Abstract
Background
In this study, we aimed to compare serum biochemical markers in patients with malignant pleural mesothelioma and pleural plaques versus healthy individuals exposed to environmental asbestos.
Methods
Between September 01, 2010 and March 31, 2011, a total of 540 participants (354 males, 186 females; mean age 61.4 years; range, 35 to 89 years) were included in the study. The participants were divided into four groups as follows: (1) patients with pleural plaques (n=277); (2) healthy individuals with normal chest X-rays who were exposed to environmental asbestos (n=121); (3) healthy individuals with normal chest X-rays who were not exposed to environmental asbestos (n=118); and (4) patients with malignant pleural mesothelioma (n=24). Serum levels of carcinoembryonic antigen, cancer antigen 125, 15-3, 19-9, free T3, free T4, thyroidstimulating hormone, vitamin B12, folate, and ferritin were measured.
Results
Serum cancer antigen 125, 15-3, folic acid, vitamin B12, and ferritin levels were higher with lower free T3 levels in Group 4 than the other groups. The areas under the curve for cancer antigen 125 and 15-3 were 0.78 and 0.67, respectively in the differential diagnosis of mesothelioma from other pathologies (p<0.001 for both). Optimal limits of these biomarkers were 13.63 and 18.43 ng/mL, respectively with 83% and 75% sensitivity and 69% and 48% specificity, respectively.
Conclusion
The combination or individual use of serum cancer antigen 125, 15-3, folic acid, vitamin B12, and ferritin levels may be helpful for early diagnosis and treatment of malignant pleural mesothelioma.
Keywords: Biochemical marker, ferritin, mesothelioma, pleural plaque
Introduction
Malignant mesothelioma is a rare, aggressive tumor originating from the pleura and serous layer of the peritoneum. Asbestos exposure and the chronic inhalation of asbestos fibers are the most important risk factors in the development of mesothelioma, and the incidence of mesothelioma due to asbestos exposure has shown an increase all over the world.[1] Treatment of malignant mesothelioma results in frustration, regardless of the treatment option, and the average life expectancy is usually less than one year with a five-year survival of less than 5%.[2] Therefore, early diagnosis of malignant mesothelioma is critical and novel biomarkers are needed during follow-up of diagnosis, prognosis, and treatment response. Recently, several studies have shown promising results with biomarkers such as tumor markers, osteopontin, mesothelin, fibulin-3, and N-ERC in predicting the development of mesothelioma, treatment response, and prognosis.[3-6]
Our study was conducted in the central Anatolia in the countryside of Sivas province of Turkey where the exposure to environmental asbestos is intense.[7,8] In a study in which asbestos fibers were evaluated in bronchoalveolar lavage, it was reported that asbestos fibers due to environmental asbestos exposure were detected in Sivas.[9] Our previous studies also reported asbestos exposure in rural regions of Sivas province.[4,7,10]
In the present study, we aimed to compare serum biochemical markers in patients with malignant pleural mesothelioma (MPM) and pleural plaques versus healthy individuals exposed to environmental asbestos and to investigate whether these markers could be used in the early diagnosis of malignant mesothelioma and in predicting prognosis and response to treatment.
Patients and Methods
This study was carried in Sivas province located in the central Anatolia region of Turkey between September 01, 2010 and March 31, 2011. We defined age of ?35 years and a requirement to have lived in the same village for 20 years as the eligibility criteria for the study. We made a list of surviving patients with MPM who were diagnosed between 2009 and 2010 using the Cancer Registry of the Health Directorate. Group 2 and Group 3 were recruited from 48 of 68 villages of Yildizeli and Sivas within a 10 km range of ophiolite units. The simple randomization method was performed to select participants, sampling 15% from each village. Accordingly, a total of 3,127 individuals were screened. Among them, 16 were excluded from the study for the following reasons: costodiaphragmatic sinus blunting (n=5), reticulonodular opacities (n=4), tuberculosis sequelae with pleural calcification (n=1), bilateral pleural effusion with cardiomegaly (n=3), chronic effusion of unknown etiology (n=1), suspicious pleural thickness (n=1), and round calcification (n=1). To create more precise groups with normal chest X-ray from the asbestos-exposed population, we decided to exclude those who lived in villages with ?7% plaque rates. The group comprising healthy individuals who were not exposed to asbestos was recruited from six villages of the Hafik district, which are farther than 25 km from ophiolitic areas, with a similar distance to the Sivas city center and who shared a similar lifestyle. A database search of the Sivas Health Directorate confirmed that no patients from the control villages had asbestos-related disease within the last decade. This data is also consistent with our three different studies published in the English literature.[4,7,10] Finally, a total of 540 participants (354 males, 186 females; mean age 61.4 years; range, 35 to 89 years) were included in the study. The participants were divided into four groups as follows: (1) patients with pleural plaques (n=277); (2) healthy individuals with normal chest X-rays who were exposed to environmental asbestos (n=121); (3) healthy individuals with normal chest X-rays who were not exposed to environmental asbestos (n=118, control group); and (4) patients with MPM (n=24). The groups other than those with mesothelioma were taken from a field-based cross-sectional study.[10] Data including demographic characteristics and anthropometric measurements of the patients, area of residence, working status and medical history were included on the day of chest X-ray and blood sampling.
Chest X-ray examination The first interpretation of the chest X-rays was performed on the examination day by one of the pulmonologists and thoracic surgeon to decide on any further investigation. The final interpretations were conducted by three study investigators who were blinded to the residencies of the participants. Discrete dense pleural opacities or linear structures localized on the chest wall, diaphragm, pericardium or mediastinum were considered. An informed consent was obtained from each participant. The study protocol was approved by the local Ethics Committee. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Serum biomarker analysis The serum samples of participants were stored at -80°C until the analysis of carcinoembryonic antigen (CEA), cancer antigen (CA)-125, CA15-3, CA19-9, free T3 (fT3), free T4 (fT4), thyroid-stimulating hormone (TSH), cobalamin (vitamin B12), folate (folic acid), and ferritin using a micro enzyme-linked immunosorbent assay (ELISA). The levels of CEA, CA125, CA15-3, CA19-9, fT3, fT4, TSH, vitamin B12, folic acid, and ferritin were determined using commercial ELISA kits (Mesomark; Fujirebio Diagnostics and Raybio) according to the manufacturer"s instructions. All measurements were performed in duplicate and in a random order. Laboratory personnel were blinded to the clinical status of the study participants.
Statistical analysis Statistical analysis was performed using the SPSS version 14.0 software (SPSS Inc., Chicago, IL, USA). Descriptive data were expressed in mean ± standard deviation (SD), median (min-max) values or number and frequency. Continuous variables were compared using analysis of variance (ANOVA), if normally distributed. The Kruskal-Wallis test and Bonferroni correction were used to compare multiple variables, if they were not normally distributed. Categorical variables were analyzed using the chi-square test.
Receiver operating characteristic (ROC) curves were generated to evaluate the ability of serum CEA, CA125, CA15-3, CA19-9, fT3, fT4, TSH, vitamin B12, folic acid, and ferritin to discriminate between MPM and each of the other groups. All other groups combined were estimated based on the sensitivity and specificity at different cut-off values. Area under the ROC curves (AUC) were reported with 95% confidence interval (CI). A two-tailed p value of <0.05 was considered statistically significant.
Results
Of the MPM patients, 47.8% never smoked and/or stopped smoking and 4.3% were still active smokers. In these patients, the body mass index (BMI) was lower compared to the other groups. There was no significant difference in the mean ages of the four groups. Baseline demographic and anthropometric characteristics of the study population are shown in Table 1.
Table 1. Baseline demographic and anthropometric characteristics of study population.
| Group 1 Pleural plaque (n=277) | Group 2 Healthy individuals exposed to asbestos | Group 3 Healthy individuals not | Group 4 Mesothelioma (n=24) | |
| exposed to asbestos | ||||
| (n=121) | (n=118, control group) | |||
| % Mean±SD | % Mean±SD | % Mean±SD | % Mean±SD | |
| Age (year) | 63.1±11.5 | 63.3±9.8 | 61.6±10.8 | 57.8±12.7 |
| Gender Male | ||||
| 65,5 | 69,1 | 61,6 | 70,8 | |
| Smoking | ||||
| Current | 15,2 | 12,2 | 19,1 | 4,3 |
| Former | 25,3 | 28,6 | 19,1 | 47,8 |
| Never | 59,4 | 59,1 | 61,6 | 47,8 |
| BMI (kg/m2) | 26.5±5.2 | 27.2±4.8 | 27.3±4.8 | 24.9±6.6 |
| SD: Standard deviation; BMI: Body mass index. | ||||
The median levels of serum CA125, CA15-3, folic acid, vitamin B12, and ferritin were significantly higher in the MPM group, compared to those with pleural plaques and those with and without exposure to asbestos (p<0.001 for each) (Table 2).
Table 2. Tumor and biochemical markers.
| Group 1 Pleural plaque (n=277) | Group 2 Healthy individuals exposed to asbestos(n=121) | Group 3 Healthy individuals not exposed to asbestos (n=118, control group) | Group 4 Mesothelioma (n=24) | ||
| % | % | % | % | p | |
| CEA (ng/mL) | 2,17 | 2,23 | 2,37 | 2,17 | 0,626 |
| CA 15-3 (U/mL) | 20,6 | 17,5 | 21,5 | 31,1 | <0.001 |
| CA 19-9 (U/mL) | 10,6 | 10,7 | 12,3 | 13,8 | 0,276 |
| CA 125 (U/mL) | 14,23 | 11,74 | 12,6 | 40,2 | <0.001 |
| Folic acid (ng/mL) | 4,53 | 4,73 | 4,49 | 5,81 | 0,002 |
| Vitamin B12 (pg/mL) | 276 | 264 | 296 | 587 | <0.001 |
| Free T3 (pg/mL) | 3,25 | 3,26 | 3,27 | 1,89 | <0.001 |
| Free T4 (ng/dL) | 1,14 | 1,14 | 1,16 | 1,23 | 0,19 |
| TSH (mIU/mL) | 1,77 | 1,66 | 1,42 | 1,44 | 0,145 |
| Ferritin (ng/mL) | 89 | 71 | 89 | 371 | <0.001 |
| CEA: Carcinoembryonic antigen; CA: Cancer antigen; TSH: Thyrotropin-stimulating hormone. | |||||
The AUC of the ROC for CA125 and CA15-3 were 0.78 and 0.68, respectively in the differential diagnosis of mesothelioma from other pathologies (p<0.001 for both) (Figure 1). The optimal cut-off value for CA125 was 13.63 nmol/L with 83.3% sensitivity and 69.6% specificity. For CA15-3, the optimal cut-off value was 18.43 nmol/L with 75% sensitivity and 48.4% specificity (Table 3). The sensitivity and specificity to discriminate mesothelioma from other pathologies were 70% and 49.6%, respectively.
Figure 1. Receiver operating characteristic curves of CA125 and CA15-3 in the diagnosis of malignant pleural mesothelioma. CA: Cancer antigen.
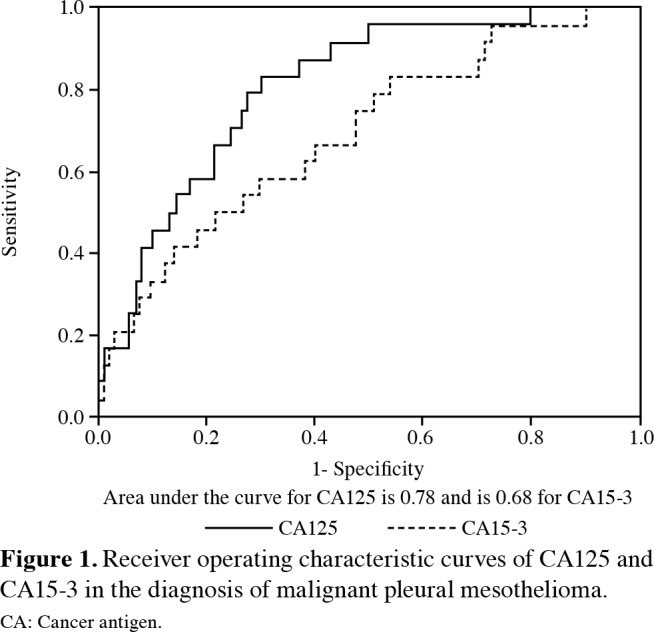
Table 3. Sensitivity and specificity of CA125 and CA15-3 according to different limit values.
| Limit value | Sensitivity | Specificity | Accuracy | |
| % | % | % | % | |
| 12,37 | 87,5 | 62,6 | 63,7 | |
| CA 125 (U/mL) | 13.63* | 83,3 | 69,6 | 70,2 |
| 14,025 | 79,2 | 72 | 72,6 | |
| 17,8 | 79,2 | 45,3 | 46,9 | |
| CA 15-3 (U/mL) | 18.43* | 75 | 48,4 | 49,6 |
| 19,205 | 70,8 | 52 | 53 | |
| CA: Cancer antigen; * Optimal limit values. | ||||
Discussion
In the comparison of blood levels of 10 different biomarkers among the groups, blood levels of CA125, CA15-3, folic acid, vitamin B12, and ferritin in the MPM group were statistically higher than the other groups, and blood levels of fT3 were lower in the MPM group than the other groups. However, there was no statistically significant difference between the groups in terms of blood levels of CEA, CA19-9, fT4, and TSH.
For early diagnosis and differential diagnosis of MPM, many biochemical markers have been investigated in the English literature, and some of them have been found to be elevated in serum and/or pleural fluids of patients with MPM.[4,11-14] In our study, different from other studies, 10 biochemical markers were measured in the blood and compared among the groups.
In our study, we found that the MPM group had lower BMI and fT3 levels compared to the other groups. This finding indicates that there may be a relationship between low BMI and low fT3 levels in MPM, and the autoimmune system may suppress the release of fT3 in response to low BMI as a defense mechanism.
In recent years, Wang et al.[15] measured biochemical tumor markers (CEA, CA125, CA15-3, CA19-9) to examine their predictive value in identifying the cause of malignant pleural effusion and reported that CA15-3 was the most potent biomarker in identifying lung cancer-related malignant pleural effusions, and CEA was a good biomarker in the differentiation of MPM and lymphoma-related pleural effusions. In our study, in contrast to the study of Wang et al.,[15] serum levels of CA125 and CA15-3 were significantly higher, although serum levels of CEA did not increase in patients with MPM. In addition, serum levels of CA19-9 were not significantly different between MPM patients and other groups. In another study, Antonangelo et al.[16] showed that CEA, CA15-3, CA125, and serum cytokeratin fragment 21.1 (CYFRA 21.1) values were higher in malignant pleural effusion than in benign pleural effusion, regardless of cytologic findings, whereas CA19-9 and CA72-4 levels did not provide information in differentiating malignant and benign pleural effusions. The results of both the aforementioned studies[15,16] and our study suggest that serum levels of CA19-9 are not informative in differentiating malignant and benign pleural effusions. In our study, the values of CA125 and CA15-3 in the ROC analysis for the diagnosis of MPM were found to be statistically significant. The AUC of the ROC for CA125 and CA15-3 were 0.78 and 0.68, respectively in discriminating mesothelioma from other pathologies (p<0.001 for both). The optimal cutoff value for CA125 was 13.63 nmol/L with 83.3% sensitivity and 69.6% specificity, and the optimal cut-off value for CA15-3 was 18.43 nmol/L with 75% sensitivity and 48.4% specificity. The sensitivity and specificity to discriminate mesothelioma from other pathologies were 70.2% and 49.6%, respectively.
The elevated ferritin levels found in our study in the MPM group can be attributed to the fact that ferritin is an acute phase reactant. Sezgi et al.[17] showed that serum levels of oxidative stress markers and acute phase reactants increased in patients who developed mesothelioma after environmental asbestos exposure and they could be used in asbestosassociated malignancies. In our study, similar to the aforementioned study, the serum level of ferritin was 371 ng/mL in the MPM patients, 89 ng/mL in the control and pleural plaque group, and 71 ng/mL in healthy individuals who were exposed to asbestos. According to these results, ferritin might have been elevated as an acute phase reactant in patients with MPM. In another study, the importance of a ferritin increase in predicting performance and prognosis in patients with primary lung cancer and worse prognosis in patients with ferritin level >300 ng/L were reported.[18] In future studies, thus, ferritin may be used as a marker in the differential diagnosis of patients with MPM or other malignancies.
A similar case is valid for vitamin B12. Previous studies showed that vitamin B12 levels increased in solitary tumors, hematological malignancies, autoimmune diseases, and liver and spleen diseases. It was suggested that the increase in B12 level might be due to impaired influx or movement of vitamin B12 to the tissues and this might be a warning in the early diagnosis of such diseases.[19,20] Oh et al.[21] reported that vitamin B12 level was an independent prognostic factor in patients with metastatic cancer; higher B12 level was associated with shorter life expectancy and, therefore, vitamin B12 level could be used to predict life expectancy in patients with metastatic solitary cancer. In another study, higher vitamin B12 levels in gallbladder adenocarcinoma patients were a strong predictor of mortality and were associated with rapid metastasis and shorter life expectancy.[22] In our study, vitamin B12 level was significantly higher (587 pg/mL) in the MPM group than the other groups, which could be an indicator of shorter life expectancy in these patients. As shown in recent studies, using biomarkers combined or alone is useful and promising in the early diagnosis of MPM, in predicting prognosis, and in monitoring response to treatment in patients who have been exposed to asbestos.[23-25]
Recent studies in the literature have also examined the diagnostic value of tenascin XB (TNXB) and soluble mesothelin-related peptide (SMRPs) for MPM. Nakayama et al.[26] suggested that TNXB was a novel diagnostic biomarker for malignant mesothelioma. A combination of detecting TNXB and calretinin may be useful for the differential diagnosis of malignant mesothelioma from lung adenocarcinoma. In another study, Gao et al.[27] investigated the diagnostic value of SMRPs in pleural effusion for MPM and reported that, although the sensitivity of SMRPs was low, pleural effusion-SMRPs can be a good indicator of the existence of MPM. We believe that novel biomarkers are needed in the early diagnosis of MPM, in predicting prognosis, and in monitoring response to treatment and further studies would provide more comprehensive data to the existing body of knowledge.
In conclusion, our study results showed that serum CA125, CA15-3, folic acid, vitamin B12, and ferritin levels were higher in malignant pleural mesothelioma than benign asbestos-related diseases and asbestosexposed individuals. In addition, free T3 levels of malignant pleural mesothelioma were lower. The combination or individual analysis of serum CA125, CA15-3, folic acid, vitamin B12, and ferritin levels may be helpful for early diagnosis and treatment of malignant pleural mesothelioma, although other tumor and biochemical markers are clinically irrelevant.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 2.Helland Å, Solberg S, Brustugun OT. Incidence and survival of malignant pleural mesothelioma in norway: a populationbased study of 1686 cases. J Thorac Oncol. 2012;7:1858–1861. doi: 10.1097/JTO.0b013e318275b346. [DOI] [PubMed] [Google Scholar]
- 3.Bruno F, Baratti D, Martinetti A, Morelli D, Sottotetti E, Bonini C, et al. Mesothelin and osteopontin as circulating markers of diffuse malignant peritoneal mesothelioma: A preliminary study. Eur J Surg Oncol. 2018;44:792–798. doi: 10.1016/j.ejso.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Bayram M, Dongel I, Akbaş A, Benli I, Akkoyunlu ME, Bakan ND. Serum biomarkers in patients with mesothelioma and pleural plaques and healthy subjects exposed to naturally occurring asbestos. Lung. 2014;192:197–203. doi: 10.1007/s00408-013-9526-9. [DOI] [PubMed] [Google Scholar]
- 5.Pass HI, Levin SM, Harbut MR, Melamed J, Chiriboga L, Donington J, et al. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N Engl J Med. 2012;367:1417–1427. doi: 10.1056/NEJMoa1115050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mori T, Tajima K, Hirama M, Sato T, Kido K, Iwakami S, et al. The N-ERC index is a novel monitoring and prognostic marker for advanced malignant pleural mesothelioma. J Thorac Dis. 2013;5:145–148. doi: 10.3978/j.issn.2072-1439.2013.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayram M, Dongel I, Bakan ND, Yalççn H, Cevit R, Dumortier P, et al. High risk of malignant mesothelioma and pleural plaques in subjects born close to ophiolites. Chest. 2013;143:164–171. doi: 10.1378/chest.11-2727. [DOI] [PubMed] [Google Scholar]
- 8.Berk S, Yalcin H, Dogan OT, Epozturk K, Akkurt I, Seyfikli Z. The assessment of the malignant mesothelioma cases and environmental asbestos exposure in Sivas province, Turkey. Environ Geochem Health. 2014;36:55–64. doi: 10.1007/s10653-013-9518-y. [DOI] [PubMed] [Google Scholar]
- 9.Dumortier P, Coplü L, de Maertelaer V, Emri S, Baris I, De Vuyst P. Assessment of environmental asbestos exposure in Turkey by bronchoalveolar lavage. Am J Respir Crit Care Med. 1998;158:1815–1824. doi: 10.1164/ajrccm.158.6.9712119. [DOI] [PubMed] [Google Scholar]
- 10.Döngel I, Bayram M, Bakan ND, Yalçın H, Gültürk S. Is living close to ophiolites related to asbestos related diseases. Cross-sectional study. Respir Med. 2013;107:870–874. doi: 10.1016/j.rmed.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Brims FJ, Lee YC, Creaney J. The continual search for ideal biomarkers for mesothelioma: the hurdles. J Thorac Dis. 2013;5:364–366. doi: 10.3978/j.issn.2072-1439.2013.04.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pass HI, Lott D, Lonardo F, Harbut M, Liu Z, Tang N, et al. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N Engl J Med. 2005;353:1564–1573. doi: 10.1056/NEJMoa051185. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez Portal JA, Rodríguez Becerra E, Rodríguez Rodríguez D, Alfageme Michavila I, Quero Martínez A, Diego Roza C, et al. Serum levels of soluble mesothelinrelated peptides in malignant and nonmalignant asbestos-related pleural disease: relation with past asbestos exposure. Cancer Epidemiol Biomarkers Prev. 2009;18:646–650. doi: 10.1158/1055-9965.EPI-08-0422. [DOI] [PubMed] [Google Scholar]
- 14.Gube M, Taeger D, Weber DG, Pesch B, Brand P, Johnen G, et al. Performance of biomarkers SMRP, CA125, and CYFRA 21-1 as potential tumor markers for malignant mesothelioma and lung cancer in a cohort of workers formerly exposed to asbestos. Arch Toxicol. 2011;85:185–192. doi: 10.1007/s00204-010-0580-2. [DOI] [PubMed] [Google Scholar]
- 15.Wang XF, Wu YH, Wang MS, Wang YS. CEA, AFP, CA125, CA153 and CA199 in malignant pleural effusions predict the cause. Asian Pac J Cancer Prev. 2014;15:363–368. doi: 10.7314/apjcp.2014.15.1.363. [DOI] [PubMed] [Google Scholar]
- 16.Antonangelo L, Sales RK, Corá AP, Acencio MM, Teixeira LR, Vargas FS. Pleural fluid tumour markers in malignant pleural effusion with inconclusive cytologic results. Curr Oncol. 2015;22:336–341. doi: 10.3747/co.22.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sezgi C, Taylan M, Sen HS, Evliyaoğlu O, Kaya H, Abakay O, et al. Oxidative status and acute phase reactants in patients with environmental asbestos exposure and mesothelioma. ScientificWorldJournal. 2014;2014:902748–902748. doi: 10.1155/2014/902748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milman N, Pedersen LM. The serum ferritin concentration is a significant prognostic indicator of survival in primary lung cancer. Oncol Rep. 2002;9:193–198. [PubMed] [Google Scholar]
- 19.Chiche L, Jean R, Romain F, Roux F, Thomas G, Canavese S, et al. Clinical implications of high cobalamin blood levels for internal medicine. Rev Med Interne. 2008;29:187–194. doi: 10.1016/j.revmed.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Andrès E, Serraj K, Zhu J, Vermorken AJ. The pathophysiology of elevated vitamin B12 in clinical practice. QJM. 2013;106:505–515. doi: 10.1093/qjmed/hct051. [DOI] [PubMed] [Google Scholar]
- 21.Oh HK, Lee JY, Eo WK, Yoon SW, Han SN. Elevated Serum Vitamin B12 Levels as a Prognostic Factor for Survival Time in Metastatic Cancer Patients: A Retrospective Study. Nutr Cancer. 2018;70:37–44. doi: 10.1080/01635581.2018.1397711. [DOI] [PubMed] [Google Scholar]
- 22.Aloreidi K, Zamulko A. Elevated Vitamin B12: A Rare Presentation for Gallbladder Adenocarcinoma. S D Med. 2018;71:171–173. [PubMed] [Google Scholar]
- 23.Arnold DT, De Fonseka D, Hamilton FW, Rahman NM, Maskell NA. Prognostication and monitoring of mesothelioma using biomarkers: a systematic review. Br J Cancer. 2017;116:731–741. doi: 10.1038/bjc.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonotti A, Foddis R, Landi S, Melaiu O, De Santi C, Giusti L, et al. A Novel Panel of Serum Biomarkers for MPM Diagnosis. Dis Markers. 2017;2017:3510984–3510984. doi: 10.1155/2017/3510984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cristaudo A, Bonotti A, Guglielmi G, Fallahi P, Foddis R. Serum mesothelin and other biomarkers: what have we learned in the last decade. J Thorac Dis. 2018;10:353–359. doi: 10.21037/jtd.2017.10.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakayama K, Seike M, Noro R, Takeuchi S, Matsuda K, Kunugi S, et al. Tenascin XB Is a Novel Diagnostic Marker for Malignant Mesothelioma. Anticancer Res. 2019;39:627–633. doi: 10.21873/anticanres.13156. [DOI] [PubMed] [Google Scholar]
- 27.Gao R, Wang F, Wang Z, Wu Y, Xu L, Qin Y, et al. Diagnostic value of soluble mesothelin-related peptides in pleural effusion for malignant pleural mesothelioma: An updated meta-analysis. Medicine (Baltimore) 2019;98:14979–14979. doi: 10.1097/MD.0000000000014979. [DOI] [PMC free article] [PubMed] [Google Scholar]


