Abstract
BACKGROUND:
Because most HBV/HIV co-infected patients on combination antiretroviral therapy (cART) have suppressed HBV DNA and normal liver enzymes, the histologic spectrum of liver disease in HBV/HIV coinfection is poorly defined. To address this gap in knowledge, we conducted a prospective study to comprehensively characterize liver disease severity assessed by liver biopsy in a well-defined cohort of HBV/HIV patients in North America receiving cART.
METHODS:
Adult HIV/HBsAg positive patients on stable cART were recruited. Demographic, clinical, serological, and virological data were collected. Liver histology was assessed by a central pathology committee. The association of demographic, clinical, serologic, and virologic characteristics with liver histology was assessed using logistic regression.
RESULTS:
In this cross-sectional analysis, the mean age of the cohort (N = 139) was 49 years; 92% were male, 51% were non-Hispanic black, 7% had at-risk alcohol use with a median duration of infections of 14 years. The median ALT was 28 IU/L and CD4 count was 568 cells/mm3. Almost all (99%) were on cART. Three-fourths (75%) had undetectable HIV RNA (<20 copies/mL). HBeAg was positive in 62%, HBV DNA was below the limit of quantification (<20 IU/mL) in 57% and <1000 IU/ mL in 80%; 7% had incomplete viral suppression (HBV DNA≥1000 IU/mL and HIV RNA<20 copies/mL). Liver histology (available in n = 114) showed significant periportal, lobular, and portal inflammation (scores ≥2) in 14%, 31%, and 22% respectively. Over a third (37%) had significant fibrosis (Ishak stage ≥2); 24% had advanced fibrosis (Ishak stage ≥3). Higher ALT (adjusted OR 1.19 per 10 IU/L; 95% CI [1.01, 1.41]; p = 0.03) and lower platelet count (adjusted OR 0.81 per 20,000 mm3; 95% CI [0.67–0.97]; p = 0.02) but not HBV DNA were independently associated with advanced fibrosis.
CONCLUSIONS:
In this cohort of patients with HBV/HIV coinfection receiving long-term cART with viral suppression, we observed significant fibrosis in more than one-third of patients.
INTRODUCTION
Hepatitis B virus (HBV) and human immunodeficiency virus (HIV) infect 257 million and 42 million persons worldwide, respectively (1). Due to shared routes of transmission, coinfection with HBV and HIV is common. Cohorts of HIV-infected individuals assembled in Europe and the US have noted HBV coinfection in 5–15% (2-7), and antibody to HIV is associated with a 25-fold increase HBV coinfection (8). Initially, the morbidity and mortality associated with HIV infection far exceeded the complications of HBV infection. However, with major advances in combination antiretroviral therapy (cART), liver-related mortality has supplanted acquired immunodeficiency syndrome (AIDS)-related mortality as a major complication in HBV-HIV coinfection (9). Despite the widespread use of antiviral agents active against both HBV and HIV, liver-related mortality remains the second leading cause of death among coinfected patients (6,9).
Current HIV treatment guidelines from the Department of Health and Human Services recommend that all persons with HBV-HIV coinfection should be treated with cART containing tenofovir (TF) plus lamivudine (3TC) or emtricitabine (FTC) (2-4,10,11). Despite the well-established guidelines on antiviral regimens in the management of individuals with HBV–HIV coinfection (2-4,10,11), there are limited prospective data on the extent of HBV control (12). Furthermore, because liver biopsy is rarely performed in HBV–HIV patients, the histologic spectrum of disease severity in the era of TF-containing cART is unknown. To address this knowledge gap, the HBV–HIV Cohort, an NIHfunded ancillary study of the Hepatitis B Research Network (HBRN) (13) was developed. The main aim of this multicenter cohort study was to assess the histologic spectrum of liver disease and its clinical and virologic correlates in a well-defined, geo-graphically diverse cohort of HBV–HIV patients in North America. The aim of this report is to describe, in cross-sectional nature, the baseline data from this cohort.
PATIENTS AND METHODS
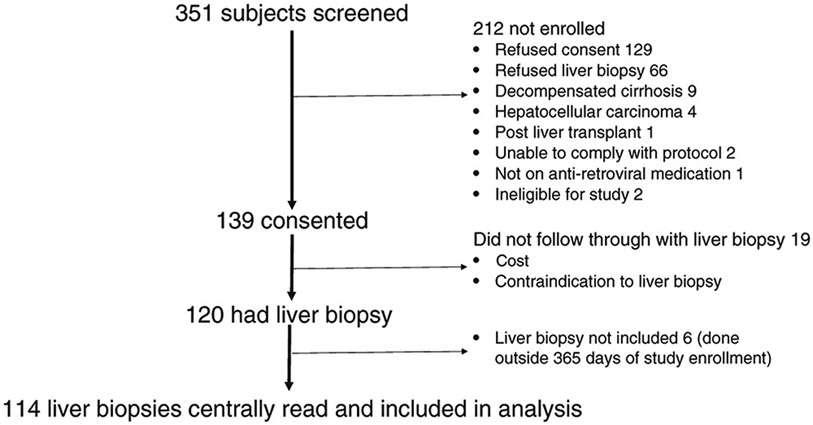
Adult patients with HIV/HBV were recruited from 8 HBRN sites in the US and Canada (Virginia Commonwealth University, University of California, San Francisco, University of Texas, Southwestern, Johns Hopkins University, University Health Network, Toronto, Washington University Saint Louis, Massachusetts General Hospital, NIDDK, National Institute of Health) to participate in this prospective observational study. Inclusion and exclusion criteria for the study are listed in the Appendix. All included subjects were anti-HIV positive and hepatitis B surface antigen positive (HBsAg) for at least 6 months. Those with hepatitis C virus (HCV) RNA, decompensated cirrhosis, or hepatocellular carcinoma (HCC) were excluded. Study participants agreed to undergo liver biopsy at study entry regardless of clinical or laboratory tests as long as they met inclusion/exclusion criteria. By close of enrollment 139 participants attended a research assessment, 120 of whom had a liver biopsy (Fig. 1). Of these, 114 subjects had a liver biopsy within 12 months of enrollment with central reading, which was required for this report. The institutional review board at each center approved the protocol, and participants gave written informed consent. The study is registered at ClinicalTrials.gov ().
Figure 1.
Flow of patients from screening to enrollment
Standardized research assessments were conducted by study personnel (clinical research investigators and their research staff) based on a common research protocol operations manual.
Demographic data
Data on demographics (age, gender, race/ethnicity, continent of birth, education) and health behaviors (e.g., alcohol use, risk factors for HIV, and HBV) were self-reported at the time of enrollment. Duration of HIV and/or HBV as well as current and past cART use was collected but was unable to be verified in many subjects due to the fragmented care received from different health providers at various sites between diagnosis and enrollment. Alcohol use was assessed with the alcohol use disorder identification test (AUDIT). Increased-risk was defined as an AUDIT score of 8–15, high-risk as a score ≥16 (14). Clinical assessment included waist circumference, height and weight, utilized to calculated body mass index (BMI), presence of diabetes mellitus (DM), and evidence of lipodystrophy (mild, moderate or severe) (15).
Laboratory data
Laboratory testing by local site included hematology, basic metabolic, hepatic panel including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP), phosphate, platelet count, and CD4/CD8. Normal ALT was defined as ≤30 U/L in men and <20 U/L in women (16). Laboratory data were collected within 12 weeks of liver biopsy in all but 10 subjects in whom liver biopsy was performed 13–38 weeks prior to enrollment.
HIV stage (1-4) was defined by CD4 count at entry (≥500, 350– 499, 200–349, and <200 cells/mm3, respectively) according to 2005 World Health Organization Guidelines (17). Data were entered by study coordinators or the central lab and transmitted the HBRN Data Coordinating Center (DCC, University of Pittsburgh).
Virologic data
Results of local viral testing (HBsAg, HBV e antigen/antibody, HBV DNA, HCV antibody, HIV RNA, hepatitis delta antibody) were recorded. Serum was collected for central testing, stored at the NIDDK biorepository and sent to the HBRN Central Laboratory (University of Washington) for quantitative HBsAg (qHBsAg) and quantitative hepatitis e antigen (qHBeAg), HBV DNA and genotype, and HBV precore/basal core promoter (PC/BCP) mutations (Roche, Branchburg, NJ).
Histologic data
Liver biopsy was performed in the standard fashion. Unstained slides were submitted for H&E and Masson trichrome staining by a central lab. Histological findings were scored blindly with respect to clinical data, including HIV status by the HBRN Pathology Committee (DEK, chair). Total length of biopsy was recorded. While the number of portal tracts was not recorded, each biopsy was assessed as adequate or inadequate by the central pathology committee with a minimum of three portal tracts required to be adequate. The primary outcomes for this report are necroinflammatory activity assessed by the Ishak Histologic Activity Index (HAI), and fibrosis assessed by the Ishak fibrosis score (18). The HAI is a global scale of hepatic inflammation calculated as the sum of lobular, periportal, portal inflammation, and confluent necrosis scores, with a possible range 0–18. The Ishak fibrosis score ranges from 0–6 and we have defined stage 2 and above as significant fibrosis, and stage 3 and above as advanced fibrosis. Steatosis/steatohepatitis was graded by methods similar to those used in the HALT-C trial (19). Specifically, steatosis was graded by the proportion of hepatocytes showing steatosis at low magnification (None, <5%, 5–33%, 34–66%, and >66%), and steatohepatitis was based on the presence of a distinctive pattern of injury (characteristic hepatocyte ballooning with or without Mallory-Denk bodies and perisinusoidal fibrosis).
Statistical analysis
Descriptive statistics summarize demographic, clinical, and virologic characteristics of participants at study entry (N = 139), as well as these characteristics and histologic characteristics of participants with liver biopsy within 12 months of enrollment (N = 114). Frequencies and percentages are reported for categorical data. Depending on the distribution of the data, either means and standard deviations, or medians and interquartile range (IQR), are reported for continuous data.
The primary aim of this cross-sectional analysis was to describe the histologic spectrum of liver disease (inflammation and fibrosis) at study entry. Linear regression models were used to test and estimate associations between demographic, clinical, serologic, and virologic characteristic and HAI (dependent variable). The following characteristics were first investigated in simple linear regression models: age, sex, race/ethnicity, tobacco use, marijuana use, coffee (cups per day), tea (cups per day), alcohol use, BMI, history of diabetes, ALT, AST, AST/ALT ratio, platelets, AST to platelet ratio index (APRI) (20), FIB-4 index (21), HIV stage (0–3), lipodystrophy/lipoatrophy grade (0–3), undetectable HIV RNA (<20 copies/mL), CD4, CD4%, CD8, CD8%, HBV duration, HBeAg positive, qHBeAg level, undetectable HBV DNA (<20 IU/ mL), suppressed HBV DNA (<1000 IU/mL), HBV DNA incomplete suppression (defined as HBV DNA ≥1000 IU/mL and HIV RNA <20 copies/mL), HBV DNA level, and qHBsAg level. Due to low representation, Asian ethnicity/race was collapsed with the “other” category. Likewise, due to low representation, high-risk alcohol use was collapsed with increased-risk at “at-risk alcohol use.” Since qHBeAg levels and qHBsAg were skewed, they were log-transformed (natural log for qHBeAg, 10-base for qHBsAg) to reduce the impact of skewness. Factors that were associated at p < 0.20 were then entered into a multivariable regression model to identify factors independently associated with inflammation; variables that had p < 0.10 were removed using a step-wise variable selection method. Quantitative HBeAg level was not considered for multivariable models as it was only available for a subgroup of HBeAg positive patients. Given the high correlation between ALT and AST, the AST/ALT ratio was considered. In addition, separate models evaluated each with the additional independent variables that met the multivariable model criteria. Results are reported as regression coefficients and corresponding 95% confidence intervals. This methodology was repeated using logistic regression to evaluate associations with the binary outcome of advanced fibrosis. Finally, models starting with APRI and FIB-4, respectively, were constructed to determine associations with advanced fibrosis, independent of these biomarkers. Separate models evaluated each with additional independent variables that met the multivariable model criteria, but were not used to calculate these measures (e.g., ALT and platelet for APRI). The results are reported as odds ratios and 95% confidence interval and p-values.
Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA). All reported p -values are two-sided; p-values <0.05 are considered to be statistically significant.
RESULTS
Characteristics of the cohort
Among the enrolling sites, 351 patients were screened, of which 139 were consented. The most common reasons for not participating were refused/no consent (n = 129), refused liver biopsy (n = 66), hepatic decompensation (n = 9), and liver cancer (n = 4) (Fig. 1). Of those enrolled, 19 did not undergo biopsy due to logistical (transportation and scheduling conflicts) or financial reasons. Six subjects underwent liver biopsy either more than 365 days prior (n = 5) or after (n = 1) enrollment, leaving 114 subjects with histology data. Liver biopsies were performed a median (IQR) of 21 days (0–47) from the date of the enrollment research assessment.
Table 1 shows the major demographic and clinical features of the enrolled participants and the histology analysis sample. Among enrolled participants, the mean (SD) age was 49 (8.8), 92% were male, 32% were non-Hispanic white, and 51% were non-Hispanic Black. Most (84%) had had at least a high school education, 64% were never married, and 65% were not working. Increased risk alcohol use was observed in 7%; no participants had high-risk use. Only 12 (9%) reported history of having diabetes. The presumed primary mode of HIV and HBV infection was sexual transmission (accounting for ≥92% of both infections), with 91% of such transmission among men having sex with men (MSM). The median (IQR) duration of co-infection was 14 (8-20) years. Most participants had stage 1 (68%) or 2 (16%) HIV disease and 11 (9%) had CD4 <200 cells/mm3. Almost all participants (99%) used cART which included anti-HBV coverage as part of cART in 91% with either tenofovir with or without FTC or 3TC (78%) or entecavir (14%). Lipodystrophy was observed in 16%.
Table 1.
Baseline characteristics of HIV-HBV co-infected participants. Unless otherwise stated in the characteristics description, numbers represent median (IQR) for continuous variables and n (percent) for categorical variables
| Characteristics | All participants (N = 139a) |
Participants with liver biopsy (N = 114a) |
|---|---|---|
| Age (years, mean (SD)) | N = 13,948.7(8.8) | N = 11,449.3 (9.0) |
| Gender | N = 139 | N = 114 |
| Male | 128 (92.1%) | 106 (93.0%) |
| Race/Ethnicity | N = 138 | N = 113 |
| Non-Hispanic White | 44 (31.8%) | 37 (32.7%) |
| Non-Hispanic Black | 70 (50.7%) | 58 (51.3%) |
| Non-Hispanic Asian | 5 (3.6%) | 5 (4.4%) |
| Other | 19 (13. 8%) | 13 (11.5%) |
| Weight (kg), male | N = 11,079.4 (67.3: 93.6) |
N = 9179.2 (66.2: 93.6) |
| Weight (kg), female |
N = 1094.0 (75.3: 112.5) |
N = 798.2 (82.5: 112.6) |
| Body mass index (kg/m2) | N = 11,825.8 (22.0: 31.2) | N = 9726.3 (22.0: 30.8) |
| Tobacco use | N = 139 | N = 114 |
| Never use a tobacco product | 32 (23.0%) | 26 (22.8%) |
| Formerly used a tobacco product | 42 (30.2%) | 36 (31.6%) |
| Currently used a tobacco product | 65 (46.8%) | 52 (45.6%) |
| Alcohol consumption | N = 138 | N = 114 |
| Low risk | 128 (92.8%) | 106 (93.8%) |
| Increasing risk | 9 (6.5%) | 7 (6.2%) |
| High risk | 1 (0.7%) | 0 (0.0%) |
| Tea cups per day | N = 138 | N = 114 |
| None | 51 (37.0%) | 39 (34.2%) |
| Occasionally, <1 per day | 62 (44.9%) | 54 (47.4%) |
| 1 per day | 12 (8.7%) | 10 (8.8%) |
| 2 per day | 9 (6.5%) | 7 (6.1%) |
| 3 or 4 per day | 2 (1.4%) | 2 (1.8%) |
| More than 4 per day | 2 (1.4%) | 2 (1.8%) |
| History of diabetes | 12 (8.6) | 10(8.8) |
| History of sexually transmitted diseases |
N = 12,681 (64.3%) | N = 10,870 (64.8%) |
| Estimated duration of HIV infection, years |
N = 12,019.5 (10.5: 25.0) | N = 10,220.0 (10.0: 25.0) |
| Estimated duration of HBV infection, years |
N = 10,314.0 (8.0: 20.0) |
N = 8714.0 (8.0: 22.0) |
| Presumed source of HIV | N = 124 | N = 105 |
| Sexually transmitted | 118 (95.2%) | 99 (94.3%) |
| Transfusion | 1 (0.8%) | 1 (1.0%) |
| Injection drug use | 3 (2.4%) | 3 (2.9%) |
| Other | 2 (1.6%) | 2 (1.9%) |
| Presumed source of Hepatitis B | N = 116 | N = 99 |
| Vertical transmission | 1 (0.9%) | 1 (1.0%) |
| Sexually transmitted | 107 (92.2%) | 87 (90.9%) |
| Transfusion | 3 (2.6%) | 3 (3.0%) |
| Injection drug use | 3 (2.6%) | 3 (3.0%) |
| Other | 2(1.7%) | 2 (2.0%) |
| History of cART use | N = 139 | N = 114 |
| Anti-HBV use | 138 (99.3%) | 114 (100.0%) |
| Tenofovir: alone | 13 (9.6%) | 12 (10.7%) |
| Tenofovir: alone or in combination | 103 (75.7%) | 87 (77.7%) |
| Emtricitabine: alone or in combination | 93 (68.4%) | 77 (68.8%) |
| Lamivudine: alone or in combination | 21 (15.4%) | 18 (16.1%) |
| Entecavir | 18 (13.2) | 16 (14.3%) |
| Tenofovir + emtricitabine | 90 (66.2) | 75 (67.0%) |
| Tenofovir + lamivudine | 9 (6.6) | 8 (7.1%) |
| Tenofovir + entecavir | 5 (3.7) | 5 (4.5%) |
| Lipodystrophy/lipoatrophy gradeb | N = 123 | N = 102 |
| 0 | 103 (83.7%) | 88 (86.3%) |
| 1 | 13 (10.6%) | 9 (8.8%) |
| 2 | 6 (4.9%) | 4 (3.9%) |
| 3 | 1 (0.8%) | 1 (1.0%) |
| HIV stagec | N = 107 | N = 86 |
| 1 | 73 (68.2%) | 63 (73.3%) |
| 2 | 17 (15.9%) | 11 (12.8%) |
| 3 | 8 (7.5%) | 5 (5.8%) |
| 4 | 9 (8.4%) | 7 (8.1%) |
Table 2 shows the laboratory and serologic results. Among enrolled participants, median (IQR) ALT was 28 (19–41) U/L with 53% having a normal ALT. Median (IQR) AST was 29 (23–40) U/L and ALP was 84 (68–106) U/L. The median (IQR) platelet count was 200,000 (174–236,000) mm3. The median (IQR) APRI and FIB-4 scores were 0.34 (026–0.57) and 1.34 (0.99–1.86), respectively. HIV RNA was below detection (<20 copies/mL) in 75%. In those with detectable HIV RNA, proportion with levels 20–100, 100–10,000, and >10,000 copies/ml were 32%, 39%, and 23%, respectively. The median (IQR) CD4 count was 568 cells/mm3 (337–713). Only 1 patient was anti-HDV positive. HBeAg was positive in 62%, and 57% had undetectable HBV DNA (<20 IU/mL) while 80% were suppressed (HBV DNA <1000 IU/mL). Median (IQR) HBV DNA in those with detectable DNA was 933 (62–28272) IU/mL. Incomplete HBV suppression (≥1000 IU/mL) in the setting of HIV RNA suppression (<20 copies/mL) was observed in 7%. In the 97 subjects with quantifiable HBsAg, median (IQR) serum qHBsAg level was 1478 (381, 10111) IU/mL and in 65 HBeAg positive participants with detectable levels, median (IQR) qHBeAg level was 14.6 (1.8, 273.2) IU/mL. The frequency of basal core promotor (BCP) mutations A1762T and G1764A alone or in combination in those with detectable HBV DNA (n = 18) were 16%, 16%, and 16%, respectively while the frequency of G1896A was seen on only 6% and no patients had the G1899A mutation. Anti-delta antibody was only positive in 1 participant. Results are similar for the histology analysis sample (Table 2).
Table 2.
Laboratory characteristics of HIV-HBV co-infected participants. Numbers represent median (IQR) for continuous variables and n (percent) for the categorical variables
| Laboratory characteristics | All participants (N = 139a) | Participants with biopsy (N = 114) |
|---|---|---|
| ALT (IU/L) | N = 13,728.0 (19.0:41.0) | N = 11,427.0 (19.0:39.0) |
| Normal ALT (≤30 IU/L for male, <20 IU /Lfor female) | 72 (52.6) | 62 (54.4) |
| AST (IU/L) | N = 13,329.0 (23.0:40.0) | N = 11,028.0 (22.0:39.0) |
| AST-ALT ratio | N = 1331.03 (0.79, 1.28) | N = 1101.04 (0.81:1.29) |
| Alkaline phosphatase (IU/L) | N = 13,284.0 (68.0:105.5) | N = 10,985.0 (68.0:107.0) |
| Total bilirubin (mg/dL) | N = 1290.5 (0.4:0.7) | N = 1060.5 (0.3:0.6) |
| Albumin (g/dL) | N = 1334.3 (4.1:4.6) | N = 1104.3 (4.1:4.6) |
| White blood cells (x1000/mm3) | N = 1345.7 (4.5:7.0) | N = 1125.7 (4.5:7.3) |
| Platelets (x1000/mm3) | N = 134,200.0 (174.0:236.0) | N = 112,200.5 (175.0:241.0) |
| APRI | N = 1320.34 (0.26, 0.57) | N = 1100.33 (0.26, 0.50) |
| APRI>1.5(%) | 1 (0.8) | 1 (0.9) |
| FIB-4 | N = 1321.34 (0.99, 1.86) | N = 1101.35 (0.99, 1.87) |
| FIB-4≥3.25 (%) | 6 (4.6) | 4 (3.6) |
| CD4 (cells/mm3) | N = 120,567.5 (337.0:712.5) | N = 102,567.5 (366.0:718.0) |
| CD4% | N = 12,125.4 (18.0:36.3) | N = 10,325.4 (18.0:36.3) |
| CD8 (cells/mm3) | N = 80,835.0 (593.5:1199.0) | N = 67,827.0 (592.0:1222.0) |
| CD8% | N = 12,145.0 (35.0:50.3) | N = 6843.5 (35.0:51.5) |
| HIV RNA undetectable (<20 copies/mL) | N = 12,493 (75.0%) | N = 10,481 (77.9%) |
| HIV RNA level (copies/mL) in those detectable |
N = 31,137 (54:7930) | N = 23,134 (54.0:2647.0) |
| Anti-HCV antibody+ | N = 1285 (3.9%) | N = 1054 (3.8%) |
| Anti-HDV antibody+ | N = 891 (1.1%) | N = 731 (1.4%) |
| Anti-HBe antibody+ | N = 12,533 (26.4%) | N = 10,229 (28.4%) |
| HBe antigen+ | N = 13,080 (61.5%) | N = 10,767 (62.6%) |
| qHBeAg (IU/mL), in HBeAg+ and quantifiable | N = 6514.6 (1.8:273.2) | N = 5714.6 (1.8:224.7) |
| qHBsAg (IU/mL), detectable | N = 971,478.4 (380.7:10,111.2) | N = 801,483.9 (379.2:11,427.4) |
| HBV DNA undetectable (<20 IU/mL) | N = 12,971 (55.0%) | N = 10,762 (57.9%) |
| HBV DNA level (IU/mL) in those detectable (≥20 IU/mL) | N = 56,933 (62:28,272) | N = 45,986 (57:26,344) |
| HBV DNA suppressed (DNA <1000 IU/mL) | N = 129,103 (79.8%) | N = 10,785 (79.4%) |
| Incomplete HBV suppression (HBV DNA ≥1000 IU/mL and HIV RNA <20 copies/mL) | N = 1148 (7.0%) | N = 977 (7.2%) |
Ag, antigen; q, Quantitative; APRI, aspartate to platelet ratio index; FIB-4, fibrosis index based on four factors
Numbers of subjects with available data
Histologic spectrum of disease
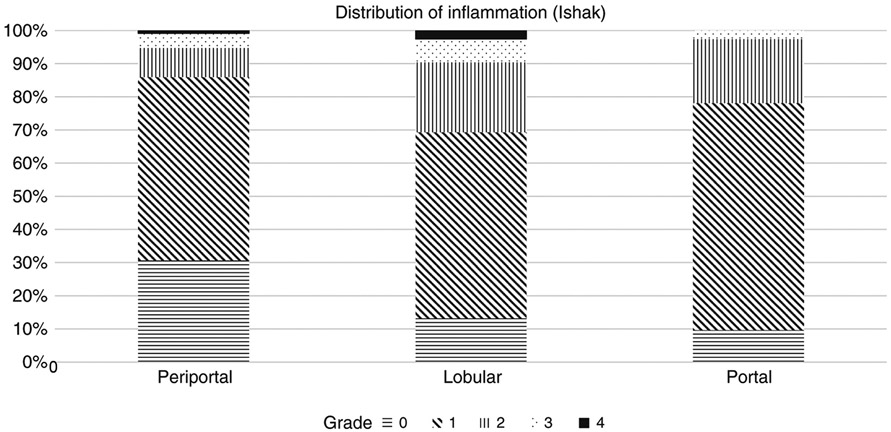
Most (99%) of liver biopsies were deemed adequate for histologic assessment by the HBRN central pathology committee. Liver biopsies were >10 mm in length in 93% of the patients and >15 mm in 72%. Figure 2 shows the proportion of participants with each grade of inflammation (0–4) by type (portal, lobular, periportal). Confluent necrosis was rare, observed in only 6 (5%) participants with one case showing multiacinar necrosis (score 6). The median (IQR) HAI was 3 (2-4). Most participants (88%) had minimal to mild inflammatory activity (total inflammation score ≤5). There was at least mild to moderate periportal, lobular, and portal inflammation (scores ≥2) in 14%, 31%, and 22% of participants, respectively.
Figure 2.
Histology: proportion grade of Ishak Inflammation (portal, peri-portal, lobular)
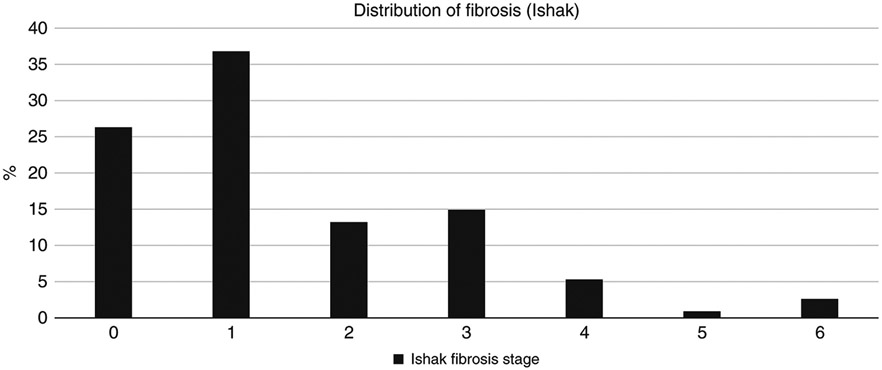
Among those with a biopsy, 36.9% had significant fibrosis (Ishak 2–6) and 23.7% had advanced fibrosis (Ishak 3–6) with only 4 patients (5%) showing cirrhosis (Ishak 5–6), Fig. 3. Over a quarter (29.0%) had >5% steatosis and 10% had NASH (8% definite and 2% probable). Of the 7 subjects (6.4%) with at-risk alcohol use 4 had <5% steatosis and 3 had grade 1 (5-33%) steatosis while none had steatohepatitis. The spectrum of disease (i.e., HAI and advanced fibrosis) did not vary by clinical site (p = 0.88 for HAI with F-test, and p = 0.21 with exact chi-square test).
Figure 3.
Histology fibrosis (Ishak 0–6)
Factors associated with inflammation and fibrosis
Table 3 shows the unadjusted and adjusted associations between demographic, clinical, and serologic characteristics with HAI. On univariate analysis, having a history of diabetes, higher ALT (continuous) or having an ALT above the upper limit of normal (ULN), higher AST, lower platelets, lower CD4%, detectable HIV RNA, and detectable HBV DNA (>20 IU/mL) were significantly (p values for all <0.05) associated with hepatic inflammation. After adjusting for other factors, platelets, CD4%, detectable HIV RNA and detectable HBV DNA were no longer significantly associated with HAI, whereas a history of diabetes (p = 0.002) and AST (p < 0.001) were. Participants with history of diabetes had on average a higher HAI (by 2.1 points) than those without any history of diabetes (95% CI, 0.8–3.4), and each 10 unit higher AST was associated with a 0.5 point higher HAI (95% CI, 0.3–0.7). In addition, tobacco use was independently associated with inflammation (p = 0.02), such that current tobacco users had on average a 1.0 point higher HAI score than never users (95% CI, 0.1–2.0). When AST was replaced by ALT in the multivariable model, the estimate (95% CI) for ALT was 0.28 (95% CI, 0.1–0.4) per 10 IU/L; p < 0.001. Sex, age, race/ethnicity, BMI, marijuana use, coffee consumption, tea consumption, at-risk alcohol use, AST/ALT ratio, HIV stage, CD4 count, CD8 count, CD8%, lipodystrophy/lipoatrophy grade, HBV duration, HBeAg status, qHBeAg, qHBsAg, suppressed HBV DNA (<1000 IU/mL), and incomplete HBV DNA suppression (HBV DNA ≥1000 IU/mL and HIV RNA <20 copies/mL) were not significantly associated with HAI in univariate or multivariable analysis.
Table 3.
Unadjusted and adjusted associations between demographic, clinical, and serologic characteristics and Ishak Histologic Activity Index. Estimates are regression coefficients, representing the estimated difference in mean HAI between the comparison group and the reference group for categorical independent variables, and estimated per unit change for continuous independent variables
| Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|
| Characteristics | Comparison group | Estimate (95% CI) | Overall P value | Estimate (95% CI) | Overall P value |
| Discrete scales | |||||
| Sex (ref = male) | Female | −0.51 (−2.1, 1.08) | 0.53 | ||
| Race/Ethnicity (ref = Non-Hispanic Black) | Non-Hispanic White | −0.26 (−1.16, 0.64) | 0.43 | ||
| Other | −0.75 (−1.90, 0.40) | ||||
| Tobacco use (ref = never smoker) | Former | −0.21 (−1.30,0.89) | 0.057 | −0.01 (−1.01, 1.00) | 0.02 |
| Current | 0.85 (−0.18, 1.87) | 1.03 (0.11, 1.95) | |||
| Marijuana (ref = never user) | Regular | −0.68 (−1.63, 0.27) | 0.36 | ||
| Occasional | −0.32 (−1.49, 0.84) | ||||
| Coffee (cups per day, ref = none or occasional) | 3 or more | −0.79 (−1.97, 0.39) | 0.32 | ||
| 1–2 | −0.52 (−1.44, 0.4) | ||||
| Tea (cups per day, ref = none or occasional) | Regular (1 or more) | 0.35 (−0.69, 1.4) | 0.51 | ||
| At-risk alcoholuse (ref = no) | Yes | −0.64 (−2.34, 1.05) | 0.45 | ||
| History of diabetes (ref = no) | Yes | 2.55 (1.19, 3.90) | <0.001 | 2.10 (0.81, 3.40) | 0.002 |
| HIV Stage (ref = 0 or 1) | 2 or above | 0.16 (−0.81, 1.13) | 0.75 | ||
| Lipodystrophy/Lipoatrophy grade (ref = 0 or 1) | 2 or above | 0.21 (−1.09, 1.51) | 0.75 | ||
| Detectable HIV RNA (ref = no) | Yes | 1.06 (0.03, 2.09) | 0.04 | ||
| Above Normal ALT (ref = no) | Yes | 0.86 (0.06, 1.66) | 0.04 | ||
| HBeAg (ref = negative) | Positive | −0.22 (−1.10, 0.67) | 0.63 | ||
| Detectable HBV DNA (ref = no) | Yes | 0.92 (0.12, 1.71) | 0.02 | ||
| HBV DNA Not Suppressed (ref = no) | Yes | 0.92 (−0.05, 1.9) | 0.06 | ||
| Incomplete HBV DNA Suppression (ref = no) | Yes | 0.6 (−1.06, 2.26)0.48 | |||
| Continuous scales | |||||
| Age, per 5 years | −0.04 (−0.26, 0.19) | 0.75 | |||
| BMI (kg/m2) | 0.00 (−0.07, 0.07) | 0.99 | |||
| ALT (per 10IU/L) | 0.32 (0.20, 0.44) | <0.001 | a | ||
| AST (per 10 IU/L) | 0.42 (0.25, 0.58) | <0.001 | 0.49 (0.26, 0.73) | <0.001 | |
| AST-ALT rat | −0.75 (−1.75, 0.24) | 0.14 | |||
| Platelets (per 20,000 mm3) | −0.18 (−0.32, −0.03) | 0.016 | |||
| CD4 (per 100) | −0.14 (−0.29, 0.01) | 0.06 | |||
| CD4% | −0.06 (−0.1, −0.03) | <0.001 | |||
| CD8 (per 100) | 0.02 (−0.08, 0.12) | 0.66 | |||
| CD8% | −0.002 (−0.04, 0.03) | 0.92 | |||
| HBV duration, years | 0.02 (−0.03, 0.07) | 0.44 | |||
| ln HBeAg quantitative (in HBeAg+) | 0.37(−0.14, 0.89) | 0.15 | |||
| log10 DNA (IU/mL) | 0.34 (0.11, 0.58) | 0.005 | |||
| log10 HBsAg Quant | −0.01 (−0.41, 0.40) | 0.98 | |||
The multivariable model selected AST, history of diabetes, and tobacco. When AST was replaced by ALT, the estimate (95% CI) was 0.28 (0.13, 0.43) per 10 IU/L of ALT with a P value<0.001; the model estimates for tobacco and diabetes remained similar to those with AST in the model
Table 4 shows unadjusted and adjusted associations between demographic, clinical, and serologic characteristics with advanced fibrosis. On univariate analysis, non-Hispanic black race, higher ALT, higher AST, lower platelets, higher APRI and FIB-4 were significantly associated with advanced fibrosis (p < 0.05). On multivariable analysis, race/ethnicity was no longer significantly associated with advanced fibrosis, whereas ALT (p = 0.03) and platelets (p = 0.02) were. Each 10 unit higher ALT was independently associated with 19% greater odds of advanced fibrosis (OR 1.19 per 10 U/L; 95% CI 1.01–1.41), and for each 20,000 mm3 higher platelets, the odds of advanced fibrosis were 19% lower (OR 0.81 per 20000 mm3; 95% CI, 0.67–0.97). In alternative models starting with either APRI or FIB-4, no other variables were independently related to advanced fibrosis. After adjusting for other variables, HBV DNA stratum (undetectable, detectable but <1000 IU/mL, or ≥1000 IU/mL at any level) was not associated with hepatic fibrosis.
Table 4.
Unadjusted and adjusted associations between demographic, clinical, and serologic characteristics and advanced fibrosis (Ishak 3–6 vs. 0–2). The odds ratio (OR) represents advanced fibrosis for the comparison group vs. the reference group for discrete independent variables. The OR per unit (unless otherwise stated) shows an increase in the continuous independent variable
| Characteristicsa | Unadjusted | Adjusted** | |||
|---|---|---|---|---|---|
| Discrete scales | Comparison group | OR (95% CI) | Overall P value | OR (95% CI) | Overall P value |
| Race/Ethnicity (ref = Non-Hispanic Black) | Non-Hispanic White | 4.26 (1.58, 11.5) | 0.02 | ||
| Other | 1.79 (0.47, 6.81) | ||||
| Tobacco use (ref = never smoker) | Former | 1.40 (0.41, 4.80) | 0.83 | ||
| Current | 1.40 (0.44, 4.47) | ||||
| Marijuana (ref = never user) | Regular | 0.42 (0.14, 1.27) | 0.13 | ||
| Occasional | 0.28 (0.06, 1.38) | ||||
| Coffee (cups per day, ref = none or occasional) | 3 or more | 0.56 (0.14, 2.24) | 0.71 | ||
| 1–2 | 0.90 (0.34, 2.36) | ||||
| Tea (cups per day, ref = none or occasional) | Regular (1 or more) | 1.37 (0.47, 4.02) | 0.56 | ||
| History of diabetes (ref = no) | Yes | 1.43 (0.34, 5.95) | 0.62 | ||
| HIV stage (ref = 0 or 1) | 2 or above | 0.96 (0.32, 2.87) | 0.93 | ||
| Lipodystrophy/lipoatrophy grade (ref = 0 or 1) | 2 or above | 2.32 (0.68, 7.93) | 0.18 | ||
| Detectable HIV RNA (ref = no) | Yes | 0.91 (0.30, 2.78) | 0.86 | ||
| Above normal ALT (ref = no) | Yes | 1.39 (0.58, 3.30) | 0.46 | ||
| HBeAg (ref = negative) | Positive | 0.56 (0.22, 1.41) | 0.22 | ||
| Detectable HBV DNA (ref = no) | Yes | 0.66 (0.26, 1.66) | 0.38 | ||
| HBV DNA not suppressed (ref = no) | Yes | 0.90 (0.30, 2.73) | 0.85 | ||
| Incomplete HBV DNA suppression (ref = no) | Yes | 0.52 (0.06, 4.64) | 0.55 | ||
| Continuous scales | |||||
| Age, per 5 years | 1.18 (0.91, 1.51) | 0.21 | |||
| BMI (kg/m2) | 1.02 (0.95, 1.10) | 0.53 | |||
| ALT (per 10 IU/L) | 1.17 (1.02, 1.35) | 0.03 | 1.19 (1.01, 1.41) | 0.03 | |
| AST (per 10 IU/L) | 1.36 (1.05, 1.77) | 0.02 | b | ||
| AST-ALT ratio | 0.62 (0.19, 2.04) | 0.43 | |||
| Platelets (per 20,000 mm3) | 0.79 (0.65, 0.95) | 0.01 | 0.81 (0.67, 0.97) | 0.02 | |
| APRI (per 0.1) | 1.32 (1.11, 1.55) | 0.001 | |||
| FIB-4 (per 1) | 2.37 (1.32, 4.26) | 0.004 | |||
| CD4 (per 100) | 0.97 (0.82, 1.15) | 0.74 | |||
| CD4% | 0.995 (0.96, 1.04) | 0.81 | |||
| CD8 (per 100) | 0.99 (0.87, 1.13) | 0.90 | |||
| CD8% | 0.998 (0.95, 1.05) | 0.93 | |||
| HBV duration, years | 1.02 (0.97, 1.08) | 0.38 | |||
| ln HBeAg Quant (in HBeAg+) | 0.71 (0.38, 1.33) | 0.29 | |||
| log10 DNA IU/mL | 1.08 (0.85, 1.37) | 0.52 | |||
| log10 HBsAg Quant | 0.80(0.54, 1.20) | 0.28 | |||
Because of the low frequencies or absence of advanced fibrosis in females and in persons with at-risk alcohol consumptions, we could not evaluate respective associations
The multivariable model selected ALT and platelet counts. If we replaced ALT with AST, the OR (95% CI) for AST in the multivariable model was 1.34 (1.02, 1.77) per 10IU/L with a P value of 0.04 with similar OR and P values for platelet counts
DISCUSSION
Coinfection of HBV with HIV has been associated with a markedly increased risk of liver-related mortality compared to HIV infection alone (22). HIV treatment guidelines recommend that all HIV-infected persons with active HBV infection (HBsAg+) undergo treatment of both infections with cART including tenofovir (2-4,10,11). However, the impact of these potent, dually active antiviral regimens on HBV-related liver disease is incompletely understood because liver biopsy is rarely performed in HBV–HIV patients (12). In this context, our data provides novel and clinically important findings regarding the spectrum of liver disease in persons living with HIV–HBV coinfection.
In our cohort, the magnitude of liver inflammation was mild, consistent with suppressed HBV replication. Importantly however, the degree of hepatic fibrosis was unexpectedly high with over a third (37%) of participants with significant fibrosis (Ishak 2–6) and 24% with advanced fibrosis (i.e., bridging fibrosis or cirrhosis) despite the majority having suppressed HIV and HBV at the time of biopsy. This finding compares to the 2003 report from Lacombe and colleagues who reported histologic findings on 104 HBV–HIV patients and found F2-4 fibrosis in 67% (23). A separate study from France in 59 HBV–HIV patients (33 of whom were on 3TC or FTC combined with TDF), found that 40% had inflammation (A1–A3), 34% with advanced fibrosis, and 10% had >10% steatosis (24). However, our findings were similar to a more recent study using vibration controlled transient elas-trography in a cohort of 92 HIV–HBV subjects on cART (12). The slightly lower proportion of advanced fibrosis in our cohort may reflect differences in demographics, duration of infection, duration of anti-HBV therapy, and/or duration of HBV prior to cART containing TDF.
Multivariate analysis revealed that higher levels of liver enzymes (AST or ALT) and lower platelets were associated with advanced fibrosis and are likely to reflect ongoing low level inflammatory activity, possibly related to low level intrahepatic HBV replication and transcription. Similarly, higher APRI and FIB-4 reflected the presence of advanced fibrosis. However, our observation that few patients had high APRI or FIB-4 most likely reflects the high proportion with normal ALT due to HBV suppression with cART. The observation of more advanced fibrosis also raises the possibility that HIV coinfection itself alters the intrahepatic milieu to promote fibrogenesis, as has been described for other forms of liver disease (25-28). These include increases in TGF-β production and signaling, increased oxidative stress, and increased hepatocyte apoptosis (26). Concurrent liver diseases, such as hepatic steatosis and steatohepatitis, may also have a role in the pathogenesis of fibrosis in patients with HIV/HBV coinfection. We found that nearly 30% of participants had evidence of steatosis involving more than 5% of hepatocytes and 10% had histologic features of steatohepatitis. To further explore the factors associated with steatosis and elevated liver enzymes despite HBV suppression in our cohort, additional testing for insulin resistance and dyslipidemia by a central laboratory are planned. However, our findings suggest that non-alcoholic fatty liver disease may contribute to liver disease in HIV-infected patients similar to those with HBV mono-infection (29). Although self-reported past-year at-risk alcohol use was uncommon and not related to hepatic inflammation or advanced fibrosis in our sample, may have been underreported or not reflective of longer-term alcohol use. Thus, alcohol use may also play a role. Taken together, these findings underscore the silent, potentially insidious nature of HBV related liver disease in HIV infected persons, even those with ostensible viral control. Our data suggest that enhanced vigilance to exclude advanced fibrosis should be performed in all persons with HBV and HIV coinfection.
We observed that incomplete HBV suppression was associated with liver inflammation and, if fibrogenesis is driven by incomplete control of HBV infection, the addition of other antiviral drugs may be indicated for persons with incomplete HBV suppression. However, it is uncertain that the addition of entecavir to tenofovir would be effective since most HIV-infected patients are treated with tenofovir in combination with lamivudine or emtricitabine which, like entecavir, are nucleoside(tide)s that target HBV polymerase. Data is also needed on the role of tenofovir alafenamide (TAF) since participants in this cohort were largely treated with TDF. Interestingly, in studies directly comparing these two forms of tenofovir, patients treated with TAF were more likely to normalize serum ALT levels (30,31). While modifications or intensification of nucleos(t)ide analog treatment warrants further study in this population, our findings highlight the need for novel agents and approaches to control HBV infection, including those that lead to functional cures (e.g., anti-HBs seroconversion).
Prior to effective cART, Colin and colleagues compared HBV DNA and liver histology in 67 anti-HIV negative HBV+ patients with 65 HBV-HIV co-infected patients and found that coinfected patients had lower ALT (103 vs. 188 U/L; p = 0.0001), higher HBV DNA (p = 0.01), and were more likely to have cirrhosis (28% vs. 13%; p = 0.04) despite similar inflammation scores (32). In contrast to the study by Colin (32), our cohort included patients on established cART. More recently, Lacombe and colleagues identified HBV genotype G (OR 12.6), use of efavirenz (OR 3.55), and HIV duration >9.5 years (OR 3.86) as independent predictors of significant fibrosis; use of “anti-HBV drugs” (3TC, TDF, or interferon) was not related (23). Associations with HBV DNA or HBV e antigen/antibody were not evaluated. Because most of our cohort had suppressed HBV DNA, we were not able to confirm the association of HBV genotype with fibrosis. A later report from the same French group of 134 HBV-HIV co-infected patients found HCV and HDV coinfections, but not use of 3TC or TDF, to be associated with advanced fibrosis (33). We excluded those with active HCV and did not have enough participants with HDV to evaluate associations with this co-infections.
These studies highlight the limited data on the histologic spectrum of liver disease in HBV–HIV patients treated with long-term TDF (34,35) and lack of data from North America, especially in the era of cART (36,37). One study from France suggested that HBV viremia was undetectable in the majority (71% of 205) on cART (30) while a more recent studies from the Netherlands (38) and Spain (12) showed 92 and 89% had undetectable HBV DNA after several years of cART containing anti-HBV therapy. In our study, 56% had detectable HBV DNA while HIV RNA was below detection (local laboratory) in 75%. In those with detectable HIV RNA, proportion with levels >10,000 copies/ml was 23%, suggesting that non-compliance with cART is only partially responsible for incomplete HBV suppression. In a longitudinal study of 165 coinfected patients starting cART, HBV DNA was detected in 21% of subjects despite reporting >95% adherence (39). To address the role of cART compliance on HBV suppression, we identified 7% as having incomplete suppression based on our predefined criteria of an undetectable HIV RNA in the setting of a detectable HBV DNA >1000 IU/L. Interestingly, after adjusting for other variables, HBV DNA stratum (undetectable, detectable but <1000 IU/mL, or ≥1000 IU/mL at any level) was not associated with hepatic fibrosis. Prior studies of HBV mutations in untreated HBV-HIV patients identified similar BCP mutations A1762T and G1764A compared to HBV mono-infected patients when adjusted for HBV genotype (40). Although we did not analyze BCP mutations by HBV genotype, the prevalence of these mutations were similar in our cohort of treated patients with detectable HBV DNA suggesting other factors associated with incomplete suppression. HBV deep sequencing analysis is underway to help elucidate viral resistant mutations to tenofovir in these patients.
The strengths of our study are the sample size and the central reading of liver biopsies by the HBRN pathology committee. Conversely, potential limitations of our study include the cross-sectional design and the self-selection of patients who were willing to undergo liver biopsy who therefore may not reflect all of adults with HBV–HIV coinfection. In addition, similar to other studies (12), we do not have baseline data prior to cART and could not account for duration of infections, nadir CD4 counts, impact or duration of anti-HBV therapy alone or as part of prior cART, or timing of prior HBeAg seroconversion as many patients received care by other providers at other sites prior to enrollment. Incomplete data on cART exposure on all patients, use of older HIV medications (such as nucleoside reverse transciptase inhibitors) or HIV itself that might have accelerate liver disease, and changes in cART exposure over time may have impacted the natural history of HBV. Furthermore, the time from liver biopsy and baseline data collection may have been up to 52 weeks. However, in our experience, laboratory tests and liver histology do not significantly change over that interval. As with any liver biopsy study, there may have been sampling error. Lastly, we were not able to compare liver histology in our HBV/HIV cohort to those with HBV alone on tenofovir to determine if those with HBV/ HIV have worse liver disease outcomes. Notwithstanding these limitations, our study remains one of the largest on liver histology in this understudied population.
In summary, in this North American cohort of HBV–HIV coinfected subjects on dually active cART, a comprehensive histologic evaluation reveals that one third have significant fibrosis with low rates of cirrhosis despite low liver enzymes and HBV viral suppression with excellent control of both infections. Additional analyses of this cohort are underway to better define histologic progression and/or regression associated with cART that includes tenofovir (41) by paired liver biopsy to determine if factors other than HBV suppression, such as specific components of cART, identify predictors of steatosis and to determine non-invasive assessments to predict which patients might need to undergo liver biopsy to identify significant fibrosis. Until then, HBV–HIV patients require close monitoring for disease severity.
Study Highlights.
WHAT IS KNOWN
Chronic hepatitis B virus (HBV) is common in those with HIV.
Most, if not all, coinfected patients are on combination antiviral therapy that includes treatment for HBV.
As such, liver biopsy is rare performed in these patients.
WHAT IS NEW HERE
Despite HBV viral suppression, significant fibrosis was common.
Hepatic fibrosis was associated with higher liver enzymes and lower platelets.
Hepatic fibrosis was not related to HBV suppression, HBeag status, or quantified HBsag levels.
ACKNOWLEDGEMENTS
We would like to thank our patients for their participation, the HBRN Steering Committee for their guidance, Roche Diagnostics and Roche Molecular for supplying test kits, and NIDDK for funding this R01. This research was also supported in part by the Intramural Research Program of the NIH, National Cancer Institute. In addition to the authors, the HBV–HIV Cohort Study of the HBRN would like to acknowledge the contributions of the following: Massachusetts General Hospital: Nifasha Rusibamayila, BA, Cara Foley, BA, Jenna Gustafson, MS, Atul Bhan, MD (Boston, MA); Johns Hopkins University: Kathleen Ward, MSPH, CHES (Baltimore, MD); Washington University School of Medicine: Rachel Presti, MD, PhD, Lisa Kessels, RN, BSN, Warren Seyfried, PhD, Michael K. Klebert, Ph.D, R.N, ANP-BC (St. Louis, MO); University Health Network: Bryan Boyachuk, RN, CCRP (Toronto, Ontario); University of California at San Francisco: Norah Terrault, MD, Annie Luetkemeyer, MD, Rageshree Ramachandran, MD, PhD, Anna Trujillo, BS, Claudia Ayala, MS, Ivy Lau, BS, Ashley Shobe, MS (San Francisco, CA); University of Texas Southwestern: Minerva Santos, Kranthi Vysyaraju, MS, Tianna Petersen, MS (Dallas, TX); Virginia Commonwealth University: Paula Smith, RN, BSN, Charlotte Hofmann, RN, Michael Idowu, MD (Richmond, VA); Liver Diseases Branch, NIDDK, NIH: Vanessa Haynes-Williams, RN, PhD, Nancy E. Fryzek, BSN, MBA, Elenita Rivera, BSN, Wen-Chun A. Huang, BS, BSN, Nevitt Morris, BS, BSN, Varun Takyar, MD, Chunwei W. Lai, MD, PhD (Bethesda, MD); Liver Disease Research Branch, NIDDK, NIH: Jay H. Hoofnagle, MD, Averell H. Sherker, MD, Edward Doo, MD (Bethesda, MD); University of Pittsburgh Graduate School of Public Health Data Coordinating Center: Steven Belle, PhD, MScHyg, Michelle Danielson, PhD, Tamara Haller, Stephanie Kelley, MS, Sharon Lawlor, MBA, Melissa Weiner, MPH (Pittsburgh, PA).
Financial support: This study was funded by NIDDK (R01-DK94818) as an ancillary study of the Hepatitis B Research Network to Dr. Richard K. Sterling.
APPENDIX: INCLUSION AND EXCLUSION CRITERIA
Inclusion criteria
≥18 years of age;
serologic evidence of HIV infection by HIV antibody positivity or history of positive HIV-RNA prior to screening;
serologic evidence of chronic hepatitis B infection by HBsAg positivity;
currently receiving any type of antiretroviral therapy for HBV or HIV; and
willingness to provide informed consent.
Exclusion criteria
estimated life expectancy of <1 year based on clinical judgment of the investigator;
history of hepatic decompensation based on clinical or laboratory criteria;
hepatocellular carcinoma (HCC);
HCV RNA positive within 6 months prior to the baseline biopsy;
history of solid organ or bone marrow transplantation;
pregnant women;
medical or social condition which, in the opinion of the study physician, would make the patient unsuitable for the study or interfere with or prevent follow-up per protocol;
unable or unwilling to return for follow-up visits;
contraindications to liver biopsy.
Footnotes
Conflicts of interest: Richard K. Sterling: Gilead, AbbVie, Merck, Roche, Pfizer, and Baxter. Abdus S. Wahed: none. Wendy C. King: none. David K. Kleiner: none. Mandana Khalili: Gilead, Intercept Pharmaceuticals, and AbbVie. Mark Sulkowski: Gilead (consultant, research support). Raymond T. Chung: Gilead, BMS, Janssen, AbbVie, Boehringer, and Merck. Mamta Jain: Gilead, Janssen, Merck, and GSK. Mauricio Lisker-Melman: Abbvie, Gilead, Merck, and Simply Speaking. David K. Wong: Gilead, BMS, Vertex, and Boehringer. Marc Ghany: none
REFERENCES
- 1.Sorrell MF, Belongia EA, Costa J, et al. National Institutes of Health Consensus Development Conference Statement: management of hepatitis B. Ann Intern Med. 2009;150:104–10. [DOI] [PubMed] [Google Scholar]
- 2.Benhamou Y Hepatitis B in the HIV-coinfected patient. J Acquir Immune Defic Syndr. 2007;45(Suppl 2):S57–65. discussion S66-7 [DOI] [PubMed] [Google Scholar]
- 3.Soriano V, Puoti M, Peters M, et al. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV-Hepatitis B Virus International Panel. AIDS. 2008;22:1399–410. [DOI] [PubMed] [Google Scholar]
- 4.Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol. 2008;48:353–67. [DOI] [PubMed] [Google Scholar]
- 5.Ockenga J, Tillmann HL, Trautwein C, Stoll M, Manns MP, Schmidt RE. Hepatitis B and C in HIV-infected patients. Prevalence and prognostic value. J Hepatol. 1997;27:18–24. [DOI] [PubMed] [Google Scholar]
- 6.Thio CL. Hepatitis B in the human immunodeficiency virus-infected patient: epidemiology, natural history, and treatment. Semin Liver Dis. 2003;23:125–36. [DOI] [PubMed] [Google Scholar]
- 7.Gaglio PJ, Sterling R, Daniels E, Tedaldi E. Hepatitis B virus and HIV coinfection: results of a survey on treatment practices and recommendations for therapy. Clin Infect Dis. 2007;45:618–23. [DOI] [PubMed] [Google Scholar]
- 8.Ioannou GN. Hepatitis B. virus in the United States: infection, exposure, and immunity rates in a nationally representative survey. Ann Intern Med. 2011;154:319–28. [DOI] [PubMed] [Google Scholar]
- 9.Smith C Factors associated with specific causes of death amongst HIVpositive individuals in the D:A:D Study. AIDS. 2010;24:1537–48. [DOI] [PubMed] [Google Scholar]
- 10.Rockstroh JK, Bhagani S, Benhamou Y, et al. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. HIV Med. 2008;9:82–8. [DOI] [PubMed] [Google Scholar]
- 11.Iser DM, Sasadeusz JJ. Current treatment of HIV/hepatitis B virus coinfection. J Gastroenterol Hepatol. 2008;23:699–706. [DOI] [PubMed] [Google Scholar]
- 12.Martin-Carbonero L, Teixeira T, Poveda E, et al. Clinical and virological outcomes in HIV-infected pateints with chronic hepatitis B on long-term nucleos(t)ide analogues. AIDS. 2011;25:73–79. [DOI] [PubMed] [Google Scholar]
- 13.Ghany MG, Perrillo R, Li R, et al. Research Network; Hepatitis B Research Network. Characteristics of adults in the hepatitis B research network in North America reflect their country of origin and hepatitis B virus genotype. Clin Gastroenterol Hepatol. 2015;13:183–92. [Epub ahead of print July 8, 2014.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423–32. [DOI] [PubMed] [Google Scholar]
- 15.Carr A, Law M. Objective lipodystrophy severity grading scale derived from the lipodystrophy case definition score. J Acquir Immune Defic Syndr. 2003;33:571–6. [DOI] [PubMed] [Google Scholar]
- 16.Prati D, Taioli E, Zanella A,et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. [DOI] [PubMed] [Google Scholar]
- 17.WHO. Report of the consensus meeting on WHO antiretroviral therapy guidelines for adults and adolescents 2009. Available at: http://www.who.int/hiv/topics/treatment/art_consensus_meeting_091016.pdf.
- 18.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–9. [DOI] [PubMed] [Google Scholar]
- 19.Lok AS, Everhart JE, Chung RT, et al. Hepatic steatosis in hepatitis C: comparison of diabetic and nondiabetic patients in the hepatitis C antiviral long-term treatment against cirrhosis trial. Clin Gastroenterol Hepatol. 2007;5:245–54. [DOI] [PubMed] [Google Scholar]
- 20.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. [DOI] [PubMed] [Google Scholar]
- 21.Sterling RK, Lissen E, Clumeck N. et al. APRICOT Clinical Investigators Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- 22.Klein MB, Althoff KN, Jing Y, et al. North American AIDS Cohort Collaboration on Research and Design of IeDEA; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Risk of end-stage liver disease in HIV-Viral hepatitis coinfected persons in North America from the early to modern antiretroviral therapy eras. Clin Infect Dis. 2016;63:1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lacombe K, Massari V, Girard PM, et al. Major role of hepatitis B genotypes in liver fibrosis during coinfection with HIV. AIDS. 2006;20:419–27. [DOI] [PubMed] [Google Scholar]
- 24.Miailhes P, Pradat P, Chevallier M, et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBVcoinfected patients. J Viral Hepat. 2011;18:61–9. [DOI] [PubMed] [Google Scholar]
- 25.Lin W, Wu G, Li S, et al. HIV and HCV cooperatively promote hepatic fibrogenesis via induction of reactive oxygen species and NFkappaB. J Biol Chem. 2011;286:2665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang JY, Shao RX, Lin W, et al. HIV infection increases HCV-induced hepatocyte apoptosis. J Hepatol. 2011;54:612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin W, Weinberg EM, Tai AW, et al. HIV increases HCV replication in a TGF-beta1-dependent manner. Gastroenterology. 2008;134:803–11. [DOI] [PubMed] [Google Scholar]
- 28.Chen JY, Feeney ER, Chung RT. HCV and HIV co-infection: mechanisms and management. Nat Rev Gastroenterol Hepatol. 2014;11:362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobson IM, Washington MK, Buti M, et al. Factors associated with persistent increase in level of alanine aminotransferase in patients with chronic hepatitis B receiving oral antiviral therapy. Clin Gastroenterol Hepatol. 2017;15:1087–94. [DOI] [PubMed] [Google Scholar]
- 30.Buti M, Gane E, Seto WK. et al. GS-US-320-0108 Investigators. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:196–206. [DOI] [PubMed] [Google Scholar]
- 31.Chan HL, Fung S, Seto WK. et al. GS-US-320-0110 Investigators Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:185–95. [DOI] [PubMed] [Google Scholar]
- 32.Colin JF, Cazals-Hatem D, Loriot MA, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology. 1999;29:1306–10. [DOI] [PubMed] [Google Scholar]
- 33.Lacombe K, Boyd A, Desvarieux M, et al. Impact of chronic hepatitis C and/or D on liver fibrosis severity in patients co-infected with HIV and hepatitis B virus. AIDS. 2007;21:2546–9. [DOI] [PubMed] [Google Scholar]
- 34.Piroth L, Pol S, Lacombe K, et al. Management and treatment of chronic hepatitis B virus infection in HIV positive and negative patients: The EPIB 2008 study. J Hepatol. 2010;53:1006–12. [DOI] [PubMed] [Google Scholar]
- 35.Sonderup MW, Wainwright H, Hall P. et al. A clinicopathological cohort study of liver pathology in 301 patients with human immunodeficiency virus/acquired immune deficiency syndrome. Hepatology. 2015;61: 1721–9. [DOI] [PubMed] [Google Scholar]
- 36.Boyd A, Gozlan J, Maylin S, et al. Persistent viremia in human immunodeficiency virus/hepatitis B coinfected patients undergoing long-term tenofovir: virological and clinical implications. Hepatology. 2014;60: 497–507. [DOI] [PubMed] [Google Scholar]
- 37.Lada O, Gervais A, Branger M, et al. Long-term outcome of primary nonresponders to tenofovir therapy in HIV/HBV-co-infected patients: impact of HBV genotype G. Liver Int. 2012;32:93–101. [DOI] [PubMed] [Google Scholar]
- 38.de Vries-Sluijs TE, Reijnders JG, Hansen BE, et al. Long-term therapy with tenofovir is effective for patients co-infected with human immunodeficiency virus and hepatitis B virus. Gastroenterology. 2010; 139:1934–41. [DOI] [PubMed] [Google Scholar]
- 39.Matthews GV, Seaberg EC, Avihingsanon A, et al. Patterns and causes of suboptimal response to tenofovir-based therapy in individuals coinfected with HIV and hepatitis B virus. Clin Infect Dis. 2013;56:e87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Audsley J, Littlejohn M, Yuen L, et al. HBV mutations in untreated HIV-HBV co-infection using genomic length sequencing. Virology. 2010;405: 539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–75. [DOI] [PubMed] [Google Scholar]