Abstract
Transfer and metabolism of vitamin D across the human placenta is required for fetal development. However, these fundamental mechanisms are not well understood and model systems are required to help understand them. The BeWo choriocarcinoma cell line is derived from extravillous trophoblast but is used as a model for villous syncytiotrophoblast and the placental barrier. Questions have been raised about the suitability of the BeWo cell line as a model for villous trophoblast. This study compares the expression of amino acid transporters and vitamin D related genes in human term placenta with the BeWo and human embryonic kidney (HEK)293 cell lines. HEK293 cells, as transporting epithelium may be more similar to placenta. Gene expression in term placenta was much more similar to HEK293 than BeWo. This study provides further evidence that the BeWo cell line is not an appropriate model for villous trophoblast and a model that more closely represents the human placenta is now required to investigate the effects of vitamin D on the placenta ex-vivo.
Keywords: vitamin D, placenta, amino acid transporters
Introduction
The placenta as the interface between the mother and fetus transports nutrients to the developing fetus. Vitamin D (calciferol) cannot be synthesised by the fetus, therefore maternal calciferol or its biologically significant metabolites 25-hydroxyvitamin D [25(OH)D] and/or 1,25-dihydroxyvitamin D [1,25(OH)2D] must be transferred across the placenta. However, the transfer and metabolism of vitamin D and its metabolites across the human placenta and the effects of vitamin D on the placenta are not well understood and model systems are required to help understand this.
During pregnancy maternal serum 1,25(OH)2D and vitamin D binding protein (DBP) levels rise, while there is no change in serum 25(OH)D. Maternal 25(OH)D levels in pregnancy show positive relationships with fetal growth [1], a factor related to risk of perinatal mortality and postnatal poor health such as obesity [2]. Specifically, low maternal 25(OH)D levels have been shown to associate with childhood fat mass [3], which in turn increases the risk of adulthood obesity. Maternal vitamin D status may affect fetal growth indirectly, via effects on placental function, or directly, following placental transfer to the fetus. Evidence that both maternal plasma 25(OH)D and DBP levels are associated with the expression of placental genes suggests that vitamin D may affect placental function [4].
Vitamin D may affect fetal growth by regulating placental function via altered gene expression. To regulate gene expression 25(OH)D is hydroxylated into 1,25(OH)2D via 1-alpha-hydroxylase (gene CYP27B1). 1,25(OH)2D binds the vitamin D receptor (VDR), which heterodimerizes with other nuclear hormone receptors such as retinoid X receptors (RXR). This mediates transcription of genes with vitamin D response elements (VDRE) in their control regions. The amount of 25(OH)D and 1,25(OH)2D available is controlled via breakdown by 24-hydroxylase (gene CYP24A1). All of these genes must be expressed in order for a tissue to respond to vitamin D. Genes whose placental expression is affected by vitamin D includes amino acid transporters [4]. These mediate placental transfer of amino acids, which are required by all fetal tissues but specifically for the formation of bone matrix. The facilitated amino acid transporters TAT1, LAT3 and LAT4 are key to placental amino acid transport as they provide net fetal amino acid transport, and their placental gene expression is associated with fetal growth [5]. Indeed, the gene expression of these was related to maternal serum 25(OH)D and/or DBP levels, as was gene expression of the accumulative transporters SNAT1 and SNAT and the exchange transporter ASCT1 [4]. The regulation of placental amino acid transporter gene expression is complex but is therefore potentially via 1,25(OH)2D activating VDR.
A suitable system or cell line is therefore required to investigate the direct effects of vitamin D on gene expression in human placenta. Primary placental trophoblast cell culture provides a system for studying these effects in human placenta, however is not always practical to use. The BeWo choriocarcinoma cell line is therefore often used as a model for villous trophoblast despite its extravillous trophoblast origin [6, 7]. However, questions have been raised about their suitability as a model of villous syncytiotrophoblast which is a transporting epithelium [8]. Other cell lines, such as human embryonic kidney (HEK)293 cells may be more similar to the transporting epithelium of placenta.
The aim of this study was to characterise the expression of genes involved in vitamin D metabolism and amino acid transport in BeWo cells and HEK293 cells.
We hypothesise that the BeWo cell line is not an appropriate model for villous trophoblast and a model that more closely represents the human placenta will be required to investigate the effects of vitamin D on the placenta ex-vivo.
Methods
Placental Samples
Term human placentas (n=102) were collected within 30 min of natural or caesarean delivery from uncomplicated term pregnancies (mean gestational age (SD), 39.8 (1.3) weeks) with written informed consent and ethical approval from the Southampton and Southwest Hampshire Local Ethics Committee (11/SC/0529).
The placenta had surrounding membranes trimmed away and the amnion removed from the basal plate. Five villous tissue samples were selected from each placenta using a stratified random sampling method (to ensure that the selected samples were representative of the placenta as a whole); the maternal decidua was cut off of each sample. Samples were snap frozen in liquid nitrogen and stored at -80°C. Prior to RNA extraction, the five samples were pooled and powdered in a frozen tissue press for each placenta. The cDNA from the 102 placental samples was pooled to make a placental tissue stock.
Primary term human cytotrophoblast culture
Cytotrophoblast cells were isolated using an adaptation of the method developed by Kliman et al. (1986) [9] as described previously [10]. Isolated cells were plated in culture medium (Dulbecco's modified Eagle's medium and Ham's F - 12 1:1, 10% heat inactivated fetal calf serum, 0.6% glutamine and the antibiotics 1% gentamicin, 0.2% penicillin and 0.2% streptomycin) onto 35 mm culture dishes (Nunc), at a density of 2.5 × 106, and were maintained in primary culture for up to 90 h at 37°C in a humidified incubator (95% air–5% CO2).
Cell Lines
BeWo and HEK293 cells were cultured at 2.5 x 105 cells per 32 mm well in Dulbecco’s modified Eagle’s medium and Ham’s F-12 1:1, with 10% Fetal Bovine Serum, plus L-glutamine, penicillin and streptomycin (Lonza, Switzerland), at 37°C in 5% CO2.
HEK293 cells were cultured with 1,25(OH)2D3 (Cayman Chemical; 0.1 nmol/l, 1 nmol/l, 10 nmol/l and 50 nmol/l), ethanol vehicle control or control media for 48 h to investigate the effect of vitamin D on target gene expression. Three independent cell culture experiments were carried out with conditions in triplicate for each experiment. Cell culture media was removed, and RNAzol® was added to the well. Cells were scrapped into RNAzol®, transferred to an eppendorf and stored at -80ºC until RNA extraction.
RNA extraction and cDNA synthesis
Total RNA was extracted from ~80% confluent cells using RNAzol (Sigma, USA) and 30 mg powdered placental tissue using the miRNeasy kit (Qiagen) according to the manufacturer’s instructions. RNA integrity was confirmed by gel electrophoresis. Total RNA (0.2 μg) was reverse transcribed with 0.5 μg random hexamer primer, 200 units (u) M-MLV reverse transcriptase, 25 u recombinant RNasin ribonuclease inhibitor and 0.5 mM each of dATP, dCTP, dGTP and dTTP in a final reaction volume of 25 μl in 1x MMLV reaction buffer (Promega, Wisconsin, USA).
Gene Expression
RT-PCR
PCR was used to determine the presence or absence of specific genes within the placenta or cell samples. Intron-spanning primers were designed using Primer3 software (National Human Genome Research Institute, USA, http://primer3.ut.ee/). PCR reactions containing 3.2 ng cDNA, 1 μmol/l forward and reverse primers, 12.5 μl of 2 X PCR Master Mix and ddH2O to a final volume of 25 μl were run on a 96 well thermal cycler. Cycling conditions were 94°C for 3 min; 40 cycles of 94°C for 30 s, primer specific annealing temperature for 30 s and 72°C for 30 s; and 72°C for 7 min. Samples were visualised by gel electrophoresis.
qRT-PCR
Reference gene stability and mRNA expression level differed between BeWo, HEK293 and term placenta. UBC was the only gene whose mRNA levels were not different between placenta, BeWo and HEK293 [11]. ATP5B and SDHA were used for normalising vitamin D treated HEK293 samples, as these did not change with vitamin D treatment.
Reference gene (Primer Design Perfect Probe) and genes of interest (Roche Probe Library) mRNA levels of were measured by qRT-PCR using a LightCycler 480 with 2X Master Mix (Roche, UK). For each PCR assay, samples (4 ng cDNA) were run alongside a standard curve and negative controls in triplicate. For Roche Universal Probe Library probes, the cycle parameters were 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. For Primer Design Perfect Probes the cycle parameters were 95°C for 10 min, followed by 40 cycles of 95°C for 10 s and 60°C and 72°C for 15 s. Intra-assay CV’s for each gene were 5-8%.
Data Analysis
Data are presented as mean ± SEM. mRNA expression was normalised to the reference gene UBC or ATP5B and SDHA as determined using the geNorm algorithm [12] with qbasePLUS software (v.3.4 Biogazelle BE, Belgium). Data were tested for normal distribution and log transformed if not normally distributed. Differences between mRNA expression levels were assessed using one-way ANOVA with a Dunnett’s post-hoc test. P < 0.05 was considered statistically significant.
Results
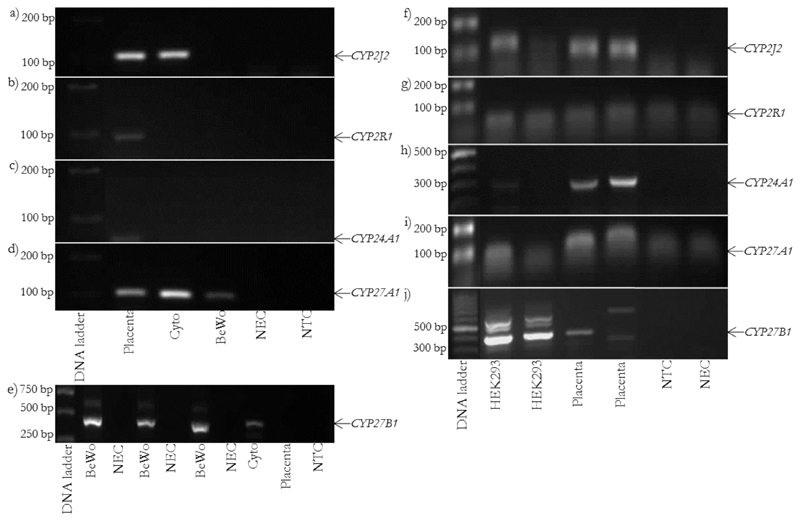
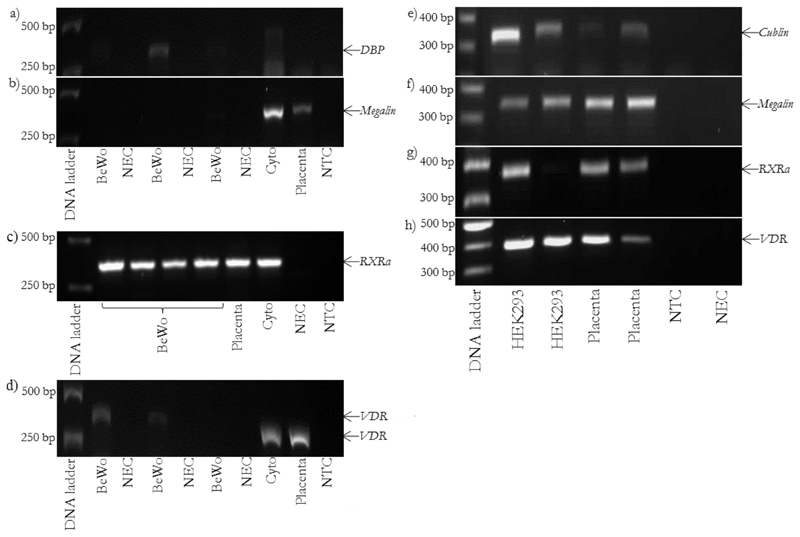
Genes involved in vitamin D metabolism and transport are expressed in human placenta, cytotrophoblast and HEK293 cells but not BeWo cells (Figure 1 & 2).
Figure 1.
Genes involved in vitamin D metabolism are expressed in placenta, cytotrophoblast and HEK293 cells but not BeWo cells. rt-PCR images for BeWo: a) CYP2J2, product size 109 bp, b) CYP2R1, product size 96 bp, c) CYP24A1, product size 66 bp, d) CYP27A1, product size 105 bp and e) CYP27B1, product size 438 bp. rt-PCR images for HEK293: f) CYP2J2, product size 109 bp, g) CYP2R1, product size 96 bp, h) CYP24A1, product size 329 bp, i) CYP27A1, product size 105 bp and j) CYP27B1, product size 438 bp. Cyto = placental cytotrophoblast.
Figure 2.
Genes involved in vitamin D transport and signalling are expressed in placenta, cytotrophoblast and HEK293 cells but not BeWo cells. rt-PCR images for BeWo: a) DBP, product size 336 bp, b) megalin, product size 344 bp, c) RXRα, product size 352 bp and d) VDR, product size 384 bp. rt-PCR images for HEK293: e) cubilin, product size 305 bp, f) megalin, product size 344 bp, g) RXRα, product size 352 bp and h) VDR, product size 384 bp. Cyto = placental cytotrophoblast.
BeWo cells lacked some major components required to metabolise and respond to vitamin D, such as VDR and CYP24A1.
Relative mRNA expression levels of the transporters LAT3 and LAT4 differed between BeWo, HEK293 and placenta (Figure 3). LAT3 mRNA expression was higher in HEK293 compared to placenta (p < 0.05) and BeWo (p < 0.001), and lower in BeWo compared to placenta (p < 0.001). LAT4 mRNA expression was lower in HEK293 (p < 0.001) and BeWo (p < 0.05) compared to placenta. TAT1 mRNA expression was lower in BeWo compared to placenta (p < 0.001).
Figure 3.
Comparison of facilitated amino acid transporter genes in BeWo HEK293 and placenta. LAT3 (a) and LAT4 (b) relative mRNA expression levels differed in BeWo and HEK293 cells compared to placenta as measured by qRT-PCR. c) TAT1 relative mRNA expression levels differed in BeWo cells compared to placenta and HEK293. Data are mean ± SD. BeWo n = 10, HEK293 n = 9, placenta n = 10. * p < 0.05, *** p < 0.001.
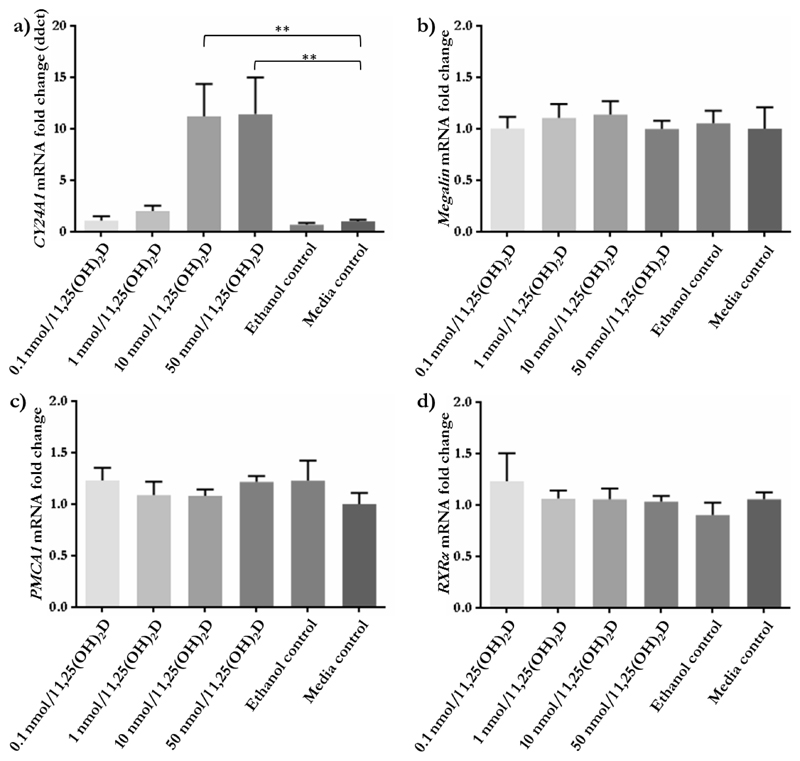
As HEK293 cells were identified as expressing most of the components required for vitamin D transport and signalling, these were used to investigate the effect of vitamin D (1,25(OH)2D3) on nutrient transporter and vitamin D-related gene expression. Treatment of HEK293 cells with the active form of vitamin D showed little impact on mRNA expression. Of the genes involved in vitamin D and calcium function that were tested, only CYP24A1 showed increased expression at the two highest concentrations of 1,25(OH)2D3 (p < 0.01; Figure 4), while none of the amino acid transporters investigated showed alterations to mRNA expression (Figure 5).
Figure 4.
Relative mRNA expression of vitamin D and calcium related genes in response to 1,25(OH)2D3 in HEK293 cells. a) CYP24A1 mRNA expression was significantly increased by 10 and 50 nmol/l 1,25(OH)2D3 compared to media control. b) Megalin mRNA was not altered in response to 1,25(OH)2D3. c) PMCA1 mRNA levels were not affected by 1,25(OH)2D3. d) RXRα mRNA levels were unaffected by 1,25(OH)2D3 treatment. 0.1 nmol/l 1,25(OH)2D3 n = 8, 1 nmol/l 1,25(OH)2D3 n = 9, 10 nmol/l 1,25(OH)2D3 n = 7, 50 nmol/l 1,25(OH)2D3 n = 8, ethanol control n = 8, media control n = 8. ** p < 0.01. Data presented as mean fold change + SEM.
Figure 5.
Relative mRNA expression of amino acid transporters in response to 1,25(OH)2D3 in HEK293 cells. mRNA expression of a) ASCT1, b) LAT3, c) LAT4, d) SNAT1, e) SNAT2 and f) TAT1 was unaffected by 1,25(OH)2D3. 0.1 nmol/l 1,25(OH)2D3 n = 8, 1 nmol/l 1,25(OH)2D n = 9, 10 nmol/l 1,25(OH)2D3 n = 7, 50 nmol/l 1,25(OH)2D3 n = 8, ethanol control n = 8, media control n = 8. Data presented as mean fold change + SEM.
Discussion
The transfer and metabolism of vitamin D and its metabolites across the human placenta and the effects of vitamin D on the placenta are not well understood and model systems are required to help understand this. We demonstrate that BeWo cells are a poor model for studying specific placental amino acid transporter and vitamin D related genes. The non-trophoblast HEK293 cells, which like syncytiotrophoblast have an epithelial phenotype, provided a better match to placental gene expression levels than BeWo cells for amino acid transporter, reference and vitamin D handling genes.
Primary placental trophoblast cell culture provides a system for studying human placenta. However, this is not always practical to use, as the method is reliant on the availability of fresh human placental tissue and an efficient, lengthy trophoblast cell isolation procedure. The BeWo cell line is often used as a model for villous trophoblast despite originating from choriocarcinoma derived from extravillous trophoblast. This study shows that BeWo cells have a very low expression of amino acid transporters involved in transepithelial amino acid transport. In contrast, the epithelial derived HEK293 cells more closely matched the facilitated amino acid transporter expression profile observed in placenta, potentially making it a better model to study the regulation of these transporters than BeWo, which may not require a nutrient transport system due to their extravillous trophoblast origins.
VDR expression is essential for cells to respond to vitamin D and initiate vitamin D mediated changes to gene expression. We could not detect VDR expression in BeWo cells, nor could we detect expression of megalin and cubilin which may be important for vitamin D transport [13, 14]. Lack of VDR expression has previously been described in BeWo [15] and suggests BeWo may not respond to vitamin D. In contrast HEK293 more closely resembled placenta in terms of the vitamin D handling genes that were detected.
Our findings support other studies showing components normally expressed in placenta, such as the renin-angiotensin system, are not expressed in BeWo cells. This may be because of altered methylation within BeWo due to its cancerous origins [8, 16]. HEK293 cells, although not placental in origin, are an epithelial barrier like placenta with potentially similar nutrient transport systems and thus may provide a model for placental nutrient transport.
However, culture of this cell line with vitamin D showed no effects on nutrient transporter expression. It is currently unclear whether this is a cell-specific effect in the HEK293 cell line or whether this also applies to human placenta. Differences in the response to vitamin D could arise due to the different functions of the placenta and kidney. A model that more closely represents the human placenta is now required, such as human placental villous fragment culture, to explore this idea further.
In conclusion, BeWo did not match placenta in terms of reference, amino acid transport or vitamin D related gene expression, meaning they are not a suitable model for studying vitamin D mediated effects on placental gene expression. The epithelial HEK293 cells are a better match for placenta in terms of these genes and it may be that cells with a similar function provide a more appropriate model for villous trophoblast than extravillous trophoblast cell lines. This highlights the need for better placental villous trophoblast cell models and that researchers must consider the question being asked when using BeWo cells as a model for human placental transport.
References
- [1].Harvey NC, Holroyd C, Ntani G, Javaid K, Cooper P, Moon R, Cole Z, Tinati T, Godfrey K, Dennison E, Bishop NJ, et al. Vitamin D supplementation in pregnancy: a systematic review. Health Technol Assess. 2014;18(45):1–190. doi: 10.3310/hta18450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiological reviews. 2014;94(4):1027–76. doi: 10.1152/physrev.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Crozier SR, Harvey NC, Inskip HM, Godfrey KM, Cooper C, Robinson SM. Maternal vitamin D status in pregnancy is associated with adiposity in the offspring: findings from the Southampton Women's Survey. Am J Clin Nutr. 2012;96(1):57–63. doi: 10.3945/ajcn.112.037473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cleal JK, Day PE, Simner CL, Barton SJ, Mahon PA, Inskip HM, Godfrey KM, Hanson MA, Cooper C, Lewis RM, Harvey NC. Placental amino acid transport may be regulated by maternal vitamin D and vitamin D-binding protein: results from the Southampton Women's Survey. The British journal of nutrition. 2015;113(12):1903–10. doi: 10.1017/S0007114515001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cleal JK, Glazier JD, Ntani G, Crozier SR, Day PE, Harvey NC, Robinson SM, Cooper C, Godfrey KM, Hanson MA, Lewis RM. Facilitated transporters mediate net efflux of amino acids to the fetus across the basal membrane of the placental syncytiotrophoblast. The Journal of physiology. 2011;589(Pt 4):987–97. doi: 10.1113/jphysiol.2010.198549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fang J, Mao D, Smith CH, Fant ME. IGF regulation of neutral amino acid transport in the BeWo choriocarcinoma cell line (b30 clone): evidence for MAP kinase-dependent and MAP kinase-independent mechanisms. Growth hormone & IGF research : official journal of the Growth Hormone Research Society and the International IGF Research Society. 2006;16(5–6):318–25. doi: 10.1016/j.ghir.2006.08.002. [DOI] [PubMed] [Google Scholar]
- [7].Jones HN, Ashworth CJ, Page KR, McArdle HJ. Cortisol stimulates system A amino acid transport and SNAT2 expression in a human placental cell line (BeWo) Am J Physiol Endocrinol Metab. 2006;291:E596–E603. doi: 10.1152/ajpendo.00359.2005. [DOI] [PubMed] [Google Scholar]
- [8].Wang Y, Pringle KG, Chen YX, Zakar T, Lumbers ER. Regulation of the renin-angiotensin system (RAS) in BeWo and HTR-8/SVneo trophoblast cell lines. Placenta. 2012;33(8):634–9. doi: 10.1016/j.placenta.2012.05.001. [DOI] [PubMed] [Google Scholar]
- [9].Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., 3rd Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118(4):1567–82. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- [10].Desforges M, Whittaker H, Farmer E, Sibley CP, Greenwood SL. Effects of taurine depletion on human placental syncytiotrophoblast renewal and susceptibility to oxidative stress. Advances in experimental medicine and biology. 2015;803:63–73. doi: 10.1007/978-3-319-15126-7_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Simner C, Novakovic B, Lillycrop KA, Bell CG, Harvey NC, Cooper C, Saffery R, Lewis RM, Cleal JK. DNA methylation of amino acid transporter genes in the human placenta. Placenta. 2017;60:64–73. doi: 10.1016/j.placenta.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalizarion of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology. 2002;3(7):1–12. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Chrsitensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- [14].Nykjaer A, Fyfe JC, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, de la Chapelle A, Moestrup SK, Ray R, et al. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3) Proceedings of the National Academy of Sciences of the United States of America. 2001;98(24):13895–900. doi: 10.1073/pnas.241516998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pospechova K, Rozehnal V, Stejskalova L, Vrzal R, Pospisilova N, Jamborova G, May K, Siegmund W, Dvorak Z, Nachtigal P, Semecky V, et al. Expression and activity of vitamin D receptor in the human placenta and in choriocarcinoma BeWo and JEG-3 cell lines. Molecular and cellular endocrinology. 2009;299(2):178–87. doi: 10.1016/j.mce.2008.12.003. [DOI] [PubMed] [Google Scholar]
- [16].Wang Y, Pringle KG, Lumbers ER. The effects of cyclic AMP, sex steroids and global hypomethylation on the expression of genes controlling the activity of the renin–angiotensin system in placental cell lines. Placenta. 2013;34(3):275–280. doi: 10.1016/j.placenta.2012.12.018. [DOI] [PubMed] [Google Scholar]