Abstract
Main objective
To prospectively assess the cost-consequence of a standardized diagnostic strategy as to compared to an open one for the etiological diagnosis of uveitis.
Design
This was a prospective, non-inferiority, multicentre, randomized controlled trial.
Methods
We included all consecutive patients with uveitis who had visited at least one of the Departments of Ophthalmology. In the standardized group, patients had a minimal work-up regardless of the type of uveitis (including evaluation of the CBC, ESR, C-reactive protein, tuberculin skin test, syphilis serology and chest X-ray). Depending on ophthalmological findings, further investigations could be performed. In the open strategy, ophthalmologists were free to order any kind of investigation. The main outcome was the mean cost per patient of each strategy.
Results
903 uveitis patients were included from January, 2010 to May, 2013. The mean cost per patient of the standardized strategy was 182.97 euros [CI 95% (173.14; 192.80)], and the mean cost per patient of the open strategy was 251.75 euros [CI 95% (229.24; 274.25)]. Therefore, the mean cost per patient of the standardized strategy was significantly lower than the mean cost per patient of the open strategy (p<0.001). There were significantly fewer visits (p<0.001), fewer radiological procedures (p<0.004) and fewer laboratory investigations (p<0.001) in the standardized group.
Conclusion
A standardized strategy is a cost-saving approach for the etiological diagnosis of uveitis.
Introduction
Uveitis, which can be defined as an inflammation of the uveal tract, can be caused by many infectious and non-infectious disorders such as systemic diseases, ocular specific disorders or may be drug-induced. However, it remains idiopathic in 25–45% of the cases [1–7] in Western countries. Uveitis accounts for approximately 5–10% of preventable blindness in the US and up to 15% worldwide [8–11], which is why it is important to search for a specific aetiology in order to start an appropriate treatment. The etiological diagnosis of uveitis remains a challenge due to the wide variety of diagnoses. An accurate history and detailed physical examination are the first steps in evaluating a patient with uveitis [12]. Then, on the basis of clinical findings, the physician may order various diagnostic tests. However, a non-selective approach to testing can be very costly, and is not always efficient, since many tests have a low diagnostic yield [13]. For example, a Canadian study showed that ophthalmologists ordered more diagnostic tests than those recommended by evidence-based guidelines for investigating anterior uveitis, including tests with low diagnostic yields. When applied to the Canadian population, this was responsible for an additional cost of 600,000 dollars/year to the Canadian health care system [14,15]. Physicians might have a broad approach leading to unnecessary investigations for fear of missing a diagnosis. However, performing many tests, which are not supported by clinical, or paraclinical findings, may lead to misinterpretation of false positive results and unnecessary supplementary investigations or treatments.
In the ULISSE study, a controlled trial that has evaluated the benefits of a standardized strategy for the etiologic diagnosis of uveitis [16], we prospectively assessed the costs of a standardized approach, in which all patients had a minimal work-up regardless of the type of uveitis (CBC, ESR, C-reactive protein, tuberculin skin test, syphilis serology, and chest X-ray) followed by more complex investigations, ordered by ophthalmologists, if needed. This standardized strategy was compared to an open one in which ophthalmologists could order any kind of investigation. In this study, the standardized strategy appeared to be a cost-saving diagnostic approach for the etiological diagnosis of uveitis.
Economic evaluations are useful to assess the cost of current practice patterns, and to determine the potential cost savings of establishing new approaches. Unfortunately, there are few studies evaluating the diagnostic yield of investigations and the cost-consequence of a strategy for the etiological diagnosis of uveitis.
Therefore, the main aim of this study was to assess the cost-consequence of the standardized diagnostic approach evaluated in the ULISSE study, compared to the open strategy.
Material and methods
Ethics
The ULISSE study was approved by a French institutional review board (Comité de Protection des Personnes Sud-Est IV). It was conducted in accordance with the Declaration of Helsinki. Patients included in the study have provided their written informed consent. The ULISSE study is registered under the unique ID #NCT01162070 at www.clinicaltrials.gov.
Design
The study design has been reported in detail previously [16]. Briefly, it was a multicentre, non-inferiority, prospective, randomized controlled trial evaluating two strategies for the etiological diagnosis of uveitis; an open strategy vs. a standardized one. In the open strategy, ophthalmologists were free to order any investigation and to refer the patient to the internal medicine department. Conversely, in the standardized strategy, regardless of the type of uveitis, a minimal work-up was performed after careful examination of the patient by both the ophthalmologist and the internist. Then, depending on clinical or paraclinical findings, extra diagnostic tests could be ordered. When no diagnosis was done at the end of the standardized strategy, physicians could perform free investigations. However, such a result was considered as a failure of the standardized strategy.
In the present study, we compared the cost-consequence of both strategies.
Patients
Inclusion and exclusion criteria have been reported previously [16]. Briefly, we included consecutive patients, who visited at least one of the participating departments of ophthalmology, for a diagnosis of uveitis, between June, 2010 and May, 2013. The diagnosis of uveitis was always established after careful ophthalmological examination and the anatomical localization was classified according the Standardization of Uveitis Nomenclature [17]. Ophthalmologists or internists had to retain an etiological diagnosis at month 6 whenever possible. In the absence of a diagnosis, the internist had to perform a new examination of the patient at month 12 to look for new signs or symptoms (except when uveitis was an acute anterior one).
Outcomes
The primary outcome was the mean cost per patient of the standardized strategy as compared to the open one. Secondary outcomes were the mean cost per patient of each step and the cost of extra free investigations.
Evaluation of costs
To evaluate the direct costs of each diagnostic strategy, we first estimated the cost of diagnostic tests (laboratory, radiological, endoscopic, and microsurgery procedures), as well as the cost of visits to specialists. The costs of laboratory investigations were estimated with the current tariffs of the National Biology Table (accessed at http://www.codage.ext.cnamts.fr/codif/nabm/). The costs of radiological, endoscopic, and microsurgery procedures were evaluated with the current tariffs of the Common Classification of Medical Acts and the costs of visits to specialists were evaluated with the current tariffs of the general classification system for professional activities. The costs of antibiotic treatments, hospitalizations and work stoppages were not estimated in the analyses:
Data on antibiotic treatments were not sufficient to allow their evaluation. We had no information on the dose prescribed, the dose administered, or the treatment duration.
To estimate the costs of hospitalizations, all the medical information departments of the participating institutions were solicited (n = 23), but only eight establishments responded. This did not allow us to estimate the hospitalization costs of the patients included in the ULISSE study.
With regards to work stoppages, no data was reported.
Statistical analysis
We performed non-parametric tests with Fischer exact test on costs data. Then, we wanted to test the mean annual costs per patient. So we compared the means between groups with a Student t-test, because the number of patient was sufficient. All analyses were performed using a IBM SPSS statistics version 19.
Data sharing statement
All data are available on request. Due to the date of study conception (2010), patients of the present study have not been informed and therefore have not consented that their data could be publicly shared.
Results
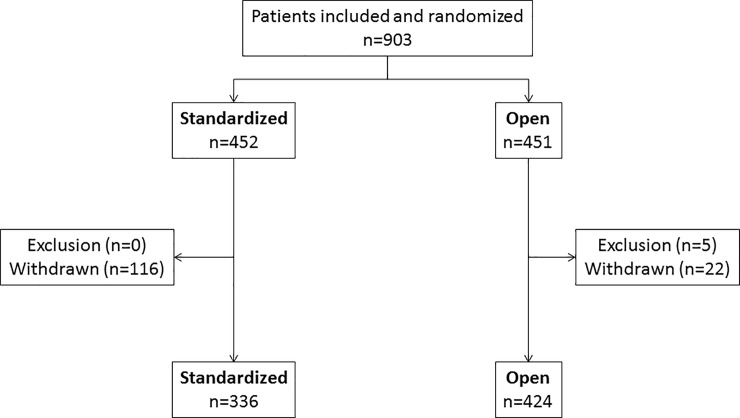
Over the study period, 903 patients with uveitis were included and randomized. Five patients were excluded and 138 were withdrawn, therefore there were 336 patients in the standardized strategy and 424 in the open strategy (Fig 1). In the standardized group, 25.6% of the patients were lost to follow-up [16].
Fig 1. Study flow chart.
In the standardized strategy, the mean cost per patient of the first step’s investigations was 43.86 euros [95% CI (43.62; 44.09)]. The chest X-ray accounted for 56% of the overall cost for this step, the complete blood count for 22%, the syphilis serology for 12%, and the C-reactive protein/erythrocyte sedimentation rate for 10%.
Among the 151 patients who underwent the second step’s investigations, the mean cost per patient was 60.62 euros [95% CI (56.65; 64.60)]. The HLA-B27 determination accounted for 74.7% of the overall cost, the chest computed tomography for 13%, and the angiotensin-converting enzyme for 6.6%.
Finally, among the 65 patients who underwent the third step’s investigations, the mean cost per patient was 42.14 euros [95% CI (31.48; 52.80)]. The 18F-fluorodeoxyglucose positron emission tomography accounted for 51% of the overall cost, the salivary gland biopsy for 25%, and the lumbar puncture for 12%.
In addition, in the standardized strategy, 335 patients had investigations guided by clinical or paraclinical findings. The mean cost per patient of these investigations was 72.23 euros [95% CI (63.48; 80.99)].
Furthermore, 59 patients had extra free investigations, with a mean cost per patient of 64.51 euros [95% CI (48.05; 80.97)].
Therefore, the mean cost per patient of the standardized strategy was 182.97 euros [CI 95% (173.14; 192.80)]. Visits accounted for 26.84% of the overall cost, radiological procedures for 20.85%, laboratory investigations for 42.75%, and other investigations for 9.56%.
The mean cost per patient of the open strategy was 251.75 euros [CI 95% (229.24; 274.25)]. Visits accounted for 12.62% of the overall cost, radiological procedures for 11.60%, laboratory investigations for 68.37%, and other investigations for 7.41% (Table 1). More specifically, HLA determination accounted for 43% of the overall cost, radiological procedures for 18%, microbiology for 14%, biochemistry for 10%, immunological tests for 9%, and invasive procedures (anterior chamber tap, vitrectomy and lumbar puncture) for 6%.
Table 1. Frequently ordered investigations in the open group (>20%).
Values are n (%).
| Laboratory investigations | |
| Complete blood count | 337 (90.35) |
| Erythrocyte sedimentation rate | 322 (86.33) |
| Serum electrolytes | 319 (85.52) |
| Syphilis serology | 308 (82.57) |
| C-reactive protein | 305 (81.77) |
| Angiotensin converting enzyme | 286 (76.68) |
| Electrophoresis of serum proteins | 254 (68.10) |
| Serum calcium | 250 (67.02) |
| Tuberculin skin test | 240 (64.34) |
| Hepatic tests | 200 (53.62) |
| Antinuclear antibodies | 159 (42.63) |
| IGRA | 152 (40.48) |
| HLA B27 | 143 (38.34) |
| Lysozyme | 137 (36.73) |
| Lyme disease serology | 132 (35.39) |
| HLA determination | 113 (30.29) |
| Hepatitis C serology | 105 (28.15) |
| Antineutrophil cytoplamic antibodies | 102 (27.35) |
| Rheumatoid factor | 100 (26.81) |
| Hepatitis B serology | 99 (26.54) |
| Herpes simplex virus 1 and 2 serology | 93 (24.93) |
| Creatinine | 93 (24.93) |
| Radiological procedures | |
| Chest CT | 174 (46.65) |
| Chest X-ray | 156 (41.82) |
| Brain MRI | 80 (21.45) |
Among the 760 patients included in this study, the mean cost per patient varied depending on the type of uveitis: 268.06€ for posterior uveitis (227.95€ for diagnostic tests and 40.11€ for diagnostic visits), 254.35€ for intermediate uveitis (212.11€ for diagnostic tests and 42.24€ for diagnostic visits), 252.27€ for panuveitis (210.98€ for diagnostic tests and 41.29€ for diagnostic visits) and 198.84€ for anterior uveitis (160.51€ for diagnostic tests and 38.33€ for diagnostic visits) (Table 2). The cost of diagnostic tests for granulomatous and non-granulomatous anterior uveitis were 167.33 € and 158.59€ respectively. There were no statistical differences between both groups except for anterior uveitis: the total cost per patient was significantly lower in the standardized group (163.73 euros vs 233.08 euros, p<0,001) (Table 3).
Table 2. Mean cost per patient according to type of uveitis.
| Anterior (n = 478) | Intermediate (n = 58) | Posterior (n = 89) | Panuveitis (n = 128) | |
|---|---|---|---|---|
| Visits | 38.33€ | 42.24€ | 40.11€ | 41.29€ |
| Diagnostic tests | 160.51€ | 212.11€ | 227.95€ | 210.98€ |
| Total | 198.84€ | 254.35€ | 268.06€ | 252.27€ |
Table 3. Mean cost per patient according to type of uveitis in each group.
| Standardized | Open | p-value | |
|---|---|---|---|
| Anterior | 163.73€ | 233.08€ | <0.001 |
| Intermediate | 232.88€ | 266.53€ | 0.599 |
| Posterior | 232.83€ | 281.07€ | 0.560 |
| Panuveitis | 221.55€ | 273.98€ | 0.218 |
There were fewer investigations in the standardized strategy than in the open one (3759 vs 5371, p<0.001), with a mean of 12.41 investigations per patient in the standardized strategy versus 15.39 in the open one (p<0.001). There were significantly fewer visits (p<0.001), fewer radiological procedures (p<0.004) and fewer laboratory investigations (p<0.001) in the standardized group. The mean cost per patient of the standardized strategy was significantly lower than the mean cost per patient of the open strategy (p<0.001)
Discussion
Here, we report a prospective study that assesses the cost-consequence of using a standardized strategy during the etiological work-up for uveitis. The mean cost per patient was 182.97 euros in the standardized strategy [CI 95% (173.14; 192.80)] and 251.75 euros in the open strategy [CI 95% (229.24; 274.25); p<0.001]. Overall, the mean cost per patient is significantly lower in the standardized strategy, and there were significantly fewer investigations in this group. In addition, the mean costs of visits, imaging tests, and laboratory investigations were significantly different between both groups.
There are few medico-economic studies on uveitis. Some studies have evaluated direct costs (hospitalizations, visits, prescription drug use) and indirect costs (disability days) of non-infectious uveitis [18]. However, almost none have evaluated the cost related to diagnostic procedures.
Only Adan-Civera et al. [19] showed that the total cost per patient of non-infectious uveitis in Spain in 2011 ranged from 2811.17 euros for acute anterior uveitis to 18 922.35 euros for posterior uveitis. Diagnostic visits accounted for 9.3% of the overall cost and diagnostic tests for 2.86%. The cost of diagnostic tests and visits in adults varied depending on the type of uveitis: panuveitis and posterior uveitis were the most costly (983€ per patient for visits and 395€ for diagnostic tests) followed by intermediate uveitis (983€ for visits and 303€ for diagnostic tests), acute anterior uveitis (655€ for visits and 156.21€ for diagnostic tests) and chronic anterior uveitis (655€ for visits and 121.89€ for diagnostic tests). In our study, we also found that the cost of diagnostic tests varied depending on the type of uveitis (anterior uveitis being the less costly) but this was not true for the cost of diagnostic visits. In addition, although the cost of diagnostic tests for anterior uveitis was similar in our study, the cost of diagnostic tests for the other types of uveitis was lower in our study. Finally, the cost of visits was much lower in our study.
More recently, Lee et al. reported a web-based survey on 13 patient scenarios in order to examine the range of practice in laboratory testing [13]. The patterns of test utilization were studied and the cost of the testing was calculated based on Noridian Medicare reimbursable rates for Seattle. Eighty-six per cent (12/14) members of the American Uveitis Society executive committee and trustees answered the survey. A total of 45 different tests (laboratory tests, imagings and/or diagnostic procedures) were ordered. The mean number of tests ordered was 5.47 ±2.71 per scenario and per provider whereas in our study there was an average of 12.41 investigations per patient in the standardized group and 15.39 in the open one. The average cost of testing was $282.80 per scenario per provider. In our study, the mean cost of diagnostic tests per patient in the open group was similar (219.97€) but the mean cost in the standardized group was lower (133.86€). In Lee’s study, imaging tests (fluorescein angiography, MRI, chest X-ray, and chest CT) contributed for 59.9% of the total costs of tests, whereas in our study, imaging tests contributed for 13.3 and 28.5% of the total cost of diagnostic tests in the open and in the standardized groups respectively. However, in our study, we did not include the cost of fluorescein angiography unlike Lee et al. In our study, more tests were ordered, especially more laboratory investigations, but the cost per patient in the standardized group was still lower.
A similar cross-sectional survey (comprising 4 anterior uveitis scenarios) was conducted among practicing ophthalmologists, fellows, and residents in Canada [15]. Similar to Lee’s et al. results a wide range of tests were ordered by the 498 responders and many of the tests were found to be of low diagnostic yield by the authors. Cost minimization and sensitivity analyses showed that applying the guidelines may lead to cost savings when compared with current practice patterns across the different scenarios that were evaluated (p<0.01) [15]. The maximal additional cost was observed with non-granulomatous anterior uveitis investigation (minimal additional cost of $75/patient). The additional cost was $40 for granulomatous uveitis whereas it was $36 when sarcoidosis was suspected. In Noble’s study, the cost of granulomatous and non-granulomatous anterior uveitis was $121 and $101, respectively. In our study, the cost of diagnostic tests for granulomatous and non-granulomatous anterior uveitis was higher (167.33€ and 158.59€, respectively).
The main limit of this study is that we assumed that the two diagnostic strategies were equivalent with respect to the clinical effectiveness of diagnosing uveitis. However, in the ULISSE study, an etiological diagnosis was established in 54% of cases in the open group and 50% in the standardized group (non-significant, p = 0.20). The difference between both strategies (standardized minus open) was -4.9% [95% CI (-12.5%; 2.6%)]. The comparison of both strategies remained inconclusive because the standardized strategy was neither inferior nor non-inferior to the open one [16]. Furthermore, as developed previously [16], in the standardized group, 25.6% of the patients were lost to follow-up, limiting the power of our study as well as its validity. Most of these patients were young, active men with acute anterior uveitis, a condition that may decrease follow-up adherence.
In addition, the cost-effectiveness ratio could not be evaluated in this study, since the only costs assessed were those of medical procedures. Investigators did not report the costs related to hospitalizations, treatments and work stoppages. It is therefore not possible to calculate this ratio representing a difference in overall costs of care and efficiency.
In conclusion, this ancillary analysis of the ULISSE multicentre, randomized study evaluating the cost-consequence of a standardized strategy vs. an open one shows that significantly fewer investigations were performed in the standardized group resulting in a significantly lower mean cost per patient. Such an approach can therefore lead to important cost savings.
Acknowledgments
The authors thank Corinne Dot (Department of Ophthalmology, Desgenettes Hospital, Lyon, France), Guillaume Gondran (Department of Internal Medicine, Hospital, Limoges, France), Julie Gueudry (Department of Ophthalmology, Hospital, Rouen, France), Gilles Kaplanski (Department of Internal Medicine, Conception Hospital, Marseille, France), Amadou Konaté (Department of Internal Medicine, Hospital, Montpellier, France), Marc Labetoulle (Department of Ophthalmology, Bicêtre Hospital, Paris, France), Jean- Christophe Lega (Department of Internal Medicine, South Hospital, Lyon, France), Isabelle Marie (Department of Internal Medicine, Hospital, Rouen, France), Martine Maugey-Faysse (Department of Ophthalmology, Rabelais Center, Lyon, France), Sarah Perignon (Department of Ophthalmology, Montchanin, France), Antoinette Perlat (Department of Internal Medicine, Hospital, Rennes, France), Pierre-Yves Robert (Department of Ophthalmology, Hospital, Limoges, France), Antoine Rousseau (Department of Ophthalmology, Bicêtre Hospital, Paris, France), Elisabeth Salles-Thomasson (Department of Internal Medicine, Hospital, Chalon sur Soâne, France), Michel Weber (Department of Ophthalmology, Hospital, Nantes, France), and Frédéric Mura (Department of Ophthalmology, Hospital, Montpellier, France) who contributed to the acquisition of the work and Laure Buscarlet (Hospices Civils de Lyon, Pole IMER Lyon, France) who coordinated this study.”
Data Availability
All data are available on request due to ethical restrictions imposed by the French Comité de Protection des Personnes Sud-Est IV. Due to the date of study conception (2010), patients of the present study have not been informed and therefore have not consented that their data could be publicly shared. Interested researchers may contact (drci.hcl@chu-lyon.fr) for data access.
Funding Statement
This study is part of a previous study that was supported by the French Ministry of Health (PHRC 2009) coordinated by PS.
References
- 1.Barisani-Asenbauer T, Maca SM, Mejdoubi L, Emminger W, Machold K, Auer H. Uveitis- a rare disease often associated with systemic diseases and infections- a systematic review of 2619 patients. Orphanet J Rare Dis. 2012;7:57 10.1186/1750-1172-7-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llorenç V, Mesquida M, Sainz de la Maza M, Keller J, Molins B, Espinosa G, et al. Epidemiology of uveitis in a Western urban multiethnic population. The challenge of globalization. Acta Ophthalmol (Copenh). 2015 [DOI] [PubMed] [Google Scholar]
- 3.Bajwa A, Osmanzada D, Osmanzada S, Khan I, Patrie J, Xin W, et al. Epidemiology of uveitis in the mid-Atlantic United States. Clin Ophthalmol Auckl NZ. 2015;9:889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCannel CA, Holland GN, Helm CJ, Cornell PJ, Winston JV, Rimmer TG. Causes of uveitis in the general practice of ophthalmology. UCLA Community-Based Uveitis Study Group. Am J Ophthalmol. 1996;121(1):35–46. 10.1016/s0002-9394(14)70532-x [DOI] [PubMed] [Google Scholar]
- 5.Merrill PT, Kim J, Cox TA, Betor CC, McCallum RM, Jaffe GJ. Uveitis in the southeastern United States. Curr Eye Res. 1997;16(9):865–74. 10.1076/ceyr.16.9.865.5048 [DOI] [PubMed] [Google Scholar]
- 6.Mercanti A, Parolini B, Bonora A, Lequaglie Q, Tomazzoli L. Epidemiology of endogenous uveitis in north-eastern Italy. Analysis of 655 new cases. Acta Ophthalmol Scand. 2001;79(1):64–8. 10.1034/j.1600-0420.2001.079001064.x [DOI] [PubMed] [Google Scholar]
- 7.Oruc S, Kaplan AD, Galen M, Kaplan HJ. Uveitis referral pattern in a Midwest University Eye Center. Ocul Immunol Inflamm. 2003;11(4):287–98. 10.1076/ocii.11.4.287.18270 [DOI] [PubMed] [Google Scholar]
- 8.Bodaghi B, Cassoux N, Wechsler B, Hannouche D, Fardeau C, Papo T, et al. Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine (Baltimore). 2001;80(4):263–70. [DOI] [PubMed] [Google Scholar]
- 9.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990. October;14(5–6):303–8. 10.1007/bf00163549 [DOI] [PubMed] [Google Scholar]
- 10.Darrell RW, Wagener HP, Kurland LT. Epidemiology of uveitis. Incidence and prevalence in a small urban community. Arch Ophthalmol Chic Ill 1960. 1962;68:502–14. [DOI] [PubMed] [Google Scholar]
- 11.Dick AD, Tundia N, Sorg R, Zhao C, Chao J, Joshi A, et al. Risk of Ocular Complications in Patients with Noninfectious Intermediate Uveitis, Posterior Uveitis, or Panuveitis. Ophthalmology. 2016;123(3):655–62. 10.1016/j.ophtha.2015.10.028 [DOI] [PubMed] [Google Scholar]
- 12.Sève P, Cacoub P, Bodaghi B, Trad S, Sellam J, Bellocq D, et al. Uveitis: Diagnostic work-up. A literature review and recommendations from an expert committee. Autoimmun Rev. 2017;16(12):1254–64. 10.1016/j.autrev.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 13.Lee CS, Randhawa S, Lee AY, Lam DL, Van Gelder RN. Patterns of Laboratory Testing Utilization Among Uveitis Specialists. Am J Ophthalmol. 2016;170:161–7. 10.1016/j.ajo.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forooghian F, Gupta R, Wong DT, Derzko-Dzulynsky L. Anterior uveitis investigation by Canadian ophthalmologists: insights from the Canadian National Uveitis Survey. Can J Ophthalmol J Can Ophtalmol. 2006;41(5):576–83. [DOI] [PubMed] [Google Scholar]
- 15.Noble J, Hollands H, Forooghian F, Yazdani A, Sharma S, Wong DT, et al. Evaluating the cost-effectiveness of anterior uveitis investigation by Canadian ophthalmologists. Can J Ophthalmol J Can Ophtalmol. 2008;43(6):652–7. [DOI] [PubMed] [Google Scholar]
- 16.De Parisot A, Kodjikian L, Errera M-H, Sedira N, Heron E, Pérard L, et al. Randomized Controlled Trial Evaluating a Standardized Strategy for Uveitis Etiologic Diagnosis (ULISSE). Am J Ophthalmol. 2017;178:176–85. 10.1016/j.ajo.2017.03.029 [DOI] [PubMed] [Google Scholar]
- 17.Standardization of Uveitis Nomenclature for Reporting Clinical Data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–16. 10.1016/j.ajo.2005.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorne JE, Skup M, Tundia N, Macaulay D, Revol C, Chao J, et al. Direct and indirect resource use, healthcare costs and work force absence in patients with non-infectious intermediate, posterior or panuveitis. Acta Ophthalmol (Copenh). 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adán-Civera AM, Benítez-Del-Castillo JM, Blanco-Alonso R, Pato-Cour E, Sellas-Fernández A, Bañares-Cañizares A. Burden and direct costs of non infectious uveitis in Spain. Reumatol Clin. 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available on request due to ethical restrictions imposed by the French Comité de Protection des Personnes Sud-Est IV. Due to the date of study conception (2010), patients of the present study have not been informed and therefore have not consented that their data could be publicly shared. Interested researchers may contact (drci.hcl@chu-lyon.fr) for data access.