Abstract
Since 1976, melanoma-prone families have been followed at the National Cancer Institute to identify etiologic factors for melanoma. We compared risks of melanoma and other cancers in 1,226 members of 56 families followed for up to 4 decades with population rates in the Surveillance, Epidemiology, and End Results program. All families were tested for mutations in CDKN2A and CDK4; 29 were mutation-positive and 27 mutation-negative. We compared rates of invasive melanomas, both first and second, by family mutation status, with Surveillance, Epidemiology, and End Results program. Comparing three calendar periods of the study, risk of first primary melanoma decreased slightly. Risks of melanoma after first examination, however, were approximately one-third the risks prior to the first examination in both mutation-positive and mutation-negative families. Among patients with melanoma, risk of a second melanoma was increased 10-fold in all families; risk was somewhat higher in mutation-positive families. Risks of other second cancers were increased only for pancreatic cancer after melanoma in mutation-positive families. Over 4 decades, prospective risk of melanoma has decreased substantially in both mutation-positive and mutation-negative families, when melanoma has greatly increased in the general population. Trial Registration: NCI 02-C-0211, (ClinicalTrials.gov ID .
Introduction
Familial aggregation of melanoma was initially reported in 1820 (Norris, 1820). Since 1976, families at increased risk for melanoma have been prospectively followed at the National Cancer Institute to study melanoma etiology (Goldstein et al., 1994, Goldstein et al., 2000, Reimer et al., 1978, Tucker et al., 1993). Primary goals of this observational study were to identify familial risk factors for melanoma. In this study, two high-risk susceptibility genes were identified, CDKN2A (Hussussian et al., 1994) and CDK4 (Goldstein et al., 2000, Zuo et al., 1996). Characterization of these genes and the search for other high-risk as well as lower-penetrance genes has been the major focus of the international Melanoma Genetics Consortium (GenoMEL; http://www.genomel.org). Previously, GenoMEL showed that penetrance of CDKN2A mutations in high-risk families varies by geography, with higher risks of melanoma in higher UV areas (Bishop et al., 2002), but among melanoma cases unselected for family history, penetrance did not differ by latitude (Cust et al., 2011). Percentage of melanoma-prone families with CDKN2A mutations also varies by geographic area, ranging from 20% in Australia to 45% in North America to 57% in Europe (Goldstein et al., 2007). Variations in pigmentation genes, particularly MC1R, also increase risk of melanoma in CDKN2A mutation carriers (Demenais et al., 2010, Goldstein et al., 2005). Additionally, family studies have revealed increased risk of pancreatic cancer and tobacco-related cancers associated with mutations in CDKN2A (de Snoo et al., 2008, Ghiorzio et al., 1999, Goldstein et al., 1995, Goldstein et al., 2006, Helgadottir et al., 2014, Vasen et al., 2000).
We assessed risks for melanoma and other cancers over 4 decades in American families at increased risk for melanoma. The current study expands previous evaluations conducted in more limited sample sets over shorter time intervals (Goldstein et al., 1994, Goldstein et al., 2004, Tucker et al., 1993).
Results
Demographic characteristics of the study population are shown in Table 1. There were no substantive differences between earlier two-proband families versus later three-proband families. All risk analyses were conducted using data from 1,226 examined individuals, excluding probands. Females were slightly more likely to have been examined than males; family members of all ages participated, the greatest proportion were young adults. All families were predominantly Caucasian; all families had individuals with clinically diagnosed dysplastic nevi. A slightly higher proportion of earlier ascertained families had identified mutations. Age range at first melanoma diagnosis was similar in the two sets of families, but appeared slightly higher among those not examined (Table 1).
Table 1.
Description of study population
| Variable | 2 Probands | 3 Probands | All Families |
|---|---|---|---|
| Family accrual date | 1976–1989 | 1990–2009 | 1976–2009 |
| No. of families | 32 | 24 | 56 |
| No. of examined individuals1 | 867 | 452 | 1319 |
| Male, n (%) | 410 (47) | 208 (46) | 618 (47) |
| Female, n (%) | 457 (53) | 244 (54) | 701 (53) |
| No. of examined individuals excluding probands | 832 | 394 | 1226 |
| Age range, y, at initial exam1 | <1–94 | 4–81 | <1–94 |
| <10 y, n (%) | 74(8) | 29(6) | 103 (8) |
| 10–19 y, n (%) | 180 (21) | 93 (21) | 273 (21) |
| 20–49 y, n (%) | 451 (52) | 219 (48) | 670 (51) |
| 50+ y, n (%) | 162 (19) | 111 (25) | 273 (21) |
| Initial exam date of individual family members | 1976–2012 | 1992–2012 | 1976–2012 |
| Dysplastic nevus status1 | |||
| Indeterminate (age), n (%) | 235 (27) | 122 (27) | 357 (27) |
| No | 371 (43) | 197 (44) | 568 (43) |
| Yes | 261 (30) | 133 (29) | 394 (30) |
| Families witli identified mutations, n (%) | 18 (56) | 11 (46) | 29 (52) |
| Families without identified mutations, n (%) | 14 (44) | 13 (54) | 27 (48) |
| Age at first melanoma, y, mean (range) | |||
| Examined individuals | |||
| Proband | 36.7 (21–67) | 40.6 (15–74) | 39.1 (15–74) |
| Retrospective | 33.7 (12–76) | 39.5 (21–63) | 35.2 (12–76) |
| Prospective | 39.4 (10–95) | 35.6 (18–66) | 38.S (10–95) |
| Not examined individuals | |||
| Proband | 40.2 (19–71) | 44.2 (13–69) | 41.5 (13–71) |
| Retrospective | 37.0 (17–76) | 44.2 (25–68) | 39.1 (17–76) |
Includes examined probands; not all probands were alive at study entry of the families.
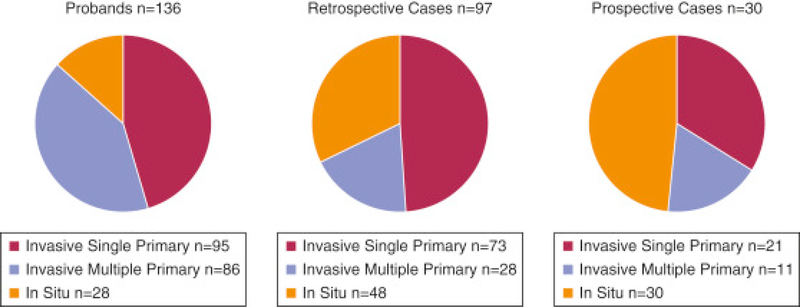
Total numbers of melanomas occurring in all 263 patients classified as probands (n = 136), retrospective (n = 97), or prospective (n = 30) cases appear in Figure 1. Distribution of invasive single, multiple primary, and in situ melanomas changed during the study, as reflected in proband, retrospective, and prospective cases (P < 0.0001). Of 136 probands, 36 (25 in 2+ and 11 in 3+ families) were deceased when their families were initially examined; 11 of these were diagnosed by metastases. Three deceased probands had multiple primary melanomas. Of 100 living probands, 7 were not examined because 4 were older than age 80 years, 1 was too ill with metastatic melanoma, and 2 were unable to be scheduled. Among these seven, three had histologic evidence of dysplastic nevi and three had multiple primary melanomas (one with both). Among the 93 examined probands, 35 developed 1−5 additional melanomas (n = 69) and 1−4 in situ melanomas (n = 28). Ten examined probands developed subsequent other cancers: two lung, two prostate and one each of pancreas, parotid, renal, colon, oropharynx, and neurofibrosarcoma.
Figure 1.
Invasive, multiple primary, and in situ melanomas in all cases. Total number of melanomas in probands (n = 136, left), retrospective cases (n = 97, middle), and prospective cases (n = 30, right). Each circle shows the distribution of invasive single primary, invasive multiple primary, and in situ melanomas for each case group, comprising all melanomas with tumor data available, including cases not clinically examined. Most cases were either probands (defining their families as eligible for study) or retrospective cases (individuals with first melanoma prior to study participation). There were many fewer prospective cases, individuals who developed their first melanoma after first study examination. The distribution of invasive single primary, invasive multiple primary, and in situ melanomas across the three case groups was significantly different (P < 0.0001).
Of 97 retrospective cases, 73 were examined and included in risk analyses. Twenty-four individuals were not examined, 20 of whom were deceased before family ascertainment. Five deceased were diagnosed by metastases, one had a sinus mucosal melanoma and three developed multiple primary melanomas. None of the deceased developed other cancers after melanoma. Two of the four not clinically examined declined examination; two were too ill with advanced melanoma.
We evaluated first invasive melanoma risk in examined non-probands by three calendar periods reflecting the history of the study (Table 2). Melanoma risk was somewhat higher in families with mutations in CDKN2A or CDK4 than in families without mutations in each period, but all risks were significantly elevated and confidence intervals (CIs) overlapped, particularly in the first calendar period. Risks in the latest calendar period appeared somewhat lower than the previous two periods, with no overlap in CIs for all families or mutation-positive families between the second and third calendar periods.
Table 2.
Prospective risk of first melanoma in examined individuals by calendar and study periods and family mutation status
| Family Type1 | Period | O | E | O/E (95% Cl) | Persons | P-Y |
|---|---|---|---|---|---|---|
| By calendar time | ||||||
| All | Before 1976 | 15 | 0.68 | 22.1 (12.4–36.4) | 885 | 22,137 |
| 1976–1989 | 36 | 1.17 | 30.8 (21.6–42.6) | 1,106 | 12,882 | |
| 1990–2014 | 44 | 3.39 | 13.0 (9.4–17.4) | 1,069 | 15,576 | |
| Mutation- | Before 1976 | 4 | 0.25 | 16.3 (4.4—41.7) | 270 | 7,360 |
| 1976–1989 | 6 | 0.39 | 15.3 (5.6–33.3) | 326 | 3,747 | |
| 1990–2014 | 9 | 1.15 | 7.8 (3.6–14.8) | 310 | 4,743 | |
| Mutation+ | Before 1976 | 11 | 0.43 | 25.4 (12.7–45.4) | 615 | 14,777 |
| 1976–1989 | 30 | 0.78 | 38.6 (26.0–55.1) | 780 | 9,135 | |
| 1990–2014 | 35 | 2.24 | 15.6 (10.9–21.8) | 759 | 10,832 | |
| By study period | ||||||
| All | Before Exam | 65 | 2.34 | 27.8 (21.5–35.5) | 1,226 | 39,715 |
| After Exam | 30 | 2.90 | 10.4 (7.0–14.8) | 707 | 10,879 | |
| Mutation- | Before Exam | 14 | 0.86 | 16.2 (8.9–27.2) | 369 | 12,817 |
| After Exam | 5 | 0.93 | 5.4(1.8–12.6) | 215 | 3,033 | |
| Mutation+ | Before Exam | 51 | 1.47 | 34.6 (25.8–45.5) | 857 | 26,899 |
| After Exam | 25 | 1.97 | 12.7 (8.2–18.7) | 492 | 7,846 | |
Abbreviations: Cl, confidence interval; E, expected number of individuals developing melanoma based on age-, sex-, and calendar year-specific population rates; O, observed number of individuals who developed melanoma; O/E, standardized incidence ratio; P-Y, person-years of observation.
Mutation- families do not have an identified mutation. Mutation+ families liave a mutation in CDKN2A or CDK4.
We also evaluated first invasive melanoma risk by study period (Table 2). We estimated first invasive melanoma risk in non-proband examined individuals before initial study examination, including melanomas diagnosed at examination and excised soon after. Prior to or at first study exam, 65 individuals had a first invasive melanoma (retrospective). After initial exam, 30 individuals developed their first melanoma (prospective). Risks of first melanoma were significantly elevated before and after first exam, but risks after exam were significantly lower in all families. Risks after initial exam in all families, families without mutations, and families with mutations were roughly one-third of the risks before exam. Of the 30 prospective melanoma patients, 4 are deceased, only 1 from melanoma.
Table 3 shows non-melanoma cancer risks in examined participants among individuals without melanoma (n = 1,131) and melanoma cases after first melanoma (n = 95). Among examined family members without melanoma, there was no significant excess in other first primary cancers (Table 3) or smoking-related cancers (n = 21; observed to expected ratio [O/E] = 0.89; 95% CI = 0.55−1.36). Among 95 examined individuals with first invasive melanoma (1,028 person-years), we assessed risk of second cancers with follow-up starting 1 month after first invasive melanoma and ending at second primary (melanoma or other cancer), death, or December 31, 2014, whichever was earliest. Risks of second melanomas were significantly elevated in all families (O/E = 10.7; 95% CI = 7.4−15.0), families without known mutations (O/E = 4.8; 95% CI = 1.3−12.4), and families with known mutations (O/E = 12.8; 95% CI = 8.6−18.2). These estimates do not, however, capture the full melanoma risk because second melanoma risk is the highest order for SEER population rates. Of 34 individuals developing a second primary melanoma, 18 developed only 2 invasive melanomas, 8 of whom also developed 12 (range 1−5) melanomas in situ. The other 16 individuals developed 77 (range 2−18) additional invasive melanomas; 8 of whom developed 34 (range 1−12) melanomas in situ. After second melanoma, six individuals developed subsequent non-melanoma cancers: one each of lung, colon, rectal, and breast and two pancreas cancers. These higher-order cancers were not included in either the second cancer or tobacco-related analyses. The only second cancer other than melanoma occurring in excess was pancreas in two individuals from mutation-positive families (Table 3). Risk of second tobacco-related cancer was elevated but did not reach statistical significance (observed = 5; O/E = 2.77; 95% CI = 0.90−6.46).
Table 3.
Risk of non-melanoma cancers in examined individuals
| Site | O | E | O/E (95% Cl) | Persons | Person-Years |
|---|---|---|---|---|---|
| After first melanoma | |||||
| AH sites (excluding skin) | 13 | 7.55 | 1.72 (0.92–2.95) | 95 | 1,459 |
| Esophagus | 1 | 0.04 | 24.65 (0.62–137.4) | 95 | 1,459 |
| Colon and rectum | 2 | 0.5S | 3.44 (0.42–12.4) | 95 | 1,459 |
| Pancreas | 2 | 0.16 | 2.29 (1.49–44.4) | 95 | 1,459 |
| Lung and bronchus | 2 | 0.78 | 2.57 (0.31–9.27) | 95 | 1,459 |
| Breast | 4 | 1.28 | 3.13 (0.85–8.01) | 95 | 1,459 |
| Prostate | 2 | 1.49 | 1.34 (0.16–4.85) | 95 | 1,459 |
| In individuals without melanoma | |||||
| All sites (excluding skin) | 57 | 92.21 | 0.62 (0.47–0.80) | 1,131 | 46,801 |
| Oral cavity and pharynx | 2 | 2.48 | 0.80 (0.10–2.91) | 1,131 | 46,801 |
| Small intestine | 1 | 0.33 | 3.07 (0.08–17.10) | 1,131 | 46,801 |
| Colon and rectum | 3 | 9.46 | 0.32 (0.07–0.93) | 1,131 | 46,801 |
| Pancreas | 2 | 1.97 | 1.01 (0.12–3.66) | 1,131 | 46,801 |
| Larynx | 1 | 0.85 | 1.18 (0.03–6.58) | 1,131 | 46,801 |
| Lung and bronchus | 12 | 11.47 | 1.05 (0.54–1.83) | 1,131 | 46,801 |
| Breast | 14 | 18.26 | 0.77 (0.42–1.29) | 1,131 | 46,801 |
| Female genital | 2 | 8.56 | 0.23 (0.03–0.S4) | 1,131 | 46,801 |
| Male genital | 7 | 10.22 | 0.6S (0.28–1.41) | 1,131 | 46,801 |
| Bladder | 2 | 3.73 | 0.54 (0.06–1.94) | 1,131 | 46,801 |
| Kidney and renal pelvis | 2 | 2.33 | 0.86 (0.10–3.09) | 1,131 | 46,801 |
| Brain and nervous system | 1 | 2.11 | 0.47 (0.01–2.64) | 1,131 | 46,801 |
| Endocrine system | 1 | 2.73 | 0.37 (0.01–2.04) | 1,131 | 46,801 |
| Hodgkin lymphoma | 1 | 1.31 | 0.76 (0.02–4.24) | 1,131 | 46,801 |
| Non-Hodgkin lymphoma | 2 | 3.90 | 0.51 (0.06–1.85) | 1,131 | 46,801 |
| Myeloma | 2 | 0.92 | 2.16 (0.26–7.82) | 1,131 | 46,801 |
| ALL | 1 | 0.71 | 1.41 (0.04–7.87) | 1,131 | 46,801 |
| CLL | 1 | 0.87 | 1.15 (0.03–6.44) | 1,131 | 46,801 |
Abbreviations: ALL, acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; E, expected; to note that many of the commercially available protein and glycan metabolic labels O, observed; O/E, standardized incidence ratio.
Figure 2 shows cumulative risks of first melanoma by age and family mutation status compared to SEER. Shapes of curves for the family sets were similar but differed substantially from that of SEER (log-rank P < 0.0001). Among families, risk in mutation-positive families was significantly different from risk in mutation-negative families (log-rank P = 0.0112). Specifically, mutation-positive families had younger age at onset and more affected individuals.
Figure 2.
Age, gender, and calendar-period specific melanoma incidence in Seer9 (1975–2013), mutation-positive families, and mutation-negative families. The numbers under the x-axis reflect the number of individuals in mutation-negative families in red and mutation-positive families in blue. The orange dashed line represents the age-specific first primary melanoma incidence in SEER; the red solid line represents the age-specific risk of first melanoma in the mutation-negative families; and the blue dashed line represents the age-specific incidence in mutation-positive families; overall log-rank P < 0.0001. The log-rank P value for the comparison of the families is 0.0112. The blue and red shading represent the 95% Hall-Wellner bands for the familial data. Seer, Surveillance, Epidemiology, and End Results program.
Discussion
In this family-based study spanning 4 decades, there has been a substantial decrease in prospective relative risk of melanomas, when melanoma risk in the general population has increased rapidly. This decrease occurred in both families with identified high-risk mutations and those without known mutations. These individuals were counseled to minimize UV exposure and to conduct self-exams monthly as part of their evaluation. Less UV exposure and surveillance of nevi for changes may have had a role in decreasing the risk of melanoma. During the study, as melanoma has increased in the United States, recognition of all thicknesses of melanoma by health care professionals has improved (Shaikh et al., 2015). With population rates increasing substantially, part of the decrease in relative risk in the families may be due to increased rates in the general population. However, the number of first invasive melanomas and multiple primary melanomas also decreased substantially during the study periods, with a concomitant greater proportion of in situ melanomas. These findings are consistent with decreased familial rates.
Families participating in this study are considered high risk and therefore not representative of the general population, but they do come from across the United States with widely varying ambient UV exposure. The marked decrease in risks of melanoma after first exam could be due to several factors, including participants’ increased knowledge of sun protective practices and risk factors for melanoma leading to decreased UV exposure; regular conduct of skin self-exams; recognition of early signs of melanoma; removal of nevi changing in a worrisome manner before they become melanoma; and regular professional skin exams. Anecdotally, most of the melanomas occurring after initial exam were first recognized by study participants while conducting self-exams. These familial data add to other observations that interventions such as education and surveillance of high-risk individuals may lead to earlier detection of melanoma (Moloney et al., 2014; Robinson et al, 2016). Some are even advocating for melanoma prevention and early detection within the general population (Tripp et al., 2016). Limited data suggest that screening within a health care system is feasible, resulting in earlier detection of melanoma (Ferris et al., 2017), but more research is needed.
Cumulative risks of first melanoma for both mutation-positive and mutation-negative families were significantly different from those in SEER during the same period. All families had earlier onset and substantially higher risk than the general population, with mutation-positive families having earliest onset and highest risk. These data suggest that all melanoma-prone families should receive regular surveillance. Similarly, risks of second melanomas were significantly elevated in all, mutation-positive, and mutation-negative families. Second melanoma risks in these families were, however, similar to general population risks in SEER from parallel periods (SEER O/E = 8.80; P < 0.05) (Curtis et al., 2005). This suggests that given susceptibility and exposure to develop one melanoma, individuals continue to be at risk and need surveillance, whether or not they carry a high-risk gene mutation.
For this study, families were classified as being mutation-positive or mutation-negative based on mutations in the melanoma susceptibility genes CDKN2A and CDK4. Although additional rare high-risk genes have been identified in small numbers of melanoma-prone families (Read et al., 2016, Robles-Espinoza et al., 2014, Shi et al., 2014), no mutations in these genes were present in the families. Whether risk in the mutation-negative families results from mutations in currently unidentified high-, intermediate-, or low-risk genes remains an area of active investigation. Multiple low-risk genetic loci have been associated with melanoma risk in the general population, and likely contribute to some familial clustering because many of the loci contain genes related to pigmentation and nevus count, both risk factors for melanoma in families (Law et al., 2015).
Although risks of first and second melanoma were significantly increased in families, risk evaluations could not capture the full extent of melanoma risk. Analyses were limited to second-order cancer rates, the highest order available from SEER. Thus, current analyses could not quantify risks for individuals with more than two melanomas. Among 95 patients with melanoma, 16 developed more than two invasive melanomas. Further, six patients with at least two melanomas developed non-melanoma cancers, including two with pancreatic cancer, which were not captured in risk evaluations. Even with these limitations, as reported previously (de Snoo et al., 2008, Ghiorzio et al., 1999, Goldstein et al., 1995, Vasen et al., 2000), we found increased risk of pancreatic cancer in CDKN2A mutation-positive families. Among family members without melanoma, observed number of pancreatic cancers was what would be expected in the general population. Although the risk of pancreatic cancer has been consistently associated with CDKN2A mutation status, recent data suggest that variations in other genes related to pancreatic cancer may also contribute to this risk (Yang et al., 2016). Unlike other reports (de Snoo et al., 2008, Helgadottir et al., 2014), we did not see a significant excess of smoking-related cancers.
The study has limitations. Although our goal is to decrease melanoma mortality, we did not have consistent cause of death information to adequately assess mortality. We were unable to estimate the risk of in situ melanomas compared to the general population during the study period because SEER did not collect the information. In the United States, we cannot link individuals to national population registries to identify cancer diagnoses; we relied on confirmed family-provided information. Further, the study used family-specific mutation status rather than individual mutation status. Within some mutation-positive families, 11 individuals without the family’s mutation developed melanoma. Additionally, as an observational study, the families received skin care from local physicians, either primary care providers or dermatologists. Although we included all willing individuals in our clinical examination, education, and follow-up, family members willing to participate may be biased. To be conservative, we excluded probands defining family eligibility from all risk analyses; thus, risks for earlier calendar period and retrospective risks might be underestimated. Finally, we did not have comprehensive data on adherence to skin care guidelines among participants to quantify contribution of education and reinforcement among participants.
In summary, in this family-based study spanning 4 decades, invasive melanoma risk, estimated in comparison to population rates, decreased in both mutation-positive and mutation-negative families, while melanoma incidence has increased greatly in the general population. Melanoma risks after the initial clinical examination were about one-third the risk before clinical examination in both mutation-positive and mutation-negative families, suggesting that participation in this observational study may have helped reduce melanoma risk in these families. The risks of in situ melanomas could not be quantified, but the proportion of in situ melanomas increased over time of study participation, perhaps because changing lesions were being recognized and removed earlier. Additional studies are warranted to explore these hypotheses and to better understand causes of melanoma and interventions to reduce both incidence and mortality.
Materials and Methods
Patient and Clinical Data Collection
Since April 1976, families at increased risk of melanoma have been recruited through observational clinical trials (currently NCI 02-C-0211, ClinicalTrials.gov ID NCT00040352). The study was reviewed by the National Cancer Institute Clinical Center Institutional Review Board and all participants signed informed consent. From 1976 to 1989, eligibility criteria included documented invasive cutaneous melanoma in two or more living family members. After 1990, this criterion was changed to three or more living members with documented melanoma because of increased melanoma incidence. Pathology documentation was sought for all melanomas. Occurrence of multiple primary melanomas or pancreatic cancer in families was not an ascertainment criterion. The earliest two (or three) individuals with documented invasive melanomas in a family were “probands,” establishing family eligibility for the study. The earliest documented melanomas in the probands were “index” melanomas. Melanomas (other than index melanomas) occurring before an individual’s study examination were categorized as “retrospective” and melanomas occurring after initial examination were “prospective.” Thus, an examined proband could potentially have index, retrospective, and prospective melanomas. Individuals with first invasive melanoma prior to initial exam were “retrospective cases” and those with first invasive melanoma after initial exam were “prospective cases.”
After confirming family eligibility, all identified living family members were invited to the National Institutes of Health for detailed skin examinations to document susceptibility phenotypes, including dysplastic nevi (Greene et al., 1985, Reimer et al., 1978). If lesions suspicious for melanoma were found, participants were referred to health care providers for excision and follow-up. Participants without health care providers were aided in obtaining care. Most participants were first examined before routine use of dermatoscopy. For diagnostic consistency over time, we used original clinical diagnostic criteria for study classification of nevus type (Greene et al., 1985). Examination included full-body photography for use in future skin examinations with close-up photography of nevi of interest, usually the most dysmorphic dysplastic nevi. Participants received copies of their photographs for their own and health care provider’s use. If families/individuals could not travel to the National Institutes of Health, we arranged field trips near individuals’ homes to collect biospecimens, conduct skin examinations, and photograph nevi. Willing participants provided blood primarily for genetic studies. During initial examination, participants were taught warning signs for melanoma, recognition of dysplastic nevi and changes worrisome for melanoma, regular (monthly) use of photographs for detection of new nevi or suspicious nevus changes, importance of UV protective practices, and need for routine (at least every 6 months) skin checks by health care providers.
The study was designed to follow participants long term; they were encouraged to notify the team when they had lesions suspicious for melanoma, pigmented lesion biopsies, or other cancers. Some participants had follow-up exams at National Institutes of Health, including photography. Updated health and/or exposure data have been requested at least every 5 years, usually more frequently. Participants were regularly updated with new information about melanoma etiology, prevention, and early detection. Distribution of study participants is shown in Table 1.
Diagnosis Verification
To verify pigmented lesion diagnoses, slides of excised lesions were obtained for expert dermatopathology review. For total count of melanomas per individual, in situ melanomas were included. For risk analyses, only first invasive melanomas were included, except for multiple primary analyses. For individuals without expert pathology review of melanoma diagnoses, local pathology reports, or if unavailable, hospital or medical records were used. Diagnoses of other cancers were confirmed by pathology reports or medical records. All reports/records were reviewed and classified by a medical oncologist (MAT).
Mutation detection and testing were conducted in multiple research laboratories using stored biospecimens. Only selected individuals within families (primarily melanoma cases and obligate carriers) were included in laboratory analyses. For risk analyses, families were classified as mutation-positive if two or more individuals with melanoma shared a pathogenic mutation in CDKN2A or CDK4. Risk analyses were conducted by family mutation status. Within some families with known mutations, 11 individuals (10 examined) without the family’s identified mutation developed melanoma.
Statistical Analyses
We estimated the risk of first primary melanoma in examined bloodline individuals, excluding probands from all risk analyses, combining two-case and three-case families. Time at risk for melanoma started at birth and ended at date of first invasive melanoma, date of last follow-up, date of death, or December 31, 2014, whichever occurred earliest. SEER program melanoma incidence rates by calendar year, age, and gender were applied to the cumulated person-years of observation to estimate expected number of first primary invasive melanomas. Observed number of melanomas was compared to expected number of melanomas based on cumulated person-years (O/E ratio) with 95% CIs calculated using SEER*STAT (National Cancer Institute, 2017). The 95% CIs not including 1.0 are considered statistically significant. For person-years cumulated prior to initiation of the SEER program in 1973, age- and gender-specific rates for the earliest period of SEER were used. For estimation of melanoma risk in relation to calendar period, we divided the time into three intervals: prior to study initiation in 1976, 1976−1989, and 1990+. For estimating melanoma risk in relation to study participation, we divided time into a retrospective period before the participant’s first clinical examination (including initial examination) and a prospective period after initial examination. For estimation of other (non-melanoma) first cancer expected rates, person-years were cumulated from birth to earliest of: date of cancer, last follow-up, death, or December 31, 2014. Age, gender, and calendar period rates for all other cancers were applied to cumulated person-years. Tobacco-related cancers were evaluated together and included oral cavity, pharynx, esophagus, pancreas, larynx, lung/bronchus, bladder, and kidney. For second cancer risks, SEER rules for definitions of second primary cancers were followed (Curtis et al., 2005). Rates of second cancers (including invasive melanoma) were applied to person-years of observation after first melanoma, ending with second cancer, date of last follow-up, date of death, or December 31, 2014, whichever occurred first. Risks of additional subsequent cancers could not be quantified because population rates are not available. Cumulative risks of first melanoma by age and family mutation status for the families and by age in the general population for SEER data were calculated with the Lifetest procedure in SAS.
Acknowledgments
This research was supported entirely by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics.
We would like to acknowledge the foresight and contributions of the founders of the familial melanoma study, Mark H. Greene and Wallace H. Clark Jr. We also thank the family members for their extraordinary generosity in working with us. In addition, we thank Laura Fontaine, Sally Koutsos, Mary King, and John Crawford for their many contributions to the study.
Abbreviations
- CI
confidence interval
- O/E
observed to expected ratio
- SEER
Surveillance, Epidemiology, and End Results program
Footnotes
Conflict of Interest
The authors state no conflicts of interest.
References
- Bishop et al., 2002 Bishop DT, Demenais F, Goldstein AM, Bergman W, Bishop JN, Bressac-de Paillerets B, et al. Geographical variation in the penetrance of CDKN2A mutations for melanoma J Natl Cancer Inst, 94 (2002), pp. 894–903 [DOI] [PubMed] [Google Scholar]
- Curtis et al., 2005 Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, et al. editors. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973– 2000. NIH Publ. No. 05–5302. National Cancer Institute Bethesda, MD; 2005. [Google Scholar]
- Cust et al., 2011 Cust A, Harland M, Makalic E, Schmidt D, Dowty JG, Aitken JF, et al. Melanoma risk for CDKN2A mutation carriers who are relatives of population-based case carriers in Australia and the UK J Med Genet, 48 (2011), pp. 266–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demenais et al., 2010 Demenais F, Mohamdi H, Chaudru V, Goldstein A, Newton JA Bishop DT Bishop, et al. Association of MCIR variants and host phenotypes with melanoma risk in CDKN2A mutation carriers: a GenoMEL study J Natl Cancer Inst, 102 (2010), pp. 1568–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Snoo et al., 2008 de Snoo FA, Bishop DT, Bergman W, van Leeuwen, van der Drift C, van Nieuwpoort FA, et al. Increased risk of cancer other than melanoma in CDKN2A founder mutation (P16- Leiden) positive families Clin Cancer Res, 14 (2008), pp. 7151–7157 [DOI] [PubMed] [Google Scholar]
- Ferris et al.,2017 Ferris LK, Saul MI, Lin Y, Ding F, Weinstock MA, Geller AC, et al. A large skin cancer screening quality initiative: Description and first-year outcomes JAMA Oncol, 3 (2017), pp. 1112–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiorzio et al., 1999 Ghiorzio P, Ciotti P, Mantelli M, Heoulaine A, Queirolo P, Rainero ML, et al. Characterization of Ligurian melanoma families and risk of occurrence of other neoplasia Int J Cancer, 83 (1999), pp. 441–448 [DOI] [PubMed] [Google Scholar]
- Goldstein et al, 2006 Goldstein A, Chan M, Harland M, Gillanders EM, Hayward NK, Avril M-F, et al. High-risk melanoma susceptibility genes and pancreatic cancer, neural system tumors, and uveal melanoma across GenoMEL Cancer Res, 66 (2006), pp. 9818–9828 [DOI] [PubMed] [Google Scholar]
- Goldstein et al, 2007 Goldstein AM, Chan M, Harland M, Hayward NK, Demenais F, Bishop DT, et al. Features associated with germline CDKN2A mutations: a GenoMEL study of melanoma-prone families from three continents J Med Genet, 44 (2007), pp. 99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein et al., 1994 Goldstein AM, Fraser MC, Clark WH Jr, Tucker MA Age at diagnosis and transmission of invasive melanoma in 23 cutaneous malignant melanoma/dysplastic nevi (CMM/DN) families J Natl Cancer Inst, 86 (1994), pp. 1385–1389 [DOI] [PubMed] [Google Scholar]
- Goldstein et al., 1995 Goldstein AM, Fraser MC, Struewing JP, Hussussian CH, Ranade K, Zametkin DP, et al. Increased risk of pancreatic cancer in melanoma-prone kindreds with pl6INK4 mutations N Engl J Med, 333 (1995), pp. 970–974 [DOI] [PubMed] [Google Scholar]
- Goldstein et al., 2005 Goldstein AM, Landi MT, Tsang S, Fraser MC, Munroe D, Tucker MA Association of MC1R variants and risk of melanoma in melanoma-prone families with CDKN2A mutations Cancer Epidemiol Biomarkers Prev, 14 (2005), pp. 2208–2212 [DOI] [PubMed] [Google Scholar]
- Goldstein et al., 2000 Goldstein AM, Struewing JP, Chidambaram A, Fraser MC, Tucker MA Genotype-phenotype relationships in American melanoma-prone families with CDKN2A and CDK4 mutations J Natl Cancer Inst, 92 (2000), pp. 1006–1010 [DOI] [PubMed] [Google Scholar]
- Goldstein et al, 2004 Goldstein AM, Struewing JP, Fraser MC, Smith MW, Tucker MA Prospective risk of cancer in CDKN2A germline mutation carriers J Med Genet, 41 (2004), pp. 421–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene et al., 1985 Greene MH, Clark WH Jr, Tucker MA, Elder DE, Kraemer KH, Guerry D IV, et al. Acquired precursors of cutaneous malignant melanoma. The familial dysplastic nevus syndrome N Engl J Med, 312 (1985), pp. 91–97 [DOI] [PubMed] [Google Scholar]
- Helgadottir et al., 2014 Helgadottir H, Hoiom V, Jonsson G, Tuominen R, Ingvar C, Borg A, et al. High risk of tobacco-related cancer in CDKN2A mutation-positive melanoma families ] Med Genet, 51 (2014), pp. 545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussussian et al., 1994 Hussussian CJ, Straewing JP, Goldstein AM, Higgins PA, Ally DS, Sheahan MD, et at Germline pl6 mutations in familial melanoma Nat Genet, 8 (1994), pp. 15–21 [DOI] [PubMed] [Google Scholar]
- Law et al., 2015Law MH, Bishop DT, Lee JE, et al. Genome-wide meta-analysis identifies five new susceptibility loci for cutaneous malignant melanoma Nat Genet, 47 (2015), pp. 987–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney et al., 2014 Moloney FJ, Guitera P, Coates E, Haass NK, Ho K, Khoury R, et al. Detection of primary melanoma in individuals at extreme risk: a prospective 5-year follow-up study JAMA Dermatol, 150 (2014), pp. 819–827 [DOI] [PubMed] [Google Scholar]
- National Cancer Institute, National Cancer Institute. Statistical tools and Data for Researchers. https://www.cancer.gov/Teseaxch/resources/statistical-tools. Accessed May 5,2017.
- Norris, 1820 Norris W A case of fungoid disease Edinburgh Surg J, 16 (1820), pp. 562–565 [PMC free article] [PubMed] [Google Scholar]
- Read et al., 2016 Read J, Wadt K, Hayward N Melanoma denetics J Med Genet, 53 (2016), pp. 1–14 [DOI] [PubMed] [Google Scholar]
- Reimer et al., 1978 Reimer RR, Clark WH Jr., Greene MH, Ainsworth AM, Fraumeni JR Jr Precursor lesions in familial melanoma. A new genetic predisposition syndrome JAMA, 239 (1978), pp. 744–746 [PubMed] [Google Scholar]
- Robinson et al., 2016 Robinson JK, Wayne JD, Martini MC, Hultgren BA, Mallett KA, Turrisi R Early detection of new melanomas by patients with melanoma and their partners using a structured skin self-examination skills training intervention: a randomized clinical trial JAMA Dermatol, 152 (2016), pp. 979–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholar Robles-Espinoza et al., 2014 Robles-Espinoza CD, Harland M, Ramsay AJ, Aoude LG, Quesada V, Ding Z, et al. POT1 loss-of-function variants predispose to familial melanoma Nat Genet, 46 (2014), pp. 478–4S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh et al, 2015 Shaikh WR, Dusza SW, Weinstock MA, Oliveria SA, Geller AC, Halpern AC Melanoma thickness and survival trends in the United States, 1989–2009] Natl Cancer Inst, 108 (1) (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi et al., 2014 Shi J, Yang XR, Ballew B, Rotunno M, Calista D, Fargnoli MC, et al. Rare missense variants in POT1 predispose to familial cutaneous melanoma Nat Genet, 46 (2014), pp. 482–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp et al., 2016 Tripp MK, Watson M, Balk SJ, Swetter SM, Gershenwald JE State of the science on prevention and screening to reduce melanoma incidence and mortality; the time is now CA Cancer J Clin, 66 (2016), pp. 460–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker et al., 1993 Tucker MA, Fraser MC, Goldstein AM, Elder DE, Guerry D 4th, Organic SM The risk of melanoma and other cancers in melanoma-prone families] Invest Dermatol, 100 (1993) 350s–5 [DOI] [PubMed] [Google Scholar]
- Vasen et al., 2000 Vasen HFA, Gruis NA, Frants RR, van Der Velden PA, Hille ET, Bergman W Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of pl6 (pl6-Leiden) Int J Cancer, 87 (2000), pp. 809–811 [PubMed] [Google Scholar]
- Yang et al., 2016 Yang XR, Rotunno M, Xiao Y, Ingvar C, Helgadottir H, Pastorino L, et al. Multiple rare variants in high-risk pancreatic cancer-related genes may increase risk for pancreatic cancer in a subset of patients with and without germline CDKN2 A mutations Hum Genet, 135 (2016), pp. 1241–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo et al., 1996 Zuo L, Weger J, Yang Q, Goldstein AM, Tucker MA, Walker GJ, et al. Germline mutations in the pl6INK4a binding domain of CDK4 in familial melanoma Nat Genet, 12 (1996) 97–9 [DOI] [PubMed] [Google Scholar]