducted on all samples using an ElectroForce® test instrument (Bosel 3230, System 11-231). The cylindrical specimens compressed between two impermeable platens (Figure 1). The load cell was attached to the lower platen and had a range of −17.5 N to +22.0 N. The experiment was recorded with a camcorder (JVC Everio GZ-E200BU) and the images were used to calculate the initial diameter and thickness for each specimen and calculate the radial displacement. The radial displacement was maintained to ensure that each sample was compressed uniformly between the upper and lower platens. The initial diameter was used to obtain the initial cross-section area of each sample. One loading cycle was executed on each sample and all procedures were performed at room temperature and to ensure full contact the tissue sample was preloaded with a force of 0.01 N.
Figure 1.

Representative images of the unconfined compression mechanical test of porcine brain tissue and synthetic substitutes on 20 mm humidified platens. (a) Porcine samples including gray and white matter, (b) hydrogel based substitute material, (c) emulsion substitute material. Unloaded position read a compression force of 0.01 N and loaded position is 40% strain condition. Bottom platen remained static while top platen did the loading movement.
Precondition means that the response of the first loading (virgin or unconditioned response) is measured to be significantly stiffer than those observed for the immediate subsequent loadings. This may be due to interstitial diffusion within the tissue [15]. The lower the rate of deformation the effects appear to be attenuated [24]. Although some authors precondition their samples in order to get a standardized initial condition, Prevost et al. [24] observed that preconditioning in the tissue or altering the rate of load-unload sequence in vivo, in situ, and in vitro does not significantly impact measurements. These observations corroborate previously reported results in vitro tested uniaxial compression tests [15]. On the other hand, Kaster et al. [39] considered the second loading cycle as the most appropriate for analysis because the first cycle was “less smooth” than the following cycles. Given these prior results, we chose not to perform preconditioning in this study. The first loading cycle can sometimes induce non-reversible tissue damage at higher strains due to the tissue’s delicacy and adhesiveness. In addition, brain tissue does not experience cyclic loading inside the cranium during the surgical procedures we aim to mimic with the brain phantom because surgeons try to minimize repeated motion and handling of the tissue during surgery.
Before placing specimen on mechanical tester platens, platens were wiped out and then air dried for each test to ensure the friction between the sample and the platens was consistent. Platens and tissue were hydrated with PBS to ensure pure slip boundary between the face of each platen and the upper and bottom surfaces of the tissue sample as well as to maintain the specimen wet to limit tissue degradation. Pure slip boundary was confirmed by visual inspection of recorded images. Friction can be assumed to be zero when the sample expands uniformly during the compression test [53].
Thus, the undeformed height of the specimen was determined by the gap between the upper and lower platens after preloading was applied and used as the initial dimension of the specimen to calculate the nominal strain. Nominal stress was calculated based on the axial force and initial cross-section area of each specimen. Apparent elastic moduli E1, E2, E3, E4 and E5 was also obtained as the slope of the stress-strain curve in the strain ranges of (0-0.1, 0.1-0.2, 0.2-0.3, 0.3-0.4 and 0.4-0.5, respectively).
The displacement and force applied were acquired with the ElectroForce® test instrument. A series of compression test were carried out at three different loading rates. The velocity of the upper platen was set at 10 mm/s, 1 mm/s and 0.1 mm/s that correspond to strain rates of 1 s−1, 0.1 s−1, and 0.01 s−1, respectively. Strain rate sensitivity of brain tissue was examined as a reference point for development of brain tissue-mimicking materials.
Anatomical location and post-mortem time effects were considered for this study, as well. First, specimens in the inferior-superior direction were obtained from three different anatomical locations to check regional heterogeneity of the cerebral cortex, stress-strain responses at a strain rate of 1 s−1 were compared between specimens transected from: anterior (n = 16), mid (n = 6), and posterior (n = 16) regions. Second, to check post-mortem time effects: samples tested 24 h post-mortem at a strain rate of 1 s−1 were compared with specimens tested within 6 h post-mortem (Supplemental Figure 3). The mechanical response of brain tissue at different strain rates (1 s−1, 0.1 s−1 and 0.01 s−1) was also examined. The stress-strain relationships obtained in the regional heterogeneity experiment were used as a baseline for brain tissue-mimicking materials’ development.
Mechanical analysis of brain tissue and development of tissue mimicking materials was carried out at strain levels up to 30% of strain. Since it was demonstrated that brain tissue mechanical response was dependent of anatomical location and post-mortem time at strain levels greater than 30%.
The same experimental protocol was followed to measure mechanical properties of all materials. Specimens were stored at 4 °C; samples were placed at room temperature for 10-20 min before testing. The material temperatures were not found to vary significantly in the hour following the initial 10-20 min warm up time.
Relaxation test
Relaxation experiments were performed following compression tests. A series of ramp and hold tests were conducted on brain tissue and brain substitute materials. Specimens were compressed at 1 s−1 to two different strain levels and held for 600 s. Porcine brain tissue (n = 34), Gelatin-chromium (n = 5), and Emulsion B (n = 10) samples were tested to 50% strain. Additionally, Gelatin-agarose (n = 15) and Emulsion B (n = 10) samples were tested to 35% strain.
The time-dependent stress response, G(t), was obtained by normalizing the relaxation measurements by the maximum peak stress in each case (Eq. 1) and approximated by a sum of exponentials to compare the time course of brain tissue and brain substitute materials (Eq. 2).
| (Eq. 1) |
where σ(t) is the stress at time t and σ(0) is the peak stress.
| (Eq. 2) |
where τ1, τ2, τ3 and τ4 are time constants. The coefficients G1, G2, G3, G4 and G5 below condition (Eq. 3), because the instantaneous modulus, G(0), is unity.
| (Eq. 3) |
The time constants represent the elapsed time required for the material response to relax to zero if the relaxation response had continued to decay at the initial rate. However, due to the continuous change in the relaxation rate, the material relaxation response gets effectively decreased in value to 1/e or 36.79% of the initial value for a given step decrease. The curve fitting procedure used four exponentials and provided a good fit (R2~99.9).
Degradation test of gelatin-agarose hydrogels and emulsions
As the gelatin-agarose hydrogels and emulsions are biodegradable are prone to disintegration by bacteria, fungi or other biological means [54], the impact of storage conditions on mechanical performance and weight loss needed to be evaluated.
A series of mechanical tests were carried out on the gelatin-agarose hydrogel and emulsion materials that were either never frozen (kept at 4 °C) or were frozen (kept at −20 ° C) for a period of time (1, 8, 15, 22, or 29 days) after synthesis (Table 2).
Table 2:
Number of samples tested per material every week over the course of a month.
| Material | Storage Method | Days Stored |
||||
|---|---|---|---|---|---|---|
| 1 | 8 | 15 | 22 | 29 | ||
| Hydrogel | Frozen | 5 | 6 | 8 | 7 | 4 |
| Hydrogel | Non-Frozen | 10 | 10 | 10 | 10 | 10 |
| Emulsion B | Frozen | 10 | 10 | 11 | 10 | 10 |
| Emulsion B | Non-Frozen | 9 | 10 | 11 | 11 | 11 |
To identify if the storage conditions resulted in sample weight loss, another set of samples were prepared and their weight measured before and after storage (n = 10 for each condition and time point).
Brain phantom fabrication and storage test
Magnetic Resonance Imaging (MRI) data utilized in this study was provided by Dr. Jane E. Joseph from the Medical University of South Caroline (MUSC). This data was processed with the software package Mimics (Materialise, Belgium) and obtain a stereolithography (STL) files of each brain, which is a common 3D object file format compatible with 3D printing services. Negative molds were then created of the brain core components (brain stem and cerebellum) and the brain cortex. Finally, the molds were 3D printed using standard polylacticides (PLA) and nylon materials to be able to inject the materials (hydrogel, emulsion) and create brain-like phantoms. Two brain phantoms, one made out of hydrogel and one made out of the emulsion, were vacuumed sealed and then stored in a freezer (−20 °C) for one month (29 days) in order to see the effects that the tight packing would make to their shape.
Results
Performance of porcine brain tissue
Stress-strain behavior of samples from different anatomical location was characterized in order to investigate whether there is a regional effect at strains up to 50%. The averaged stress-strain curves of the brain tissue (all rates and regions) was concave upwards. Results were compared quantitatively across sample regions in terms of peak force level reached and elastic modulus. Results showed there is no statistical difference among samples up to 30% strain, nevertheless, the mid region was found to be different than the anterior and posterior regions at strain levels greater than 30%. This can be attributed to the high content of white matter located in the mid region. In addition, one-way analysis of variance was conducted and elastic moduli E1, E2 and E3, and peak stresses were not found to be statistically different (p-value > 0.05). Although E4 and E5 were statistically different for the mid region, the difference in the measurements remained small. Data obtained from different regions of the brain were considered homogeneous up to 30% strain, thus, combined together for further analysis. Table 3 summarizes elastic moduli for the three regions of the brain. Donnelly et al. [55] also reported that stress-strain relationship was brain-location independent using fresh human brain samples measured, 17 mm in diameter and 12 mm in thickness. Therefore, brain tissue samples with 20 mm diameter and 10 mm height tested in this study were assumed to have a isotropic response with no significant regional effects. The mean diameter of the unloaded porcine brain specimens was 19.00 ± 0.79 mm and the height was 9.82 ± 0.80 mm (n = 97). Post-mortem time test was also carried out in the tissue response resulted statistically different between 6 h and 24 h post-mortem with a decrease of ~20% and ~30% of stress at 40% and 50% strain levels, respectively (see supplemental figures).
Table 3:
Apparent elastic moduli of the three identified regions on the brain. (p-value < 0.01). Posterior (n=16), Mid (n=6), Anterior (n=15).
| Region | E1 | E2 | E3 | E4 | E5 |
|---|---|---|---|---|---|
| kPa | kPa | kPa | kPa | kPa | |
| Posterior | 5.33 ± 1.52 | 10.34 ± 2.60 | 19.04 ± 2.88 | 29.27 ± 4.36 | 33.27 ± 10.11 |
| Mid | 5.76 ± 0.63 | 12.19 ± 1.50 | 24.72 ± 2.62 | 39.77 ± 5.28 | 45.02 ± 14.85 |
| Anterior | 5.30 ± 1.52 | 10.41 ± 2.49 | 19.19 ± 4.01 | 30.85 ± 6.31 | 39.11 ± 6.14 |
The stress-strain relationship for the three strain rates tested in this study indicated the stress response to be nonlinear (Figure 3). Tissue response was found to be highly rate dependent where tissue dramatically stiffens at greater strain rates. The increase in the stress response was 70% and 40% from 0.01-0.1 s−1 and 0.1-1.0 s−1 respectively at 30% strain. One-way ANOVA tests shows there is significant difference between the apparent elastic moduli at each strain rate (p value > 0.05).
Figure 3.

Porcine brain averaged stress-strain relationships in unconfined compression test for 1s−1 (n=25), 0.1s−1 (n= 11), and 0.01s−1 (n = 10) strain rates.
Performance of the gelatin-agarose hydrogel
The diameter and height of the unloaded of gelatin-agarose specimens were 20.44 ± 0.49 mm and 9.5 ± 0.13 mm (n = 10), respectively. Figure 4 displays the averaged stress-strain relationship of some hydrogels and silicones tested at 30% strain during the evolution of our tissue-mimicking material development compared against the brain tissue mechanical behavior. Formaldehyde preserved fixed sheep brain tissue (VWR International, LLC) was also tested to have an overall picture how stiff brain tissue becomes acquiring different mechanical properties when preserved for teaching purposes (black dashed line). As can be seen in the graph, gelatin 12 g/g% and 6 g/g%, is considerably stiffer than brain tissue (black solid line). 4 g/g% gelatin had closer behavior; however, very low concentrations of gelatin resulted in gels that were extremely difficult to handle without tearing or damaging. Agarose alone is also stiffer than brain tissue and has a reduced failure point (less than 25% strain). Shore 000-35 silicone was the softest silicon tested, showing a much stiffer mechanical response compared to brain tissue, 00-20, 00-10 and 000-35 silicones (Ecoflex-series, Smooth-On Inc. Macungie, PA) peak stress at 30% strain were much higher than observed in the brain tissue (6.7, 5.6 and 4.7 times higher, respectively) and their stress-strain responses were linear instead of concave upwards as brain tissue response revealed to be. Mixed gelatin (6 v/v% and 4 v/v%) and agarose (0.6 v/v% and 0.4 v/v%) at different ratios exhibited a mechanical behavior similar in shape to that of brain tissue (Figure 5). Gelatin 4 v/v%-Agarose 0.4 v/v%, 3:1 ratio (3 v/v% gelatin- 1 v/v% agarose, green solid line) resulted the best approximation to stress-strain relationship of brain.
Figure 4.

Stress-strain relationships porcine brain tissue and synthetic materials: hydrogels, silicones, emulsions. Samples were tested under compressive loads at 35% strain and strain rate of 1 s−1.
Figure 5.

(a) Stress-strain mechanical response of brain substitute materials and brain tissue at a strain rate of 1s−1 and strain of 30% and (b) Averaged stress- strain curves of 3% gelatin- 1% agarose material at different strain rates.
Gelatin-agarose material was not able to withstand compressive strains over 35% without reaching failure point. As the other materials were tested at 50% strain, in order to be able to compare the relaxation curves among all materials and to investigate the effect of the applied strain levels, some relaxation experiments were conducted using emulsion samples. Data was obtained at two different compressive strains (50% and 35%) during a strain rate of 1 s−1.
The strength in a hydrogel is derived from the cross-links in the system, Forte et. al. [56] studied gelatin gels up to 15% w/w concentrations, tested under unconfined compression, they demonstrated that fracture strain increased as rate increased, tests performed produced failure points at strains smaller than 20% at strain rates slower than 1.0 s−1 , since gelatin was shown to be weak and not handleable at low concentrations registering low failure points, it was strengthened with chromium. Chromium acts as a cross-linker agent for gelatin, increasing the number of bonds within the polymer network, hence improving its mechanical strength. Tests of gelatin mixed with different concentrations of chromium were carried out and results showed improvement of the polymer mechanical response and reduction of brittleness allowing 4% gelatin concentrations withstand strain levels greater than 50%. Adding 1.7% of chromium to a 4% gelatin solution exhibited a similar behavior as brain tissue. However, gelatin-chromium mechanical behavior at greater strains than 30% were much different than brain tissue (Appendix A.1), thus, only gelatin-agarose will be further analyzed.
Force and displacement were obtained at three different strain rates. The force (N) was divided by the cross-sectional area to determine the compressive nominal stress. The stress strain curves resulted concave upward for all compression loading velocities. A total of 25 samples were tested at 10 mm s−1 up to 30% strain. One loading cycle was performed in each specimen that results were averaged into a single value for every strain rate. The stress response was found to be nonlinear. Mechanical behavior was found to be highly rate dependent where tissue dramatically stiffened with increasing strain rate. Stress strain relationships are summarized in Figure 5. The increase in the stress response was found to be ~20% and ~50% from 0.01-0.1 s−1 and 0.1-1.0 s−1 respectively at 30% strain. One-way ANOVA test determined there is significant difference between the apparent elastic moduli at each strain rate (p-value < 0.05).
Performance of emulsions
The actual diameter and height of the unloaded emulsion specimens were measured as 20.76 ± 0.25 mm and 9.8 ± 0.81 mm (n = 10) for Emulsion A and 20.66 ± 0.33 mm and 9.5 ± 0.30 mm (n = 10) for Emulsion B. Figure 6 depicts the averaged stress-strain relationship of emulsions A and B compared to brain tissue. As shown in the graph, Emulsion A is too stiff compared with brain tissue stress-strain curve. This is due to stearic acid that acts as a hardener, even low concentrations made a big difference in the mechanical response of the emulsion. In the other hand Emulsion B has a good approximation to brain tissue response. Thus, further analysis was made for Emulsion B only.
Figure 6.

a) Stress-strain curve of Emulsions A and B (stearic acid free) compared to brain tissue mechanical response at a strain rate of 1 s−1 and 30% strain. b) Averaged stress- strain relationships of Emulsion B mechanical response at different strain rates.
Force and displacement were obtained at three different strain rates. The stress strain curves resulted concave upward for all velocities, the same behavior shown by brain tissue and the hydrogel material. Figure 6 depicts typical responses of Emulsion B under compression loading. A total of 30 samples were tested. One loading cycle was performed on each specimen and results were averaged into a single value for every strain rate.
Strain Rate dependency
Mechanical behavior was found to be highly rate dependent where tissue stiffens at greater strain rates. Stress strain relationships are summarized in Figures 6. The increase in the stress response is ~ 22% and ~ 40% from 0.01 to 0.1 s−1 and 0.1 to 1 s−1 respectively at 30% strain. One-way ANOVA test showed there is significant difference between the apparent elastic moduli at each strain rate (p-value < 0.05). Figure 7 summarizes the different apparent elastic moduli at each strain rate for the brain tissue, hydrogel, and emulsion.
Figure 7.

Average apparent elastic moduli of brain tissue (black bars, n = 38 from 19 brains), gelatin (3%) – agarose (1%) hydrogel (red dotted bars, n =10), and emulsion B (dashed green bars, n = 10) at 1 s−1, 0.1 s−1, and 0.01 s−1 strain rates.
Temperature dependency of hydrogel, emulsions and brain tissue
A well know problem when trying to characterize the mechanical properties of materials is the disparity in protocols that will lead to variation of results in the literature, especially when working with living tissues. One aspect that will yield to differences in measurements is the temperature at that tests are carried out. Brain phantom materials used for surgical training or planning would likely be utilized at room temperature. However, many materials used to fabricate these phantoms are prone to biodegradation and dehydration if not stored properly at low temperatures (e.g., refrigerator or freezer). For instance, gelatin-based hydrogels, often used at room temperature as a phantoms for a wide range of biological tissues, change mechanical properties with temperature but have to be stored in cold condition to stave off degradation [54].
To determine the effect of temperature on the mechanical properties of our candidate materials, a series of mechanical test were conducted on hydrogel/agar (3:1) and emulsion B specimens at different times post refrigeration. Samples were maintained at 4 °C, then five samples of each material were tested at a strain rate of 1 s−1 and up to 30% strain at 0, 10, 20, 30, 40, 50, 60, 70, 240 and 400 minutes after being removed from the refrigerator and placed at room temperature. By normalizing the stress-strain responses with is peak force in each case, mechanical response of both materials could be compared with each other as well with brain tissue mechanical response. Analysis of variance was used to determine that Elastic moduli E1, E2 and E3 of oil-based material, hydrogel-based material and brain tissue are temperature dependent (p-value < 0.05). Figure 8 represents the averaged stress normalized to the peak stress versus strain for each material.
Figure 8.

Averaged stress normalized to peak stress vs. strain of emulsion (green), hydrogel based material (red) and brain tissue (black) tested at 1 s−1 strain rate.
Stress was found to change as a function of temperature. For both materials, mechanical behavior softens with increasing temperature. The peak stresses decreased to ~45% of their initial value in 60 min after samples were removed from the refrigerator. Emulsion seemed to stabilize within an hour while gelatin continued warming up. After 4 h, both materials have reached a 23 °C temperature.
Relaxation of hydrogel, emulsions and brain tissue
The mechanical response of a material can consist of both a viscoelastic and an elastic component. The plateau values of the relaxation curve are determined by the elastic part but the elastic and viscoelastic together affect the height of the peak response when loads are applied [12]. Comparing between same strain levels, gelatin with chromium, the emulsion, and brain tissue do not match. Gelatin-chromium and emulsion (50% strain) have similar plateau values but their averaged peak stresses were significantly higher than the brain tissue; the peak stresses at 50% strain were 21.75 kPa, 17.20 kPa, 10.76 kPa gelatin-chromium, emulsion and brain tissue, respectively.
During relaxation, the force changed as a function of applied strain. Nevertheless, once the relaxation curves of the emulsion at strain levels of 35% and 50% were normalized to its peak force, the emulsion relaxation response was found to be independent of the strain applied representing a time-strain separable material. In other words, the relaxation response is a function of time but does not depend on the level of strain applied.
During the stress relaxation test, the compressive force decreased very rapidly initially (within 0.5s) and continue decreasing gradually during the time range allowed. Table 4 summarizes the decrease of the change in compressive force from the initial peak force (%) after 1 s. Notice that the stress measured on the emulsion at 50% and 35% strain levels dropped about 30% and 28%, respectively, 1 s after load was applied, which demonstrates a strain independency. Based on these results, the gelatin-agarose relaxation response will be assumed independent of applied strain level. Thus, it can be compared with brain tissue and gelatin-chromium material relaxation curves tested at 50% strain.
Table 4:
Force percentage decreased after 1 s of reaching a peak stress during relaxation experiments.
| Material | % Decrease in stress |
|---|---|
| Brain | 63% |
| Gelatin-agarose | 49% |
| Gelatin-chromium | 43% |
| Emulsion (50% strain) | 30% |
| Emulsion (35% strain) | 28% |
Figure 5 depicts the normalized stress response of brain tissue and brain substitute materials. The coefficients G1, G2, G3, G4, G5, τ1, τ2, τ3 and τ4 were determined for each individual test for all materials in order to study the differences in time courses of the relaxation response G(t) of brain tissue and three brain substitute materials (Table 5). Tukey-Kramer tests were performed for each coefficient. All coefficients revealed differences across materials but are in the same magnitude order.
Table 5:
Mean parameter values for brain tissue and substitute materials’ relaxation functions of the form G(t) = G1e−t/τ1 + G2e−t/τ2 + G3e−t/τ3 + G4e−t/τ4 + G5.
| Material | Porcine Brain Tissue | Emulsion | Gelatin-chromium | Emulsion | Gelatin-agarose Hydrogel |
|---|---|---|---|---|---|
| Strain | 50% | 50% | 50% | 30% | 30% |
| G1 | 0.53 ± 0.02 | 0.31 ± 0.03 | 0.37 ± 0.03 | 0.25 ± 0.01 | 0.36 ± 0.02 |
| τ1 (s) | 0.60 ± 0.01 | 1.17 ± 0.01 | 1.23 ± 0.03 | 1.40 ± 0.02 | 0.55 ± 0.01 |
| G2 | 0.18 ± 0.01 | 0.08 ± 0.01 | 0.16 ± 0.04 | 0.08 ± 0.01 | 0.16 ± 0.01 |
| τ2 (s) | 2.03 ± 0.21 | 4.07 ± 1.02 | 2.44 ± 0.92 | 2.87 ± 1.12 | 1.02 ± 0.13 |
| G3 | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.05 ± 0.01 | 0.08 ± 0.01 | 0.04 ± 0.01 |
| τ3 (s) | 21.57 ± 0.75 | 37.53 ± 2.55 | 25.29 ± 6.26 | 43.98 ± 3.20 | 34.35 ± 4.43 |
| G4 | 0.08 ± 0.01 | 0.17 ± 0.01 | 0.13 ± 0.01 | 0.23 ± 0.01 | 0.13 ± 0.01 |
| τ4(s) | 240.35 ± 11.18 | 330.41 ± 23.78 | 507.01 ± 70.93 | 433.73 ± 36.97 | 425.66 ± 47.39 |
| G5 | 0.10 ± 0.01 | 0.31 ± 0.04 | 0.26 ± 0.02 | 0.34 ± 0.02 | 0.28 ± 0.01 |
Furthermore, the measured stress decreased rapidly within the first time constant, (τ1), ~1 s, after the load was applied (ranged from 0.5 −2.6 kPa). The long-term time constant τ2 and τ4 were found to be not statistically different between the brain phantom candidate materials but all were significantly different from brain tissue. Brain tissue at time constant τ1 resulted in significantly different to the emulsion and gelatin with chromium as well as time constant τ3 was found to be similar only to gelatin-chromium material.
Degradation testing of hydrogels, emulsions and brain tissue
The following characteristics were observed on brain substitute materials after their respective time stored and registered before mechanical testing (Table 6).
Table 6:
Macroscopic findings of oil and hydrogel-based materials after 1, 8, 15, 22 and 29 days frozen and refrigerated (Figure 11).
| Material | Storage Method | Observations |
|---|---|---|
| Gelatin-Agarose Hydrogel | Frozen | Hydrogel macroscopic structure was affected within a week. Starting at Week 1, tears were found within the sample structure, the expansion of ice crystals compromised the polymer structure. Syneresis observed. Week 3, volume reduction was observed, and surface of sample was clearly uneven. |
| Non-Frozen (refrigerated) | Clear changes appeared at Week 3, stiffening and dehydration were remarkable, and at Week 4 bumps could be observed over the surface and shrinkage of the whole structure. | |
| Emulsion | Frozen | A thin oily layer over the sample surface and a slight change of color. No shrinkage. |
| Non-Frozen (refrigerated) | At Week 3, samples felt stiffer. Shrinkage observed at Week 4. |
In general, non-frozen materials tended to stiffen through time as trending lines (green and light green) confirm. In the other hand, frozen materials did not show important changes in peak stresses over a one-month period of storage, similar behavior was found in the elastic moduli measurements. One-way ANOVA and Fisher’s Least Significant Difference (LSD) statistical methods were used to compare E1, E2 and E3 and the peak stress measured at 10%, 20% and 30% strain levels. All materials except the frozen emulsion, were significantly different in at least one measured parameter for all five replicates. Frozen emulsion was the only one condition that was not statistically different over the 29 days of the experiment. Frozen hydrogel samples fractured during the mechanical experiments and only a few samples could be tested due to the tears caused by ice crystals.
Figure 11 displays the percentage of weight loss for each material measured every week for one month. Non-frozen materials experienced a significant loss of weight at Week 2 and thereafter. The frozen hydrogel also had a significant loss of weight, especially in Week 1. In the other hand, the frozen emulsion did not show a significant change in weight during the four weeks.
Figure 11.

Percentage of weight loss of tissue mimicking materials tested at days 1, 8, 15, 22 and 29. (n=10).
Discussion:
The apparent compression properties of porcine brain are affected by the inherent variation in the composition of biological materials and the variation on the cross-sectional area due to deviation from cylindrical shape, recorded up to 8% and plotted in Figure 2a. Brain tissue response was compared to those reported in previous studies by Tamura et al. and Prevost et al. [15], [17] as plotted in Figure 2b. There were no significant differences in elastic moduli E1 E2, E3, E4 and E5 between both articles and the mean values of our collected data (supplemental Table 1), one-way analysis of variance was perform, p-value > 0.05. It should be noted that high strains are not expected to be applied during neurosurgery procedures, since previous studies [17] suggest that traumatic brain injury (TBI) occurs at strains greater than ~20% and strain rates greater than 10 s−1.
Figure 2.
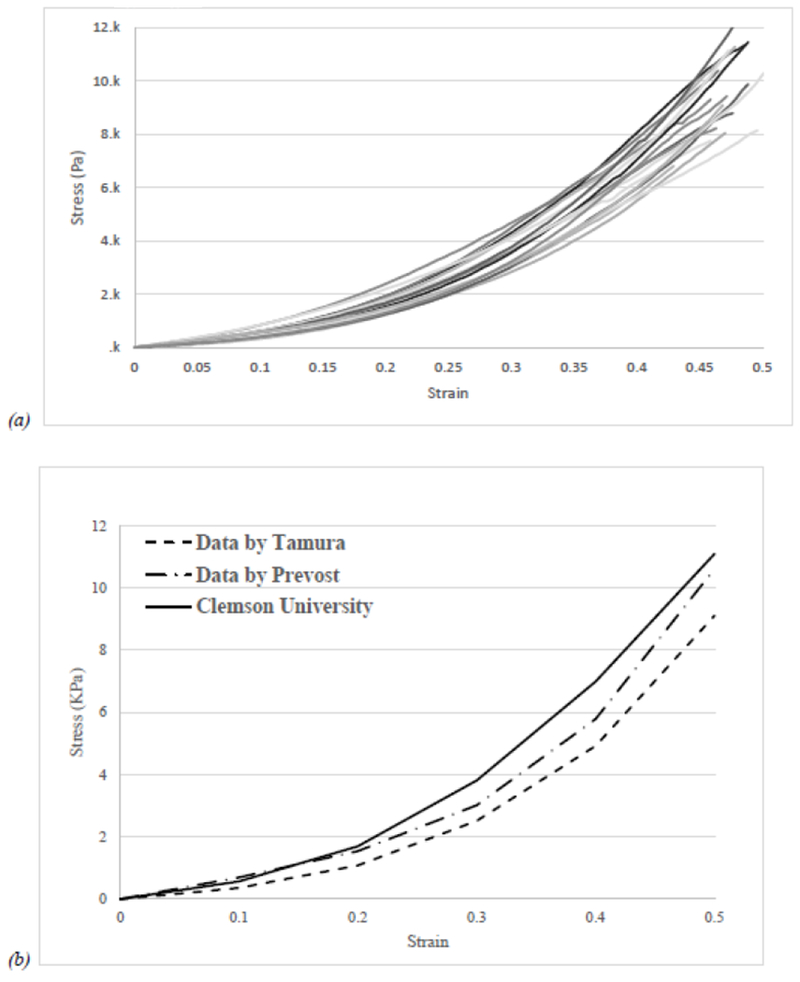
(a) Typical response of brain tissue in compression test obtained at strain rate of 1s−1 up to 50% strain. (b) Comparison of the average response of porcine brain tissue to literature reports. Tamura et al. [16] used 14 mm tall specimens to 50% strain at 1 s−1 strain rate, approximation using ImageJ and Prevost et al. [14] first load response on 9 mm tall specimens to 50% strain at 1 s−1 strain rate, approximation using ImageJ.
According to Miller [53], brain tissue tested in unconfined compression can be affected by friction from supporting platens, resulting in an apparent augmented reaction force. Thus, there is a possibility that test results in this study might be overestimated. For this reason, platens (seen in Figure 1) were kept humidified during experimentation to minimize friction.
Furthermore, we investigated the effect of hours’ post-mortem on mechanical properties. Porcine brain specimens tested under compression loadings at 6 h and 24 h post-mortem at different strain rates. In several studies tissue mechanical property variations related to post-mortem degradation and dehydration were found to be negligible up to 15 h [12], [15]–[22], [24]–[30], [33]–[35], [57]. There have been some studies that reported tissue stiffening with increasing post-mortem time [42], [58]. In studies consistent with this study at high strain levels, authors have suggested that degradation takes place with increasing post-mortem time due to autolytic process, rigor mortis or osmotic swelling [32], [37], [38], [59], [60].
Although, Prange and Margulies showed that there is significant difference between white matter and gray matter attributed to anisotropy [38], their samples were 1 mm of thick, at small dimension scales directional properties will be identified but working with large enough samples, soft tissue does not exhibit directional structure [14], [17], [20], [33], [53], [61]–[65]. in our study, measurements made at numerous samples, with different concentrations of gray matter and white matter did not appear to have an influence in the mechanical response of brain tissue under compressive loading, which is consistent with the studies made by Kaster et al. [39]. As expected, brain response reported in Figure 3 are strain rate dependent, tissue elicit stiffer properties at increased strain rates. The averaged stress-strain relationships obtained in this study showed to be similar compared to previous results obtained under moderate loadings [15], [17]. Similar behavior has been reported in other brain biomechanics studies in vivo [66], in situ [24] and in vitro [13], [14], [17], [23], [24].
Synthetic material for brain tissue phantom
We tested many material types and formulations. Our goal was to find a material with mechanical properties similar to brain tissue, which could be structurally stable at room temperature, and easy to manipulate while maintaining its structure for manufacturing. In this study, we considered silicones, hydrogels, and emulsions.
Silicone possess several properties that are relevant to those of brain matter; it is soft to the touch, sticky, has low toxicity, high heat resistance, and it can be colored; however, as shown in Figure 4 silicones are significantly stiffer than brain tissue which limits their suitability for their use in realistic surgical training and planning models. Silicones would be better suited for models where the identification and appearance of the structure represented is enough.
We did find a hydrogel and an emulsion formulation with compressive mechanical properties close to those of brain tissue, reported in Figure 5–8. The hydrogel resulted relatively easy to fabricate into complex shapes by molding. However, the hydrogels were difficult to store over long periods of time. Figure 11 shows hydrogels kept at 4 °C and −18 °C lost over 5-18% of their weight after 8-15 days, suggesting that low temperatures were not sufficient to maintain the structural integrity of the phantom. Not even vacuum sealing was able to avoid syneresis and tear marks were apparent on hydrogel phantoms. In contrast, the emulsion phantom was a bit more complicated to mold compared to the hydrogel. But the overall fabrication using the emulsion was feasible. Figure 11 shows that emulsion phantoms preserve their weight significantly better when stored at −18 °C, only modifying its weight <3%. More importantly, when emulsion was vacuum seal, no detectable changes in its shape were notice. Suggesting that the vacuum sealing does not affects the physical characteristics of the emulsion brain phantom. The current phantom has a pale yellowish color that is not relevant to the natural brain color; further studies will address this important feature to ensure that a color dye will not affect the viscoelastic properties.
Cast method has been the most common manufacturing practice to build brain models; however, novel technologies such as cryogenic 3D printing has been used successfully in creating soft materials such as chitosan [67] and polyvinyl alcohol/Phytagel /hydrogel [68], although this last may create 3D complex geometrical structures and mimic the mechanical properties of soft tissue, there is still need to achieve the convoluted shape of brain as well as full size dimensions which is of great importance when using brain phantoms for training and/or planning surgery. 3D printing enables a more spatiotemporal control on the fabrication of highly complicated organ such as the brain.
Regarding the relaxation results, the fastest relaxation occurred within the first second after load was apply and was in the range of 0.5-2.6 kPa. In terms of haptic sensation, it is unlikely that humans would be able to perceive such small differences in terms of compressive force occurring at a short time. Dargahi et al [69] reported that human has a sensitivity of 0.2 g/mm2, which is equivalent to 1.96 kPa. In addition, surgeons use double gloves and touch the brain using tools that would dampened even more their ability to distinguish between our substitute material and brain tissue.
In addition to the brain model application, our developed material has the potential to be used on surgical models for lung since Tan et al [70] has shown that brain and lung had similar behavior. Moreover, the materials can be tunable to achieve similar mechanical properties of other organs such as liver that is stiffer that brain/lung, in the order of 10 KPa. The ability to adjust the materials properties depending on the targeted organ is important for applying the model. In this area, simulations of brain and substitute materials is essential as studied elsewhere [56], [71].
It should be noted that none of the materials exhibited the full range of mechanical properties of the actual brain tissue. In particular, the materials did not replicate the viscous relaxation behavior observed; after compression, the brain tissue relaxed much more than any of our candidate phantom materials. For a material to be used for surgical planning or training application, it must ‘feel’ similar to the tissue it is mimicking. Further haptic studies are required to identify how close the viscoelastic properties need to be for the material to have the haptic feel of real tissue.
Conclusions:
In this study, we analyzed the compression properties of various materials to determine candidate phantom materials with mechanical properties close to that of brain tissue. The materials explored include silicone, gelatin (with and without chromium), hydrogels (gelatin-agarose) and oil-supplemented emulsions. Silicones were found significantly stiffer when compared to properties of brain tissue. By contrast, formulations for one of the hydrogels and an emulsion had similar elastic moduli to that of the brain tissue; however, the suitability of the hydrogel formulation as a brain phantom material was found to be limited by the poor structural integration after regular handling and storage test. The emulsion formulation developed in this study show promising application moving forward in the development of human relevant brain phantoms.
Supplementary Material
Figure 9.


(a) Averaged relaxation response of brain substitute materials and brain tissue. (b) Time curve of the averaged normalized relaxation responses of brain tissue and tissue-mimicking materials against a logarithmic time scale. (c) Averaged relaxation response of brain substitute materials and brain tissue first 2 seconds.
Figure 10.

Emulsion brain phantom 29 days after storage at freezer, ~ −18°C. No macroscopic differences could be found but a slight darker color.
Acknowledgements:
The authors would like to thank CONACYT from México, NIH P20 RR-016461, NIH K25 HL092228, NSF CAREER CBET 1254609 for partially funding for this research. This work was partially performed on the facilities supported by a grant from NIGMS of the National Institutes of Health under the award number 5P20GM103444. Professor Jane Joseph, PhD, at the Medical University of South Carolina generously provided the MRI data used in the fabrication of brain phantoms. Professor John Parrish, DVM, PhD, and attending veterinarian, for the procurement of porcine tissue.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- [1].Maddahi Y, Zareinia K, Tomanek B, and Sutherland GR, “Challenges in developing a magnetic resonance-compatible haptic hand-controller for neurosurgical training,” Proc. Inst. Mech. Eng. Part H J. Eng. Med, vol. 232, no. 12, pp. 1148–1167, Oct. 2018. [DOI] [PubMed] [Google Scholar]
- [2].Jiang Y, Li G, Qian L-X, Liang S, Destrade M, and Cao Y, “Measuring the linear and nonlinear elastic properties of brain tissue with shear waves and inverse analysis,” Biomech. Model. Mechanobiol, vol. 14, no. 5, pp. 1119–1128, 2015. [DOI] [PubMed] [Google Scholar]
- [3].Ma X, Aravind A, Pfister BJ, Chandra N, and Haorah J, “Animal Models of Traumatic Brain Injury and Assessment of Injury Severity,” Mol. Neurobiol, 2019. [DOI] [PubMed] [Google Scholar]
- [4].Ganpule S, Alai A, Plougonven E, and Chandra N, “Mechanics of blast loading on the head models in the study of traumatic brain injury using experimental and computational approaches,” Biomech. Model. Mechanobiol, vol. 12, no. 3, pp. 511–531, 2013. [DOI] [PubMed] [Google Scholar]
- [5].Ganpule S, Gu L, Alai A, and Chandra N, “Role of helmet in the mechanics of shock wave propagation under blast loading conditions,” Comput. Methods Biomech. Biomed. Engin, vol. 15, no. 11, pp. 1233–1244, 2012. [DOI] [PubMed] [Google Scholar]
- [6].Seung S et al. , “Virtual wall–based haptic-guided teleoperated surgical robotic system for single-port brain tumor removal surgery,” Proc. Inst. Mech. Eng. Part H J. Eng. Med, vol. 231, no. 1, pp. 3–19, 2017. [DOI] [PubMed] [Google Scholar]
- [7].Maddahi Y, Ghasemloonia A, Zareinia K, Sepehri N, and Sutherland GR, “Quantifying force and positional frequency bands in neurosurgical tasks,” J. Robot. Surg, vol. 10, no. 2, pp. 97–102, 2016. [DOI] [PubMed] [Google Scholar]
- [8].Cobb MI-PH, Taekman JM, Zomorodi AR, Gonzalez LF, and Turner DA, “Simulation in neurosurgery—a brief review and commentary,” World Neurosurg, vol. 89, pp. 583–586, 2016. [DOI] [PubMed] [Google Scholar]
- [9].Shuck LZ and Advani SH, “Rheological Response of Human Brain Tissue in Shear,” J. Basic Eng, vol. 94, no. 4, pp. 905–911, 1972. [Google Scholar]
- [10].Bilston LE, Liu Z, and Phan-Thien N, “Linear viscoelastic properties of bovine brain tissue in shear,” Biorheology, vol. 34, no. 6, pp. 377–385, 1997. [DOI] [PubMed] [Google Scholar]
- [11].Darvish KK and Crandall JR, “Nonlinear viscoelastic effects in oscillatory shear deformation of brain tissue,” Med. Eng. Phys, vol. 23, no. 9, pp. 633–645, 2001. [DOI] [PubMed] [Google Scholar]
- [12].Hrapko M, van Dommelen J. a W, Peters GWM, and Wismans JSHM, “The mechanical behaviour of brain tissue: large strain response and constitutive modelling.,” Biorheology, vol. 43, no. 5, pp. 623–36, 2006. [PubMed] [Google Scholar]
- [13].Forte AE, Gentleman SM, and Dini D, “On the characterization of the heterogeneous mechanical response of human brain tissue,” Biomech. Model. Mechanobiol, vol. 16, no. 3, pp. 907–920, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Miller K and Chinzei K, “Constitutive modelling of brain tissue: experiment and theory.,” J. Biomech, vol. 30, no. 11/12, pp. 1115–1121, 1997. [DOI] [PubMed] [Google Scholar]
- [15].Prevost TP, Balakrishnan A, Suresh S, and Socrate S, “Biomechanics of brain tissue,” Acta Biomater, vol. 7, no. 1, pp. 83–95, 2011. [DOI] [PubMed] [Google Scholar]
- [16].Franceschini G, Bigoni D, Regitnig P, and Holzapfel GA, “Brain tissue deforms similarly to filled elastomers and follows consolidation theory,” J. Mech. Phys. Solids, vol. 54, no. 12, pp. 2592–2620, 2006. [Google Scholar]
- [17].Tamura A, Hayashi S, Nagayama K, and Matsumoto T, “Mechanical Characterization of Brain Tissue in High-Rate Compression,” J. Biomech. Sci. Eng, vol. 2, no. 3, pp. 263–274, 2007. [Google Scholar]
- [18].Cheng S and Bilston LE, “Unconfined compression of white matter,” J. Biomech, vol. 40, no. 1, pp. 117–124, 2007. [DOI] [PubMed] [Google Scholar]
- [19].Hrapko M, Van Dommelen JAW, Peters GWM, and Wismans JSHM, “Characterisation of the mechanical behaviour of brain tissue in compression and shear,” Biorheology, vol. 45, no. 6, pp. 663–676, 2008. [PubMed] [Google Scholar]
- [20].Miller K and Chinzei K, “Mechanical properties of brain tissue in tension,” J. Biomech, vol. 35, no. 4, pp. 483–490, 2002. [DOI] [PubMed] [Google Scholar]
- [21].Velardi F, Fratemali F, and Angelillo M, “Anisotropic constitutive equations and experimental tensile behavior of brain tissue,” Biomech. Model. Mechanobiol, vol. 5, no. 1, pp. 53–61, 2006. [DOI] [PubMed] [Google Scholar]
- [22].Tamura A, Hayashi S, Nagayama K, and Matsumoto T, “Mechanical Characterization of Brain Tissue in High-Rate Extension,” J. Biomech. Sci. Eng, vol. 3, no. 2, pp. 263–274, 2008. [Google Scholar]
- [23].Van Dommelen JAW, Van der Sande TPJ, Hrapko M, and Peters GWM, “Mechanical properties of brain tissue by indentation: Interregional variation,” J. Mech. Behav. Biomed. Mater, vol. 3, no. 2, pp. 158–166, 2010. [DOI] [PubMed] [Google Scholar]
- [24].Prevost TP, Jin G, De Moya MA, Alam HB, Suresh S, and Socrate S, “Dynamic mechanical response of brain tissue in indentation in vivo, in situ and in vitro,” Acta Biomater, vol. 7, no. 12, pp. 4090–4101, 2011. [DOI] [PubMed] [Google Scholar]
- [25].Muthupillai R, Rossman PJ, Lomas DJ, Greenleaf JF, Riederer SJ, and Ehman RL, “Magnetic resonance imaging of transverse acoustic strain waves.,” Magn. Reson. Med, vol. 36, no. 2, pp. 266–274, 1996. [DOI] [PubMed] [Google Scholar]
- [26].McCracken PJ, Manduca A, Felmlee J, and Ehman RL, “Mechanical transient-based magnetic resonance elastography,”Magn. Reson. Med, vol. 53, no. 3, pp. 628–639, 2005. [DOI] [PubMed] [Google Scholar]
- [27].Hamhaber U, Sack I, Papazoglou S, Rump J, Klatt D, and Braun J, “Three-dimensional analysis of shear wave propagation observed by in vivo magnetic resonance elastography of the brain,” Acta Biomater, vol. 3, no. 1, pp. 127–137, 2007. [DOI] [PubMed] [Google Scholar]
- [28].Xu L, Lin Y, Han JC, Xi ZN, Shen H, and Gao PY, “Magnetic resonance elastography of brain tumors: preliminary results,” Acta Radiol, vol. 48, no. 3, pp. 327–330, 2007. [DOI] [PubMed] [Google Scholar]
- [29].Atay SM, Kroenke CD, Sabet A, and Bayly PV, “Measurement of the dynamic shear modulus of mouse brain tissue in vivo by magnetic resonance elastography.,” J. Biomech. Eng, vol. 130, no. 2, p. 021013, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Green MA, Bilston LE, and Sinkus R, “In vivo brain viscoelastic properties measured by magnetic resonance elastography,” NMR Biomed, vol. 21, pp. 755–764, 2008. [DOI] [PubMed] [Google Scholar]
- [31].Schiavone P, Chassat F, Boudou T, Promayon E, Valdivia F, and Payan Y, “In vivo measurement of human brain elasticity using a light aspiration device,” Med. Image Anal, vol. 13, no. 4, pp. 673–678, 2009. [DOI] [PubMed] [Google Scholar]
- [32].Metz H, McElhaney J, and Ommaya AK, “A comparison of the elasticity of live, dead, and fixed brain tissue,” J. Biomech, vol. 3, no. 4, pp. 453–458, 1970. [DOI] [PubMed] [Google Scholar]
- [33].Miller K, Chinzei K, Orssengo G, and Bednarz P, “Mechanical properties of brain tissue in-vivo: Experiment and computer simulation,” J. Biomech, vol. 33, no. 11, pp. 1369–1376, 2000. [DOI] [PubMed] [Google Scholar]
- [34].Miga MI, Paulsen KD, Hoopes PJ, Kennedy FE, Hartov a, and Roberts DW, “In vivo modeling of interstitial pressure in the brain under surgical load using finite elements.,” J. Biomech. Eng, vol. 122, no. 4, pp. 354–363, 2000. [DOI] [PubMed] [Google Scholar]
- [35].Gefen A and Margulies SS, “Are in vivo and in situ brain tissues mechanically similar?,” J. Biomech, vol. 37, no. 9, pp. 1339–1352, 2004. [DOI] [PubMed] [Google Scholar]
- [36].Brien JSO and Sampson EL, “Lipid composition of the normal human brain :,” vol. 6, no. 9, 1965. [PubMed] [Google Scholar]
- [37].Arbogast KB and Margulies SS, “Material characterization of the brainstem from oscillatory shear tests.,” J. Biomech, vol. 31, no. 9, pp. 801–807, 1998. [DOI] [PubMed] [Google Scholar]
- [38].Prange MT and Margulies SS, “Regional, directional, and age-dependent properties of the brain undergoing large deformation.,” J. Biomech. Eng, vol. 124, no. 2, pp. 244–252, 2002. [DOI] [PubMed] [Google Scholar]
- [39].Kaster T, Sack I, and Samani A, “Measurement of the hyperelastic properties of ex vivo brain tissue slices,” J. Biomech, vol. 44, no. 6, pp. 1158–1163, 2011. [DOI] [PubMed] [Google Scholar]
- [40].Chatelin S, Constantinesco A, and Willinger R, “Fifty years of brain tissue mechanical testing: from in vitro to in vivo investigations,” Biorheology, vol. 47, no. 5–6, pp. 255–276, 2010. [DOI] [PubMed] [Google Scholar]
- [41].Fischl B and Dale AM, “Measuring the thickness of the human cerebral cortex from magnetic resonance images.,”Proc. Natl. Acad. Sci. U. S. A, vol. 97, no. 20, pp. 11050–5, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nicolle S, Lounis M, and Willinger R, “Shear Properties of Brain Tissue over a Frequency Range Relevant for Automotive Impact Situations: New Experimental Results.,” Stapp Car Crash J, vol. 48, pp. 239–58, November 2004. [DOI] [PubMed] [Google Scholar]
- [43].Forte AE, Galvan S, Manieri F, y Baena FR, and Dini D, “A composite hydrogel for brain tissue phantoms,”Mater. Des, vol. 112, pp. 227–238, 2016. [Google Scholar]
- [44].Chen Q, Suki B, and An K-N, “Dynamic mechanical properties of agarose gels modeled by a fractional derivative model,” J. Biomech. Eng, vol. 126, no. 5, pp. 666–671, 2004. [DOI] [PubMed] [Google Scholar]
- [45].Karimi A and Navidbakhsh M, “Material properties in unconfined compression of gelatin hydrogel for skin tissue engineering applications,” Biomed. Eng. / Biomed. Tech, vol. 59, no. 6, pp. 479–486, 2014. [DOI] [PubMed] [Google Scholar]
- [46].Liu Q, Subhash G, and Moore DF, “Loading velocity dependent permeability in agarose gel under compression,” J. Mech. Behav. Biomed. Mater, vol. 4, no. 7, pp. 974–982, 2011. [DOI] [PubMed] [Google Scholar]
- [47].Anseth KS, Bowman CN, and Brannon-Peppas L, “Mechanical properties of hydrogels and their experimental determination,” Biomaterials, vol. 17, no. 17, pp. 1647–1657, 1996. [DOI] [PubMed] [Google Scholar]
- [48].Timmermans C et al. , “Potential of a statistical approach for the standardization of multicenter diffusion tensor data: A phantom study,” J. Magn. Reson. Imaging, 2019. [DOI] [PubMed] [Google Scholar]
- [49].Sedlacik J and Reichenbach JR, “Validation of quantitative estimation of tissue oxygen extraction fraction and deoxygenated blood volume fraction in phantom and in vivo experiments by using MRI.” Magn. Reson. Med. An Off. J. Int. Soc. Magn. Reson. Med, vol. 63, no. 4, pp. 910–921, 2010. [DOI] [PubMed] [Google Scholar]
- [50].Ford MD et al. , “PIV-measured versus CFD-predicted flow dynamics in anatomically realistic cerebral aneurysm models,” J. Biomech. Eng, vol. 130, no. 2, p. 21015, 2008. [DOI] [PubMed] [Google Scholar]
- [51].O’Brien JS and Sampson EL, “Lipid composition of the normal human brain: gray matter, white matter, and myelin,” J. Lipid Res, vol. 6, no. 4, pp. 537–544, 1965. [PubMed] [Google Scholar]
- [52].Bozan B and Temelli F, “Chemical composition and oxidative stability of flax, safflower and poppy seed and seed oils,” Bioresour. Technol, vol. 99, no. 14, pp. 6354–6359, 2008. [DOI] [PubMed] [Google Scholar]
- [53].Miller K, “Method of testing very soft biological tissues in compression,” J. Biomech, vol. 38, no. 1, pp. 153–158, 2005. [DOI] [PubMed] [Google Scholar]
- [54].Mattijssen E, Alberink I, Jacobs B, and van den Boogaard Y, “Preservation and storage of prepared ballistic gelatine,” Forensic Sci. Int, vol. 259, pp. 221–223, 2016. [DOI] [PubMed] [Google Scholar]
- [55].Donnelly BR and Medige J, “Shear properties of human brain tissue,” J. Biomech. Eng, vol. 119, no. November 1997, pp. 423–432, 1997. [DOI] [PubMed] [Google Scholar]
- [56].Forte AE, D’Amico F, Charalambides MN, Dini D, and Williams JG, “Modelling and experimental characterisation of the rate dependent fracture properties of gelatine gels,” Food Hydrocoil, vol. 46, pp. 180–190, 2015. [Google Scholar]
- [57].Pervin F and Chen WW, “Dynamic mechanical response of bovine gray matter and white matter brain tissues under compression,” J. Biomech, vol. 42, no. 6, pp. 731–735, 2009. [DOI] [PubMed] [Google Scholar]
- [58].Garo A, Hrapko M, van Dommelen JAW, and Peters GWM, “Towards a reliable characterisation of the mechanical behaviour of brain tissue: The effects of post-mortem time and sample preparation.,” Biorheology, vol. 44, no. 1, pp. 51–58, 2007. [PubMed] [Google Scholar]
- [59].Brands DWA, Bovendeerd PHM, Peters GWM, and Wismans JSHM, “The large shear strain dynamic behavior of in-vitro procine brain tissue and a silicone gel model material,” 44th Stapp car crash Conf. J, vol. 44, no. June 2016, pp. 249–260, 2000. [DOI] [PubMed] [Google Scholar]
- [60].Thibault KL and Margulies SS, “Age-dependent material properties of the porcine cerebrum: Effect on pediatric inertial head injury criteria,” J. Biomech, vol. 31, no. 12, pp. 1119–1126, 1998. [DOI] [PubMed] [Google Scholar]
- [61].Pamidi MR and Advani SH, “Nonlinear Constitutive Relations for Human Brain Tissue,” Trans. ASME. J. Biomech, vol. 100, no. 3, pp. 44–48, 1978. [Google Scholar]
- [62].Sahay KB, Mehrotra R, Sachdeva U, and Banerji AK, “Elastomechanical characterization of brain tissues,” J. Biomech, vol. 25, no. 3, pp. 319–326, 1992. [DOI] [PubMed] [Google Scholar]
- [63].Farshad M, Barbezat M, Flüeler P, Schmidlin F, Graber P, and Niederer P, “Material characterization of the pig kidney in relation with the biomechanical analysis of renal trauma,” J. Biomech, vol. 32, no. 4, pp. 417–425, 1999. [DOI] [PubMed] [Google Scholar]
- [64].Miller K, “Constitutive model of brain tissue suitable for finite element analysis of surgical procedures.,” J. Biomech, vol. 32, no. 5, pp. 531–537, 1999. [DOI] [PubMed] [Google Scholar]
- [65].Bilston LE, Liu Z, and Phan-Thien N, “Large strain behaviour of brain tissue in shear: some experimental data and differential constitutive model.,” Biorheology, vol. 38, no. 4, pp. 335–45, 2001. [PubMed] [Google Scholar]
- [66].McIlvain G, Schwarb H, Cohen NJ, Telzer EH, and Johnson CL, “Mechanical properties of the in vivo adolescent human brain,” Dev. Cogn. Neurosci, vol. 34, pp. 27–33, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Qiu K, Haghiashtiani G, and McAlpine MC, “3D printed organ models for surgical applications,” Annu. Rev. Anal. Chem, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tan Z, Parisi C, Di Silvio L, Dini D, and Forte AE, “Cryogenic 3D printing of super soft hydrogels,” Sci. Rep, vol. 7, no. 1, p. 16293, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Dargahi J and Najarian S, “Human tactile perception as a standard for artificial tactile sensing—a review.” Int. J. Med. Robot. Comput. Assist. Surg, vol. 1, no. 1, pp. 23–35, 2004. [DOI] [PubMed] [Google Scholar]
- [70].Tan Z, Dini D, y Baena FR, and Forte AE, “Composite hydrogel: A high fidelity soft tissue mimic for surgery,” Mater. Des, vol. 160, pp. 886–894, 2018. [Google Scholar]
- [71].Forte AE, Galvan S, and Dini D. Models and tissue mimics for brain shift simulations. Biomechanics and modeling in mechanobiology, 77(1), 249–261, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


