Abstract
Background:
Mycosis fungoides (MF) is associated with increased risk of second primary hematologic malignancies, but its association with second primary solid tumors is less well characterized.
Objective:
This retrospective analysis seeks to assess the risk of being diagnosed with a second primary hematologic or solid malignancy in patients with MF.
Design:
We performed an analysis of patients diagnosed with MF from 2000 through 2015 in the United States cancer registries of SEER-18 (N = 6742).
Results:
Relative risks were estimated by using standardized incidence ratios (SIRs). Among 6742 patients, there were 511 (7.5%) second cancer events (SIR, 10.15; 95% confidence interval [CI], 9.29–11.07). These included 184 (36.0%) hematologic malignancies (SIR, 39.71; 95% CI, 34.05–46.05) and 327 (64.0%) solid tumor malignancies (SIR, 7.33; 95% CI, 6.56–8.17). Patients with MF were at increased risk for non-Hodgkin lymphoma; Hodgkin lymphoma; melanoma; and lung, female breast, prostate, colon, and renal cancers. Females were at higher risk than males (P < .05). All ethnic groups showed a statistically significant elevation in SIRs. Elevation of SIRs was observed across all stages of MF.
Conclusions and Relevance:
Patients with MF are at increased risk for diagnosis of second primary malignancies and should be carefully screened for discernable signs and symptoms of second malignancies.
Keywords: CTCL, cutaneous lymphoma, cutaneous T-cell lymphoma, mycosis fungoides, non-Hodgkin lymphoma, second malignancy, SEER, Surveillance Epidemiology and End Results
CAPSULE SUMMARY
Mycosis fungoides (MF) is a rare non-Hodgkin T-cell lymphoma. Patients with MF are at increased risk for diagnosis of second primary malignancies, both hematologic and solid.
Patients MF require careful attention to signs and symptoms of second primary malignancies, as well as age-appropriate malignancy screenings.
A handful of previous studies have shown a link between mycosis fungoides (MF) and the diagnosis of a second primary malignancy. Kantor et al1 first used Surveillance, Epidemiology, and End Results (SEER) data in 1989 to show an increased risk among patients with MF for lung cancer, colon cancer, and non-Hodgkin lymphoma. In 2007, Huang et al2 used the SEER-9 database, examining cases from 1973 through 2001 and a cohort of 429 patients from Stanford University. They identified an increased risk of Hodgkin lymphoma, non-Hodgkin lymphoma, melanoma, and urinary cancer. Other studies have yielded similar results, including a study of SEER data by Amber et al (2016),3 the Finnish Cancer Registry by Väkeväa et al (2000),4 the Danish Nationwide Population-Based Registries by Lindahl et al (2014),5 and the California Cancer Registry by Ai et al (2014).6
Although the studies were informative, they were limited in terms of population selection and sample size. A number of these studies grouped MF with Sézary syndrome1,2 or even all other cutaneous T-cell lymphomas.3,4 Some included as few as 5 patients with second malignancies.6 Many included cases of MF from the 1950s through 1970s, before the advent of modern immunohistochemical staining and diagnostic techniques.1,2,4,6 We therefore studied the SEER-18 data set (2000–2015) to define the spectrum of second primary malignancies in patients with MF and to identify high-risk second malignancies to guide follow-up screening and preventative interventions.
MATERIALS AND METHODS
Patients and design
The SEER-18 cohort consisted of patients with MF diagnosed between January 2000 and December 2015 in the 18 SEER cancer registries (SEER 18 Regs excluding AK Research Data, Nov 2017 Sub [2000–2015]). Patients with MF were identified with International Classification for Diseases for Oncology, third edition, code 9700/3. Patients with Sèzary syndrome were excluded. First of 2 or more primaries was selected. Patients were excluded if they received a diagnosis of a second malignancy in the first 12 months after the initial diagnosis of MF. Cutaneous keratinocytic malignancies were not reported. Early-stage MF included stages IA through IIA; advanced-stage MF included stages IIB through IVB and IV not otherwise specified (IV-NOS). This study was exempt from institutional review board approval.
Statistical analysis
Relative risk was estimated as the standardized incidence ratio (SIR), the ratio of observed (O) and expected (E) malignancies (SIR = O/E). An SIR of 1 indicated no difference in incidence as compared to the general population. Statistical significance of the SIR was assessed based on the 95% confidence interval (CI). To calculate the number of expected malignancies, the number of person-years at risk among patients with MF was calculated from MF diagnosis until diagnosis of a second cancer, death, loss to follow-up, or end of observation, whichever came first. The total number of person-years was multiplied by age, sex, and cancer-specific incidence rates in the United States yearly, performed using the SEER*Stat software package, version 8.3.4 (National Cancer Institute, Bethesda, MD).7 Routine methods of categorical (chi-square test) and continuous (Student t test and analysis of variance) testing were applied, with statistical significance defined at an a level of .05 (Stata statistical software, release 15, 2017; StataCorp, College Station, TX). The data analysis for this paper was generated using SAS software (SAS Institute Inc, Cary, NC).8,9 When disease-specific and overall survival rates were calculated, it was assumed that all deaths due to “non-Hodgkin lymphoma” were due to MF.
RESULTS
MF and incidence of second malignancy
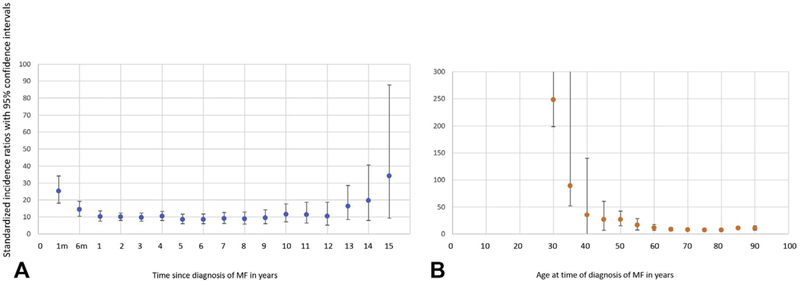
The SEER-18 MF cohort consisted of 6742 patients, of whom 511 (7.5%) received diagnosis of a second malignancy, excluding malignancies that occurred during the first year after diagnosis of MF (n = 137). A conservative latency exclusion period of 12 months was used because of an observed spike in SIR immediately after MF diagnosis (Fig 1, A); this spike is presumably attributed to initial diagnostic workup. The median age of MF diagnosis was 63 years for patients developing a second malignancy and 57 years for patients not developing a second malignancy. One second malignancy diagnosis occurred in a patient aged younger than 18 years and 4 in patients aged 18 to 30 years. The median latency between MF diagnosis and second malignancy diagnosis was 3 years (range, 0–15). When malignancies that were found during the first 12 months after MF diagnosis (n = 137) were excluded, the SIR was 10.15 (95% CI, 9.29–11.07) (Table I). This included 184 (36.0%) hematologic malignancies (SIR, 39.71; 95% CI, 34.05–46.05) and 327 (64.0%) solid tumor malignancies (SIR, 7.33; 95% CI, 6.56–8.17). The SIRs for each organ-specific malignancy are outlined in Table I.
Fig 1.
Stratified standardized incidence ratios for all malignancies (A) by time from diagnosis of mycosis fungoides and (B) by patient age at diagnosis of mycosis fungoides. MF, Mycosis fungoides.
Table I.
Spectrum of second primary malignancies in patients with MF (N = 6742) in the SEER-18 database
| Second malignancy diagnosed at <1 year (n = 137) | Second malignancy diagnosed at ≥1 year (n = 511) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Expected | SIR | 95% CI lower | 95% CI upper | Observed | Expected | SIR | 95% CI lower | 95% CI upper | |
| All sites | 137 | 10.43 | 13.13* | 11.03 | 15.53 | 511 | 50.35 | 10.15* | 9.29 | 11.07 |
| All solid tumors | 71 | 9.30 | 7.64* | 5.96 | 9.63 | 327 | 44.62 | 7.33* | 6.56 | 8.17 |
| Oral cavity and pharynx | 1 | 0.24 | 4.09 | 0.10 | 22.81 | 3 | 1.18 | 2.55 | 0.53 | 7.44 |
| Esophagus | — | — | — | — | — | 7 | 0.62 | 11.36* | 4.57 | 23.41 |
| Stomach | — | — | — | — | — | 5 | 0.91 | 5.51* | 1.79 | 12.85 |
| Colon, rectum, and anus | 8 | 1.10 | 7.30* | 3.15 | 14.38 | 36 | 5.15 | 6.99* | 4.90 | 9.68 |
| Liver | — | — | — | — | — | 5 | 0.79 | 6.37* | 2.07 | 14.86 |
| Gallbladder | — | — | — | — | — | 2 | 0.12 | 16.55* | 2.00 | 59.78 |
| Hepatic,† bile ducts, and other biliary | — | — | — | — | — | 2 | 0.32 | 6.32 | 0.77 | 22.82 |
| Pancreas | 1 | 0.29 | 3.51 | 0.09 | 19.54 | 10 | 1.46 | 6.84* | 3.28 | 12.57 |
| Larynx | — | — | — | — | — | 5 | 0.45 | 11.01* | 3.57 | 25.69 |
| Lung and bronchus | 12 | 1.62 | 7.43* | 3.84 | 12.99 | 65 | 7.79 | 8.34* | 6.44 | 10.63 |
| Melanoma of the skin | 4 | 0.42 | 9.61* | 2.62 | 24.62 | 20 | 2.22 | 9.00* | 5.50 | 13.90 |
| Other nonepithelial skin | — | — | — | — | — | 1 | 0.24 | 4.11 | 0.10 | 22.89 |
| Female breast | 9 | 0.85 | 10.59* | 4.84 | 20.10 | 47 | 4.17 | 11.28* | 8.29 | 15.00 |
| Male breast | 1 | 0.02 | 62.29* | 1.58 | 347.05 | 2 | 0.08 | 24.49* | 2.97 | 88.45 |
| Cervix uteri | — | — | — | — | — | 10 | 1.61 | 6.31 | 0.16 | 35.15 |
| Ovary | — | — | — | — | — | 1 | 0.16 | 4.84 | 0.59 | 17.5 |
| Prostate | 19 | 2.39 | 8.05* | 4.85 | 12.57 | 2 | 0.41 | 5.65* | 4.30 | 7.28 |
| Urinary bladder | 3 | 0.62 | 4.82 | 0.99 | 14.10 | 59 | 10.45 | 4.35* | 2.38 | 7.30 |
| Kidney and renal pelvis | 4 | 0.33 | 12.05* | 3.28 | 12.57 | 14 | 3.22 | 3.62* | 1.33 | 7.87 |
| Brain and other nervous system | — | — | — | — | — | 6 | 1.66 | 16.11* | 6.96 | 31.75 |
| Thyroid | 7 | 0.11 | 63.25* | 25.43 | 130.31 | 8 | 0.5 | 24.37* | 13.33 | 40.89 |
| Thymus | 1 | 0.01 | 182.18* | 4.61 | 1015.04 | 14 | 0.57 | 36.79 | 0.93 | 205.00 |
| All lymphatic and hematopoietic diseases | 62 | 0.87 | 71.31* | 54.68 | 91.42 | 184 | 7.46 | 24.66* | 21.47 | 28.18 |
| Lymphoma | 53 | 0.44 | 120.57* | 90.31 | 157.70 | 152 | 1.44 | 105.26* | 54.39 | 183.87 |
| Hodgkin lymphoma | 3 | 0.02 | 123.63* | 25.49 | 374.24 | 12 | 0.12 | 100.14* | 49.99 | 179.19 |
| Hodgkin: nodal | 3 | 0.02 | 128.06* | 26.41 | 374.24 | 11 | 0.05 | 240.29* | 6.08 | 1338.82 |
| Hodgkin: extranodal | — | — | — | — | — | 1 | 0.02 | 66.65* | 56.06 | 78.65 |
| Non-Hodgkin lymphoma | 50 | 0.42 | 120.39* | 89.35 | 158.72 | 140 | 4.59 | 30.48* | 22.06 | 41.06 |
| Non-Hodgkin: nodal | 17 | 0.28 | 60.35* | 35.15 | 96.62 | 43 | 0.31 | 140.61* | 114.02 | 171.53 |
| Non-Hodgkin: extranodal | 33 | 0.13 | 246.96* | 169.99 | 346.82 | 97 | 12.56 | 7.72* | 2.83 | 16.8 |
| Myeloma | 2 | 0.15 | 13.11* | 1.59 | 47.34 | 6 | 0.50 | 12.02* | 7 | 19.24 |
| Leukemia | 7 | 0.28 | 25.25* | 10.15 | 52.03 | 17 | 0.69 | 24.47 | 0.62 | 136.33 |
| Acute lymphocytic leukemia | — | — | — | — | — | 1 | 0.11 | 9.52* | 3.49 | 20.73 |
| Chronic lymphocytic leukemia | 6 | 0.12 | 48.59* | 17.83 | 105.76 | 6 | 0.49 | 12.16* | 3.95 | 28.38 |
| Acute myeloid leukemia | — | — | — | — | — | 5 | 0.06 | 83.35* | 10.09 | 301.01 |
CI, Confidence interval; MF, Mycosis fungoides; SIR, standardized incidence ratio (data for malignancies that did not occur in the sample set are omitted).
P < .05.
Extrahepatic and intrahepatic.
Risk of second primary malignancy by sex and race
There was no significant difference in sex distribution between patients with MF who did and did not receive with a second malignancy diagnosis (Table II). However, SIR analysis showed that female patients with MF were more likely (SIR, 13.95; 95% CI, 12.09–16.02) to have a second malignancy diagnosis than males (SIR, 8.62; 95% CI, 7.69–12.09; P < .033) (Table III). Furthermore, women with a second malignancy diagnosis were younger than men with a second malignancy (mean, 61.9 years vs 64.3; P <.05).
Table II.
Demographics of patients with MF with and without diagnosis of second primary malignancy in the SEER-18 database
| Characteristics | MF with second malignancy | MF without second malignancy | All MF | P value* | |||
|---|---|---|---|---|---|---|---|
| Mean age at diagnosis, y | 63.17 | 57.12 | 57.35 | <.0001 | |||
| n | % | n | % | n | % | ||
| Total | 511 | 7.51 | 6231 | 92.40 | 6742 | 100 | |
| Sex | |||||||
| Male | 310 | 60.67 | 3549 | 56.96 | 3859 | 57.24 | .10 |
| Female | 201 | 39.33 | 2682 | 43.04 | 2883 | 42.76 | |
| Race/ethnicity | |||||||
| Non-Hispanic white | 369 | 72.21 | 3896 | 62.53 | 4265 | 63.26 | .015 |
| Non-Hispanic black | 73 | 14.29 | 889 | 14.27 | 962 | 14.27 | |
| Non-Hispanic American Indian/Alaska Native | 3 | 0.59 | 27 | 0.43 | 30 | 0.44 | |
| Non-Hispanic Asian or Pacific Islander | 34 | 6.65 | 411 | 6.60 | 445 | 6.60 | |
| Hispanic (all races) | 32 | 6.26 | 639 | 10.26 | 671 | 9.95 | |
| Vital status | |||||||
| Alive | 268 | 52.4 | 4688 | 75.2 | 4956 | 73.5 | <.00001 |
| Dead | 243 | 47.6 | 1543 | 24.7 | 1786 | 23.4 | |
| Death due to other causes | 64 | 26.3 | 626 | 40.6 | 690 | 38.6 | <.00003 |
| Death due to malignancy | 179 | 73.7 | 917 | 59.4 | 1096 | 61.4 | |
| Non-Hodgkin lymphoma: extranodal† | 66 | 36.9 | 641 | 69.9 | 707 | 64.5 | <.00001 |
| Other malignancy | 113 | 63.1 | 276 | 30.0 | 389 | 35.5 | |
| Stage | |||||||
| IA | 152 | 29.75 | 3171 | 50.89 | 3323 | 49.29 | |
| IB | 34 | 6.65 | 900 | 14.44 | 934 | 13.85 | |
| IIA | 3 | 0.59 | 204 | 3.27 | 207 | 3.07 | |
| IIB | 20 | 3.91 | 286 | 4.59 | 306 | 4.54 | |
| IIIA | 6 | 1.17 | 134 | 2.15 | 140 | 2.08 | |
| IIIB | 3 | 0.59 | 52 | 0.83 | 55 | 0.82 | |
| IVNOS | 3 | 0.59 | 83 | 1.33 | 86 | 1.28 | |
| IVA | 4 | 0.78 | 72 | 1.16 | 76 | 1.13 | |
| IVB | 5 | 0.98 | 87 | 1.40 | 92 | 1.36 | |
| Unknown stage‡ | 90 | 17.61 | 1178 | 18.91 | 1268 | 18.81 | |
| Blank§ | 191 | 37.38 | 1245 | 19.98 | 1436 | 21.30 | |
| Stage IA-IIA | 189 | 0.82 | 4275 | 0.86 | 4464 | 0.86 | .17 |
| Stage IIB+ | 41 | 0.18 | 714 | 0.14 | 755 | 0.14 | |
| MF with second malignancy | MF without second malignancy | All MF | ||||
|---|---|---|---|---|---|---|
| Survival | OS, % | DSS, % | OS, % | DSS, % | OS, % | DSS, % |
| 2-year | 93 | 98 | 91 | 95 | 92 | 95 |
| 5-year | 77 | 91 | 84 | 92 | 84 | 92 |
| 10-year | 61 | 84 | 79 | 90 | 78 | 89 |
| 15-year | 52 | 80 | 77 | 89 | 75 | 88 |
DSS, Disease (MF)-specific survival; MF, mycosis fungoides; NOS, not otherwise specified; OS, age-adjusted overall survival.
Comparisons between patients with MF with and without a second malignancy.
Includes MF and other non-MF, non-Hodgkin malignancies.
Unknown stage indicates that stage was entered as unknown upon data entry.
Indicates that the entry for stage was left blank at the time of data entry.
Table III.
Standardized incidence ratios stratified by sex, race/ethnicity, and stage of disease
| Characteristics | Observed, n | Expected, n | SIR | 95% CI lower limit | 95% CI upper limit | Mean age at diagnosis of MF, years | Mean age at diagnosis of second malignancy, years | Latency, years* |
|---|---|---|---|---|---|---|---|---|
| Total | 511 | 50.35 | 10.15† | 9.29 | 11.07 | 63.33 | 68.14 | 4.81 |
| Sex | ||||||||
| Male | 310 | 35.94 | 8.62† | 7.69 | 9.64 | 64.28 | 68.84 | 4.56 |
| Female | 201 | 14.41 | 13.95† | 12.09 | 16.02 | 61.86 | 67.05 | 5.19 |
| Race/ethnicity | ||||||||
| Non-Hispanic white | 369 | 40.41 | 9.13† | 8.22 | 10.11 | 64.94 | 69.67 | 4.73 |
| Non-Hispanic black | 73 | 5.20 | 14.03† | 11.00 | 17.64 | 56.35 | 61.59 | 5.24 |
| Non-Hispanic American Indian/Alaska Native | 3 | 0.12 | 24.43† | 5.04 | 71.39 | 68.5 | 73.81 | 5.31 |
| Non-Hispanic Asian or Pacific Islander | 34 | 2.02 | 16.87† | 11.68 | 23.57 | 63.92 | 68.4 | 4.48 |
| Hispanic (all races) | 32 | 2.42 | 13.24† | 9.06 | 18.69 | 59.08 | 64.55 | 5.47 |
| Stage | ||||||||
| IA | 152 | 11.46 | 13.26† | 11.24 | 15.54 | 62.99 | 67.35 | 4.36 |
| IB | 34 | 1.84 | 18.51† | 12.82 | 25.87 | 62.46 | 63.73 | 1.27 |
| IIA | 3 | 0.14 | 20.99† | 4.33 | 61.34 | 57.45 | 62 | 4.55 |
| IIB | 20 | 1.53 | 13.10† | 8.00 | 20.22 | 66.77 | 71.78 | 5.01 |
| IIIA | 6 | 0.41 | 14.71† | 5.40 | 32.03 | 70.07 | 70.43 | 0.36 |
| IIIB | 3 | 0.05 | 65.78† | 13.57 | 192.25 | 66.89 | 67.89 | 1.00 |
| IVNOS | 3 | 0.20 | 14.96† | 3.08 | 43.71 | 61.96 | 69.54 | 7.58 |
| IVA | 4 | 0.01 | 281.33† | 76.65 | 720.31 | 66.67 | 67.17 | 0.50 |
| IVB | 5 | 0.11 | 43.56† | 14.14 | 101.66 | 73.37 | 75.23 | 1.86 |
| Unknown stage | 90 | 6.53 | 13.78† | 11.08 | 16.93 | 63.77 | 68.08 | 4.31 |
| Blank(s) | 191 | 28.06 | 6.81† | 5.88 | 7.84 | 62.81 | 69.04 | 6.23 |
CI, Confidence interval; MF, mycosis fungoides; NOS, not otherwise specified; SIR, standardized incidence ratio (data for malignancies that did not occur in the sample set are omitted).
The time between diagnosis of MF and diagnosis of a second primary malignancy.
P < .05.
Patients with second malignancies were disproportionately found to be non-Hispanic whites compared with patients with MF without a second malignancy (P < .037) (Table III), although this group did have the lowest SIR. All ethnic groups showed a statistically significant elevation in SIR (Table III). Non-Hispanic black patients were substantially younger at age of MF diagnosis (mean, 56.4 years) and at diagnosis of second malignancy (mean, 61.6 years) than any other ethnic group. Non-Hispanic white patients had the shortest time between diagnosis of MF and second malignancy (4.7 years).
Risk of second primary malignancy by stage
Only 45% of patients with an MF diagnosis in the SEER database had staging information. Most of these patients who received a second malignancy diagnosis had early-stage disease (189/239, 81.8%), compared with 41 (17.1%) patients with advanced-stage disease (Table I). Although elevated SIRs were observed across all stages of MF (Table III), there was no significant difference in incidence of second malignancy between the early and late stages of MF (P = .17) (Table I).
Risk of second malignancy as a function of time since MF diagnosis
Descriptive SIR analysis showed significantly higher risk of being diagnosed with second malignancy throughout all time points after MF diagnosis (Fig 1, A). Increased SIRs for second malignancy had a bimodal pattern, peaking early (1–6 months) and late (13–15 years) after MF diagnosis.
Risk of second malignancy as a function of age at the time of MF diagnosis
Patients with a second malignancy diagnosis were older than those without a second malignancy diagnosis (63.2 vs 57.1 years, P <.001). SIRs stratified by age at MF diagnosis showed statistically higher SIRs among patients 30 years or older; SIRs declined with increasing age but retained statistical significance (Fig 1, B). Risk of receiving a second malignancy diagnosis was substantially higher for 30- to 50-year-old patients than older patients. Solid tumor malignancies diagnosed in at least 20 individuals included cancers of the rectum, colon/anus, lung/bronchus, skin (melanoma), breast, and prostate. For lung/bronchus, breast, and prostate cancers, patients aged 30 to 50 years were at substantially increased risk of malignancy. This risk declined with age but remained statistically significant, followed by increased SIRs in patients 75 years and older. This pattern of time-dependent SIR changes was not observed among patients with melanoma or colorectal malignancies. Furthermore, the risk of these malignancies was not affected by patient sex.
The SEER subset analysis of patients with nodal and extranodal non-Hodgkin lymphoma showed a significant increase in risk of second malignancies among 30- to 50-year-old patients. SIRs declined subsequently with increasing age but remained significantly elevated. There was no uptick at age 75 years and older, as seen with solid tumor malignancies. The risk of these malignancies was not affected by patient sex.
Survival analysis
Patients with second malignancies had worse age-adjusted overall survival than those without second malignancy (hazard ratio [HR], 2.72; 95% CI, 2.37–3.12; P < .001) (Table I). In the Cox proportionate hazards analysis, patient age was associated with higher mortality, with an HR of 1.88 per 10-year increment (95% CI, 1.81–1.95; P < .001). Black patients with MF had a higher risk of death than whites, independent of age (HR, 1.65; 95% CI, 1.44–1.88; P < .001). Similarly, patients with advanced-stage MF (stages III-IV) had a 3.36-fold higher mortality than patients with stage I to II disease. Patients with second malignancies had worse MF-specific survival at 5, 10, and 15 years compared with patients without second malignancies, with increasing divergence over time. Patients with MF who had a second malignancy diagnosis were more likely to die during the follow-up interval than patients without second malignancy (243/511 [47.6%] vs 1543/6231 [24.7%]; P <.00001).
DISCUSSION
Previous studies have documented that patients with MF are at increased risk of lymphoid malignancy diagnoses, particularly Hodgkin lymphoma and some solid tumor malignancies.1–6 Our data from SEER-18 corroborate previous findings that patients with MF are at increased risk of hematologic malignancy, including Hodgkin and non-Hodgkin lymphomas, compared with the general population. We also found that patients were at increased risk of solid tumor malignancies, including cancers of the skin (melanoma), lung, female breast, prostate, bladder, colon, pancreas, and kidney. This study, therefore, advances our understanding of second malignancies among patients with MF by high-lighting the increased risk of not only hematologic malignancies but also solid tumor malignancies.
Our analysis of SIR by sex, age, and race/ethnicity showed several important associations. We found that female patients had a higher risk of having a second malignancy diagnosis than their male counterparts. In addition, women with a second malignancy diagnosis tended to receive the MF diagnosis at a younger age than men. Examination by race/ethnicity indicated that all groups showed a statistically significant elevation in risk but that non-Hispanic white patients had the lowest risk of all groups, as well as the shortest time between diagnosis of MF and second malignancy, which may reflect racial bias in efficiency of malignancy detection. Non-Hispanic black patients were substantially younger at the time of MF diagnosis and at the time of second malignancy diagnosis, which may suggest the need for enhanced screening of these patients.
This work raises the question of how to best identify patients with MF who are at the highest risk of a second primary malignancy diagnosis. Patients of all ages were at increased risk, although patients aged 30 through 50 years were at a greater increased risk. Patients for whom MF was diagnosed at a younger age may have genetic or environmental risk factors that predispose them to the development of other malignancies. Alternatively, malignancies are uncommon in young patients, so any increase in the number of cases may result in an exaggerated increase in SIR.
There was no significant difference in the proportion of patients with a second malignancy diagnosis based on stage when comparing early- (stage IA-IIA) and late-stage disease (stage IIB1). Overall, there was an increased risk of second malignancy for all stages of disease. This is consistent with the findings of Huang et al,2 in both their SEER cohort and their Stanford patient cohort. However, nearly one third of patients in our SEER cohort lacked stage data, which impairs our ability to make conclusions about the relationship between stage and second malignancy. This also does not account for patients with progressive disease, whose stage changed over time (listed stage data is for the most recent timepoint collected).
Survival analysis showed that patients with second malignancies were more likely to die compared with those without second malignancy, with greatly decreased 5-, 10-, and 15-year overall survival. This increased mortality underscores the importance of identifying patients at risk of a second malignancy diagnosis early and screening them appropriately.
To our knowledge, this study a more modern cohort (2000–2015) than all prior studies, more accurately reflecting current treatment and staging of MF. Although other studies have shown links to second malignancies, consistently identifying Hodgkin and non-Hodgkin lymphoma and variably identifying colon, lung, melanoma, and urinary cancer, ours is the first to identify additional solid tumor malignancies as being implicated in MF. To the best of our knowledge, this is the largest analysis of second malignancies in patients with MF. This study also offered stratification according to patient sex, race, age at MF diagnosis, time since MF diagnosis, and type of second malignancy. Furthermore, the key findings from this study raise further awareness about second cancers and are thereby highly informative for patients with MF and their caregivers.
Our findings that patients with MF are at significantly increased risk for a variety of hematologic and solid tumor malignancies raise important questions about the need to monitor patients with MF closely for second malignancies. The most common malignancies seen in patients with MF (lung, colon, female breast, melanoma, prostate, and non-Hodgkin lymphoma) are also the most common malignancies overall in the United States.10 The US Preventive Services Task Force and other groups provide screening guidelines and recommendations for many common malignancies, including colon, breast, and lung cancer. However, there are no widely accepted recommendations for screening for melanoma, prostate, non-Hodgkin lymphoma, kidney, or bladder cancers. Given our findings, it is unclear if we should be applying more rigorous screening guidelines to a population we have identified as being at high risk or adhere to guidelines designed for the general population by major groups such as the US Preventive Services Task Force.
The majority of dermatologists perform an annual total-body skin examination for all patients with MF and obtain at least a complete blood count. It is important that all health care providers verify that patients with MF are in fact having their age-appropriate cancer screenings and that careful attention is paid to any possible signs or symptoms of second malignancy. Given the increased risk of lung and bladder cancer, counseling patients with MF on smoking cessation is of utmost importance. Careful cooperation and teamwork with primary care and other providers can potentially enhance earlier detection of these second malignancies, when they can be more easily and effectively treated.
Potential mechanisms of carcinogenesis in patients with MF may include immune suppression resulting in decreased immune surveillance, treatment effect, environmental factors, and common genetic mechanisms. The most likely of these is immune suppression resulting in decreased immune surveillance. MF has been associated with immune suppression, including quantitative and qualitative defects in T-cell–mediated immunity, natural killer cells, and dendritic cells.11,12 Lymphoma-associated dendritic cells have been noted to express PD-L1 (programmed death-ligand 1), the T-cell coinhibitor ligand that indirectly impairs antitumor immunity by promoting induction of T-regulatory cells.11
Some limitations of this SEER-based analysis are inherent in its population-level data, and as such, conclusions cannot be directly applied to any given patient. Classically, SEER analyses are performed based on site rather than underlying biological entity,1–3,6 which may mask underlying biological connections. SEER does not have detailed data on methods of diagnosis or MF treatments, thereby limiting the ability to investigate further the potential causes of the increased risk of second malignancies.
In conclusion, patients with MF are at increased risk of diagnosis with a second malignancy. Clinicians should be particularly attuned to signs or symptoms of second primary malignancies in patients with MF. Further studies are needed to elucidate potential biologic mechanisms of this association and to use focused screening strategies for early detection and intervention.
We would like to thank the American Society of Hematology for supporting this study as part of the HONORS (Hematology Opportunities for the Next Generation of Research Scientists) award research grant. We appreciate Michael Franklyn’s editorial contributions.
Funding sources:
Supported by an HONORS (Hematology Opportunities for the Next Generation of Research Scientists) award research grant from the American Society of Hematology. Statistical analysis was in part supported by National Institutes of Health grant P30 CA77598 usign the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the National Institutes of Health award number UL1TR000114. This funded the statistical analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations used:
- CI
confidence interval
- HR
hazard ratio
- MF
mycosis fungoides
- SEER
Surveillance, Epidemiology, and End Results
- SIR
standardized incidence ratio
Footnotes
Conflicts of interest: None disclosed.
REFERENCES
- 1.Kantor AF, Curtis RE, Vonderheid EC, van Scott EJ, Fraumeni JF. Risk of second malignancy after cutaneous T-cell lymphoma. Cancer. 1989;63(8):1612–1615. [DOI] [PubMed] [Google Scholar]
- 2.Huang KP, Weinstock MA, Clarke CA, McMillan A, Hoppe RT, Kim YH. Second lymphomas and other malignant neoplasms in patients with mycosis fungoides and Sèzary syndrome. Arch Dermatol. 2007;143(1):45–50. [DOI] [PubMed] [Google Scholar]
- 3.Amber KT, Bloom R, Nouri K. Second primary malignancies in CTCL patients from 1992 to 2011: a SEER-based, population-based study evaluating time from CTCL diagnosis, age, sex, stage, and CD301 subtype. Am J Clin Dermatol. 2016;17(1):71–77. [DOI] [PubMed] [Google Scholar]
- 4.Väkevä L, Pukkala E, Ranki A. Increased risk of secondary cancers in patients with primary cutaneous T cell lymphoma. J Invest Dermatol. 2000;115(1):62–65. [DOI] [PubMed] [Google Scholar]
- 5.Lindahl LM, Fenger-Grøn M, Iversen L. Subsequent cancers, mortality, and causes of death in patients with mycosis fungoides and parapsoriasis: a Danish nationwide, population-based cohort study. J Am Acad Dermatol. 2014; 71(3):529–535. [DOI] [PubMed] [Google Scholar]
- 6.Ai WZ, Keegan TH, Press DJ, et al. Outcomes after diagnosis of mycosis fungoides and S ezary syndrome before 30 years of age. JAMA Dermatol. 2014;150(7):709–715. [DOI] [PubMed] [Google Scholar]
- 7.Coyte A, Morrison DS, McLoone P. Second primary cancer risk—the impact of applying different definitions of multiple primaries: results from a retrospective population-based cancer registry study. BMC Cancer. 2014;14(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clayton D, Cuzick J. Multivariate generalizations of the proportional hazards model. J R Stat Soc Ser A. 1985; 148(2):82. [Google Scholar]
- 9.Efron B Logistic regression, survival analysis, and the Kaplan-Meier curve. J Am Stat Assoc. 1988;83(402):414–425. [Google Scholar]
- 10.American Cancer Society. Cancer facts and figures 2018. Cancer Facts Fig. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf. Accessed August 19, 2019.
- 11.Lee BN, Duvic M, Tang CK, Bueso-Ramos C, Estrov Z, Reuben JM. Dysregulated synthesis of intracellular type 1 and type 2 cytokines by T cells of patients with cutaneous T-cell lymphoma. Clin Diagn Lab Immunol. 1999;6(1):79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim EJ. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115(4):798–812. [DOI] [PMC free article] [PubMed] [Google Scholar]