All major groups of Mesozoic dinosaurs exhibited metabolically mediated thermoregulation based on clumped isotopes in eggshells.
Abstract
Studying the origin of avian thermoregulation is complicated by a lack of reliable methods for measuring body temperatures in extinct dinosaurs. Evidence from bone histology and stableisotopes often relies on uncertain assumptions about the relationship between growth rate and body temperature, or the isotopic composition (δ18O) of body water. Clumped isotope (Δ47) paleothermometry, based on binding of 13C to 18O, provides a more robust tool, but has yet to be applied across a broad phylogenetic range of dinosaurs while accounting for paleoenvironmental conditions. Applying this method to well-preserved fossil eggshells demonstrates that the three major clades of dinosaurs, Ornithischia, Sauropodomorpha, and Theropoda, were characterized by warm body temperatures. Dwarf titanosaurs may have exhibited similar body temperatures to larger sauropods, although this conclusion isprovisional, given current uncertainties in taxonomic assignment of dwarf titanosaur eggshell. Our results nevertheless reveal that metabolically controlled thermoregulation was the ancestral condition for Dinosauria.
INTRODUCTION
Determining the metabolic rates of dinosaurs has remained a persistent challenge ever since the name “Dinosauria” was first coined in the 19th century (1). Arguments for and against dinosaurian endothermy have been made on the grounds of paleohistology, biophysical models, and isotopic signatures of fossil teeth and eggshell (2–6). Recent histological work has suggested that the dinosaur endothermy versus ectothermy debate is a false dichotomy and argues instead that most nonavian dinosaurs exhibited an intermediate “mesothermic” thermoregulatory strategy (4). However, the correlation between metabolic rate and parameters such as body mass and growth rate in extinct dinosaurs relies on loosely constrained assumptions about growth constants, scaling relationships, and the activation energy controlling biochemical reactions, which are often derived from extant reptiles and mammals and are used in modeling dinosaur metabolic rates (2). Variations in assumed model parameters can produce large deviations in temperature estimates (2° to 8°C), even in extant taxa (7). This uncertainty requires independent constraints on internal body temperatures from dinosaur fossils representing a broad range of sizes and taxonomic groups.
Carbonate clumped isotope paleothermometry (reported as Δ47) is based on the internal order of 13C and 18O atoms among C─O bonds within a carbonate mineral lattice, reflecting mineral formation temperature independent of the isotopic composition of the water from which the mineral formed (8). This method accurately reflects the body temperatures of living vertebrates when applied to biogenic carbonates in tooth bioapatite from reptiles, sharks, and mammals, as well as eggshell carbonate from living reptiles and birds (6, 9). With this foundation, Δ47 paleothermometry provides a means to assess the body temperatures of nonavian dinosaurs, alleviating problematic assumptions inherent to estimating temperatures from the δ18O of skeletal or dental material or through the relationship of growth rate and body temperature (4, 10). Previous Δ47-based estimates of body temperatures derived from fossil tooth enamel and eggshell of sauropods (Camarasaurus, Giraffititan, and an unspecified titanosaur) yielded estimates in the range of 35° to 38°C (5, 6), and those from oviraptorid theropod fossil eggshell yielded body temperature estimates of 32° ± 3°C (6). These body temperature estimates for enormous sauropods (which ranged in size from ~104 to 105 kg) (11) fall within the range of living endotherms (12), whereas those for the much smaller oviraptorid (~100 kg) overlap with the body temperatures of living ectothermic and endothermic vertebrates (6).
Although long-standing consensus regarding the higher-order phylogenetic relationships of dinosaurs has recently been challenged (13), Dinosauria is unequivocally subdivided into three major clades: Sauropodomorpha, Theropoda, and Ornithischia (Fig. 1) (13, 14). To date, Δ47 paleothermometry has never been applied to ornithischian dinosaurs, which represent either the sister taxon to all other dinosaurs (and thus the most distant dinosaurian relatives of birds) (14) or the sister taxon to theropods (13). Furthermore, studies applying Δ47 paleothermometry to dinosaurs have yet to control for the paleoenvironmental temperatures at which these animals lived, limiting robust metabolic interpretations. Namely, existing studies have focused on samples from low to mid (~30° to 45°) paleolatitudes, where ambient summer temperatures could have been as high as the body temperature estimates of the dinosaurs studied, thereby resulting in uncertainty with respect to thermoregulatory interpretations. For example, eggshell samples from the ectothermic-radiated tortoise (Astrochelys radiata) and bearded lizard (Pogona barbata) taken from the Los Angeles Zoo yielded temperatures within the range of living endotherms only because they came from a warm environment (6). Here, we apply Δ47 paleothermometry to assess the phylogenetic distribution of elevated core body temperatures across Dinosauria, with samples spanning the dinosaurian phylogeny (Fig. 1). Specifically, we investigate the hadrosaurid ornithischian Maiasaura peeblesorum, the paravian theropod Troodon formosus (the Mesozoic dinosaur most closely related to birds yet assessed using Δ47 paleothermometry; Fig. 1), and an eggshell potentially attributable to a “dwarf” titanosaur sauropod from Romania (see taxonomic discussion of this eggshell in Results).
Fig. 1. Simplified archosaur phylogeny illustrating the taxa investigated in this and previous clumped isotope studies.
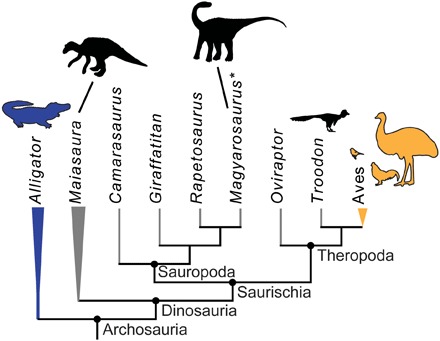
Extant ectotherms are blue; extant endotherms are orange. Maiasaura represents the major dinosaurian subclade Ornithischia, which is traditionally thought to represent the sister taxon to a Theropoda + Sauropoda clade as shown in (14) but in some recent phylogenies is placed as sister to Theropoda (13). Asterisk indicates taxonomic uncertainty for the Romanian eggshell sample, which may derive from a titanosaur sauropod (either the dwarf taxa Magyarosaurus or Paludititan or a larger titanosaurian) or the dwarf hadrosauroid Telmatosaurus (39).
Since extant endotherms—especially large-bodied ones—are often “thermal mosaics,” with their extremities approaching environmental temperatures while retaining a warmer core (10), temperature estimates from eggshells that developed near the center of a dinosaur’s body are likely to be more reliable than estimates from tooth enamel. Δ47 estimates of body temperatures for American alligators (Alligator mississippiensis) and Nile crocodiles (Crocodylus niloticus) were 1° to 3°C warmer and closer to expected temperatures when measured from the eggshell versus tooth bioapatite (6, 9). Here, we compare dinosaurian core body temperature estimates from fossil eggshells to paleoenvironmental temperatures by using Δ47 paleothermometry of co-occurring ectothermic fossil mollusks or other climatic paleotemperature estimates derived from published literature. Most of our samples come from high paleolatitude sites (Alberta, Canada), where cooler ambient temperatures facilitate linking of warm body temperature to endothermic-like metabolisms.
Geologic and climatic setting of fossil eggshell
Eggshell fragments of T. formosus, M. peeblesorum, Hypacrosaurus stebingeri, and an indeterminate lambeosaurine hadrosaur (15) were recovered from the Upper Campanian (~75 million years old) Oldman Formation of southern Alberta (localities: Devil’s Coulee, Wann’s Hill, Lost River Ranch; fig. S1). The relatively high paleolatitude of southern Alberta (~55°N; fig. S2) (16) facilitates our investigation of thermoregulation because the difference between environmental temperature estimates and endothermic body temperatures should be great enough to be analytically distinguishable by Δ47 measurements. Upper Campanian mean annual temperature (MAT) estimates at 55°N, based on fossil leaf temperature proxies and δ18Ophosphate from vertebrate fossils (teeth, turtle shell, and fish scales), are ~12° to 13°C (17, 18).
In addition, we analyzed a fossilized eggshell from the Maastrichtian (~69 million years old) Densuş-Ciula Formation of the Haţeg Basin (Southern Carpathians, Romania), a subtropical island (23°N paleolatitude) (19) within the Tethys Ocean (fig. S2). MAT estimates at this paleolatitude are variable, but range from ~30°C based on climate models (20, 21) to 18° to 25°C based on fossil leaf proxies (17) and ~25°C from δ18Ophosphate of vertebrate fossils (teeth, turtle shell, and fish scales) (18).
RESULTS
Δ47 temperature calibration
Extant eggshell samples were analyzed to corroborate previous work (6), suggesting that modern bird and nonavian reptile eggshell carbonate Δ47 values reflect internal body temperatures. For birds, internal body temperatures are largely controlled by metabolism (12). Extant bird eggshell samples were obtained from personal collections or through the Yale Peabody Museum’s (YPM’s) Ornithology Collection. Eggshells come from the following species: emu (Dromaius novaehollandiae; collected in 2012 at the Songline Emu Farm in Gill, MA), chicken (Gallus gallus; collected in 2012 in New Haven, CT), house sparrow (Passer domesticus; YPM collections uncatalogued), house wren (Troglodytes aedon; YPM collections uncatalogued), and Ruby-throated hummingbird (Archilochus colubris; YPM ORN 132487). The resting body temperatures for the birds used for the Δ47 temperature calibration (Fig. 2) are from (22). Extant American alligator eggshell (A. mississippiensis; YPM HERR 015203) was collected from the Rockefeller State Wildlife Refuge (RSWR) in Grand Chenier, LA, in June 2004. Studies of American alligators from the RSWR in June and July 2001 show that alligator body temperatures closely follow environmental temperatures (23). Environmental temperatures for June 2004 when the specific alligator egg was collected averaged 27.1°C and ranged between 23.3° and 30.9°C based on local temperature gauges (24). This average environmental temperature for June 2004 is used in the Δ47 temperature calibration (Fig. 2). The Δ47 values obtained for the hummingbird, wren, and sparrow are higher than expected based on their measured body temperatures, possibly related to facultative hypothermia, a mechanism used by small birds to conserve energy during breeding. Facultative hypothermia has been documented in several bird lineages including Passeriformes (which include wrens and sparrows) and Trochilidae (hummingbirds) (25). Our modern eggshell Δ47 values show good agreement with previous modern eggshell calibrations obtained from other laboratories (6), as well as a laboratory precipitation calibration from (8), which was generated in the same laboratory following the same analytical protocol as our eggshell data (Fig. 2).
Fig. 2. Clumped isotope [Δ47(abs)] value (calculated using the Gonfiantini parameters) versus temperature relationship of extant eggshell.
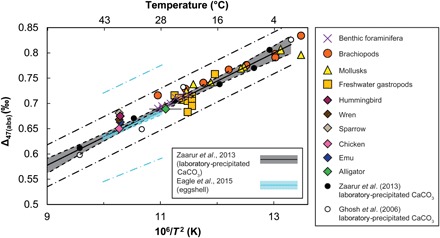
Data from this study, other biogenic carbonates analyzed between 2010 and 2018 at Yale University (8, 56–59), the calibration of laboratory precipitated carbonates from (8), and the extant eggshell carbonate calibration from (6). Error bars are SE for Δ47 (smaller than the symbols) and the range of expected body temperatures. Calibration lines, solid line; 95% confidence error envelope, shaded line; and 95% prediction envelope, dashed-dotted lines.
It has been suggested recently (26) that the isotopic parameters used to estimate 17O abundance, as part of the calculation of Δ47, require revision. Currently, the preferred method for calculating Δ47 uses the “Brand parameters” as opposed to those originally used (termed “Gonfiantini parameters”). However, when converting the Yale biogenic carbonate Δ47 values and the laboratory-precipitated carbonate data from (8) to the Brand parameters, the Δ47 values show contrasting responses (fig. S3). The result is a mismatch between biogenic and laboratory precipitation Δ47 temperature calibrations, which is unexpected given previous agreement between them (Fig. 2). Generally, for samples with δ13C and δ18O similar to that of the reference gas, the choice of 17O isotopic parameters exerts only a small effect on Δ47. However, the laboratory precipitation samples used to generate the Δ47 temperature calibration in (8) are much more depleted in 13C [δ13C of ~−30‰ Vienna Pee Dee Belemnite (VPDB)] compared with biogenic carbonates and the reference gas (δ13C of −3.640‰ VPDB). Therefore, the difference in behavior of the biogenic and laboratory precipitation samples is probably due to uncertainty in the retroactive conversion process from the Gonfiantini to the Brand parameters, related to the fact that the standards used for defining the absolute reference frame (see the Supplementary Materials for more details) were not designed for this conversion. It is likely that the 13C-depleted laboratory precipitation data are more sensitive to this uncertainty. Resolving some of this uncertainty could be achieved by repeating the laboratory precipitation experiments from (8) to produce synthetic carbonates with a range of different carbon isotope compositions.
Because of remaining questions regarding the conversion process to Brand parameters and its effect on older Gonfiantini-calculated Δ47 data, we used three possible Δ47 temperature calibrations to estimate temperature from our Brand parameter Δ47 data (see the Supplementary Materials for more details). When using any one of these three equations, the difference in temperature is on average less than 1°C relative to the results obtained using the original Gonfiantini parameters and the calibration from (8) (data S1). We therefore report our data using the Gonfiantini parameters and the Gonfiantini-based Δ47 temperature calibration (Fig. 2) from (8), as it is in agreement with our biogenic data and enables direct comparisons with previously published dinosaur body temperature estimates (5, 6).
Preservation of fossil carbonates
Before making any interpretation of dinosaur body temperature based on Δ47 paleothermometry results, we must consider the preservation of the fossil carbonates. The internal order of 13C─18O bonds within the mineral lattice may change during burial diagenesis or due to recrystallization (27), altering Δ47 signatures such that the derived temperatures may reflect the temperature of recrystallization rather than the original environmental or body temperature of carbonate formation. To account for this potential limitation, we first assessed the degree of eggshell preservation by examining the eggshell morphology and identifying any nonprimary eggshell microstructures. All fossil eggshells were imaged using a scanning electron microscope and a petrographic microscope to assess preservation of primary eggshell microstructure. Biogenic carbonates that retain fine microstructure are more likely to preserve the original mineral and therefore their original geochemical signatures, as recrystallized calcite often loses the preferential orientation of biosynthesized crystals (28). Next, we analyzed fossil carbonate for evidence of recrystallization using trace element concentrations and cathodoluminescence (CL) microscopy. In addition, we used x-ray diffraction to assess the preservation of the original aragonite in mollusk shell fossils. See Table 1 for summary of results.
Table 1. Criteria for characterization of fossil eggshell preservation.
| Eggshell | Locality |
Mn (ppm) |
Fe (ppm) |
CL image % luminescent |
Preservation test* |
Final preservation characterization |
|||
|
Low-trace metals |
% Lum. | Microstructure | 1° Aragonite | ||||||
|
Troodon formosus (Theropod) TMP 2008.75.127 |
Devil’s Coulee, AB, Canada |
60† | 76† | 0.5 | ✓✓ | ✓✓ | ✓✓ | NA | Well preserved |
|
Maiasaura peeblesorum (Hadrosaur) TMP 2009.153.1 |
Devil’s Coulee, AB, Canada |
1560 | 741 | 11.3 | ✓ | ✓ | ✓✓ | NA | Moderately preserved |
|
Hypacrosaurus stebingeri (Hadrosaur) TMP 1989.69.10 |
Devil’s Coulee, AB, Canada |
4291† | 2313† | NA | X | NA | NA | NA | Poorly preserved |
|
Troodon formosus (Theropod) TMP 1995.21.4 |
Wann’s Hill, AB, Canada |
99 | 54 | 0.6 | ✓✓ | ✓✓ | ✓✓ | NA | Well preserved |
| Indeterminate lambeosaurine (Hadrosaur) TMP 1988.121.41 |
Wann’s Hill, AB, Canada |
3265 | 1623 | 73 | X | X | X | NA | Poorly preserved |
|
Troodon formosus (Theropod) TMP 2003.81.1 |
Lost River Ranch, AB, Canada |
62 | 8 | 0 | ✓✓ | ✓✓ | ✓✓ | NA | Well preserved |
|
Magyarosaurus dacus (Sauropod) TMP 1991.175.2 |
Tuştea, Haţeg Basin, Romania |
795 | 6 | 15 | ✓ | ✓ | ✓✓ | NA | Moderately preserved |
| Gastropod W20 | Wann’s Hill, AB, Canada |
79 | 297 | NA | ✓✓ | NA | NA | ✓✓ | Well preserved |
| Bivalve TMP 2009.149.5 |
Milk River, AB, Canada |
218 | 21 | NA | ✓✓ | NA | NA | ✓✓ | Well preserved |
*If the sample passed, it received a “✓✓”; partially passed, a “✓”; or failed, a “X.” NA indicates that either test was “not applicable” (e.g., original biomineral was calcite) or “not available” (e.g., no thin sections available).
†Average of duplicates run to test stability of mass spectrometer.
Morphology of eggshells
Accurate taxonomic identification of fossil eggshell is fundamental to understand the evolutionary implications of dinosaur body temperatures when using eggshell carbonate Δ47 values. Although classification of dinosaur eggshell may be complicated by a lack of association with identifiable skeletal material, eggshell can be diagnosed much like skeletal morphology for taxonomic assignment (29). The eggshell of T. formosus (ootaxon Prismatoolithus levis) (30) has been identified based on eggs containing embryonic remains from the Campanian Two Medicine Formation of Montana (31). Troodon eggshell is about 1 mm thick, has a smooth outer surface with single- and double-pore openings, and consists of two main layers representative of the continuous/prismatic and mammillary zones of nonavian theropod dinosaurs (32). In the three Troodon samples, this diagnostic eggshell bilayer is well preserved (Fig. 3, A to C). On the basis of the primary eggshell structure, the Troodon samples pass the first criterion of being well preserved (Table 1). The excellent preservation of the Troodon microstructure could be due to the fact that these shells are the least porous of all the taxa examined here, possibly limiting exposure to pore fluids that may cause calcite dissolution and reprecipitation.
Fig. 3. Petrographic microscope images of dinosaur eggshell from this study.
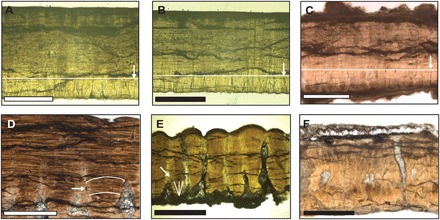
(A) Well-preserved Troodon eggshell from Devil’s Coulee, Alberta, Canada [Tyrrell Museum of Palaeontology (TMP) 2008.75.51]. (B) Well-preserved Troodon eggshell from Lost River Ranch, Alberta, Canada (TMP 2003.81.1). (C) Well-preserved Troodon eggshell fragment from Wann’s Hill, Alberta, Canada (TMP 1995.21.4). Arrows and horizontal lines point to the approximate boundary between the mammillary and prismatic layers. Presence of two calcitic layers is diagnostic of nonavian theropods. (D) Maiasaura hadrosaur eggshell from Devil’s Coulee, Alberta, Canada, with intermediate preservation (TMP 2009.153.1). The diagnostic radiating tabular units are indicated by the white arrow. (E) Romanian eggshell (oospecies Megaloolithus cf. M. siruguei, TMP 1991.175.2) from Tuştea locality, Romania, with intermediate preservation. Diagnostic radiating acicular crystals indicated by white arrow. (F) Poorly preserved lambeosaurine hadrosaur eggshell fragment from Wann’s Hill, Alberta, Canada (TMP 1988.121.41). Scale bars, 500 μm.
The eggshell diagnostic of M. peeblesorum (ootaxon Spheroolithus albertensis) (33) is over 1 mm thick and has a surface ornamentation of anastomosing ridges, irregular pore canals, and fan-shaped shell units with radiating tabular structure (Fig. 3D) (29, 33). Association between the hadrosaur Maiasaura and the ootaxon S. albertensis is based on hatchling skeletal material found in association with eggs and eggshells from the Campanian Two Medicine Formation of Montana (15). The Maiasaura eggshell is more porous than that of Troodon (Fig. 3), and coarse-grained calcite infilling is observed in the pore canals. However, the diagnostic microstructure of radiating tabular basic units is preserved, suggesting that the majority of the eggshell calcite is primary mineral, with relatively minor amounts of secondary calcite (Fig. 3D and Table 1; see below for a quantitative discussion).
Determination of the taxonomic affinity of the eggshell from Romania (ootaxon Megaloolithus cf. Megaloolithus siruguei) (34) is more complicated. Characteristics of the Romanian eggshell include nodular surface ornamentation, Y-shaped vertical pore canals, and fan-shaped shell units composed of acicular calcite crystals radiating from nucleation centers (Fig. 3E). These features are shared by fossil eggs from Auca Mahuevo, Argentina, which can be confidently identified as belonging to titanosaur sauropods based on embryos in ovo (within the egg) (35). Compatible with a titanosaur affinity for the Romanian eggs, skeletal material from two dwarf titanosaur species (the similarly sized Paludititan nalatzensis and Magyarosaurus dacus) (36, 37) and one indeterminate, large titanosaur species (38) have been recovered from the Haţeg Basin. Although multiple eggshell characteristics are compatible with a titanosaur affinity, embryonic remains of the dwarf hadrosauroid Telmatosaurus have also been recovered within the Haţeg Basin, in close proximity to a Megaloolithus cf. M. siruguei nest (39, 40). This has prompted the idea that Telmatosaurus may have convergently evolved oological characteristics such as those in sauropods, raising uncertainty of a titanosaur affinity for this Romanian eggshell (39, 40). For the purpose of this study, if these eggshells derive from the dwarf hadrosauroid Telmatosaurus, then they provide additional information on ornithischian body temperatures. However, given its numerous similarities with eggshell associated with definitive titanosaur embryos from Argentina, we consider it most plausible that these eggshells are from Romanian titanosaurs, either dwarf or giant. However, since the taxonomic identification of the eggshells is not yet definitive, we discuss the implications of both a hadrosauroid and titanosaur affinity for these eggshells.
Fossil bone histology (37) suggests that the dwarf titanosaurs of Romania had an adult body mass of ~900 kg—at least an order of magnitude smaller than titanosaurs from Argentina. In turn, the body length of Telmatosaurus is estimated to have been ~4 m, compared to the 7- to 13-m (or more) body lengths of most hadrosaurids (41). The relatively small size of these Romanian dinosaurs has been interpreted as evidence of island dwarfism, as the Haţeg Basin would have been located on an island in the Tethys during the Maastrichtian (19).
The Romanian eggshell is also porous, with diagnostic “Y-shaped” pore canals, which appear to have been filled with secondary calcite (Fig. 3E). The preservation of the primary acicular radiating calcite crystals from the nucleation centers indicates that the majority of the original eggshell calcite is preserved. The volume of secondary calcite that could fill these pore spaces is small relative to the total eggshell calcite volume (Fig. 3E; see below for a quantitative discussion).
Hadrosaur eggshells from two Alberta localities (H. stebingeri eggshell from Devil’s Coulee and indeterminate lambeosaurine eggshell from Wann’s Hill; see the Supplementary Materials for more details about geologic settings) display obvious secondary calcite overgrowths on their exterior and have lost or partially lost their original microstructure (e.g., Fig. 3F). We therefore consider these samples as poorly preserved (Table 1).
Mollusk Shell preservation
In contrast to dinosaur eggshell carbonate, which is composed of calcite, freshwater mollusk shells are originally composed of aragonite. The Wann’s Hill gastropod and Milk River bivalve mineral preservation was assessed using x-ray diffraction (fig. S4), which shows clear aragonite peaks and no calcite peaks, suggesting that these mollusk samples are well preserved and are therefore considered adequate for environmental temperature determination from isotopic analysis (Table 1). This conclusion was further tested using trace element analysis on both mollusk and eggshell samples.
Trace element analysis
Trace elements, such as Fe and Mn, are not common in eggshells of living taxa but are typically present in anoxic pore fluids in which calcite eggshell may recrystallize during diagenesis (28). Bulk concentrations of trace metals were measured in eggshell and mollusk carbonates as indicators of recrystallization and therefore likely alteration of Δ47 values. Mn concentrations in the eggshells from extant taxa were below detection limits. Fe concentrations were 5 parts per million (ppm) for extant chicken, sparrow, and alligator eggshell, and below detection limit in extant emu eggshell. Concentrations of Fe and Mn in the fossil eggshell were between ~50 and 80 ppm Fe and ~60 and 100 ppm Mn for the best-preserved samples (the three Troodon eggshell samples), and between ~1500 and 2500 ppm Fe and 1500 and 4500 ppm Mn for obviously altered hadrosaur eggshell based on their poorly preserved microstructure (Devil’s Coulee Hypacrosaurus and Wann’s Hill lambeosaurine; Table 1 and Fig. 4A). Two eggshell samples (Maiasaura and the Romanian eggshell) exhibited intermediate trace metal concentrations of ~0 to 750 ppm Fe and 800 to 1600 ppm Mn (Table 1 and Fig. 4A). See the Supplementary Materials for more details.
Fig. 4. Bulk trace metal concentrations in eggshells and mollusks with CL images of fossil eggshell.
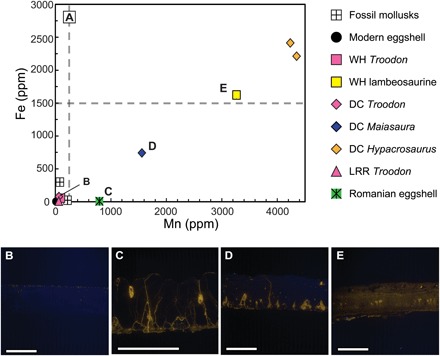
(A) Fe and Mn concentrations (ppm) in extant and fossil eggshells and fossil mollusks. Letters in the graph refer to specific CL images. (B to E) CL images of fossil eggshell thin sections in cross section used to assess preservation. (B) Well-preserved Devil’s Coulee Troodon, (C) Romanian eggshell (oospecies Megaloolithus cf. M. siruguei) with intermediate preservation, (D) Maiasaura from Devil’s Coulee with intermediate preservation, (E) poorly preserved lambeosaurine from Wann’s Hill, Alberta, Canada. Scale bars, 1 mm. Luminescence correlates with increased Mn concentrations in (A).
CL microscopy
This technique was used to characterize fossil eggshell preservation by identifying the extent of diagenetic calcite overgrowth, infilling, and replacement of the original mineral. CL microscopy identifies areas within the shell that are enriched in Mn and Fe: regions enriched in Mn luminesce, and when subsequently enriched in Fe, these areas become dimmer (28).
CL photomicrographs of Troodon eggshell samples in thin section are nonluminescent (Fig. 4B and fig. S5, G to I), consistent with their low bulk concentrations of Fe and Mn (Fig. 4A) and their preservation of primary eggshell microstructure (Fig. 3, A to C). We therefore characterize the Troodon eggshells as well preserved (Table 1) and appropriate for body temperature determination from isotopic analysis. In contrast, the Wann’s Hill lambeosaurine, in which microstructure was not preserved, was luminous throughout (Fig. 4E), likely due to both high Fe and Mn concentrations (Fig. 4A). The lambeosaurine eggshell was characterized as poorly preserved (Table 1) and inappropriate for body temperature determination from isotopic analysis.
The Devil’s Coulee Maiasaura eggshell sample exhibits well-preserved microstructure (Fig. 3D) but contains minor amounts of secondary calcite and moderately elevated Fe and Mn concentrations (Fig. 4A). This sample had bulk nonluminescent calcite but luminescent calcite filling in thin cracks and pores, consistent with secondary calcite infilling being the source of Mn and Fe (Fig. 4D). This Mn and Fe content could also be what gives the eggshell its black color. It is likely that the Δ47 value reflects a mixture of original eggshell (and therefore actual dinosaur body temperature), with a minor contribution of diagenetic secondary calcite, which in turn reflects ambient or burial temperatures. The Romanian eggshell sample also exhibits well-preserved microstructure (Fig. 3E) with minor secondary calcite filling in cracks and pores (Fig. 4C) and only moderately elevated Mn concentrations (Fig. 4A).
The CL images (Fig. 4, B to E, and fig. S5) can be used to quantify the contribution from secondary calcite. Since luminescent areas are indicative of trace metal enrichment and, thus, likely areas of recrystallization, we used the number of luminescent pixels to estimate the fraction of the eggshell that is recrystallized (see the Supplementary Materials for more details). For the Maiasaura eggshell, we estimate ~11% is recrystallized, and for the Romanian eggshell, ~16% is recrystallized (Table 1). On the basis of well-preserved microstructure and relatively low amounts of recrystallized calcite, we characterize the Maiasaura and Romanian eggshell as moderately preserved (Table 1) and adequate for body temperature determination from isotopic analysis.
Dinosaur body temperatures
The theropod Troodon eggshells yielded average (± 1 SE) Δ47-derived temperatures of 38° ± 4°C, 27° ± 4°C, and 28° ± 3°C, and the ornithischian Maiasaura eggshell yielded a temperature of 44° ± 2°C (Fig. 5). The Δ47-derived temperature obtained from the Romanian eggshell producer is 36° ± 1°C. This is within the range of living endothermic animals, such as birds and mammals (34° to 44°C) (12) and is warmer than the modeled MAT for the Haţeg Basin locality of ~30°C (20, 21). The warmest Troodon (38° ± 4°C) and Maiasaura (44° ± 2°C) body temperature estimates are also within the range of living endothermic animals and substantially exceed the warmest environmental temperatures estimated from mollusk shells at the same location (25° ± 1°C and 28° ± 2°C; Fig. 5).
Fig. 5. Log body mass (kg) versus Δ47-derived body temperature estimates of fossil dinosaurs and mollusks.
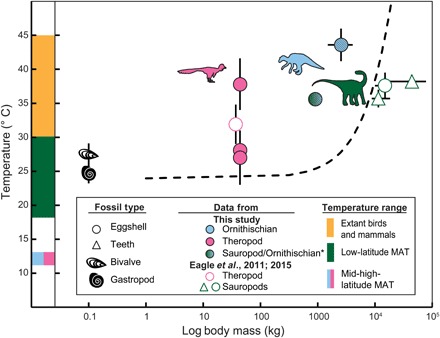
We present data from this study and (5, 6) in the context of model-based body temperature estimates from (2) (black dashed curve). Note that this model provides an illustrative example for the possibility of gigantothermy, but it has been debated (7). Diagonal-hatched symbols are specimens with moderate calcite preservation, while the rest are well preserved. Estimates of ambient MAT are taken from the literature (17, 18, 20, 21) for the latitudes of Late Cretaceous sites in Haţeg Basin, Romania (green bar), and Alberta, Canada (blue/pink bar). For Alberta, ambient warm season temperatures are also estimated from Δ47 in freshwater mollusks (shell symbols). Δ47 temperature error bars are 1 SE, and body mass error bars indicate a range of estimated sizes (11, 37, 60). Extant bird body temperature range is from (12). Asterisk indicates taxonomic uncertainty for the Romanian eggshell sample, which may derive from a titanosaur sauropod or the hadrosauroid Telmatosaurus (39).
DISCUSSION
The Δ47-derived mollusk temperatures provide a direct way for comparing dinosaur body temperature estimates to that of their environment and determine whether these dinosaurs had thermoregulatory strategies closer to modern endothermic or ectothermic animals. Considering the highly seasonal growth of extant mid-high-latitude freshwater bivalves and gastropods (42), these environmental temperatures likely reflect the warm season. Mann-Whitney tests were conducted to compare dinosaurian body temperatures to average mollusk-derived environmental temperatures. These tests indicate that the Maiasaura and Devil’s Coulee Troodon temperatures are both significantly warmer than the mollusk temperatures (Ub = 0, P = 0.0024, and Ub = 6, P = 0.0363). By contrast, the two cooler Troodon samples [Lost River Ranch (27° ± 4°C) and Wann’s Hill (28° ± 3°C)] yield estimates similar (i.e., not significantly different) to the estimated summer environmental temperatures of fossil mollusk shells from the same deposits (Ub = 8, P = 0.3196, and Ub = 17, P = 0.5953; Fig. 5). Although these estimates are consistent with those previously obtained for a Mongolian oviraptorid (32° ± 3°C) (6), the ~10°C range of body temperatures within these three Alberta Troodon samples may suggest a capacity for heterothermic metabolism (Fig. 5) (43).
The Maiasaura eggshell is not as well preserved as the Troodon eggshells (~11% recrystallization or infilling of secondary calcite in the Maiasaura sample). Consideration of several possible diagenetic scenarios allows us to assess the potential effect of this secondary calcite on Δ47 using a simple mass balance. Under one scenario, the secondary calcite could have formed from diagenetic fluids after burial. On the basis of vitrinite reflectance data from southern Alberta, the burial temperatures were likely below 80°C (44). Assuming that Δ47 of the secondary calcite reflects a formation temperature of at most 80°C, this would correspond to a Δ47 value of 0.523‰. If this estimated 11% of secondary calcite is mixed with the remaining 89% primary calcite to yield the combined measured Δ47 of 0.632‰, then we can use a simple two end-member mixing model to estimate the primary unaltered calcite Δ47 as 0.645‰. This corresponds to a body temperature of 40°C. Thus, the effect of recrystallized calcite under this scenario does not change our biological interpretation, as the estimated Maiasaura body temperature still falls within the range of extant endotherms.
A second possible burial scenario for the Maiasaura is that of surface diagenesis, in which recrystallized calcite formed from diagenetic fluids before burial and reflects temperatures close to ambient (~26°C, Δ47 0.698‰, based on our average freshwater mollusks Δ47). Similar mass balance considerations result in Δ47 of 0.623‰ for the primary calcite, corresponding to a body temperature of 46°C. Another option for a surface diagenesis scenario is to use the clearly altered Hypacrosaurus fossil from the same locality (Devil’s Coulee), with very high trace metal concentrations (Fig. 4A), as the end member for total recrystallization at this site. Hence, a 100% recrystallized shell yields Δ47 of 0.681‰ (same as the Hypacrosaurus). Mass balance calculations then result in primary calcite being 0.625‰ with a corresponding body temperature of 45°C. Under these two surface diagenesis scenarios, our Maiasaura body temperature estimate of 44°C is a minimum temperature. The diagenetic history probably involved some dissolution and reprecipitation at the surface and some at depth, but either way, the impact of any secondary calcite on our biological interpretation is minimal.
In addition to dissolution and reprecipitation or pore infilling by secondary calcite due to interactions with diagenetic fluids, it is possible that Δ47 temperatures in Maiasaura were affected by solid-state reordering due to burial heating. To rule out this possibility, we compared the Maiasaura sample with poorly preserved Hypacrosaurus eggshell fossils from the same locality. The Hypacrosaurus eggshell experienced the same thermal history during burial as the Maiasaura eggshell but yielded a Δ47 temperature estimate of ~30°C—much lower than that of the Maiasaura eggshell (Δ47 temperature estimate of ~44°C). While these samples clearly experienced different extents of recrystallization as discussed above, it is unlikely that burial heating would have initiated solid-state reordering in the Maiasaura eggshell but not in that of Hypacrosaurus. Thus, these results support the use of isotopic temperatures from the Maiasaura eggshell to infer body temperatures, which in turn suggests their capacity for metabolic thermoregulation.
The Romanian eggshell is derived either from the dwarf hadrosauroid Telmatosaurus or from a titanosaur sauropod. This eggshell yielded a Δ47-derived temperature of 36° ± 1°C (Fig. 5). Preservation quality is a consideration also when interpreting body temperatures estimated from the Romanian eggshell. This specimen has slightly elevated Mn concentrations, likely from secondary calcite pore infilling, as indicated by luminous areas on photomicrographs (~16% of the material; Fig. 4C). The Maastrichtian Densuş-Ciula Formation was buried under as little as 500 m of overlying sediment for the past 65 million years (45). With an average geothermal gradient of ~25°C/km (46) and assuming the maximum MAT of 30°C (20, 21), the burial temperature would be 42.5°C. This gives a primary calcite Δ47 value of 0.665‰ (using similar mass balance considerations as those for Maiasaura, above) and a corresponding body temperature estimate of 34°C in a burial diagenesis scenario. Alternatively, using the MAT of 30°C (20, 21) as the surface diagenetic temperature, the primary calcite Δ47 value would be 0.656‰ and correspond to a body temperature of 37°C. Under both scenarios, the change is small and does not affect our biological interpretation.
The presence of rare large-bodied sauropod remains in the Haţeg Basin precludes the definitive assignment of the Romanian eggshell to a dwarf titanosaur. However, remains of the dwarf taxa Magyarosaurus and Paludititan greatly outnumber those of large-bodied sauropods in the region (37). Hence, the implications of this eggshell deriving from one of these dwarf titanosaur taxa (either Magyarosaurus or Paludititan)—a clade composed predominantly of giant sauropods—is worth exploring. If the Romanian eggshell material is hadrosaurid in origin, then the results provide additional evidence (beyond Maiasaura) of ornithischian body temperatures within the range of extant birds. By contrast, if this material originates from a large titanosaur species, then the body temperature estimate of 36° ± 1°C is comparable to previous Δ47-derived body temperature estimates of ~35° to 38°C for giant titanosaurs, based on fossil teeth and eggshell (5, 6). If the Romanian eggshell is derived from a dwarf titanosaur, then it would allow testing of the hypothesis that the large body size of sauropods is responsible for their high body temperatures, as has previously been suggested (47). Body mass estimates for the dwarf titanosaurs of the Haţeg Basin suggest that they weighed only ~900 kg (37), much smaller than their colossal relatives (104 to 105 kg) (11). The concept of “gigantothermy” or “inertial homeothermy” is based on the link between low surface area–to–volume ratios and heat retention in animals and is modeled from the relationship between body mass and temperature in living crocodilians (2), although the accuracy of this model has recently been called into question (7). If the Romanian eggshell is derived from a dwarf titanosaur, then the similarity in body temperatures with that of giant sauropods (~35° to 38°C) (5, 6), despite an at least 10-fold difference in body mass, would be inconsistent with an inertial homeothermy thermoregulatory model (dashed curve, Fig. 5) (2). Our results from the Romanian eggshell—tentative though they are—are consistent with a recent critique of the inertial homeothermy model that suggested no relationship between size and body temperature in sauropods (7). The modeled MAT for the Haţeg Basin is ~30°C (Fig. 5) (20, 21). Seasonal models of Maastrichtian climate are not available, but a Campanian model with four times preindustrial levels of CO2 gives an approximate summer temperature of ~33°C at ~23°N (48). Our body temperature estimates from the Romanian eggshell (36° ± 1°C) are warmer than either mean annual or summer paleoenvironmental temperature estimates, thus favoring an interpretation of endothermy for this eggshell producer.
Our inferred dinosaur body temperatures, combined with previous work on oviraptorosaurs and large-bodied sauropods (5, 6), indicate that representatives of all three major dinosaurian lineages exhibited elevated body temperatures relative to environmental temperatures, suggesting that a capacity for metabolic control of internal body temperatures was ancestral for Dinosauria. The variable body temperatures of Troodon (range of ~10°C; Fig. 5) suggest that some Mesozoic dinosaurs may have exhibited thermoregulatory strategies similar to those of extant mesothermic or heterothermic taxa. Many extant birds and mammals are heterothermic (43); they can lower their internal body temperatures to save energy during periods of environmental stress or during reproductive periods before laying eggs (43). Extant mesotherms, such as leatherback sea turtles (47), use a higher metabolism to raise their internal body temperatures above those of their surroundings but do not necessarily maintain a fixed internal body temperature such as homeothermic animals (4). Our data therefore expand on previous Δ47 results from theropods and sauropods in suggesting that these dinosaurs exhibited at least some metabolic control over their body temperatures to raise them above ambient temperatures, independent of their body size. Maiasaura, with body temperature estimates within the range of extant birds (44° ± 2°C; Fig. 5), extends this conclusion to Ornithischia.
The observed high body temperatures across all three major dinosaur clades point toward a potential decoupling of the proposed relationship between mass-specific growth rates and metabolic rates in Mesozoic dinosaurs. Although many crownward stem avians, such as Archaeopteryx (49), exhibited slower growth rates than extant birds, our results indicate that these extinct avian taxa would have inherited an ancestral capacity for thermoregulation. Hence, estimates of growth rates and body size may be insufficient to delineate metabolic modes in the fossil record. Heat retention scales negatively with size—a consequence of smaller animals exhibiting higher surface area–to–volume ratios than larger animals (50). Insulation would have been increasingly important throughout the protracted period of body size reduction along the evolutionary lineage toward extant birds (51, 52). Therefore, the acquisition of dense plumage among Mesozoic dinosaurs, which may have arisen independently in theropods (53) and ornithischians (54) or deeper still along the lineage subtending pterosaurs and dinosaurs (55), may have been related to selection for body heat retention in smaller-bodied animals before being co-opted for sexual display or flying potential.
MATERIALS AND METHODS
Trace metal analysis and CL microscopy
Extant bird, alligator, and fossil dinosaur eggshells were crushed using a mortar and pestle. Powdered samples (~6 mg each) were weighed into 1.5-ml acid-cleaned centrifuge tubes and sequentially leached with 0.35 N HCl. The combined supernatants from each leaching step were dried and redissolved in enough 5% HNO3 to reach a dilution factor of 1000, along with a 2-ppb indium spike. These solutions were analyzed using a Thermo Element XR ICP-MS and compared to in-house trace element analytical standards and two geostandards with a Ca spike to match our sample solutions. CL photomicrographs of eggshell thin sections were imaged using a RELIOTRON VII CL apparatus. For more details, see the Supplementary Materials and Methods.
Clumped isotope (Δ47) analysis
The clumped isotope analytical procedure at the Yale Analytical and Stable Isotopic Center is described in detail in the Supplementary Materials and Methods. Powdered carbonate samples (3.5 to 5 mg) were reacted with 105% phosphoric acid (H3PO4) overnight at 25°C. The extracted CO2 was cryogenically purified on a vacuum line and passed through a gas chromatography column (Supelco Q-plot, 30 m by 0.53 mm) at −20°C to remove volatile organic compounds. Clean CO2 samples were analyzed for δ13C, δ18O, and Δ47 using Thermo MAT-253 optimized to measure mass/charge ratio of 44 to 49. We report our Δ47 data using the Gonfiantini parameters in the main text and give Δ47 data that were calculated using the Brand parameters in the Supplementary Materials (data S1).
Supplementary Material
Acknowledgments
We thank and remember M. Pagani for the discussion and guidance during the course of this project. We also thank the staff of the Yale Analytical and Stable Isotope Center, a Yale Institute for Biospheric Studies research center. Last, we thank K. Zyskowski of the YPM for access to extant eggshell samples. Funding: We thank support by The John F. Enders Grant and Yale University (R.R.D.), The Isaac Newton Trust and U.K. Research and Innovation Future Leaders Fellowship (MR/S032177/1 to D.J.F.), The Sloan Research Fellowship (P.M.H.), the Natural Sciences and Engineering Research Council Discovery Grant (D.K.Z.), and Israel Science Foundation grant 171/16 (H.P.A.). Author contributions: R.R.D., D.J.F., and H.P.A. designed the study. F.T., D.K.Z., and D.J.F. collected the fossil samples. R.R.D. performed the laboratory work with supervision and assistance from H.P.A. and P.M.H. All authors analyzed the data and wrote the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/7/eaax9361/DC1
Supplementary Materials and Methods
Fig. S1. Fossil localities in southern Alberta, Canada.
Fig. S2. Paleogeographic map.
Fig. S3. Clumped isotope [Δ47(abs)] value versus temperature relationship of laboratory-precipitated carbonate calibration data.
Fig. S4. X-ray diffraction spectra of fossil mollusks.
Fig. S5. Photomicrographs of dinosaur eggshell samples.
Table S1. Specimen descriptions, localities, and isotopic values.
Data S1. Raw data for both carbonate samples and standards and CO2 standards.
REFERENCES AND NOTES
- 1.Owen R., Report on British fossil reptiles. Rep. Brit. Assn. Adv. Sci. 11, 60–204 (1841). [Google Scholar]
- 2.Gillooly J. F., Allen A. P., Charnov E. L., Dinosaur fossils predict body temperatures. PLOS Biol. 4, 1467–1469 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amiot R., Lécuyer C., Buffetaut E., Escarguel G., Fluteau F., Martineau F., Oxygen isotopes from biogenic apatites suggest widespread endothermy in Cretaceous dinosaurs. Earth Planet. Sci. Lett. 246, 41–54 (2006). [Google Scholar]
- 4.Grady J. M., Enquist B. J., Dettweiler-Robinson E., Wright N. A., Smith F. A., Evidence for mesothermy in dinosaurs. Science 344, 1268–1272 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Eagle R. A., Tütken T., Martin T. S., Fricke H. C., Connely M., Cifelli R. L., Eiler J. M., Dinosaur body temperatures determined from isotopic (13C-18O) ordering in fossil biominerals. Science 333, 443–445 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Eagle R. A., Enriquez M., Grellet-Tinner G., Perez-Huerta A., Hu D., Tütken T., Montanari S., Loyd S. J., Ramirez P., Tripati A. K., Kohn M. J., Cerling T. E., Chiappe L. M., Eiler J. M., Isotopic ordering in eggshells reflects body temperatures and suggests differing thermophysiology in two Cretaceous dinosaurs. Nat. Commun. 6, 8296 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Griebeler E. M., Body temperatures in dinosaurs: what can growth curves tell us? PLOS ONE 8, e74317 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaarur S., Affek H. P., Brandon M. T., A revised calibration of the clumped isotope thermometer. Earth Planet. Sci. Lett. 382, 47–57 (2013). [Google Scholar]
- 9.Eagle R. A., Schauble E. A., Tripati A. K., Tütken T., Hulbere R. C., Eiler J. M., Body temperatures of modern and extinct vertebrates from 13C-18O bond abundances in bioapatite. Proc. Natl. Acad. Sci. U.S.A. 107, 10377–10382 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.A. Chinsamy, J. Willem, in The Dinosauria, D. B. Weishampel, D. Peter, H. Osmólska, Eds. (University of California Press, 2004), pp. 643–659.
- 11.Sander P. M., Christian A., Clauss M., Fechner R., Gee C. T., Griebeler E. M., Gunga H.-C., Hummel J., Mallison H., Perry S. F., Preuschoft H., Rauhut O. W. M., Remes K., Tütken T., Wings O., Witzel U., Biology of the sauropod dinosaurs: the evolution of gigantism. Biol. Rev. 86, 117–155 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke A., Rothery P., Scaling of body temperature in mammals and birds. Funct. Ecol. 22, 58–67 (2008). [Google Scholar]
- 13.Baron M. G., Norman D. B., Barrett P. M., A new hypothesis of dinosaur relationships and early dinosaur evolution. Nature 543, 501–506 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Gauthier J. A., Saurischian monophyly and the origin of birds. Mem. California Acad. Sci. 8, 1–55 (1986). [Google Scholar]
- 15.Hirsch K. F., Quinn B., Eggs and eggshell fragments from the Upper Cretaceous Two Medicine Formation of Montana. J. Vertebr. Paleontol. 10, 491–511 (1990). [Google Scholar]
- 16.van Hinsbergen D. J. J., de Groot L. V., van Schaik S. J., Spakman W., Bijl P. K., Sluijs A., Langereis C. G., Brinkhuis H., A paleolatitude calculator for paleoclimate studies. PLOS ONE 10, e0126946 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spicer R. A., Herman A. B., The late Cretaceous environment of the Arctic: A quantitative reassessment based on plant fossils. Palaeogeogr. Palaeoclimatol. Palaeoecol. 295, 423–442 (2010). [Google Scholar]
- 18.Amiot R., Lécuyer C., Buffetaut E., Fluteauc F., Legendre S., Martineau F., Latitudinal temperature gradient during the cretaceous upper campanian–middle maastrichtian: δ18O record of continental vertebrates. Earth Planet. Sci. Lett. 226, 255–272 (2004). [Google Scholar]
- 19.Panaiotu C. G., Panaiotu C. E., Palaeomagnetism of the upper cretaceous sânpetru formation (Haţeg Basin, South Carpathians). Palaeogeogr. Palaeoclimatol. Palaeoecol. 293, 343–352 (2010). [Google Scholar]
- 20.Hunter S. J., Valdes P. J., Haywood A. M., Markwick P. J., Modelling Maastrichtian climate: investigating the role of geography, atmospheric CO2 and vegetation. Clim. Past Discuss. 4, 981–1019 (2008). [Google Scholar]
- 21.Upchurch G. R., Kiehl J., Shields C., Scherer J., Scotese C., Latitudinal temperature gradients and high-latitude temperatures during the latest Cretaceous: Congruence of geologic data and climate models. Geology 43, 683–686 (2015). [Google Scholar]
- 22.Prinzinger R., Preβmar A., Schleucher E., Body temperature in birds. Comp. Biochem. Physiol. 99, 499–506 (1991). [Google Scholar]
- 23.Seebacher F., Elsey R. M., Trosclair III P. L., Body temperature null distributions in reptiles with nonzero heat capacity: seasonal thermoregulation in the American Alligator (Alligator mississippiensis). Physiol. Biochem. Zool. 76, 348–359 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Southern Regional Climate Center, Rockefeller Wildlife Refuge Station Information, (Louisiana State University, Baton Rouge, LA, 2017); https://www.srcc.lsu.edu/.
- 25.McKechnie A. E., Lovegrove B. G., Avian facultative hypothermic responses: A review. Condor 104, 705–724 (2002). [Google Scholar]
- 26.Daëron M., Blamart D., Peral M., Affek H. P., Absolute isotopic abundance ratios and the accuracy of Δ47 measurements. Chem. Geol. 422, 83–96 (2016). [Google Scholar]
- 27.Ghosh P., Adkins J., Affek H., Balta B., Guo W., Schauble E. A., Schrag D., Eiler J. M., 13C–18O bonds in carbonate minerals: A new kind of paleothermometer. Geochim. Cosmochim. Acta 70, 1439–1456 (2006). [Google Scholar]
- 28.Montanari S., Cracking the egg: the use of modern and fossil eggs for ecological, environmental and biological interpretation. R. Soc. Open Sci. 5, 180006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.K. E. Mikhailov, Palaeontological Association., Fossil and Recent Eggshell in Amniotic Vertebrates: Fine Structure, Comparative Morphology and Classification, Special papers in palaeontology, (Palaeontological Association, London, 1997).
- 30.Zelenitsky D. K., Hills L. V., An egg clutch ofPrismatoolithus levisoosp. nov. from the Oldman Formation (Upper Cretaceous), Devil's Coulee, southern Alberta. J. Can. Earth Sci. 33, 1127–1131 (1996). [Google Scholar]
- 31.Varricchio D. J., Horner J. R., Jackson F. D., Embryos and eggs for the Cretaceous theropod dinosaurTroodon formosus. J. Vertebr. Paleontol. 22, 564–576 (2002). [Google Scholar]
- 32.Zelenitsky D. K., Modesto S. P., Currie P. J., Bird-like characteristics of troodontid theropod eggshell. Cretac. Res. 23, 297–305 (2002). [Google Scholar]
- 33.Zelenitsky D. K., Hills L. V., Normal and pathological eggshells of Spheroolithus albertensis, oosp. nov., from the Oldman formation (Judith river group, late Campanian), southern Alberta. J. Vertebr. Paleontol. 17, 167–171 (2010). [Google Scholar]
- 34.Van Itterbeeck J., Sasaran E., Codrea V., Sasaran L., Bultynck P., Sedimentology of the Upper Cretaceous mammal- and dinosaur-bearing sites along the Râul Mare and Barbat rivers, Haeg Basin, Romania. Cretac. Res. 25, 517–530 (2004). [Google Scholar]
- 35.Grellet-Tinner G., Codrea V., Folie A., Higa A., Smith T., First evidence of reproductive adaptation to "island effect" of a dwarf Cretaceous Romanian Titanosaur, with embryonic integument in ovo. PLOS ONE 7, e32051 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Csiki Z., Codrea V., Jipa-Murzea C., Godefroit P., A partial titanosaur (Sauropoda, Dinosauria) skeleton from the Maastrichtian of Nălaţ-Vad, Haţeg Basin, Romania. Neues Jahrbuch Fur Geologie Und Palaontologie-Abhandlungen 258, 297–324 (2010). [Google Scholar]
- 37.Stein K., Csiki Z., Rogers K. C., Weishampel D. B., Redelstorff R., Carballido J. L., Sander P. M., Small body size and extreme cortical bone remodeling indicate phyletic dwarfism in Magyarosaurus dacus (Sauropoda: Titanosauria). Proc. Natl. Acad. Sci. 107, 9258–9263 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Loeuff J., Romanian late cretaceous dinosaurs: big dwarfs or small giants? Hist. Biol. 17, 15–17 (2005). [Google Scholar]
- 39.Grigorescu D., The “Tuştea puzzle” revisited: Late Cretaceous (Maastrichtian)Megaloolithuseggs associated withTelmatosaurushatchlings in the Haţeg Basin. Hist. Biol. 29, 627–640 (2016). [Google Scholar]
- 40.Botfalvai G., Csiki-Sava Z., Grigorescu D., Vasile Ş., Taphonomical and palaeoecological investigation of the Late Cretaceous (Maastrichtian) Tuştea vertebrate assemblage (Romania; Haţeg Basin) – insights into a unique dinosaur nesting locality. Palaeogeogr. Palaeoclimatol. Palaeoecol. 468, 228–262 (2017). [Google Scholar]
- 41.Benton M. J., Csiki Z., Grigorescu D., Redelstorff R., Sander P. M., Stein K., Weishampel D. B., Dinosaurs and the island rule: The dwarfed dinosaurs from Haţeg Island. Palaeogeogr. Palaeoclimatol. Palaeoecol. 293, 438–454 (2010). [Google Scholar]
- 42.Versteegh E. A. A., Vonhof H. B., Troelstra S. R., Kaandorp R. J. G., Kroon D., Seasonally resolved growth of freshwater bivalves determined by oxygen and carbon isotope shell chemistry. Geochem. Geophys. Geosyst. 11, Q08022 (2010). [Google Scholar]
- 43.McAllan B. M., Geiser F., Torpor during reproduction in mammals and birds: dealing with an energetic conundrum. Integr. Comp. Biol. 54, 516–532 (2014). [DOI] [PubMed] [Google Scholar]
- 44.T. D. J. England, The University of British Columbia, Vancouver, Canada (1984).
- 45.Stilla A., Géologie de la région de Haţeg-Cioclovina-Pui-Băniţa (Carpathes Méridionales). Anuarul Instituţului de Geologie şi Geofizica 66, 91–173 (1985). [Google Scholar]
- 46.Sclater J. G., Jaupart C., Galson D., The heat flow through oceanic and continental crust and the heat loss of the Earth. Rev. Geophys. 18, 269–311 (1980). [Google Scholar]
- 47.Paladino F. V., O’Connor M. P., Spotila J. R., Metabolism of leatherback turtles, gigantothermy, and thermoregulation of dinosaurs. Nature 344, 858–860 (1990). [Google Scholar]
- 48.Super J. R., Chin K., Pagani M., Li H., Tabor C., Harwood D. M., Hull P. M., Late Cretaceous climate in the Canadian Arctic: Multi-proxy constraints from Devon Island. Palaeogeogr. Palaeoclimatol. Palaeoecol. 504, 1–22 (2018). [Google Scholar]
- 49.Erickson G. M., Rauhut O. W. M., Zhou Z. H., Turner A. H., Inouye B. D., Hu D. Y., Norell M. A., Was dinosaurian physiology inherited by birds? reconciling slow growth in Archaeopteryx. PLOS ONE 4, e7390 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.K. Schmidt-Nielsen, Scaling: Why is Animal Size so Important? (Cambridge Univ. Press., New York, NY, 1984).
- 51.Lee M. S. Y., Cau A., Naish D., Dyke G. J., Sustained miniaturization and anatomical innovation in the dinosaurian ancestors of birds. Science 345, 562–566 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Brusatte S. L., Lloyd G. T., Wang S. C., Norell M. A., Gradual assembly of avian body plan culminated in rapid rates of evolution across the dinosaur-bird transition. Curr. Biol. 24, 2386–2392 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Chen P.-J., Dong Z.-m., Zhen S.-n., An exceptionally well-preserved Theropod dinosaur from the Yixian Formation of China. Nature 391, 147–152 (1998). [Google Scholar]
- 54.Godefroit P. S., Sofia M., Dhouailly D., Bolotsk Y. L., Sizov A. V., McNamara M. E., Benton M. J., Spagna P., A Jurassic ornithischian dinosaur from Siberia with both feathers and scales. Science 345, 451–455 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Yang Z., Jiang B., McNamara M. E., Kearns S. L., Pittman M., Kaye T. G., Orr P. J., Xu X., Benton M. J., Pterosaur integumentary structures with complex feather-like branching. Nat. Ecol. Evol. 3, 24–30 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Douglas P. M. J., Affek H. P., Ivany L. C., Houben A. J. P., Sijp W. P., Sluijs A., Schouten S., Pagani M., Pronounced zonal heterogeneity in Eocene southern high-latitude sea surface temperatures. Proc. Natl. Acad. Sci. U.S.A. 111, 6582–6587 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Came R. E., Brand U., Affek H. P., Clumped isotope signatures in modern brachiopod carbonate. Chem. Geol. 377, 20–30 (2014). [Google Scholar]
- 58.Zaarur S., Affek H. P., Stein M., Last glacial-Holocene temperatures and hydrology of the Sea of Galilee and Hula Valley from clumped isotopes in Melanopsis shells. Geochim. Cosmochim. Acta 179, 142–155 (2016). [Google Scholar]
- 59.Evans D., Sagoo N., Renema W., Cotton L. J., Muller W., Todd J. A., Saraswati P. K., Stassen P., Ziegler M., Pearson P. N., Valdes P. J., Affek H. P., Eocene greenhouse climate revealed by coupled clumped isotope-Mg/Ca thermometry. Proc. Natl. Acad. Sci. U.S.A. 115, 1174–1179 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Werner J., Griebeler E. M., New insights into non-avian dinosaur reproduction and their evolutionary and ecological implications: linking fossil evidence to allometries of extant close relatives. PLOS ONE 8, e72862 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eberth D. A., Brinkman D. B., Paleoecology of an estuarine, incised-valley fill in the Dinosaur Park Formation (Judith River Group, Upper Cretaceous) of Southern Alberta, Canada. Palaios 12, 43–58 (1997). [Google Scholar]
- 62.Eberth D. A., Hamblin A. P., Tectonic, stratigraphic, and sedimentologic significance of a regional discontinuity in the Upper Judith River Group (Belly River Wedge) of Southern Alberta, Saskatchewan, and Northern Montana. Can. J. Earth Sci. 30, 174–200 (1993). [Google Scholar]
- 63.F. Therrien, D. K. Zelenitsky, K. Tanaka, W. J. Sloboda, in Hadrosaurs, D. A. Eberth, D. C. Evans, Eds. (Indiana Univ. Press, Bloomington, 2014), pp. 532–539.
- 64.J. R. Horner, P. J. Currie, in Dinosaur Eggs and Babies, K. F. H. Ken Carpenter, John R. Horner, Ed. (Cambridge Univ. Press, 1994), chap. 21, pp. 312–336.
- 65.Henkes G. A., Passey B. H., Grossman E. L., Shenton B. J., Perez-Huerta A., Yancey T. E., Temperature limits for preservation of primary calcite clumped isotope paleotemperatures. Geochim. Cosmochim. Acta 139, 362–382 (2014). [Google Scholar]
- 66.Therrien F., Palaeoenvironments of the latest Cretaceous (Maastrichtian) dinosaurs of Romania: Insights from fluvial deposits and paleosols of the Transylvanian and Haţeg basins. Palaeogeogr. Palaeoclimatol. Palaeoecol. 218, 15–56 (2005). [Google Scholar]
- 67.Rueden C. T., Schindelin J., Hiner M. C., Dezonia B. E., Walter A. E., Arena E. T., Eliceiri K. W., ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18, 529 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.-Y., White D. J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A., Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Affek H. P., Eiler J. M., Abundance of mass 47 CO2 in urban air, car exhaust, and human breath. Geochim. Cosmochim. Acta 70, 1–12 (2006). [Google Scholar]
- 70.Huntington K. W., Eiler J. M., Affek H. P., Guo W., Bonifacie M., Yeung L. Y., Thiagarajan N., Passey B., Tripati A., Daëron M., Came R., Methods and limitations of ‘clumped’ CO2 isotope (Δ47) analysis by gas-source isotope ratio mass spectrometry. J. Mass Spectrom. 44, 1318–1329 (2009). [DOI] [PubMed] [Google Scholar]
- 71.Dennis K. J., Affek H. P., Passey B. H., Schrag D. P., Eiler J. M., Defining an absolute reference frame for ‘clumped’ isotope studies of CO2. Geochim. Cosmochim. Acta 75, 7117–7131 (2011). [Google Scholar]
- 72.Hammer Ø., Harper D. A. T., Ryan P. D., PAST:Paleontological Statistics software package for education and data analysis. Palaeontol. Electron. 4, 9 (2001). [Google Scholar]
- 73.Kluge T., Affek H. P., Quantifying kinetic fractionation in Bunker Cave speleothems using Δ47. Quat. Sci. Rev. 49, 82–94 (2012). [Google Scholar]
- 74.Kim S.-T., Mucci A., Taylor B. E., Phosphoric acid fractionation factors for calcite and aragonite between 25 and 75 °C: Revisited. Chem. Geol. 246, 135–146 (2007). [Google Scholar]
- 75.Kelson J. R., Huntington K. W., Schauer A. J., Saenger C., Lechler A. R., Toward a universal carbonate clumped isotope calibration: Diverse synthesis and preparatory methods suggest a single temperature relationship. Geochim. Cosmochim. Acta 197, 104–131 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/7/eaax9361/DC1
Supplementary Materials and Methods
Fig. S1. Fossil localities in southern Alberta, Canada.
Fig. S2. Paleogeographic map.
Fig. S3. Clumped isotope [Δ47(abs)] value versus temperature relationship of laboratory-precipitated carbonate calibration data.
Fig. S4. X-ray diffraction spectra of fossil mollusks.
Fig. S5. Photomicrographs of dinosaur eggshell samples.
Table S1. Specimen descriptions, localities, and isotopic values.
Data S1. Raw data for both carbonate samples and standards and CO2 standards.


