Abstract
Microbial keratitis is a potentially blinding condition that must be treated emergently to preserve vision. Although long recognized as a significant cause of corneal blindness, our understanding of its true global scale, associated burden of disease, and etiological patterns remains somewhat limited. Current epidemiological data suggest that microbial keratitis may be epidemic in parts of the world—particularly within South, South-East, and East Asia—and may exceed 2 million cases per year worldwide. Etiological patterns vary between economically developed and developing countries, with bacterial predominance in the former and fungal predominance in the latter. The key to effective management lies in timely diagnosis; however, the current gold standard of stain and culture remains time consuming and often yields no clinically useful results. For this reason, there are attempts to develop highly sensitive and accurate molecular diagnostic tools to provide rapid diagnosis, inform treatment decision making, and minimize the threat of antimicrobial resistance. We provide an overview of these key areas and of avenues for further research toward the goal of more effectively addressing the problem of microbial keratitis on both an individual and public health level.
Keywords: microbial keratitis, bacterial keratitis, fungal keratitis, epidemiology, etiology, antimicrobial resistance
1. Introduction
Microbial keratitis (MK) is an ocular emergency that may result in sight loss for which the prospect of visual rehabilitation is often poor. Clinically, MK is diagnosed by the presence of corneal ulceration with or without stromal infiltration, hypopyon and anterior chamber reaction, or signs of conjunctival injection. Historically, MK refers to nonviral causes of corneal infection caused by bacteria, fungi, and/or protozoa. Beyond the visual disability associated with MK, our understanding of its global epidemiology and associated health care and socioeconomic costs remains incomplete. Furthermore, the diagnostic tools used currently, including Gram stain and culture, are dated and often inadequate, highlighting the need for replacement by more accurate and rapid methods, which in turn would focus patient management, minimize treatment failures, and limit the challenge of antimicrobial resistance. As the treatment of MK has been discussed in other excellent reviews,8,72 we focus rather on currently understudied aspects of MK, to inform future efforts to prevent and lessen the global societal burden of MK.
2. Global epidemiology and burden
Up to 5% of all blindness may be attributed to the consequences of ocular trauma and resulting infection.104,115 Global estimates of MK as a cause of unilateral blindness range from 1.5 to 2 million cases per year, although this is likely conservative due to underreporting in economically less developed countries.116 Capturing epidemiological data for MK is difficult because most data are reported under the term “corneal blindness” that itself comprises a range of traumatic, infectious, inflammatory, and inherited conditions. For this reason, rigorous data on MK epidemiology are scant and derived from a limited number of published studies (Table 1; Fig. 1). Three of these studies included only contact lens wearers.18,83,97
Table 1 -.
Incidence studies for microbial keratitis, including study populations, and studies involving CL-wearing populations
| Study | Location | Years of study | Study population (population size) | Overall incidence per 100,000 persons | Incidence among contact lens (CL) wearers and predisposed populations per 100,000 persons |
|---|---|---|---|---|---|
| Overall incidence studies (North America and Europe) | |||||
| Ibrahim et al (2012)44 | United Kingdom | 1997–2003 2006 |
Portsmouth, UK 1997–2003 (489,391)— retrospective study 2006 (499,100)— prospective study | 2006—40.3 1997–2003—52.1 |
- |
| Jeng et al (2010)45 | USA | 1998–1999 | Northern California (1,093,210) | 27.6 per 100,000 person-years | 130.4 per 100,000 person-years HIV-positive persons—238.1 per 100,000 person-years |
| Seal et al (1999)88 | Scotland | 1995 | West Scotland (2,945,810) | 3.6 | 18.1 |
| Erie et al (1993)28 | USA | 1950–1988 | Olmsted County, Minnesota (46,650 in 1950 to 105,720 in 1988) | 1950s—2.5 1980s—11.0 |
- |
| Overall incidence studies (Asia) | |||||
| World Health Organization (2004)121 | Bhutan | NA | Unstated | 339 | - |
| World Health Organization (2004)121 | Burma | NA | Unstated | 710 | - |
| Lam et al (2002)59 | Hong Kong, China | 1997–1998 | Hong Kong (2,489,701) | 6.3 | 33.9 |
| Upadhyay et al (2001)107 | Nepal | 1992–1993 | Bhaktapur, Kathmandu Valley (34,902) | 799 | - |
| Gonzales et al (1996)35 | India | 1993 | Madurai, Tamil Nadu (3,558,056) | 113 | - |
| Targeted contact lens use studies | |||||
| Stapleton et al (2008)97 | Australia | 2003–2004 | Australia (13,381,800–13,568,400) | - | Overall (all CL): 42 Daily-wear rigid gas- permeable: 12 Daily soft: 19 |
| Cheng et al (1999)18 | The Netherlands | 1996 | The Netherlands (13,188,000) | - | Daily-wear rigid gas- permeable: 11 Daily soft: 35 Extended wear soft: 200 |
| Poggio et al (1989)83 | USA | 1987 | New England-Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont (8,021,000) | - | Hard lens: 20 Rigid gas-permeable: 40 Daily-wear soft: 41 Extended-wear soft: 209 |
Fig. 1 -.
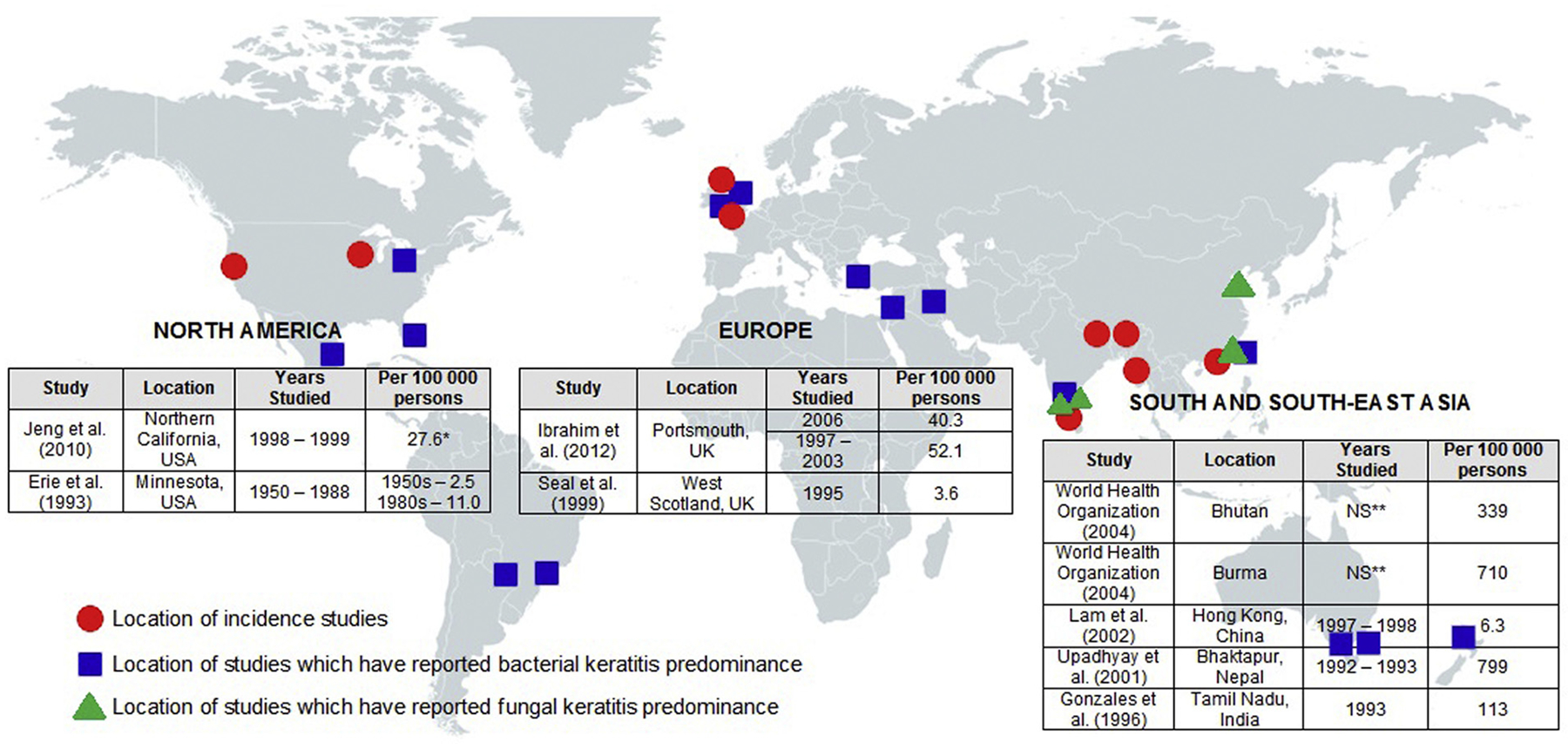
World incidence and etiological predominance of MK at a glance, grouped into region showing wide disparity in incidence between European and Northern American studies and those from South and South-East Asia, as well as relative paucity in available worldwide epidemiological data. Circles have been placed showing the location of incidence studies. Location of studies that reported bacterial keratitis (squares) and fungal keratitis (triangles) predominance has also been marked. *Per 100,000 person-years. ** NS, not stated.
2.1. Microbial keratitis in the developed world
There is a clear distinction between the incidence of MK within developing and developed countries (Fig. 1). The estimated incidence in the United States was first extrapolated by Erie and coworkers who followed the population of Olmsted County in Minnesota from 1950 to 1988, reporting an incidence rate of 2.5 per 100,000 persons in the 1950s compared to 11.0 per 100,000 in the 1980s, a rise driven largely by increased contact lens use.28 Another more recent study by Jeng and coworkers focused on a population within Northern California from September 1998 to August 1999 and reported an incidence of 27.6 per 100,000 person-years overall, with 130.4 cases per 100,000 person-years in the contact lens–wearing population.45 Data from the United Kingdom are similar, with Seal and coworkers reporting an incidence of 3.6 per 100,000 in Scotland from a study population in 1995, rising to 40.3 per 100,000 as reported by Ibrahim and coworkers who studied the population of Portsmouth, England, in 2006.44,88 In addition, 1 study by Lam and coworkers from the highly industrialized, developed city of Hong Kong reported an incidence of 6.3 per 100,000.59
2.2. Microbial keratitis in the developing world
By comparison, epidemiological data from South Asia demonstrate that MK has reached epidemic levels in this part of the world (Fig. 1) and presents a major public health threat alongside other causes of corneal blindness such as leprosy and trachoma, both of which have been targets of large-scale successful World Health Organization campaigns. In parts of the world with difficulties regarding access to health care, poorer health indices, and a higher proportion of workers within high-risk professions such as farming and agriculture, rates of MK are as high as 113 per 100,000 in Madurai, Tamil Nadu, India,35 339 per 100,000 in Bhutan,121 710 per 100,000 in Burma,121 and 799 per 100,000 in Nepal.107 Whitcher and Srinivasan deduced that there may be in excess of 800,000 cases of MK per year in India alone, tenfold greater than that reported in the United States.115 Extrapolating similar figures to other similarly underresourced parts of the world, it would be reasonable to expect that in similar regions still unstudied, rates may resemble those found currently in South Asia. Overall it is likely, owing to scarcity of current data, that the scope of MK has been largely underrealized, particularly in economically less developed countries.
2.3. Burden of disease
In the United States, according to a Morbidity and Mortality Weekly Report by Collier and coworkers, infectious keratitis results in approximately 1 million clinical visits to health practitioners and 58,000 registered emergency departments per annum.21 In all, MK costs the US health care system an estimated 175 million dollars in direct health expenditures and approximately 70 million dollars in Medicare- and Medicaid-related costs. It is difficult, however, to provide a definite estimate of the global burden of MK, other than to suggest that it is likely higher than reported and also likely affects poor rural and agricultural farm-based populations in a disproportionate manner. Furthermore, it is likely that the true burden of MK is underestimated because the current WHO guidelines define “blindness” as a visual acuity of <3/60 corrected in the better eye, and MK often causes visual disability that is significant, but falls short of this standard. Already, corneal ulceration is the most common cause of corneal blindness in China according to a nationwide survey91 and the second most common cause of all blindness, behind cataract, in children aged 0–15 years in Uganda.113 Global health care and socioeconomic costs from MK are difficult to ascertain as no such data exist, to our knowledge, from the developing world; however, it is reasonable to conclude that the costs of MK are magnified insofar as it affects the poorest populations and often during their most productive years.117 Although these costs may derive in part from poor access to health care, they also may reflect nontraditional patterns of managing MK at a patient’s first contact with an eye care provider. Fulminant infection and therefore increased burden of disease have been associated with delays in presentation,15 underdosage of empirical antibiotics, inappropriate use of corticosteroids, and the impracticalities of obtaining diagnostic cultures, particularly within private practice set-tings.108 Furthermore, the estimated burden of disease would be even higher if cases were included for which cultures were either not considered to be indicated or not performed, including cases with small peripheral ulcers that might have been infectious, but were successfully treated with empirical antibiotics in the absence of any diagnostic measures.
3. Global etiology in the context of risk factors
3.1. Literature search for microbial keratitis etiology
Global variations in MK etiology largely reflect patient-based risks such as population demographic, occupation, contact lens use, concomitant ocular and systemic illness, as well as environmental factors such as geographical location, climate, and virulence of causative organisms. In June 2018, our search of PubMed and its subsidiary MEDLINE, EMBASE, and Web of Science databases using iterations of the key words, “microbial keratitis,” “bacterial keratitis,” “fungal keratitis,” “infective keratitis,” and “acanthamoeba keratitis” revealed 6,226 articles, from which 65 were deemed to be original, unduplicated studies published after 2000, written in English, with 200 or more patients, and described the results of corneal scrapings from individual cases of clinically suspected MK. The captured articles were then sorted according to region of study and sample sizes. Table 2 shows the 3 largest etiological studies on nonherpetic MK published since 2000 for 7 distinct regions: South Asia, East and South-East Asia, Europe, North America, South America, Australia and Oceania, and the Middle East and Africa.
Table 2 -.
Literature search of large case series reporting infectious agents involved in MK, grouped by region
| Study | Study period | Location | Corneal scrapes | Culture positivity (%) | Pure bacterial cases (%) | Pure fungal cases (%) | Mixed bacterial and fungal (%) | Acanthamoeba cases (%) | Gram (+) isolates (%) | Gram (-) isolates (%) | Most common bacterial isolates (%) | Most common fungal isolates (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Southern Asia | ||||||||||||
| Lalitha et al (2016)58 | 2002–2013 | Tamil Nadu, India | 17,948 | 56.9 | 35.4 | 60.9 | 1.1 | 2.6 | NA | NA |
S. pneumoniae (32.7) P. aeruginosa (24.3) Nocardia spp. (6.7) |
NA |
| Gopinathan et al (2009)37 | 1991–2001 | Hyderabad, India | 5,897 | 60.4 | 51.9 | 38.2 | NA | 2.4 | 82.1 | 17.9 |
S. epidermidis (32.5) Corynebacterium spp. (14.5) S. pneumoniae (13.9) |
Fusarium spp. (35.6) Aspergillus spp. (29.0) Curvularia spp. (5.5) |
| Bharathi et al (2007)14 | 1999–2002 | Tamil Nadu, India | 3,183 | 70.6 | 46.4 | 48.7 | 3.4 | 1.5 | 72.6 | 27.4 |
S. pneumoniae (35.9) P. aeruginosa (19.9) S. epidermidis (18.2) |
Fusarium spp. (41.9) Aspergillus spp. (25.0) Cladosporium spp. (6.8) |
| East Asia | ||||||||||||
| Lin et al (2017)69 | 2009–2013 | Guangzhou, China | 2,973 | 46.1 | 41.9 | 44.6 | 13.6 | NA | 75.4 | 24.6 |
S. epidermidis (31.9) P. aeruginosa (12.4) S. simulans (5.5) |
Fusarium spp. (29.3) Aspergillus spp. (24.1) Helminthosporium spp. (18.2) |
| Hsiao et al (2016)41 | 2003–2012 | Linkou, Taiwan | 2,012 | 49.3 | 81.1 | 16.0 | NA | 1.1 | 51.0 | 49.0 |
P. aeruginosa (24.4) CoNS (16.6) Propionibacterium spp. (9.1) |
NA |
| Xie et al (2006)123 | 1999–2004 | Shandong, China | 1,056 | 75.6 | 16.2 | 77.9 | 4.0 | 1.9 | NA | NA | NA |
Fusarium spp. (73.3) Aspergillus spp. (12.1) Alternaria spp. (3.2) |
| Europe | ||||||||||||
| Ting et al (2018)105 | 2008–2017 | Sunderland, UK | 914 | 44.5 | 91.0 | 4.2 | NA | 4.8 | 70.8 | 29.2 | CoNS (28.5) S. aureus (14.9) Streptococcus spp. (13.3) |
Yeasts (50.0) Filamentous fungi (50.0) |
| Tan et al (2017)100 | 2004–2015 | Manchester, UK | 4,229 | 32.6 | 90.6 | 7.1 | NA | 2.3 | 69.9 | 30.1 | CoNS (26.9) S. aureus (16.7) Streptococcus spp. (14.7) |
Yeasts (53.2) Fusarium spp. (25.7) |
| Ibrahim et al (2009)a,43 | 1997–2003 | Portsmouth, UK | 1,254 | 63.8 | 85.4 | 0 | 1.0 | 1.4 | 71.1 | 28.9 |
S. epidermidis (31.7) P. aeruginosa (12.0) S. aureus (11.5) |
NA |
| North America | ||||||||||||
| Hernandez- Camarena et al (2015)40 | 2002–2011 | Mexico City, Mexico | 1,638 | 37.6 | 88.3 | 11.7 | NA | NA | 75.7 | 24.2 |
S. epidermidis (27.4) P. aeruginosa (12.1) S. aureus (9.0) |
Fusarium spp. (50.0%) Aspergillus spp. (19.4) Candida spp. (8.3) |
| Lichtinger et a’ (2012)” | 2000–2010 | Toronto, Canada | 1,701 | 57.4 | 91.8 | 6.0 | NA | 2.2 | 76.2 | 23.8 | CoNS (36.5) Streptococcus spp. (17.4) S. aureus (17.2) |
NA |
| Alexandrakis et al (2000)5 | 1990–1998 | Miami, USA | 2,920 | 50.3 | 91.1 | NA | NA | NA | 47.6 | 49.6 |
P. aeruginosa (25.7) S. aureus (19.4) S. marcescens (7.6) |
NA |
| South America | ||||||||||||
| Cariello et al (2011)16 | 1975–2007 | Sao Paulo, Brazil | 6,804 | 48.6 | 78.9 | 11.0 | 1.1 | 7.4 | 71.7 | 26.5 | CoNS (26.3) S. aureus (21.1) P. aeruginosa (11.8) |
Fusarium spp. (51.9) Candida spp. (14.3) Aspergillus spp. (9.1) |
| Laspina et al (2004)60 | 1998–2001 | Asuncion, Paraguay | 660 | 79.4 | 51.0 | 26.0 | 23.1 | NA | NA | NA | CoNS (25.1) S. aureus (23.7) P. aeruginosa (10.7) |
Acremonium spp. (37.8) Fusarium spp. (19.6) Aspergillus spp. (17.7) |
| Middle East and Africa | ||||||||||||
| Politis et al (2016)84 | 2002–2014 | Jerusalem, Israel | 943 | 47.9 | 91.8 | 8.2 | NA | NA | NA | NA | CoNS (32.8) Pseudomonas spp. (19.3) S. pneumoniae (13.0) |
NA |
| Al-Sharkarchi et al (2007)2 | 2002–2005 | Baghdad, Iraq | 396 | 58.6 | 69.8 | 31.9 | 1.7 | NA | 54.3 | 45.7 |
Pseudomonas spp. (42.0) CoNS (24.1) Coagulase-positive Staphylococcus (19.1) |
Aspergillus spp. (56.8) Fusarium spp. (27.0) Candida spp. (5.4) |
| Yilmaz et al (2007)124 | 1990–2005 | Izmir, Turkey | 620 | 36.3 | 77.8 | 22.2 | NA | NA | 88.6 | 11.4 |
S. epidermidis (34.3) S. aureus (31.4) S. pneumoniae (20.0) |
Fusarium spp. (50.0) Candida spp. (30.0) Aspergillus spp. (20.0) |
| Australia and Oceania | ||||||||||||
| Pandita and Murphy (2011)79 | 2003–2007 | Waikato, New Zealand | 265 | 65.6 | 98.3 | 1.7 | NA | NA | 79.5 | 20.5 | CoNS (41.5) S. aureus (11.7) S. pneumoniae (7.6) |
Fusarium spp. (66.7) Candida spp. (33.3) |
| Keay et al (2006)49 | 2001–2003 | Melbourne, Australia | 291 | 49.2 | 90.2 | 6.3 | NA | 3.6 | 71.3 | 28.7 | CoNS (45.5) P. aeruginosa (9.9) S. aureus (9.9) |
NA |
| Mallari et al (2000)71 | 1991–1999 | Melbourne, Australia | 277 | 100% (only positive cultures included) | 100 (all bacterial) | NA | NA | NA | 76.7 | 23.3 | CoNS (26.0) S. aureus (25.6) Pseudomonas spp. (11.5) |
NA |
CoNS, coagulase-negative Staphylococcus.
- Culture positivity considered number of positive cultures over total collected.
- Percentage of bacterial, fungal, mixed bacterial and fungal, and Acanthamoeba cases calculated as percentage of positive cultures, or total isolates.
- Percentage of gram positive (including Actinomycetes such as Nocardia spp.) and gram negative organisms calculated as a percentage of total bacterial and bacterial plus fungal cultures, or total number of bacterial isolates retrieved, whichever was provided by the study.
- Percentage of individual bacterial and fungal organisms calculated as percentage of total bacterial and fungal isolates, respectively.
Mixed viral and bacterial cultures accounted for 64 (5.1%) cases.
3.2. Preponderance of keratitis etiologies is dependent on region
Among all cases of MK, the highest proportions of bacterial etiology have been reported in Europe,43,100,105 North America,5,40,66 Australia, and Oceania,49,71,79 (range 85.4–91.0%, 88.3–91.8%, and 90.2–98.3%, respectively), locations that also have the highest prevalence of contact lens wear. By comparison, in other parts of the world, proportions of MK attributable to fungal organisms reach parity with bacterial causes. In some studies, fungal keratitis rates exceed bacterial keratitis, as in Asia (range 56.1–82.0%).58,61,123 The Asia Cornea Society Infectious Keratitis Study recently published the results of a prospective, international multicenter study that studied etiological patterns of infectious keratitis, including viral disease, from 13 tertiary centers throughout Asia.52 The study included over 6,500 eyes, reporting fungal predominance in India and China for nonviral MK, both from study populations consisting of urbanized and rural communities. The largest known case series on microbial keratitis was published by Lalitha and coworkers58 from the Aravind Eye Hospital in Madurai, India. In this study, the results of corneal cultures taken from 17,948 patients presenting with corneal ulceration from 2002 to 2013 were reviewed, with 6,218 of 10,207 (60.9%) culture positive patients determined to have a fungal etiology. Similarly, 2 large Chinese studies by Lin and coworkers69 and Xie and coworkers123 reported proportions of 44.6% and 77.9%, respectively. In contrast to variations seen in relative rates of bacterial and fungal keratitis, worldwide cases of Acanthamoeba keratitis appear to dwell between 1% and 3% of all MK, although the proportion of Acanthamoeba keratitis relative to total MK case numbers was 7.4% in a large case series of over 6,800 patients from Brazil.16
3.3. Fungal keratitis
In South Asia, the disparity in etiology, as well as the preponderance of fungal to bacterial keratitis, may be explained by several factors. Mycotic ocular infections are seen predominantly in areas with a large agricultural and manual labor workforce, especially among men who are exposed to foreign body trauma from plant material. In many studies, trauma to the eye caused by vegetable matter (including paddy, wheat, and maize stalks), and sand or mud, ranked among the top antecedent foreign body insults leading to subsequent infection.10,12,14,47,80 The use of traditional eye medicines, prescribed by alternative medicine practitioners, and often containing vegetable matter and unpasteurized dairy products, has been found to be a risk factor in up to 27.5% in several case series and may exacerbate the keratitis.47,80 The preponderance of filamentous fungi such as Fusarium spp. and Aspergillus spp., and to a lesser extent dematiaceous molds such as Bipolaris spp. and Curvularia spp. that thrive amid tropical conditions, suggests that climate and geography also play important roles. Despite perennially warm conditions, seasonal variations in fungal keratitis have been previously observed with some studies demonstrating uneven yearly distributions of fungal keratitis with peaks coinciding, for instance, with the windy and harvest seasons within Tamil Nadu, India,68 Qingdao, China,123 and with the monsoon season in Taiwan.17 Yeast-associated keratitis, including cases caused by Candida spp., are uncommon in tropical climates but more common in temperate zones in patients with preexisting ocular surface and/or predisposing systemic disease.31,103
3.4. Bacterial keratitis
Despite local and regional variations in bacterial keratitis etiology, the most commonly reported causative organisms appear consistent worldwide, with Table 2 demonstrating a higher proportion of gram-positive isolates (range 47.6–88.6%; median 72.2%) than gram-negative isolates (range 11.4–49.6%; median 27.0%); however, we interpret these figures with caution because most eyelid and ocular surface commensal organisms are gram positive and more likely to contaminate samples. In the absence of standardized methods of specimen collection and laboratory reporting, a significant proportion of reported gram-positive isolates could very well be false positives. Nonetheless, among gram-positive isolates, coagulase-negative Staphylococcus species including Staphylococcus epidermidis featured among the top 3 bacterial isolates within 17 out of 20 studies for which a breakdown of pathogens is available (range 16.6–45.5%; median 28.5%).2,14,16,37,40,41,43,49,60,66,69,71,79,84,100,105,124 Staphylococcus aureus featured among the top 3 isolates within 12 of 20 studies (range 9.0–31.4%; median 17.0%).5,16,40,43,49,60,66,71,79,100,105,124 Streptococcus species including Streptococcus pneumoniae were less common (range 7.6–35.9%, median 14.7%).14,37,58,66,79,84,100,105,124
Among gram-negative isolates, Pseudomonas spp. including Pseudomonas aeruginosa ranked among the 3 most common isolates in 13 of these 20 studies (range 9.9–42.0%; median 12.4%).2,5,14,16,40,41,43,49,58,60,69,71,84 As current data are skewed toward gram-positive organisms for reasons listed previously, these figures are likely a substantial underrepresentation of the true proportion of MK caused by Pseudomonas spp., which is almost always considered pathogenic when cultured from the cornea. Although the exact degree of underrepresentation is difficult to ascertain, Pseudomonas spp. is undoubtedly a major causative organism as it has been identified as the most common singular culprit in studies from major centers based in the United States,5,76,106 United Kingdom,89 and Asia.30,41,59,99,101,127 Most notably, Pseudomonas aeruginosa was the second most common pathogen isolated from the Asia Cornea Society Infectious Keratitis Study study, behind Fusarium spp., and the most common bacteria isolated in participating centers in the Philippines, Taiwan, Thailand, and Singapore.52 Historically, Pseudomonas keratitis has been associated with contact lens wear,6,24,67 possibly owing to favorable contact lens case colonization,96 a survival niche created between the corneal surface and the lens which allows for microbial replication,95 and biofilm production.73,122 Among other less common gram-negative isolates, the Enterobacteriaceae family that includes Escherichia spp., Klebsiella spp., and Serratia spp. typically account for <10% of all bacterial isolates from MK patients.26,58,66
It is important to also mention several emerging causes of MK, with speciation made possible with more contemporary laboratory isolation methods. For instance, the aerobic, nonfermenting gram-negative rods Achromobacter xylosoxidans and Stenotrophomonas maltophilia, now regarded as important causes of MK, were once typically included as “other” uncommon gram-negative etiologies and easily mistaken with their more common relative, P. aeruginosa75,93; however, both are now recognized as important causes of contact lens–related MK.62,118 This can be critical in the absence of sensitivity testing because both are frequently resistant to fluoroquinolones and aminoglycosides. Another example of an emerging important MK etiology are Actinomycetes including Nocardia spp., which is an uncommon cause of MK in the economically developed world, but which accounted for 6.7% of all bacterial isolates found in the study by Lalitha and coworkers and 11.1% of 500 participants of the Steroids for Corneal Ulcers (SCUT) trial, most of whom were recruited from Tamil Nadu, India.58,94
3.5. Pediatric microbial keratitis
Although pediatric MK is uncommon, it presents unique clinical and diagnostic challenges owing to difficulties associated with achieving a thorough ophthalmic history and examination, and the challenge of obtaining corneal scrapings when indicated. For children, the risk of complications such as vision loss and amblyopia may be compounded by a general tendency to present in the later stages of disease. Data from small, local epidemiological studies (Table 3) suggest a general shift in the predisposing factors associated with pediatric MK over time. For studies published before 2000, irrespective of geographic location, preceding trauma, systemic disease, ocular surface disease, and anterior segment surgery accounted for 21.1–44.0%,20,22,56,109 14.0–30%,20,22,78,109 17.7–22.7%,56,78 and 8.8–24.0%22,56 of cases, respectively. By contrast, studies published after 2000 demonstrate that contact lens use is now the predominant risk factor in the developed world, identified in 35.3–83.3%42,63,77,87,119,126 of cases, while trauma still predominates in less developed areas.3,80,90,92 The rise in nocturnal orthokeratology lens use among children for the treatment of myopia is now recognized as a strong risk factor for the development of MK.114,125
Table 3 -.
Case series of pediatric keratitis, ordered by chronology
| Study | Location | Years | Patients (eyes) | Culture positivity (%) | Risk factors (%) | Pathogens (%) | Outcomes and complications (%) |
|---|---|---|---|---|---|---|---|
| Rossetto et al (2017)87 | Miami, USA | 1992–2015 | 107 (108) | 52/89 (58.4) | CL wear (77.6) Ocular trauma (8.4) Systemic disease (4.7) |
P. aeruginosa (46.2) Stenotrophomonas maltophilia (19.3) Fusarium spp. (13.5) |
|
| Noureddin et al (2016)77 | Vancouver, Canada | 2006–2011 | 16 (17) | 13/17 (76.5) | CL wear (35.3) Ocular surface disease (35.3) Systemic disease (17.6) |
CoNS (38.5) Acanthamoeba (30.8) P. aeruginosa (15.4) |
|
| Lee et al (2014)63 | Linkou, Taiwan | 2008–2012 | 67 (68) | 36/63 (57.1) | CL wear (52.9) Trauma (16.2) Systemic disease (16.2) |
P. aeruginosa (30.6) CoNS (13.9) Fungi (13.9) |
|
| Young et al (2013)126 | Hong Kong, China | 2001–2010 | 18 (18) | 16/18 (88.8) | CL wear (83.3) Ocular surface disease (11.1) Trauma (5.5) |
Pseudomonas spp. (62.5) CoNS (31.2) Corynebacterium spp. (12.5) |
|
| Al Otaibi et al (2012)3 | Riyadh, Saudi Arabia | 2000–2010 | 68 (68) | 34/68 (50) | Trauma (39.7) Systemic disease (20.5) CL wear (16.1) |
S. pneumoniae (23.5) S. epidermidis (20.5) S. aureus (17.6) |
|
| Song et al (2012)92 | Shandong, China | 2000–2009 | 76 (80) | 39/80 (48.8) | Trauma (58.8) Ocular disease (10.0) Anterior segment surgery (5.0) |
Fusarium spp. (38.5) CoNS (35.9) Aspergillus spp. (10.3) |
|
| Wong et al (2011)119 | Hong Kong, China | 2005–2010 | 50 (50) | 43/50 (86.0) | CL wear (82.0) Trauma (12.0) Blepharitis and eyelid abnormalities (6.0) |
CoNS (36.0) P. aeruginosa (22.0) Acanthamoeba (12.0) |
|
| Hsiao et al (2007)42 | Linkou, Taiwan | 1998–2002 | 78 (81) | 47/81 (58.0) | CL wear (40.7) Trauma (21.0) Ocular disease (14.8) |
P. aeruginosa (44.7) S. aureus (19.1) Fungi (6.4) |
|
| Parmar et al (2006)80 | Tiruchirappalli, India | 2003 | 26 (26) | 15/26 (57.7) | Trauma (57.7) Traditional eye medicine use (34.6) Prior ocular surgery, ocular disease, and topical steroids (3.8 each) |
S. epidermidis (26.7) Bacillus spp. (26.7) S. pneumoniae (20.0) |
|
| Singh et al (2006)90 | Coimbatore, India | 1997–2004 | 97 (97) | 97/310 (31.3) | Trauma (69.0) CL (1.0) Unknown (29.8) |
P. aeruginosa (17.8) Fusarium spp. (16.8) S. epidermidis (15.8) |
|
| Vajpayee et al (1999)109 | New Delhi, India | 1993–1995 | 50 (50) | 35/50 (70.0) | Trauma (38.0) Systemic disease (24.0) Previous eye disease (12.0) |
CoNS (60) P. aeruginosa (14.5) S. aureus (8) |
|
| Kunimoto et al (1998)56 | Hyderabad, India | 1991–1995 | 107 (113) | 64/113 (56.6) | Trauma (21.1) Ocular surface disease (17.7) Systemic disease (15.9) |
S. epidermidis (23.4) S. aureus (20.4) S. pneumoniae (18.8) |
|
| Clinch et al (1994)20 | Philadelphia, New Orleans, USA | 1986–1991 | 28 (29) | 22/29 (75.9) | Trauma (34.5) Systemic disease (27.6) CL wear (24.1) |
S. epidermidis (20.8) P. aeruginosa (8.3) S. viridans and S. pneumoniae (both 8.3) |
|
| Cruz et al (1993)22 | Miami, USA | 1980–1991 | 50 (51) | 44/51 (86.3) | Trauma (44) Anterior segment surgery (24) Systemic disease (14) CL wear (12) |
P. aeruginosa (34.1) S. aureus (20.5) Fusarium solani (11.4) |
NS |
| Ormerod et al (1986)78 | Los Angeles, USA | 1972–1983 | 44 (47) | 41/47 (87.2) | Systemic disease (30.0) Exposure (25.0) Ocular surface disease (22.7) CL wear (6.8) |
Pseudomonas aeruginosa (24) S. pneumoniae (20) S. aureus and S. epidermidis (both 17) |
|
- Culture positivity considered number of positive cultures over total collected.
- Percentage of bacterial, fungal, mixed bacterial and fungal, and Acanthamoeba cases calculated as percentage of positive cultures, or total isolates.
- Percentage of individual bacterial and fungal organisms calculated as percentage of total bacterial and fungal isolates, respectively.
Although it is not surprising that the pathogens associated with MK in these cases are linked to specific risk factors, with organisms such as P aeruginosa, CoNS, and S aureus the most common isolates found in relation to contact lens use, there appears to be a higher incidence of atypical infections in the pediatric population. For example, an unusually high percentage of Acanthamoeba has been isolated in cases from Vancouver, Canada77 and Hong Kong, China,119 at 30.8 and 12.0%, respectively. The largest case series of pediatric MK published in the last decade, of over 108 eyes from Miami, Florida, found Stenotrophomonas maltophilia and Fusarium spp. in 19.3 and 13.5% of culture positive cases, respectively.87 Whether unusual causative organisms are truly more common in children with MK as compared to adults, or a reflection of selection bias, is not known due to the paucity of data from children. We speculate that, if an association exists, it may relate to factors such as more advanced disease at the time of presentation and therefore potentially higher diagnostic yield from cultures, less stringent care and use of contact lenses, and perhaps even differences in ocular surface immunity and/ or the pediatric microbiome, particularly in children with systemic illnesses.
4. The challenge of diagnosis
4.1. Stain, microscopy, and culture
The current gold standard for diagnosis and determination of a causative agent in MK remains stains and culture. The most common stains are Gram and Giemsa for bacteria, potassium hydroxide for fungi, and calcofluor white where there is clinical suspicion of Acanthamoeba. Commonly utilized culture media include blood agar (sheep, horse, chocolate), Sabouraud agar for suspected fungal pathogens, and nonnutrient agar with gram-negative seeding to culture amoeba. These culturing methods have been used for many decades to supplement clinicians’ history and examination, which remain notoriously inaccurate in predicting the causative organisms for most MK cases.23 In vivo confocal microscopy may be a helpful clinical adjunct but is heavily observer dependent and often lacks sufficient resolution to attain definitive diagnostic results.39,51 Latest models of in vivo confocal microscopy have reported axial and lateral resolutions of up to 7.6 μm and 1 μm, respectively,19 which, while potentially useful in determining the presence of Acanthamoeba cysts (10–20 μm) and fungal hyphae (>200 μm) within the cornea, are not sufficient to visualize bacteria (0–5 μm), which may appear as indistinct hyperreflective lesions within a sea of inflammatory cells.7 In vivo confocal microscopy is still not available everywhere, however, and older devices may lack sufficient resolution to reliably identify cysts and hyphae.
Unfortunately, the overall yield from stain and culture remains unsatisfactory even if remarkably consistent worldwide. As shown in Table 2, the median culture positivity rate from clinically diagnosed cases of MK is 50.3% (range 32.6–79.4). Staining methods alone are similarly ineffective, achieving diagnosis in only 27.3–61.6% of cases.16,30,50,61 The relative insensitivity of these methods may relate to prior antibiotic use, the technical difficulties in growing organisms from small samples, and the challenges to immediate incubation of culture plates to optimize diagnostic yield.48 These obstacles are not unique to corneal infections, except for the relatively small quantity of infected material in a cornea, as compared to other infected sites. In MK, for which time is vision, culture is time consuming and may generate negative results in spite of the patient having a clinical diagnosis of MK. This is often the case with fastidious organisms such as Streptococcus spp. and Propionibacterium spp. Furthermore, lack of timely susceptibility and resistance data means that clinicians often fall back on treating patients with broad spectrum, fortified antibiotics, or a late-generation fluoroquinolone, against which we are now witnessing the emergence of resistance.5,64,89 Therefore, despite having satisfactory specificity, the utility of stain and culture are limited by poor sensitivity. Finally, polymicrobial keratitis presents unique diagnostic challenges, as it can be difficult to distinguish from culture contamination.
4.2. Molecular diagnosis
Novel molecular methods have been developed as a possible means of complementing Gram stain and culture in the diagnosis of MK. The hope with such efforts is to work toward developing a rapid, highly sensitive, and accurate diagnostic tool for determining with reasonable confidence the etiology of corneal ulceration that can direct antimicrobial therapy. Polymerase chain reaction (PCR) has been seen as a potential adjunct or frank alternative to current diagnostic methods. Briefly, PCR involves the cyclical amplification of minute quantities of deoxyribonucleic acid (DNA) via the processes of denaturation, primer hybridization, and elongation.11,55 Denaturation of sampled genetic material is achieved with the application of heat, separating DNA into its 2 complementary strands. Primers that anneal to their complementary sequences are used to start the synthesis of new complementary strands of DNA by a thermostable DNA polymerase. This process is typically repeated for over 30 cycles until adequate amounts of DNA (usually over 2 billion copies) are synthesized to permit detection. Quantitative PCR allows for estimation of the amount of DNA in the initial sample. Multiplex PCR makes it possible to simultaneously test for multiple pathogens from a single sample.98
4.3. PCR in ocular infectious diseases
Use of PCR to diagnose eye infection until now has primarily been limited to the detection of viral pathogens in suspected ocular infections, such as Herpes simplex virus in herpetic keratitis and cytomegalovirus, varicella-zoster virus, and Herpes simplex virus in posterior segment uveitis. In the setting of microbial keratitis, multiple studies have investigated the diagnostic utility of PCR particularly in the differentiation of bacterial and fungal pathologies that are not always distinct on clinical examination. In most studies, 16S for bacteria and 18S for fungi rDNA primers are used as they are universally conserved in these organisms. The sensitivity and specificity of this approach for detecting pathogens (Table 4) have been calculated against different reference standards, either by comparison with a definitive clinical diagnosis or with culture. The sensitivity and specificity of all forms of PCR, using clinical diagnosis as the standard, range from 70.0–98.0%9,34,54,102,112,128 and 56.7–100%,9,34,112,128 respectively. Articles that included culture as the reference standard reported similar sensitivities and specificities of 73.3–90.9%1,27,32,46,81 and 94.7–98.0%,27,46,81 respectively. In the largest PCR-based study, conducted by Kim and coworkers,54 108 consecutive corneal ulcer specimens were analyzed by Gram and potassium hydroxide staining, culture, and PCR. That study identified 25 culture-positive bacterial cases and 31 culture-positive cases for fungi. By comparison, PCR was positive for 19 bacterial and 29 fungal cases, resulting in a sensitivity of 76% and 93.5%, respectively. Using clinical diagnosis as the standard reference, the sensitivity of PCR for bacterial and fungal keratitis was similar at 75.0% and 87.9%, respectively. The concordance between PCR and culture was 89% for fungi, but just 63% for bacteria, possibly from amplification by PCR of commensal ocular surface bacteria.
Table 4 -.
Summary of selected studies investigating PCR in MK diagnosis from collected corneal scrapes, ordered by chronology, published following 2000
| Study | Organism(s) of interest | Sample size | Technique | Sensitivity (%) | Specificity (%) | Concordance with culture (%) | Number of PCR cycles | Duration of test (hours) |
|---|---|---|---|---|---|---|---|---|
| Goh et al (2018)34 | Acanthamoeba | 14 | Real-time PCR: 18S rDNA | 71.4 | 100 | - | 40 | - |
| Zhao et al (2014)128 | Bacteria and fungi | 67 | PCR: 5.8S rRNA for fungi and 16S rRNA for bacteria | 98.0 | 81.8 | 51.6 | 35 | 3 |
| Tananuvat et al (2012)102 | Fungi | 30 | Semi-nested PCR: pan-fungal primers | 93.3 | 91.7 | 35 | ||
| Abu Eleinen et al (2012)1 | Bacteria | 88 | Broad-range PCR: 16S rRNA primer | 87.9** | - | - | 30 | 4–8 |
| Fungi | Broad-range PCR: universal fungal primer | 90.9** | 35 | |||||
| Badiee et al (2010)9 | Fungi | 38 | Nested PCR: universal fungal primer | 75.0 | 70.0 | 81.6 | - | - |
| Vengayil et al (2009)112 | Fungi | 40 | PCR: 28S primer | 70.0 | 56.7 | - | 50 | 4–8 |
| Kim et al (2008)54 | Bacteria | 108 | PCR: 16S rDNA primer | 75.0* | - | 63.2 | 40 | >20 |
| Fungi | PCR: 18S rDNA primer | 87.9* | - | 89.7 | 45 | |||
| Embong et al (2008)27 | Fungi | 30 | Semi-nested PCR: 18S rRNA primer | 90.9** | 94.7** | - | 30 | - |
| Joseph et al (2006)46 | Fungi (microsporidia) | 31 | PCR: 16S rRNA pan- Microsporidian primer |
83.0** | 98.0** | - | - | - |
| Pasricha et al (2003)81 | Acanthamoeba | 53 | PCR: 18S rRNA primer | 87.5** | 97.8** | - | 40 | - |
| Gaudio et al (2002)32 | Fungi | 30 | PCR: 18S primer | 73.3** | - | - | 30 | 4 |
Sensitivity and specificity as stated in articles, using proven diagnoses as the reference standard; marked * if calculated by authors.
Sensitivity and specificity calculated against culture and microscopy, if this was the only figure provided in text.
PCR-based assays that use alternative postamplification methods for microbial detection have also been developed to provide rapid MK diagnosis. Kuo and coworkers57 developed a dot hybridization assay to diagnose fungal keratitis, using PCR to first amplify the highly conserved fungal 5.8S rRNA gene before adding it to immobilized oligonucleotide probes specific for fungi fixed to a nylon membrane. Detection by this dot assay, which could be seen with the naked eye, was reported to have been 100% sensitive and 96.7% specific for fungi identification, although the sample size was small, with only 20 verified fungal keratitis specimens. Although this technique does not give the specific genus of the offending fungus, it does highlight the potential of such an assay in determining its presence in a sample. In a patient’s initial workup, diagnosis at the bacterial/fungal level may have substantial impact because the decision to commence empirical anti-fungal therapy is often made on clinical impression alone. Overall, PCR as a diagnostic tool in MK warrants further validation, including development of operational protocols for proper sample collection, defined diagnostic thresholds, and cost reduction, before full adoption into clinical practice.
4.4. Next generation sequencing
Next generation sequencing (NGS) is a euphemism currently used to describe recently developed technologies for very high throughput DNA sequence determination. NGS is now being coupled with bioinformatic analysis to detect matches between a sample and large databases of reference genome sequences. This has the potential to allow for rapid and highly accurate identification of an etiologic agent, as well as its antimicrobial susceptibility properties. Importantly, genome sequencing bypasses a well-documented limitation of PCR as PCR often requires a priori clinical suspicion to determine which primer sets to use to detect a suspected microbe. Numerous approaches have been developed for NGS, with 2 commonly used short-read methods including sequencing by ligation and sequencing by synthesis.36 The former involves the addition of a fluorophore-bound probe ligated to its complementary oligonucleotide, from which emission spectra are used to detect the presence of annealed sequences at respective time points.74 The latter involves the detection of singularly fluorophore-bound nucleotides to elongating strands, mediated by the addition of a polymerase. Both methods typically generate millions of nucleotide sequences.
To date, only 1 study has investigated the feasibility of using undirected DNA sequencing to identify suspected pathogens in MK. Li and coworkers used NGS in an effort to determine the etiology in 16 infected corneas by comparison to organisms recovered from 4 noninfected controls.65 Their NGS reactions generated 20–46 million separate sequences, which were then analyzed using 2 metagenomics database search algorithms, Kraken120 and Centrifuge.53 From the infected samples (which included specimens derived from 14 penetrating keratoplasties), a pathogen or pathogens were identified in 11 of 16 (Kraken) and 14 of 16 (Centrifuge) specimens. Combining the data, a putative culprit organism was identified in all bacterial cases, 5 of 6 fungal cases, and all 3 Acanthamoeba cases. Despite the great diagnostic potential of NGS, including the potential to aid in early identification of resistance genes in a range of pathogens, this study also shows that metagenomics databases and search algorithms themselves require additional refinement. As for generic PCR, calibration is essential. It will be important to determine threshold levels of NGS sequence reads consistent with infection diagnosis, as opposed to background levels from normal ocular flora and or contaminants. In addition, search algorithms such as Kraken, which only include complete genomes, may result in cleaner results, but a more a limited range of identifiable organisms. Furthermore, the analysis performed in this study required the use of a statistical filter for Centrifuge, which includes partially assembled genomes that widen diagnostic possibilities, but invites greater potential for contamination and ambiguity in the targets identified.
5. The emergence of antimicrobial resistance
Antimicrobial resistance has become one of the major public health threats of the 21st century. In a so-called “post-antibiotic” world,4 it has become incumbent on clinicians to be judicious in the use of antimicrobial therapy to treat infections. Indiscriminate antimicrobial use selects for the proliferation of microbial lineages with resistance to commonly prescribed antibiotics and antifungals.25,38 In bacterial keratitis, there exists a clear trend toward resistance to commonly prescribed empirical antibiotics, which include fluoroquinolones and fortified antibiotics, often a combination of a cephalosporin or glycopeptide and aminoglycoside (e.g., ceftazidime or vancomycin and tobramycin or gentamicin). In patients enrolled into the SCUT trial for instance, a 3.48-fold higher minimum inhibitory concentration was found for bacteria isolated from patients who had been pretreated with topical fluoroquinolones, compared to treatment-naive patients.86 Similarly, an important sub-analysis from the Mycotic Ulcer Treatment Trial I (MUTT I), with over 300 fungal keratitis patients randomized to topical natamycin versus voriconazole, found a 2.14 fold increase in mean minimum inhibitory concentration per year after adjusting for causative organism.85
The relative impact of systemic versus topical antibiotic use in selection for resistance is the subject of considerable controversy. Moxifloxacin is a leading fourth-generation fluoroquinolone often used as empirical monotherapy to treat bacterial keratitis, and ocular topical preparations have only been commercially available since the early 2000s. Despite its relatively recent availability, increased resistance has been observed globally. In India, susceptibility to moxifloxacin for coagulase-negative Staphylococcus species and methicillin-sensitive Staphylococcus has been reported as low as 61.2% and 53.1%, respectively.58 In the United States, moxifloxacin resistance has been documented in 26% of all organisms cultured at Wills Eye Hospital, Philadelphia,76 and in approximately 35% of all Staphylococcus and Streptococcus species isolated in a study from the Francis I. Proctor Foundation, San Francisco.82 These results were consistent with findings from an earlier study from Bascom Palmer Eye Institute, Miami, where 28% of Staphylococcus aureus isolates were resistant to ofloxacin or ciprofloxacin, both second-generation fluo-roquinolones.5 Ocular use of advanced fluoroquinolones also parallels and generally follows introduction for systemic use.13 As microbes such as Staphylococcus and Streptococcus species can also asymptomatically colonize patients, systemic use of antibiotics for any type of infection leaves the patient at increased risk of low level colonization by resistant microbes and thereby predisposed to antibiotic resistant infection. Therefore, the extent to which ocular application of antibiotics contributes to the actual genesis of antibiotic resistance, as opposed to simply selecting for the outgrowth of existing antibiotic resistant microbes, remains unclear. In either case, prudent use of antibiotics is essential for preserving their utility.
Compounding the challenge of MK treatment is the dilemma now posed by multidrug resistant (MDR) organisms, which is defined as having acquired nonsusceptibility to at least 1 agent in 3 or more antimicrobial classes.70 Although Pseudomonas aeruginosa susceptibility to either ciprofloxacin or moxifloxacin still hovers around 80% worldwide,47‘58,66,76,82 MDR Pseudomonas aeruginosa is emerging as problematic cause of MK, especially in South Asia,29,111 and demonstrates the possibility of common bacterial etiologies becoming increasingly resistant to frontline topical antimicrobials. In addition, MDR organisms not traditionally associated with MK are being isolated from keratitis because of the acquisition of virulence factors that extend their pathogenicity to the ocular surface. Our institution recently reported an unusual case of extended-spectrum β-lactamase–producing Escherichia coli keratitis in a patient residing in an aged-care facility who had been prescribed a long-term course of moxifloxacin and erythromycin for recurrent MK.110 Genotyping of this E. coli variant revealed that it was a multilocus sequence type 131 (ST131) strain with a novel mutation that confers a mucoid phenotype that impedes clearance by phagocytic cells of innate immunity. This strain exhibited resistance to nearly all b-lactams, aminoglycosides, and fluoroquinolones and was likely acquired as a result of prior hospitalization and antibiotic therapy for nonocular infection. The emergence of MDR organisms looms as a particularly frightening threat to patient care because the only agents to which many are now susceptible are toxic, expensive, and not widely available as topical medications.
6. Future directions and conclusions
MK is a complex disease with far-reaching health and socioeconomic costs. The epicenters of MK include South, South East, and East Asia where, in some instances, vision loss has surpassed that attributable to other historically leading causes of corneal blindness. Compounding the challenge, MK disproportionately afflicts poor, underresourced communities for whom access to specialized care is limited and ocular medications are often prohibitively expensive. The success of public health interventions with topical antimicrobial prophylaxis of corneal abrasions in Bhutan33 and Nepal107 may offer hope. Further clarification of the true incidence and trends in etiology of MK outside Asia would improve our understanding of the burden of disease and could influence resource distribution. From a clinical standpoint, such data are vital in determining empirical therapies in the acute setting. It is still uncommon for topical antifungals, for instance, to be included in empirical treatment regimens in Southern Asia.
We continue to face many challenges in the diagnosis of MK and the prevention of antimicrobial resistance among key pathogens. Although stain and culture have formed the cornerstone of MK diagnosis for many decades, with the emergence of new molecular-based technologies, older methodologies are no longer adequate as they often fail to provide the timely diagnosis necessary to salvage vision. Molecular diagnostics in MK offer substantial promise for the future but will require substantial cost reductions, validation of specific technologies, and development of clinically practical diagnostic thresholds before they can be fully incorporated into practice. Although technical challenges remain, molecular based techniques including PCR and NGS show great promise as tools to detect etiological agents and direct antimicrobial therapy. Moreover, as the costs of these technologies fall, there is hope that their utility will extend to the regions of the world where they are needed most.
7. Method of literature search
In June 2018, systematic literature searches were completed using PubMed and its subsidiary MEDLINE, EMBASE, and Web of Science for the components of this review. For the etiology of MK, iterations of the key words, “microbial keratitis,” “bacterial keratitis,” “fungal keratitis,” “infective keratitis,” and “acanthamoeba keratitis” were entered into these prospective databases, revealing 6,226 related articles. Given the volume of literature recovered, as well as the potential for patient duplication within multiple case series, our inclusion criteria included publication following 2000 in the English language, with over 200 patients and/or corneal scrapes for which identification of etiological agents was available. While restriction to the English language may have limited our search results, such was the breadth of literature, an overall appreciation of global etiological patterns was still achievable. Articles were carefully read, and case series from single institutions were carefully screened to ensure only the largest case series was included.
A more specific search was required to obtain studies investigating molecular diagnostic techniques for MK. The terms “molecular” or “PCR” or “next generation sequencing” and “keratitis” or “corneal ulcer” in the aforementioned databases revealed 192 potentially relevant results overall. Emphasis was placed on studies which utilized PCR or NGS to identify bacterial, fungal, and/or amoebic keratitis, and we restricted our results to those published following 2000 to ensure studies were contemporaneous to our discussion of potential future diagnostic techniques. Given the novel nature of these technologies, there was no distinction made between etiologies as the aim of this section of our review was to critically appraise whether it may be an avenue of research worthy of future pursuit.
8. Disclosures
Lawson Ung, Paulo J.M. Bispo, and Swapna S. Shanbhag have nothing to disclose. Michael S. Gilmore: This review has been supported in part by the National Eye Institute research project grant, EY024285–01, “Molecular Basis for Ocular Surface Tropism in Conjunctivitis”. James Chodosh: This review has been supported in part by an unrestricted grant to the Department of Ophthalmology, Harvard Medical School, from Research to Prevent Blindness, NY, NY.
REFERENCES
- 1.Abu Eleinen KG, Mohalhal AA, Elmekawy HE, et al. Polymerase chain reaction-guided diagnosis of infective keratitis–a hospital based study. Curr Eye Res. 2012;37(11):1005–11 [DOI] [PubMed] [Google Scholar]
- 2.Al-Shakarchi F. Initial therapy for suppurative microbial keratitis in Iraq. Br J Ophthalmol. 2007;91(12):1583–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Otaibi AG, Allam K, Damri AJ, et al. Childhood microbial keratitis. Oman J Ophthalmol. 2012;5(1):28–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alanis AJ. Resistance to antibiotics: are we in the postantibiotic era? Arch Med Res. 2005;36(6):697–705 [DOI] [PubMed] [Google Scholar]
- 5.Alexandrakis G, Alfonso EC, Miller D. Shifting trends in bacterial keratitis in South Florida and emerging resistance to fluoroquinolones. Ophthalmology. 2000;107(8):1497–502 [DOI] [PubMed] [Google Scholar]
- 6.Alfonso E, Mandelbaum S, Fox MJ, Forster RK. Ulcerative keratitis associated with contact lens wear. Am J Ophthalmol. 1986;101(4):429–33 [DOI] [PubMed] [Google Scholar]
- 7.Alzubaidi R, Sharif MS, Qahwaji R, et al. In vivo confocal microscopic corneal images in health and disease with an emphasis on extracting features and visual signatures for corneal diseases: a review study. Br J Ophthalmol. 2016;100(1):41–55 [DOI] [PubMed] [Google Scholar]
- 8.Austin A, Lietman T, Rose-Nussbaumer J. Update on the Management of Infectious Keratitis. Ophthalmology. 2017;124(11):1678–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badiee P, Nejabat M, Alborzi A, et al. Comparative study of Gram stain, potassium hydroxide smear, culture and nested PCR in the diagnosis of fungal keratitis. Ophthalmic Res. 2010;44(4):251–6 [DOI] [PubMed] [Google Scholar]
- 10.Bandyopadhyay S, Das D, Mondal K, et al. Epidemiology and laboratory diagnosis of fungal corneal ulcer in the Sundarban Region of West Bengal, eastern India. Nepalese J Ophthalmol. 2012;4(1):29–36 [DOI] [PubMed] [Google Scholar]
- 11.Bartlett JM, Stirling D. A short history of the polymerase chain reaction In: Bartlett JM (ed) Stirling D: PCR protocols. Totowa, NJ, Humana Press; 2003, pp 3–6 [DOI] [PubMed] [Google Scholar]
- 12.Basak SK, Basak S, Mohanta A, Bhowmick A. Epidemiological and microbiological diagnosis of suppurative keratitis in Gangetic West Bengal, eastern India. Indian J Ophthalmol. 2005;53(1):17–22 [DOI] [PubMed] [Google Scholar]
- 13.Bertino JS Jr. Impact of antibiotic resistance in the management of ocular infections: the role of current and future antibiotics. Clin Ophthalmol (Auckland, NZ). 2009;3:507–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bharathi MJ, Ramakrishnan R, Meenakshi R, et al. Microbial keratitis in South India: influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007;14(2):61–9 [DOI] [PubMed] [Google Scholar]
- 15.Burton MJ, Pithuwa J, Okello E, et al. Microbial keratitis in East Africa: why are the outcomes so poor? Ophthalmic Epidemiol. 2011;18(4):158–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cariello AJ, Passos RM, Yu MCZ, Hofling-Lima AL. Microbial keratitis at a referral center in Brazil. Int Ophthalmol. 2011;31(3):197–204 [DOI] [PubMed] [Google Scholar]
- 17.Chang C-W, Ho C-K, Chen Z-C, et al. Fungi genus and concentration in the air of onion fields and their opportunistic action related to mycotic keratitis. Arch Environ Health An Int J. 2002;57(4):349–54 [DOI] [PubMed] [Google Scholar]
- 18.Cheng KH, Leung SL, Hoekman HW, et al. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet. 1999;354(9174):181–5 [DOI] [PubMed] [Google Scholar]
- 19.Chidambaram JD, Prajna NV, Larke NL, et al. Prospective study of the diagnostic accuracy of the in vivo laser scanning confocal microscope for severe microbial keratitis. Ophthalmology. 2016;123(11):2285–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinch TE, Palmon FE, Robinson MJ, et al. Microbial keratitis in children. Am J Ophthalmol. 1994;117(1):65–71 [DOI] [PubMed] [Google Scholar]
- 21.Collier SA, Gronostaj MP, MacGurn AK, et al. Estimated burden of keratitis—United States, 2010. Morbidity Mortality Weekly Rep. 2014;63(45):1027–30 [PMC free article] [PubMed] [Google Scholar]
- 22.Cruz OA, Sabir SM, Capo H, Alfonso EC. Microbial keratitis in childhood. Ophthalmology. 1993;100(2):192–6 [DOI] [PubMed] [Google Scholar]
- 23.Dahlgren MA, Lingappan A, Wilhelmus KR. The clinical diagnosis of microbial keratitis. Am J Ophthalmol. 2007;143(6):940–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dart J. Predisposing factors in microbial keratitis: the significance of contact lens wear. Br J Ophthalmol. 1988;72(12):926–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74(3):417–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deorukhkarl S, Katiyarl R, Sainil S. Epidemiological features and laboratory results of bacterial and fungal keratitis: five-year study at tertiary-care hospital in western Maharashtra, India. Singapore Med J. 2012;53(4):264–7 [PubMed] [Google Scholar]
- 27.Embong Z, Hitam WHW, Yean CY, et al. Specific detection of fungal pathogens by 18S rRNA gene PCR in microbial keratitis. BMC Ophthalmol. 2008;8(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erie JC, Nevitt MP, Hodge DO, Ballard DJ. Incidence of ulcerative keratitis in a defined population from 1950 through 1988. Arch Ophthalmol. 1993;111(12):1665–71 [DOI] [PubMed] [Google Scholar]
- 29.Fernandes M, Vira D, Medikonda R, Kumar N. Extensively and pan-drug resistant Pseudomonas aeruginosa keratitis: clinical features, risk factors, and outcome. Graefes Arch Clin Exp Ophthalmol. 2016;254(2):315–22 [DOI] [PubMed] [Google Scholar]
- 30.Fong C-F, Tseng C-H, Hu F-R, et al. Clinical characteristics of microbial keratitis in a university hospital in Taiwan. Am J Ophthalmol. 2004;137(2):329–36 [DOI] [PubMed] [Google Scholar]
- 31.Galarreta DJ, Tuft SJ, Ramsay A, Dart JK. Fungal keratitis in London: microbiological and clinical evaluation. Cornea. 2007;26(9):1082–6 [DOI] [PubMed] [Google Scholar]
- 32.Gaudio P, Gopinathan U, Sangwan V, Hughes T. Polymerase chain reaction based detection of fungi in infected corneas. Br J Ophthalmol. 2002;86(7):755–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Getshen K, Srinivasan M, Upadhyay M, et al. Corneal ulceration in South East Asia. I: A model for the prevention of bacterial ulcers at the village level in rural Bhutan. Br J Ophthalmol. 2006;90(3):276–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goh JW, Harrison R, Hau S, et al. Comparison of In Vivo Confocal Microscopy, PCR and Culture of Corneal Scrapes in the Diagnosis of Acanthamoeba Keratitis. Cornea. 2018;37(4):480–5 [DOI] [PubMed] [Google Scholar]
- 35.Gonzales CA, Srinivasan M, Whitcher JP, Smolin G. Incidence of corneal ulceration in Madurai district, South India. Ophthalmic Epidemiol. 1996;3(3):159–66 [DOI] [PubMed] [Google Scholar]
- 36.Goodwin S, McPherson JD, McCombie WR. Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet. 2016;17(6):333–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gopinathan U, Sharma S, Garg P, Rao GN. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: experience of over a decade. Indian J Ophthalmol. 2009;57(4):273–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gross PA, Patel B. Reducing antibiotic overuse: a call for a national performance measure for not treating asymptomatic bacteriuria. Clin Infect Dis. 2007;45(10):1335–7 [DOI] [PubMed] [Google Scholar]
- 39.Hau SC, Dart JK, Vesaluoma M, et al. Diagnostic accuracy of microbial keratitis with in vivo scanning laser confocal microscopy. Br J Ophthalmol. 2010;94(8):982–7 [DOI] [PubMed] [Google Scholar]
- 40.Hernandez-Camarena JC, Graue-Hernandez EO, Ortiz-Casas M, et al. Trends in microbiological and antibiotic sensitivity patterns in infectious keratitis: 10-year experience in Mexico City. Cornea. 2015;34(7):778–85 [DOI] [PubMed] [Google Scholar]
- 41.Hsiao C-H, Sun C-C, Yeh L-K, et al. Shifting trends in bacterial keratitis in Taiwan: a 10-year review in a tertiary-care hospital. Cornea. 2016;35(3):313–7 [DOI] [PubMed] [Google Scholar]
- 42.Hsiao C-H, Yeung L, Ma DH, et al. Pediatric microbial keratitis in Taiwanese children: a review of hospital cases. Arch Ophthalmol. 2007;125(5):603–9 [DOI] [PubMed] [Google Scholar]
- 43.Ibrahim YW, Boase DL, Cree IA. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: the Portsmouth corneal ulcer study. Br J Ophthalmol. 2009;93(10):1319–24 [DOI] [PubMed] [Google Scholar]
- 44.Ibrahim YW, Boase DL, Cree IA. Incidence of infectious corneal ulcers, Portsmouth study. UK J Clin Exp Ophthalmol. 2012;6(7). S6–001. [DOI] [PubMed] [Google Scholar]
- 45.Jeng BH, Gritz DC, Kumar AB, et al. Epidemiology of ulcerative keratitis in Northern California. Arch Ophthalmol. 2010;128(8):1022–8 [DOI] [PubMed] [Google Scholar]
- 46.Joseph J, Sharma S, Murthy SI, et al. Microsporidial keratitis in India: 16S rRNA gene-based PCR assay for diagnosis and species identification of microsporidia in clinical samples. Invest Ophthalmol Vis Sci. 2006;47(10):4468–73 [DOI] [PubMed] [Google Scholar]
- 47.Kaliamurthy J, Kalavathy CM, Parmar P, et al. Spectrum of bacterial keratitis at a tertiary eye care centre in India. Biomed Research International. 2013;2013 181564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaye SB, Rao PG, Smith G, et al. Simplifying collection of corneal specimens in cases of suspected bacterial keratitis. J Clin Microbiol. 2003;41(7):3192–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keay L, Edwards K, Naduvilath T, et al. Microbial keratitis: predisposing factors and morbidity. Ophthalmology. 2006;113(1):109–16 [DOI] [PubMed] [Google Scholar]
- 50.Khanal B, Deb M, Panda A, Sethi HS. Laboratory diagnosis in ulcerative keratitis. Ophthalmic Res. 2005;37(3):123–7 [DOI] [PubMed] [Google Scholar]
- 51.Kheirkhah A, Syed ZA, Satitpitakul V, et al. Sensitivity and specificity of laser-scanning in vivo confocal microscopy for filamentous fungal keratitis: role of observer experience. Am J Ophthalmol. 2017;179:81–9 [DOI] [PubMed] [Google Scholar]
- 52.Khor W-B, Prajna VN, Garg P, et al. The Asia Cornea Society Infectious Keratitis Study: A Prospective Multicenter Study Of Infectious Keratitis In Asia. Am J Ophthalmol. 2018;195:161–70 [DOI] [PubMed] [Google Scholar]
- 53.Kim D, Song L, Breitwieser FP, Salzberg SL. Centrifuge: rapid and sensitive classification of metagenomic sequences. Genome Res. 2016;26(12):1721–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim E, Chidambaram JD, Srinivasan M, et al. Prospective comparison of microbial culture and polymerase chain reaction in the diagnosis of corneal ulcer. Am J Ophthalmol. 2008;146(5):714–23 [DOI] [PubMed] [Google Scholar]
- 55.Kubista M, Andrade JM, Bengtsson M, et al. The real-time polymerase chain reaction. Mol Aspects Med. 2006;27(2–3):95–125 [DOI] [PubMed] [Google Scholar]
- 56.Kunimoto DY, Sharma S, Reddy MK, et al. Microbial keratitis in children. Ophthalmology. 1998;105(2):252–7 [DOI] [PubMed] [Google Scholar]
- 57.Kuo M-T, Chang H-C, Cheng C-K, et al. A highly sensitive method for molecular diagnosis of fungal keratitis: a dot hybridization assay. Ophthalmology. 2012;119(12):2434–42 [DOI] [PubMed] [Google Scholar]
- 58.Lalitha P, Manoharan G, Karpagam R, et al. Trends in antibiotic resistance in bacterial keratitis isolates from South India. Br J Ophthalmol. 2016;101(2):108–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lam D, Houang E, Fan D, et al. Incidence and risk factors for microbial keratitis in Hong Kong: comparison with Europe and North America. Eye. 2002;16(5):608–18 [DOI] [PubMed] [Google Scholar]
- 60.Laspina F, Samudio M, Cibils D, et al. Epidemiological characteristics of microbiological results on patients with infectious corneal ulcers: a 13-year survey in Paraguay. Graefes Arch Clin Exp Ophthalmol. 2004;242(3):204–9 [DOI] [PubMed] [Google Scholar]
- 61.Leck A, Thomas P, Hagan M, et al. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br J Ophthalmol. 2002;86(11):1211–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee B, Cai CX, Srikumaran D, Woreta FA. Severe Achromobacter xylosoxidans keratitis with deep corneal involvement. Am J Ophthalmol Case Rep. 2018;11:128–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee YS, Tan HY, Yeh LK, et al. Pediatric microbial keratitis in Taiwan: clinical and microbiological profiles, 1998–2002 versus 2008–2012. Am J Ophthalmol. 2014;157(5):1090–6 [DOI] [PubMed] [Google Scholar]
- 64.Leibovitch I, Lai T, Senarath L, et al. Infectious keratitis in South Australia: emerging resistance to cephazolin. Eur J Ophthalmol. 2005;15(1):23–6 [PubMed] [Google Scholar]
- 65.Li Z, Breitwieser FP, Lu J, et al. Identifying corneal infections in formalin-fixed specimens using next generation sequencing. Invest Ophthalmol Vis Sci. 2018;59(1):280–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lichtinger A, Yeung SN, Kim P, et al. Shifting trends in bacterial keratitis in Toronto: an 11-year review. Ophthalmology. 2012;119(9):1785–90 [DOI] [PubMed] [Google Scholar]
- 67.Liesegang TJ, Forster RK. Spectrum of microbial keratitis in South Florida. Am J Ophthalmol. 1980;90(1):38–47 [DOI] [PubMed] [Google Scholar]
- 68.Lin CC, Prajna L, Srinivasan M, et al. Seasonal trends of microbial keratitis in South India. Cornea. 2012;31(10):1123–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin L, Lan W, Lou B, et al. Genus distribution of bacteria and fungi associated with keratitis in a large eye center located in southern China. Ophthalmic Epidemiol. 2017;24(2):90–6 [DOI] [PubMed] [Google Scholar]
- 70.Magiorakos AP, Srinivasan A, Carey R, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81 [DOI] [PubMed] [Google Scholar]
- 71.Mallari PLT, McCarty DJ, Daniell M, Taylor H. Increased incidence of corneal perforation after topical fluoroquinolone treatment for microbial keratitis. Am J Ophthalmol. 2001;131(1):131–3 [DOI] [PubMed] [Google Scholar]
- 72.McDonald EM, Ram FS, Patel DV, McGhee CN. Topical antibiotics for the management of bacterial keratitis: an evidence-based review of high quality randomised controlled trials. Br J Ophthalmol. 2014;98(11):1470–7 [DOI] [PubMed] [Google Scholar]
- 73.McLaughlin-Borlace L, Stapleton F, Matheson M, Dart J. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. J Appl Microbiol. 1998;84(5):827–38 [DOI] [PubMed] [Google Scholar]
- 74.Metzker ML. Sequencing technologies—the next generation. Nat Rev Genet. 2010;11(1):31–46 [DOI] [PubMed] [Google Scholar]
- 75.Newman P, Hider P, Waring G, et al. Corneal ulcer due to Achromobacter xylosoxidans. Br J Ophthalmol. 1984;68(7):472–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ni N, Nam EM, Hammersmith KM, et al. Seasonal, geographic, and antimicrobial resistance patterns in microbial keratitis: 4-year experience in eastern Pennsylvania. Cornea. 2015;34(3):296–302 [DOI] [PubMed] [Google Scholar]
- 77.Noureddin GS, Sasaki S, Butler AL, et al. Paediatric infectious keratitis at tertiary referral centres in Vancouver, Canada. Br J Ophthalmol. 2016;100(12):1714–8 [DOI] [PubMed] [Google Scholar]
- 78.Ormerod LD, Murphree AL, Gomez DS, et al. Microbial keratitis in children. Ophthalmology. 1986;93(4):449–55 [DOI] [PubMed] [Google Scholar]
- 79.Pandita A, Murphy C. Microbial keratitis in Waikato, New Zealand. Clin Exp Ophthalmol. 2011;39(5):393–7 [DOI] [PubMed] [Google Scholar]
- 80.Parmar P, Salman A, Kalavathy C, et al. Microbial keratitis at extremes of age. Cornea. 2006;25(2):153–8 [DOI] [PubMed] [Google Scholar]
- 81.Pasricha G, Sharma S, Garg P, Aggarwal RK. Use of 18S rRNA gene-based PCR assay for diagnosis of Acanthamoeba keratitis in non-contact lens wearers in India. J Clin Microbiol. 2003;41(7):3206–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peng MY, Cevallos V, McLeod SD, et al. Bacterial Keratitis: Isolated Organisms and Antibiotic Resistance Patterns in San Francisco. Cornea. 2018;37(1):84–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poggio EC, Glynn RJ, Schein OD, et al. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N Engl J Med. 1989;321(12):779–83 [DOI] [PubMed] [Google Scholar]
- 84.Politis M, Wajnsztajn D, Rosin B, et al. Trends of Bacterial Keratitis Culture Isolates in Jerusalem; a 13- Years Analysis. PLoS One. 2016;11(11):e0165223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prajna NV, Lalitha P, Rajaraman R, et al. Changing azole resistance: A Secondary analysis of the MUTT I randomized clinical trial. JAMA Ophthalmol. 2016;134(6):693–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ray KJ, Prajna L, Srinivasan M, et al. Fluoroquinolone treatment and susceptibility of isolates from bacterial keratitis. JAMA Ophthalmol. 2013;131(3):310–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rossetto JD, Cavuoto KM, Osigian CJ, et al. Paediatric infectious keratitis: a case series of 107 children presenting to a tertiary referral centre. Br J Ophthalmol. 2017;101(11):1488–92 [DOI] [PubMed] [Google Scholar]
- 88.Seal D, Kirkness C, Bennett H, et al. Population-based cohort study of microbial keratitis in Scotland: incidence and features. Cont Lens and Anterior Eye. 1999;22(2):49–57 [DOI] [PubMed] [Google Scholar]
- 89.Shalchi Z, Gurbaxani A, Baker M, Nash J. Antibiotic resistance in microbial keratitis: ten-year experience of corneal scrapes in the United Kingdom. Ophthalmology. 2011;118(11):2161–5 [DOI] [PubMed] [Google Scholar]
- 90.Singh G, Palanisamy M, Madhavan B, et al. Multivariate analysis of childhood microbial keratitis in South India. Ann Acad Med Singapore. 2006;35(3):185–9 [PubMed] [Google Scholar]
- 91.Song X, Xie L, Tan X, et al. A multi-center, cross-sectional study on the burden of infectious keratitis in China. PLoS One. 2014;9(12):e113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song X, Xu L, Sun S, et al. Pediatric microbial keratitis: a tertiary hospital study. Eur J Ophthalmol. 2012;22(2):136–41 [DOI] [PubMed] [Google Scholar]
- 93.Spierer O, Miller D, O’brien TP. Comparative activity of antimicrobials against Pseudomonas aeruginosa, Achromobacter xylosoxidans and Stenotrophomonas maltophilia keratitis isolates. Br J Ophthalmol. 2018;102(5):708–12 [DOI] [PubMed] [Google Scholar]
- 94.Srinivasan M, Mascarenhas J, Rajaraman R, et al. Corticosteroids for bacterial keratitis: the Steroids for Corneal Ulcers Trial (SCUT). Arch Ophthalmol. 2012;130(2):143–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stapleton F, Carnt N. Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye. 2012;26(2):185–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stapleton F, Dart J, Seal D, Matheson M. Epidemiology of Pseudomonas aeruginosa keratitis in contact lens wearers. Epidemiol Infect. 1995;114(3):395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008;115(10):1655–62 [DOI] [PubMed] [Google Scholar]
- 98.Strommenger B, Kettlitz C, Werner G, Witte W. Multiplex PCR assay for simultaneous detection of nine clinically relevant antibiotic resistance genes in Staphylococcus aureus. J Clin Microbiol. 2003;41(9):4089–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun X, Deng S, Li R, et al. Distribution and shifting trends of bacterial keratitis in north China (1989–98). Br J Ophthalmol. 2004;88(2):165–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tan S, Walkden A, Au L, et al. Twelve-year analysis of microbial keratitis trends at a UK tertiary hospital. Eye. 2017;31(8):1229–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tananuvat N, Punyakhum O, Ausayakhun S, Chaidaroon W. Etiology and clinical outcomes of microbial keratitis at a tertiary eye-care center in northern Thailand. J Med Assoc Thai. 2012;95(Suppl 4):S8–17 [PubMed] [Google Scholar]
- 102.Tananuvat N, Salakthuantee K, Vanittanakom N, et al. Prospective comparison between conventional microbial work-up vs PCR in the diagnosis of fungal keratitis. Eye. 2012;26(10):1337–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thomas P. Fungal infections of the cornea. Eye. 2003;17(8):852–62 [DOI] [PubMed] [Google Scholar]
- 104.Thylefors B. Epidemiological patterns of ocular trauma. Clin Exp Ophthalmol. 1992;20(2):95–8 [DOI] [PubMed] [Google Scholar]
- 105.Ting DSJ, Settle C, Morgan SJ, et al. A 10-year analysis of microbiological profiles of microbial keratitis: the North East England Study. Eye. 2018;32(8):1416–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Truong DT, Bui M-T, Cavanagh HD. Epidemiology and outcome of microbial keratitis: private university versus urban public hospital care. Eye & contact lens. 2016;44(Suppl 1): S82–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Upadhyay M, Karmacharya P, Koirala S, et al. The Bhaktapur eye study: ocular trauma and antibiotic prophylaxis for the prevention of corneal ulceration in Nepal. Br J Ophthalmol. 2001;85(4):388–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vajpayee RB, Dada T, Saxena R, et al. Study of the first contact management profile of cases of infectious keratitis: a hospital-based study. Cornea. 2000;19(1):52–6 [DOI] [PubMed] [Google Scholar]
- 109.Vajpayee RB, Ray M, Panda A, et al. Risk factors for pediatric presumed microbial keratitis: a case-control study. Cornea. 1999;18(5):565–9 [PubMed] [Google Scholar]
- 110.Van Tyne D, Ciolino JB, Wang J, et al. Novel Phagocytosis-Resistant Extended-Spectrum b-Lactamase-Producing Escherichia coli From Keratitis. JAMA Ophthalmol. 2016;134(11):1306–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vazirani J, Wurity S, Ali MH. Multidrug-resistant Pseudomonas aeruginosa keratitis: risk factors, clinical characteristics, and outcomes. Ophthalmology. 2015;122(10):2110–4 [DOI] [PubMed] [Google Scholar]
- 112.Vengayil S, Panda A, Satpathy G, et al. Polymerase chain reaction-guided diagnosis of mycotic keratitis: a prospective evaluation of its efficacy and limitations. Invest Ophthalmol Vis Sci. 2009;50(1):152–6 [DOI] [PubMed] [Google Scholar]
- 113.Waddell KM. Childhood blindness and low vision in Uganda. Eye. 1998;12(2):184–92 [DOI] [PubMed] [Google Scholar]
- 114.Watt K, Swarbrick HA. Microbial keratitis in overnight orthokeratology: review of the first 50 cases. Eye Contact Lens. 2005;31(5):201–8 [DOI] [PubMed] [Google Scholar]
- 115.Whitcher JP, Srinivasan M. Corneal ulceration in the developing world—a silent epidemic. Br J Ophthalmol. 1997;81(8):622–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–21 [PMC free article] [PubMed] [Google Scholar]
- 117.Whitcher JP, Srinivasan M, Upadhyay MP. Prevention of corneal ulceration in the developing world. Int Ophthalmol Clin. 2002;42(1):71–7 [DOI] [PubMed] [Google Scholar]
- 118.Wiley L, Bridge DR, Wiley LA, et al. Bacterial biofilm diversity in contact lens-related disease: emerging role of Achromobacter, Stenotrophomonas, and Delftia. Invest Ophthalmol Vis Sci. 2012;53(7):3896–905 [DOI] [PubMed] [Google Scholar]
- 119.Wong VW, Lai TY, Chi SC, Lam DS. Pediatric ocular surface infections: a 5-year review of demographics, clinical features, risk factors, microbiological results, and treatment. Cornea. 2011;30(9):995–1002 [DOI] [PubMed] [Google Scholar]
- 120.Wood DE, Salzberg SL. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014. March 3;15(3):R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.World Health Organization. Guidelines for the management of corneal ulcer at primary, secondary and tertiary care health facilities in the South-East Asia region. [cited 2018 Jun 18]. World Health Organization; 2004. Available from: http://www.who.int/iris/handle/10665/205174. [Google Scholar]
- 122.Wu YT, Zhu H, Willcox M, Stapleton F. Removal of biofilm from contact lens storage cases. Invest Ophthalmol Vis Sci. 2010;51(12):6329–33 [DOI] [PubMed] [Google Scholar]
- 123.Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113(11):1943–8 [DOI] [PubMed] [Google Scholar]
- 124.Yilmaz S, Ozturk I, Maden A. Microbial keratitis in West Anatolia, Turkey: a retrospective review. Int Ophthalmol. 2007;27(4):261–8 [DOI] [PubMed] [Google Scholar]
- 125.Young AL, Leung AT, Cheng LL, et al. Orthokeratology lens-related corneal ulcers in children: a case series. Ophthalmology. 2004;111(3):590–5 [DOI] [PubMed] [Google Scholar]
- 126.Young AL, Leung KS, Tsim N, et al. Risk factors, microbiological profile, and treatment outcomes of pediatric microbial keratitis in a tertiary care hospital in Hong Kong. Am J Ophthalmol. 2013;156(5):1040–4 [DOI] [PubMed] [Google Scholar]
- 127.Zhang C, Liang Y, Deng S, et al. Distribution of bacterial keratitis and emerging resistance to antibiotics in China from 2001 to 2004. Clin Ophthalmol (Auckland, NZ). 2008;2(3):575–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao G, Zhai H, Yuan Q, et al. Rapid and sensitive diagnosis of fungal keratitis with direct PCR without template DNA extraction. Clin Microbiol Infect. 2014;20(10):776–82 [DOI] [PubMed] [Google Scholar]


