Abstract
Objectives
To evaluate patients’ preferences for sarcopenia outcomes.
Design
Discrete-choice experiment (DCE).
Setting and participants
Community-dwelling individuals aged over 65 years suffering from sarcopenia recruited in Belgium, France, Germany, Italy, Spain and Switzerland, who visited the clinic and were cognitively able to understand and fill out the survey.
Methods
In the DCE survey, participants were repetitively asked to choose which one of two patients suffering from sarcopenia deserves the most a treatment. The two patients presented different levels of risk for five pre-selected sarcopenia outcomes: quality of life, mobility, domestic activities, fatigue and falls. The DCE included 12 choice sets. Mixed logit panel model was used to estimate patients’ preferences and latent class model was conducted to identify profiles of responses.
Results
A total of 216 sarcopenic persons were included for the analysis (68% women; mean age 78 years). All five pre-selected sarcopenia outcomes were shown to be significant. Overall, the most important sarcopenia outcome was mobility (30%) followed by the ability to manage domestic activities (22%), the risk of falls (18%), fatigue (17%) and quality of life (14%). The latent class model identified two classes of respondents. In the first class (probability of 56%), participants valued mobility the most (42%) followed by ability to manage domestic activities (23%) and risk of falls (17%). In the second class, fatigue was the most important outcome (27%) followed by domestic activities (19%) and risk of falls (19%). No statistically significant associations between the latent classes and socio-demographic characteristics were found.
Conclusion/implications
This study suggests that all five pre-selected outcomes were important for sarcopenic older individuals. Overall, the most important outcomes were mobility and ability to manage domestic activities, although variations in preferences were observed between respondents. This could help in incorporating patient preferences when designing appropriate solutions for individuals with sarcopenia.
Keywords: Discrete-Choice Experiment, Sarcopenia, Outcomes, Patient Preferences
Introduction
It is now largely acknowledged that sarcopenia represents an individual as well as a considerable public health burden 1–3 that can lead to a plethora of health consequences. Recently, a systematic review tried to provide a valid list of outcomes associated with sarcopenia identified through published studies 4. Little is however known about how the patients themselves value these outcomes. Understanding which sarcopenia outcomes are the most important is highly relevant for clinicians when trying to understand patients` concerns. In addition, improved insights into patients’ preferences on sarcopenia outcomes might and should have an impact on the design of future treatments and of the necessary clinical studies (e.g. incorporation of primary endpoints). Product development and acceptance can benefit from knowledge about what patients value and what they prefer in the context of their disease and available treatment options 5.
To get insight into important sarcopenia outcomes, as a first step, we identified and prioritized the five most important outcomes for patients with sarcopenia based on consecutively a systematic review, focus groups with patients and expert discussions 6. As a next step, it is important to know how patients make trade-offs between these outcomes. This study aimed therefore to assess the preferences of participants across Europe for sarcopenia outcomes using a discrete-choice experiment (DCE).
Methods
In the DCE survey, participants were presented with a series of choices and asked in each to select the patient among two hypothetical patients suffering from sarcopenia which one needed the most a treatment. The hypothetical patients were described by a set of attributes which were further specified by attributes` levels. Good research practices for stated-preference studies were followed 7, 8.
Attributes and levels
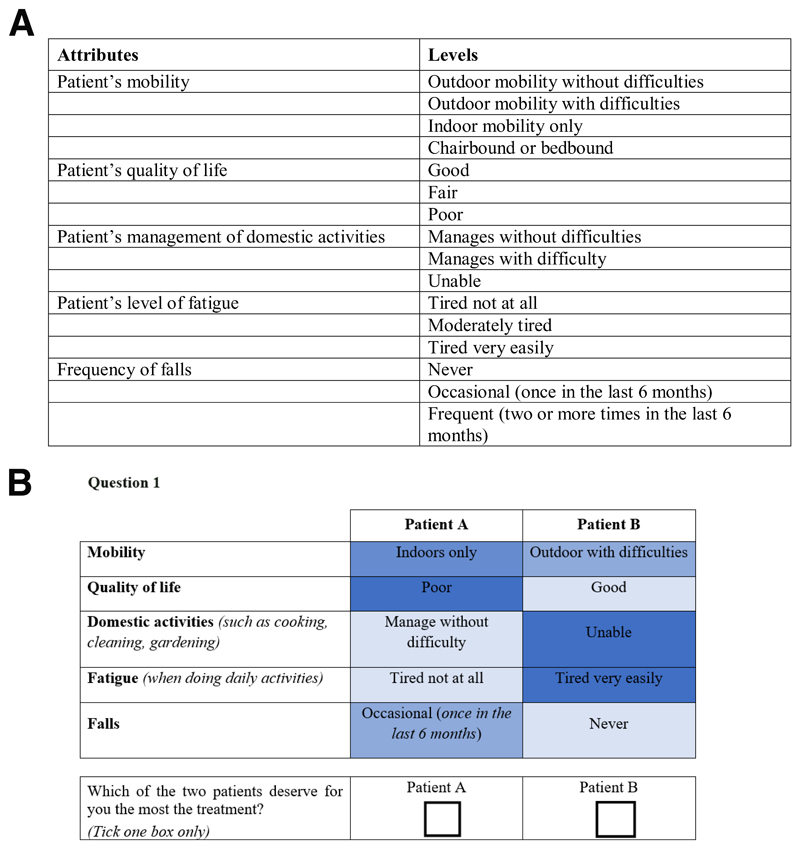
The identification and prioritization of sarcopenia outcomes was conducted following a 4-step procedure: a literature review, an expert consultation, focus groups with participants suffering from sarcopenia and an expert meeting. More details about these four stages are presented in Beaudart et al. 6. The five sarcopenia outcomes included in the DCE were mobility, quality of life, ability to manage domestic activities, level of fatigue and risk of falls (see Figure 1A).
Figure 1.
A: Attributes and levels included in the DCE
B: Example choice set of the DCE questionnaire
Experimental design
A subset of choice sets to be presented to the respondents was selected based on efficient design using Ngene software (Version 1.1.1, http://www.choice-metrics.com). A total of 24 choice tasks was designed and blocked into two versions of the questionnaire containing 12 choice tasks each. A dominance test - a choice set with one hypothetical patient that is clearly better than the other - was added to assess reliability of respondents’ choices 9. An example of a choice task is shown in Figure 1B.
Questionnaire
The questionnaire was paper-based. Data on participants’ demographics and socioeconomic characteristics were also collected. The English version of the questionnaire was pilot tested with 10 sarcopenia experts/clinicians and 20 sarcopenic older persons to check interpretation problems, face validity and length of the questionnaire. Only minor changes to layout were made. The questionnaire was then translated into additional languages. The questionnaire is available upon request from the corresponding author.
Subject selection and data collection
The study was conducted in six European countries (between November 2017 and December 2018) in community-dwelling persons 65 years of age and older suffering from sarcopenia and visiting the clinic. Sarcopenia was diagnosed according to valid published definitions (EWGSOP, FNIH, IWGS) 10–12. Only participants who were cognitively able to understand and fill out the questionnaire were included. The questionnaire was completed by the participant at the clinic or at home. In line with common rules-of-thumb for minimum sample size 13, a minimum of 200 respondents was targeted.
Approval for this study was obtained from the Medical Ethics Committee of the University of Liège that coordinated the project and in participating centers that required ethics approval for a DCE questionnaire study.
Statistical analyses
Data analysis was carried out using Nlogit software, version 5.0. Data of participants who failed the dominance test were excluded.
First, a panel mixed logit model (estimated using 1,000 Halton draws) was used which allows to capture heterogeneity by estimating the standard deviation of the parameter’s distribution. A standard deviation significantly different from zero is interpreted as evidence of significant preference heterogeneity for the attribute/level in the sample. Analyses were conducted for the whole sample as well as per country. All variables were included as effects-coded categorical variables that were normally distributed. Using effect coding, mean attributes are normalized to zero and preference weights are relative to the mean effect of the different levels of the attribute.
Using the range method 8, the relative importance of attributes was calculated by measuring the difference between the highest and lowest coefficient for the levels of the respective attribute. The relative importance is then calculated by dividing the attribute-specific level range by the sum of all attribute level ranges.
Second, a latent class model was used to determine preference profiles of respondents 14. To determine the number of classes, we selected the model with the best fit based on the Akaike information criterion. To investigate if the latent classes differed according to patients’ characteristics, Chi squared tests and multinomial logistic regression were used to test whether parameters significantly differed across latent classes. These analyses were conducted with IBM SPSS 24™.
Finally, subgroup analyses were conducted to investigate potential differences between countries and socio-demographics variables. The mean age was used to create a dummy variable, and high education level included participants with a diploma from secondary school, college or university. To assess if preferences are significantly different between subgroups, a joint model taking scale heterogeneity into account 15 was estimated using interaction terms to capture systematic differences in preference between subgroups.
Results
Participants’ characteristics
A total of 245 questionnaires were completed and returned. Of those, 29 participants failed the dominance test and were excluded from the final analysis. Participants who failed the dominance test did not differ in age, gender and education level, and inclusion of these patients in an additional analysis did not affect the results and conclusions. The final sample consisted of 216 participants (46 from Belgium, 30 from France, 18 from Germany, 50 from Italy, 39 from Spain and 33 from Switzerland). The respondents had a mean age of 77.9 years, and 68% were female. Sample characteristics are shown in Appendix Table 1. On average, the task difficulty was seen as moderate with an average score of 4.22 (SD: 1.46), based on responses to a seven-point scale (one for extremely easy).
Mixed logit models
The panel mixed logit model results are presented in Table 1. All five pre-selected sarcopenia outcomes were significant and thus important for respondents. All coefficients had the expected sign. Overall, the most important sarcopenia outcome was mobility (30%) followed by the ability to manage domestic activities (22%), the risk of falls (18%), fatigue (17%) and quality of life (14%). Given the significant standard deviation for most coefficients (with the exception of quality of life), variations in preferences between participants were observed for all attributes.
Table 1. Results from the panel mixed logit model.
| Attributes and levels | Estimate (95% CI) | Standard deviation a | Relative importance |
|---|---|---|---|
| Constant | |||
| Patient’s mobility | 29.9% | ||
| Outdoor mobility without difficulties | -1.1532*** (-0.94;1.37) | 0.9327*** | |
| Outdoor mobility with difficulties | 0.0246 (-0.13;0.18) | 0.6002*** | |
| Indoor mobility only | 0.1702* (-0.01;0.35) | 0.6120*** | |
| Chairbound or bedbound | 0.9584*** (0.64;1.27) | -- | |
| Patient’s quality of life | 13.7% | ||
| Good | -0.4732*** (-0.59;0.35) | 0.0271 | |
| Fair | -0.0213 (-0.11;0.07) | --- | |
| Poor | 0.4945***(0.37;0.62) | 0.1413 | |
| Patient’s management of domestic activities | 21.7% | ||
| Managed without difficulties | -0.8571***(-1.01;0.71) | 0.2275** | |
| Managed with difficulty | 0.1811**(0.07;0.29) | --- | |
| Unable | 0.6760***(0.53;0.82) | 0.2424*** | |
| Patient’s level of fatigue | 16.6% | ||
| Tired not at all | -0.5233***(-0.65;-0.40) | 0.2258*** | |
| Moderately tired | -0.1253**(-0.22;-0.03) | --- | |
| Tired very easily | 0.6486***(0.51;0.79) | 0.2419** | |
| Frequency of falls | 18.1% | ||
| Never | -0.6711***(-0.80;-0.54) | 0.0828*** | |
| Occasional (once in the last 6 months) | 0.0627(-0.03;0.16) | --- | |
| Frequent (≥ 2 in the last 6 months) | 0.6083***(0.47;0.75) | 0.3428*** | |
Standard deviations correspond to the random component of the model coefficients,
**P<0.05, ***P<0.01
The relative importance of attributes per country is shown in Appendix Figure 1. Mobility was the most important sarcopenia outcome in five out of the six countries. In Spain, ability to manage domestic activities was the most important outcome, followed by risk of falls and mobility. In all countries, all five pre-selected sarcopenia outcomes were significant and some variations in preferences between respondents were observed, especially for mobility.
Latent class model
The latent class model identified two classes of respondents with class probabilities of 56% and 44%, respectively (see Table 2). In the first class, participants valued mobility the most (42%) while fatigue was the most important outcome (27%) in the second class. When assessing the differences of the individual patient characteristics between the latent classes, no statistical significant differences were found.
Table 2. Latent class analysis and association between patients’ characteristics and latent class membership.
| Latent class 1 (56%) Mobility 42% Quality of life 10% Domestic activities 23% Fatigue 9% Falls 17% |
Latent class 2 (44%) Mobility 18% Quality of life 17% Domestic activities 19% Fatigue 27% Falls 19% |
|
|---|---|---|
| Belgium | 20% | 22% |
| France | 14% | 14% |
| Germany | 7% | 9% |
| Italy | 32% | 18% |
| Spain | 18% | 18% |
| Switzerland | 9% | 19% |
| Older age | 46% | 53% |
| High education | 47% | 50% |
| Women | 62% | 71% |
Subgroup analyses
Some significant differences between countries and subgroups were observed (see Appendix Table 2). In comparison with Belgium (the reference country), respondents from France, Germany and Spain has a significant lower preference for the ability to manage domestic activities. Quality of life was significantly more important in Switzerland than in Belgium. Age and gender did not have a significant effect on respondents’ preferences. Participants with high education level gave more importance to ability to manage domestic activities.
Discussion
This study suggests that all five pre-selected sarcopenia outcomes included in the DCE were important for participants. As sarcopenic older persons are affected with regard to their muscle mass, muscle strength and physical performance, mobility is often restricted in these patients. In a previous work dedicated to develop a health-related quality of life questionnaire in individuals suffering from sarcopenia, 18 out of the 55 items of the scale were targeting mobility 16. It is therefore not surprising that this outcome is of huge importance in our study. The second most important outcome is “ability to manage domestic activities”. The loss of muscle strength can impact several activities of daily living such as household tasks (like opening a bottle or jar, carrying and storing heavy objects), and older adults know that not being able to manage domestic activity may eventually mean admission to nursing home. The latent class model identified also a profile of respondent with a preference for the outcome “fatigue”. In our previous publication aiming to identify the attributes to include into this DCE 6, the outcome “fatigue” was not identified based on literature review and experts` opinion, but only during focus groups with sarcopenic older persons. Amelioration of fatigue should thus be considered as a very important therapy outcome for sarcopenic patients, as also found in patients with rheumatoid arthritis 17. This finding further highlights the need and importance to involve patients into research planning and to investigate patients’ preferences.
Although this study attempted to follow good research practices, some potential limitations exist. First, patients in this survey are younger on average than the typical sarcopenia patient. Given that we were collecting data from the patient’s perspective, we had to make sure that they were cognitively intact and reliable, so the selection of a younger cohort could be partially explain by these factors. Despite the fact that patients need to be able to understand the questionnaire, they were otherwise absolutely typical to our sarcopenia population. Selection bias and limitations in generalizability of our results can therefore not be excluded. On the other hand, older sarcopenic patients are usually disabled and that disability, in many cases due to multi morbidity, may have an impact on the results that do not reflect sarcopenia, but other conditions. Second, different number of samples and gender participants from each country were collected which could also limit generalizability. Exclusion and refusals were also not systematically collected. Third, back and forward translations of the questionnaire were not done, and a pilot study was not conducted in all countries. Fourth, although a sound methodology was used to select and define attributes, it cannot be excluded that additional attributes may play a role, at least in some countries. To maintain consistency across countries, the same list of attributes as well as levels and the same design was used in all countries. In addition, other important covariates should also be acknowledged like the severity of sarcopenia in each participant which was not systematically collected in our study. Fifth, we were unable to understand the causes of the differences between countries. Finally, while DCEs are widely used, an inherent limitation is that respondents are evaluating hypothetical options. Therefore, what respondents declare they will do may differ from what they would actually do if faced with the choice in real life.
Conclusion and implications
In conclusion, this study suggests that all five pre-selected sarcopenia outcomes were highly relevant for sarcopenia patients and that the most important outcomes were mobility and ability to manage domestic activities, although variations in preferences were observed between respondents. Assessing sarcopenic older persons’ preferences offers support to health professionals that want to improve sarcopenia management, to facilitate shared-decision making, and finally, those outcomes could further be useful when designing and evaluating appropriate healthcare programs.
Supplementary Material
Brief summary.
This study revealed patient preferences in sarcopenia outcomes, suggesting some variations in preference choices. This could help in involving patients when designing appropriate solutions.
Acknowledgements
We thank the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) for support. The authors are further grateful to the Prince Mutaib Chair for Biomarkers of Osteoporosis, King Saud University, Riyadh, Saudi Arabia, for its support, and would like to thank all patients for their participation as well as all doctors and research assistants for helping us in recruiting patients.
Funding
This work was supported by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO).
Footnotes
Conflict of interest
CB, OB and JYR are shareholders of SarQoL sprl, a spin-off of the University of Liège. The other authors have no conflict of interest relevant to the content of this study.
References
- 1.Bruyere O, Beaudart C, Ethgen O, et al. The health economics burden of sarcopenia: a systematic review. Maturitas. 2019;119:61–69. doi: 10.1016/j.maturitas.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Visser M, Schaap LA. Consequences of Sarcopenia. Clin Geriatr Med. 2011;27(3):387. doi: 10.1016/j.cger.2011.03.006. + [DOI] [PubMed] [Google Scholar]
- 3.Beaudart C, Rizzoli R, Bruyere O, et al. Sarcopenia: burden and challenges for public health. Arch Public Health. 2014;72(1):45. doi: 10.1186/2049-3258-72-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaudart C, Zaaria M, Pasleau FEO, et al. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. Plos One. 2017;12(1) doi: 10.1371/journal.pone.0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ijzerman MJ, Steuten LM. Early assessment of medical technologies to inform product development and market access: a review of methods and applications. Appl Health Econ Health Policy. 2011;9(5):331–347. doi: 10.2165/11593380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Beaudart C, Bruyère O, Cruz-Jentoft A, et al. Patient’s engagement in the identification of critical outcomes in sarcopenia. doi: 10.1016/j.jamda.2019.09.004. In submission in the JAMDA. [DOI] [PubMed] [Google Scholar]
- 7.Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health--a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–413. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Hauber AB, Gonzalez JM, Groothuis-Oudshoorn CGM, et al. Statistical Methods for the Analysis of Discrete Choice Experiments: A Report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value in Health. 2016;19(4):300–315. doi: 10.1016/j.jval.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Janssen EM, Marshall DA, Hauber AB, et al. Improving the quality of discrete-choice experiments in health: how can we assess validity and reliability? Expert Rev Pharm Out. 2017;17(6):531–542. doi: 10.1080/14737167.2017.1389648. [DOI] [PubMed] [Google Scholar]
- 10.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Bekker-Grob EW, Donkers B, Jonker MF, et al. Sample Size Requirements for Discrete-Choice Experiments in Healthcare: a Practical Guide. Patient. 2015;8(5):373–384. doi: 10.1007/s40271-015-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou M, Thayer WM, Bridges JFP. Using Latent Class Analysis to Model Preference Heterogeneity in Health: A Systematic Review. Pharmacoeconomics. 2018;36(2):175–187. doi: 10.1007/s40273-017-0575-4. [DOI] [PubMed] [Google Scholar]
- 15.Swait J, Louviere J. The Role of the Scale Parameter in the Estimation and Comparison of Multinomial Logit-Models. J Marketing Res. 1993;30(3):305–314. [Google Scholar]
- 16.Beaudart C, Biver E, Reginster JY, et al. Development of a self-administrated quality of life questionnaire for sarcopenia in elderly subjects: the SarQoL. Age Ageing. 2015;44(6):960–966. doi: 10.1093/ageing/afv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz P. Fatigue in Rheumatoid Arthritis. Curr Rheumatol Rep. 2017;19(5):25. doi: 10.1007/s11926-017-0649-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.