Summary
Vascular smooth muscle cells (VSMCs) can be derived in large numbers from human induced pluripotent stem cells (hiPSCs) for producing tissue engineered vascular grafts (TEVGs). However, hiPSC-derived TEVGs are hampered by low mechanical strength and significant radial dilation after implantation. Here we report generation of hiPSC-derived TEVGs with mechanical strength comparable to native vessels used in arterial bypass grafts by utilizing biodegradable scaffolds, incremental pulsatile stretching, and optimal culture conditions. Following implantation into a rat aortic model, hiPSC-derived TEVGs show excellent patency without luminal dilation and effectively maintain mechanical and contractile function. This study provides a foundation for future production of non-immunogenic, cellularized hiPSC-derived TEVGs comprised of allogenic vascular cells, potentially serving needs to a considerable number of patients whose dysfunctional vascular cells preclude TEVG generation via other methods.
eTOC Blurb
Luo et al. generated tissue engineering vascular grafts (TEVGs) using human induced pluripotent stem cell (hiPSC)-derived vascular smooth muscle cells. These hiPSC-derived TEVGs displayed comparable mechanical strength to that of native vessels used clinically as vascular grafts and maintained excellent patency and mechanical function following implantation into a rat model.
Grphical Abstract
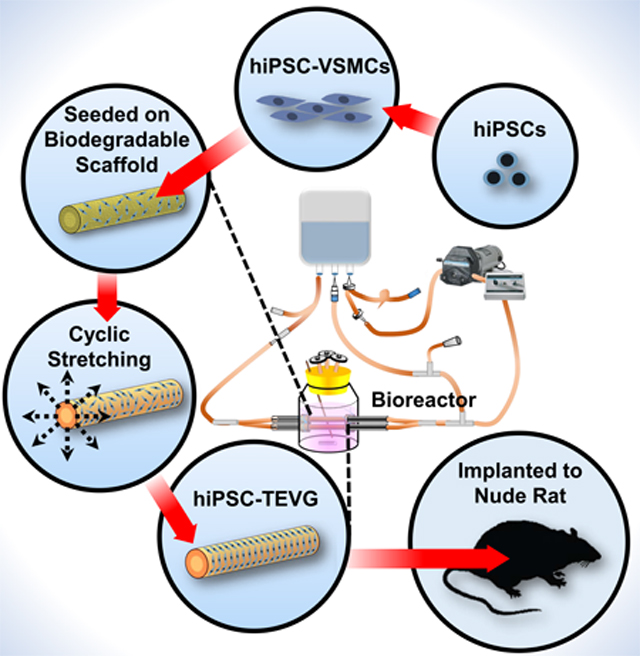
Introduction
Mechanically robust vascular grafts are in urgent clinical demand for treating cardiovascular diseases or providing hemodialysis access. While autologous or synthetic vascular grafts are clinically employed, the lack of suitable native vessels from patients or the potential risk of thrombosis and infection from synthetic materials hampers their application and efficacy (Akoh and Patel, 2010; Conte, 2013). The application of human allograft vessels from cadavers (Madden et al., 2005) has also been reported. However, potential aneurysm, calcification, and thrombosis hinder their wide-spread clinical utilization. Tissue-engineered vascular grafts (TEVGs) provide an alternative source of vascular grafts, and TEVGs with remarkable mechanical strength have been generated from human primary vascular smooth muscle cells (VSMCs) or fibroblasts (Dahl et al., 2011; McAllister et al., 2009; Syedain et al., 2017). To date, TEVGs from primary VSMCs coupled with decellularization have achieved promising results for hemodialysis access in clinical trials (Lawson et al., 2016). Acellular TEVGs, therefore, offer a readily available option for emergent vascular intervention (Elliott et al., 2019; Lawson et al., 2016; Wu et al., 2012), but should also allow for effective ingrowth and vascular remodeling for long-term engraftment and ultimate replacement of the implanted graft by host vascular tissue. However, vascular cells from a substantial number of patients who may need TEVGs could have defective proliferation or vascular remodeling due to advanced age or diseases such as diabetes (Poh et al., 2005; Spinetti et al., 2008), and as a result, may not benefit from acellular TEVGs. As such, current acellular TEVGs may not work efficaciously for a considerable patient population in need.
Alternatively, human induced pluripotent stem cells (hiPSCs) may fundamentally address the above challenges (Song et al., 2018). hiPSCs, derived from reprogramming somatic cells by ectopic expression of stem cell factors (Takahashi et al., 2007), are self-renewable and capable of differentiating into virtually any cell type in the body, including VSMCs. VSMCs have previously been generated from hiPSCs (hiPSC-VSMCs) and used for tissue fabrication and disease modeling (Atchison et al., 2017; Bajpai et al., 2012; Cheung et al., 2012; Dash et al., 2016; Eoh et al., 2017; Patsch et al., 2015; Wang et al., 2014). Moreover, recent studies reported “universal” or immunocompatible pluripotent stem cells can be produced by modulating the expression of human leukocyte antigens (HLAs) (Deuse et al., 2019; Gornalusse et al., 2017; Xu et al., 2019). This theoretically makes it possible to establish minimal or non-immunogenic hiPSC lines, with the ultimate goal of allowing allogeneic transplantation of hiPSC-derived tissue immunocompatible to any patient recipient. Thus, with the support of short-term storage for live tissue and multi-site manufacturing coordination, TEVGs generated on a large scale from “universal” hiPSC-VSMCs could provide promptly available, functional cell-based vascular grafts for patients with defective vascular remodeling in the future. To date, hiPSC-derived TEVGs (hiPSC-TEVGs) have been reported, but their continued development encountered a major obstacle in low mechanical strength and causative radial dilation in a rat aortic transplantation model (Gui et al., 2016), and would therefore not qualify for clinical transplantation. Thus, augmentation of mechanical strength is a priority before the future clinical application of hiPSC-TEVGs.
Herein, we cultured hiPSC-VSMCs onto biodegradable polyglycolic acid (PGA) scaffolds and developed hiPSC-TEVGs with remarkable mechanical strength approaching that of native vessels applied as typical arterial bypass grafts. The mechanical properties of TEVGs can be heightened by mechanical stretching to enhance collagen synthesis and proliferation of VSMCs (Kim et al., 1999; Niklason et al., 1999). We found that the incremental addition of pulsatile radial stress at 110–120 beats per minute (bpm) significantly heightened the mechanical properties of the hiPSC-TEVGs (Figure 1A). Moreover, after a four-week implantation as an interpositional aortic graft in a nude rat model, our hiPSC-TEVGs remained patent, without radial dilation, longitudinal elongation, or teratoma formation. Our current, mechanically robust hiPSC-TEVGs provide a critical foundation for addressing patients with defective vascular remodeling and pave the way for the production of “universal” hiPSC-TEVGs in the future as promptly available therapies (Figure 1B). Additionally, for patients with functional vascular remodeling, functionally comparable hiPSC-VSMCs could be produced and stocked on a large scale, eliminating the donor-donor functional variability of primary cells, which could allow for more efficient production of decellularized hiPSC-TEVGs as an off-the-shelf therapy (Figure 1C).
Figure 1. Strategies for Generating Human Induced Pluripotent Stem Cell-based Tissue Engineered Vascular Grafts.
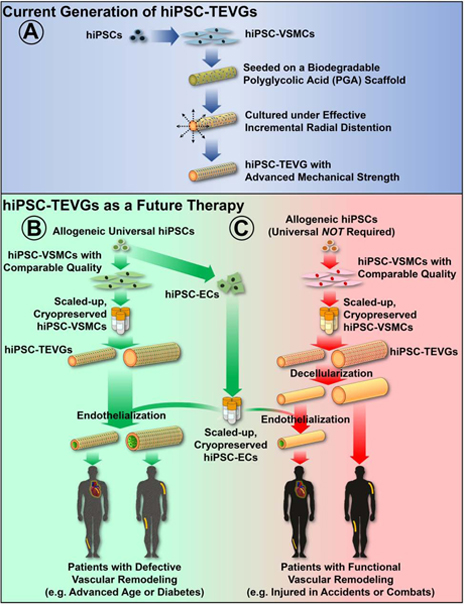
(A) Current generation of hiPSC-TEVGs and (B-C) strategies for hiPSC-TEVGs as a future therapy. For small-diameter (2–4 mm) bypass applications (e.g. coronary artery or below the knee), allogeneic, universal hiPSC-ECs would be used to coat the grafts to prevent coagulation and luminal stenosis before implantation. Large diameter (≥6 mm) TEVGs could be immediately available for vascular intervention without the need for endothelialization (e.g. above the knee or hemodialysis). Note that short-term storage and multi-site coordination for graft production and usage based on estimated clinical need are feasible and necessary for cell containing TEVG applications in Panels B and C.
Results
Robust, Large-Scale Generation of Functional hiPSC-VSMCs
Generation of hiPSC-TEVGs requires the large-scale production of hiPSC-VSMCs (Figure 1). We first optimized a method of deriving hiPSC-VSMCs (Figure S1A). In comparison with previous dispase-assisted embryoid body (EB) formation, more uniform EBs with healthier morphology were derived by EDTA-mediated hiPSC dissociation coupled with an extended medium transition from mTeSR1 (hiPSC self-renewal medium) to EB differentiation medium (Figure S1B; details see STAR Methods). After 7–10 days of culture in SmGM-2 (VSMC growth medium), hiPSC-VSMCs in a proliferative state were derived (hiPSC-VSMCs-P). To validate VSMC properties, hiPSC-VSMCs-P were induced into a mature phenotype (hiPSC-VSMCs-M) by a maturation medium. Similar to human primary VSMCs, hiPSC-VSMCs-M exhibited higher expression levels of VSMC markers including α-smooth muscle actin (α-SMA), calponin (CNN1), and smooth muscle myosin heavy chain (MYH11) as well as extracellular matrix (ECM) markers such as collagen type I (COL1) and elastin (ELN), compared with hiPSC-VSMCs-P (Figure S1C-E). hiPSC-VSMCs did not express OCT4, while hiPSCs were OCT4-positive but VSMC marker-negative (Figure S1C-E). In addition, hiPSC-VSMCs following maturation significantly reduced cell surface area in response to vasoconstrictor (carbachol), indicating their functional contractility (Figure S1F-G). As expected, hiPSC-VSMCs-M showed increased contractility compared with hiPSC-VSMCs-P.
As growth factors like FGF2 and EGF in SmGM-2 medium may reduce VSMC contractile function and collagen production (Chen et al., 2016; Schlumberger et al., 1991), both of which are essential for vascular graft engineering, we “primed” hiPSC-VSMCs-P in DMEM containing 10% FBS without exogenous FGF2 or EGF, and named them generation 2 (Gen 2) hiPSC-VSMCs-10% FBS (Figure S1H). Gen 2 hiPSC-VSMCs-10% FBS showed an enhanced expression of VSMC and ECM markers, but a similar growth rate, compared with hiPSC-VSMCs-P (Figure S1H-J). In addition, compared with those Gen 1 hiPSC-VSMCs obtained via our previous approach (Gui et al., 2016), Gen 2 hiPSC-VSMCs-10% FBS exhibited a higher proliferative rate and an enhanced expression of VSMC and ECM markers (Figure S1H-J). Thus, we elected to use Gen 2 hiPSC-VSMCs-10% FBS (“primed hiPSC-VSMCs”) in subsequent TEVG studies. We routinely attained 181.8 ± 10.3 million VSMCs from ~4.5 million hiPSCs in around 4 weeks, a ~9-fold increase of VSMC yield versus our previous approach (Gui et al., 2016).
We also evaluated the potential of lineage-specific hiPSC-VSMCs for vascular engineering (Cheung et al., 2012). However, lineage-specific hiPSC-VSMCs appeared to be less proliferative than those derived from our EB-based approach (Figure S1K). As tissue engineering requires cells to promptly expand and fill the void space in the scaffolds before the addition of mechanical stimuli, we chose to use EB-derived hiPSC-VSMCs for vascular engineering herein. Future efforts are warranted to obtain lineage specific VSMCs with enhanced proliferative capacity for vessel engineering.
Determination of Culture Medium for hiPSC-TEVGs
Our previous medium for culturing hiPSC-TEVGs contained VSMC lineage-specifying growth factors TGF-β1 and PDGF-BB (Gui et al., 2016). However, such medium led to TEVGs with limited mechanical strength. We next investigated the effects of TGF-β1 and PDGF-BB on collagen deposition, cell viability, and preservation of the VSMC phenotype in hiPSC-VSMCs grown on biodegradable PGA scaffolds. The “primed” hiPSC-VSMCs were seeded onto PGA meshes (5 mm x 5 mm) and cultured in TEVG medium containing TGF-β1 and PDGF-BB (T/P), TGF-β1 only (T/-), PDGF-BB only (-/P), or no growth factor (−/−), respectively, for three weeks to form engineered tissues (Figure 2A). Cells effectively populated tissues under all 4 medium conditions, and the tissues cultured in T/P and T/- media appeared to deposit more collagen than those grown in -/P and −/− media (Figure 2B-C). Hydroxyproline assay further confirmed that hiPSC-VSMCs produced more collagen per mesh in T/P and T/- media (Figure 2D). T/P and T/- media also appeared to induce higher expression of VSMC markers in hiPSC-VSMCs (Figure 2E and S1L). Moreover, engineered tissues in T/P or -/P medium presented a higher ratio of apoptotic cells (Figure 2F-G), suggesting that PDGF-BB may decrease the survival of hiPSC-VSMCs cultured on PGA scaffolds. Thus, a TEVG medium containing TGF-β1 without PDGF-BB (T/-) may provide optimal support for collagen production, cell viability, and maintenance of VSMC properties of hiPSC-VSMCs. In addition, hiPSC-VSMCs in T/- medium readily contracted when exposed to carbachol (Figure S1M-N). Based on the above results, we selected to focus on the T/- medium in our vessel engineering studies.
Figure 2. Optimization of TEVG Medium by Generating Engineered Tissues from Culturing hiPSC-VSMCs on Biodegradable Polyglycolic Acid Scaffolds.
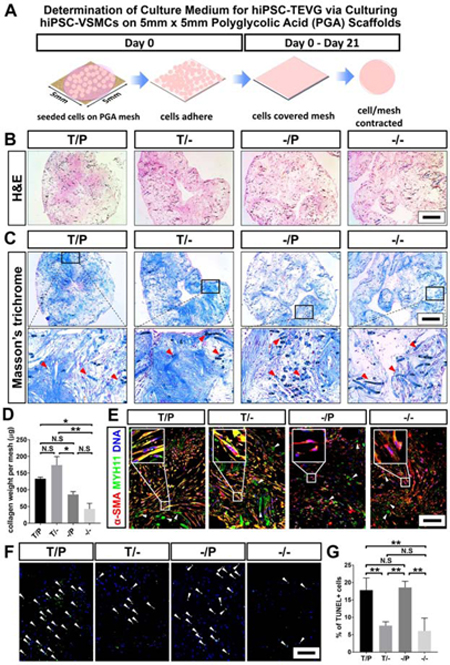
(A) Schematic illustration for developing tissue patches from “primed” hiPSC-VSMCs grown on PGA scaffolds. (B and C) H&E and Masson’s Trichrome staining of the engineered tissues derived from hiPSC-VSMCs seeded onto PGA scaffold. Red arrow heads indicate PGA remnants. Scale bars: 500 μm. (D) Collagen weight per mesh of engineered tissue via hydroxyproline assay. (E) Immunostaining of the engineered tissues from hiPSC-VSMCs. Sections were stained for VSMC markers MYH11 and α-SMA. White arrow heads indicate PGA remnants. Scale bar: 100 μm. (F) TUNEL staining of the engineered tissues from hiPSC-VSMCs. DNA was counterstained by DAPI. White arrow heads indicate the TUNEL-positive apoptotic cells. Scale bar: 100 μm. (G) Quantification of percentage of TUNEL-positive cells in engineered tissues. All data was presented as Mean ± S.E.M and analyzed using a One-way ANOVA with Tukey’s multiple comparisons test; n=3; *: p<0.05; **: p<0.01; N.S: not significant.
Generation and Characterization of hiPSC-TEVGs
We next investigated the effect of the improved TEVG medium (T/-) on the ECM production and cytoskeletal and metabolic alterations of hiPSC-VSMCs under a workload via mechanical stretching. hiPSC-VSMCs were cultured in the presence of the T/- or T/P medium under static or uniaxial cyclic stretching (2.5% distention at 2.75 Hz) with a FlexCell FX-6000T™ Tension System. Results suggest that both T/- medium and a 48-hour stretching period enhanced the expression of VSMC and ECM markers and the formation of filamentous actin bundles (phalloidin) with a preferred alignment perpendicular to the direction of stretching (Figure S2A-C). Hydroxyproline assay further revealed that both T/- medium and stretching promoted collagen deposition in hiPSC-VSMCs (Figure S2D). Moreover, stretching alone increased the expression of focal adhesion (vinculin) or adherens junction (N-cadherin) markers in hiPSC-VSMCs (Figure S2A-C).
Mechanical stretching also resulted in an increase in energy production. Since hiPSC-VSMCs were cultured in a TEVG medium based on high-glucose DMEM basal medium (~25 mM glucose), glucose was hypothesized to be the predominant energy source and further investigated. Results showed that stretching increased glucose consumption and cellular ATP production (Figure S2E-F). These findings were also reflected transcriptionally with significant upregulation of pivotal glucose metabolism-associated genes under stretching, including the glucose transporters-1 and −4 (GLUT1 and GLUT4), citrate synthase (CS; involved in mitochondrial TCA cycle metabolism), and PGC1α (a transcriptional cofactor of mitochondrial biogenesis) (Figure S2A), suggesting a degree of metabolic maturation was induced in these hiPSC-VSMCs. These results suggest that both mechanical stretching and an optimized TEVG medium would potentially promote the generation of hiPSC-based TEVGs with advanced mechanical strength.
We then fabricated TEVGs from hiPSC-VSMCs and augmented their mechanical strength with the inclusion of pulsatile radial stretching. As illustrated in Figure 3A, PGA scaffolds were sewn over silicone tubing (~3.2 mm outer diameter) and connected in bioreactors. Scaffolds were seeded with hiPSC-VSMCs and cultured statically for 1 week. PBS was then flowed by a peristaltic pump into distensible silicone tubing inserted through the vessel lumen, and pulsatile radial stress was applied to the vessels for 7 weeks. Radial stress with 1–2.5% radial distention (strain) has been applied to develop TEVGs from human primary VSMCs (Dahl et al., 2011; Poh et al., 2005). However, acute addition of 2% strain repeatedly led to the disintegration of the engineered tissue after three weeks of culture, suggesting that abrupt exposure to this level of mechanical stress may be detrimental to hiPSC-VSMCs. This phenomenon prompted us to add radial stress incrementally from week 2 to week 4, and then maintain the strain unchanged until the completion of culture. Using this mechanical regimen, hiPSC-TEVGs successfully maintained integrity during the 8-week culture. Previous studies revealed a pulse rate (bpm) of radial distention at ~165 bpm resulted in optimal mechanical strength of human or porcine primary VSMC-derived TEVGs (Dahl et al., 2011; Solan et al., 2009). We evaluated the rupture pressure of hiPSC-TEVGs developed in the presence of 1% radial distention at several pulse rates (110–120, 150–160, and 190–200 bpm). Surprisingly, distinct from human primary VSMCs, 110–120 bpm resulted in the highest rupture pressure of hiPSC-TEVGs, and rupture pressure declined dramatically along with an increase in bpm (Figure 3B). Thus, pulsatile radial stress of 110–120 bpm was applied in our hiPSC-TEVGs.
Figure 3. Generation and Characterization of TEVGs from hiPSC-VSMCs under Cyclic Stretch in a Bioreactor with Pulsatile Flow.
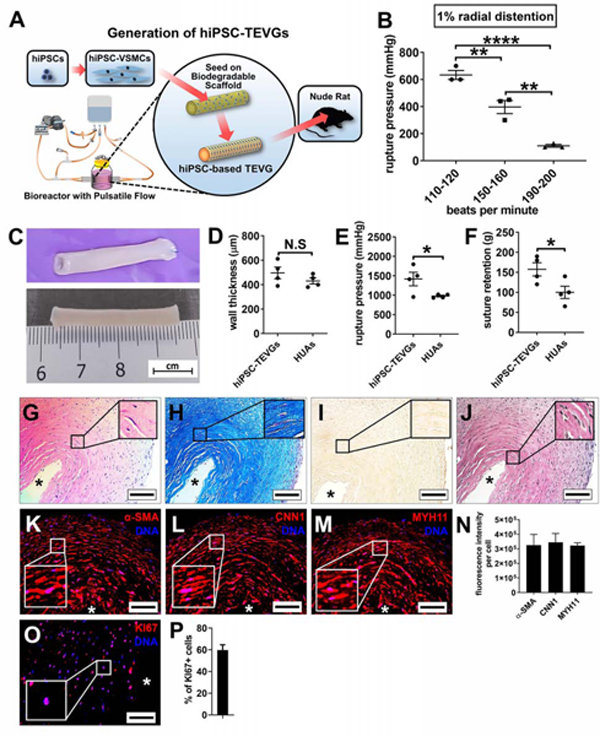
(A) Schematic illustration for generation and implantation of small caliber (3.2 mm of inner diameter) hiPSC-TEVGs. (B) The rupture pressures of hiPSC-TEVGs developed after 8 weeks of culture in the presence of pulsatile radial stress with 1% ultimate strain at different rates of 110–120, 150–160 and 190–200 bpm (One-way ANOVA with Tukey’s multiple comparisons test). Filled dots, squares and triangles indicate the values of rupture pressure for individual hiPSC-TEVGs. (C-F) Generation of hiPSC-TEVGs with 8 weeks of culture in the presence of pulsatile radial stress with 3% ultimate strain at 120 bpm: (C) Representative images of hiPSC-TEVGs after 8 weeks of culture. Scale bar: 1 cm; (D-F) The wall thickness (D), rupture pressure (E), and suture retention strength (F) of hiPSC-TEVGs and native human umbilical arteries HUAs (Two-tailed unpaired Student’s T-test was performed in Panels D-F; Dots indicate the values of results for individual hiPSC-TEVGs or HUAs). (G-J) Histological examination (H&E staining [G], Masson’s trichrome staining [H], Alizarin Red staining [I], and EVG staining [J]) of hiPSC-TEVGs were performed. Asterisk indicates the lumen of the graft. Scale bar: 100 μm. (K-N) Immunostaining of sections of hiPSC-TEVGs (K-M). Sections were stained for VSMC markers α-SMA, CNN1, and MYH11. DNA was counterstained by DAPI. Asterisk indicates the lumen of the graft. Scale bar: 100 μm; (N) Quantification of fluorescence intensity (gray value) of α-SMA, CNN1, and MYH11 per cell from 3 representative sections (>100 cells/section) in each hiPSC-TEVG. (O-P) Immunostaining of KI67 in hiPSC-TEVGs. Asterisk indicates the lumen of the graft. Scale bar: 100 μm; (P) Quantification of percentage of KI67-positive cells from 3 representative sections (>100 cells/section) in each hiPSC-TEVG. All data was presented as Mean ± S.E.M; n≥3; *: p<0.05; **: p<0.01; ****: p<0.0001; N.S: not significant.
Next, we increased the ultimate strain to 3% and maintained the pulse rate at 110–120 bpm to generate hiPSC-TEVGs. At the end of the 8-week culture period, hiPSC-TEVGs appeared opaque, similar to native vessels (Figure 3C). The wall thickness of hiPSC-TEVGs was 496.8 ± 48.0 μm (Figure 3D). Importantly, hiPSC-TEVGs showed robust mechanical properties indicated by rupture pressure (1419.0 ± 174.4 mmHg) and suture retention strength (157.5 ± 16.5 g) (Figure 3E and 3F) comparable to those of native vessels widely used as coronary artery bypass grafts (saphenous veins, rupture pressure: 1599 ± 877 mmHg; suture retention strength: 196 ± 29 g) (Dahl et al., 2011). Moreover, the mechanical strength of the hiPSC-TEVGs was markedly higher than that of native human umbilical arteries (HUA, rupture pressure: 930.4 ± 23.3 mmHg; suture retention strength: 102.5 ± 17.6 g), while the thickness was similar (Figure 3D-F). In addition, the amount of collagen in hiPSC-TEVGs was 43.1 ± 4.4% (n=4) of the dry weight of the engineered vessels, which was comparable to that of primary human VSMC-derived TEVGs (31 ± 7%) (Quint et al., 2012) or native blood vessels (HUA, 36 ± 12%) (Gui et al., 2016). In comparison to the mechanical properties of hiPSC-TEVGs previously developed without pulsatile radial stress (rupture pressure: 500 mmHg; suture retention strength: 30 and 70 g; collagen amount: 12 ± 4% of the dry weight of engineered vessels) (Gui et al., 2016), our current results revealed that the addition of incremental pulsatile radial stretching coupled with the optimized TEVG medium markedly improved the biomechanical properties of hiPSC-TEVGs.
Similar to a human native blood vessel (HUA, Figure S3A-J), histological analysis showed hiPSC-TEVGs were highly cellularized (Figure 3G), exhibited robust accretion of collagen (Figure 3H), and lacked obvious calcium deposition (Figure 3I). Similar to previous TEVGs derived from primary or hiPSC-VSMCs (Gui et al., 2016; Niklason et al., 1999), mature ELN fibers were absent in hiPSC-TEVGs (Figure 3J). PGA remnants were seldom observed in hiPSC-TEVGs (Figure 3G-J), indicating thorough scaffold degradation. Typical VSMC markers were present in the cells within the hiPSC-TEVGs (Figure 3K-N), similar to those expressed within native HUAs (Figure S3E-H). Interestingly, contrary to the minimal number of cells with positivity for the proliferation marker KI67 in native HUAs (Figure S3I-J), a significant number of cells in the hiPSC-TEVGs were positive for KI67 (Figure 3O-P), potentially due to the cell growth-promoting nature of the TEVG medium. Importantly, cells in hiPSC-TEVGs became largely quiescent under physiological conditions after in vivo implantation into the rat abdominal aorta (Figure 4O-P). These data suggest that hiPSC-TEVGs displayed the typical histological and cellular phenotypes of engineered vessels.
Figure 4. Characterization of hiPSC-TEVGs in Nude Rats 30 Days Post-Operation.
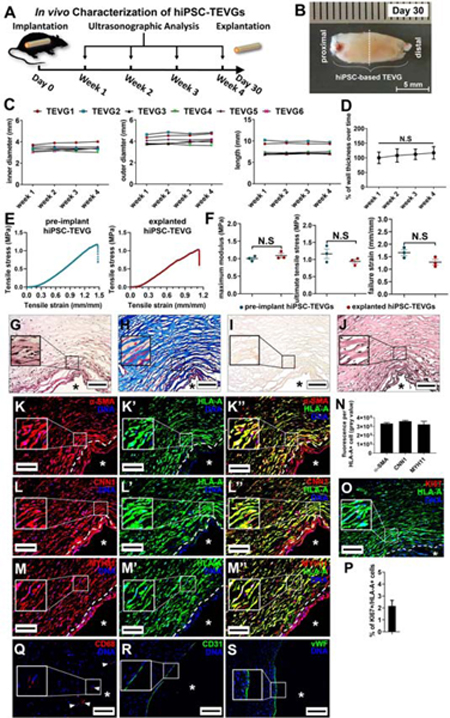
(A) Schematic illustration for implantation of hiPSC-TEVGs into nude rats and ultrasonographic analysis. (B) Representative image of explanted TEVG graft (TEVG2) 30 days post-operation. The dashed line indicates the position of sectioning. Scale bar: 5cm. (C) The inner diameters, outer diameters, and length of the 6 implanted hiPSC-TEVGs over time during 30 days of implantation by ultrasonographic analysis. (D) Wall thickness of 6 hiPSC-TEVGs during 30 days of implantation by ultrasonographic analysis (One-way ANOVA with Tukey’s multiple comparisons test). Note that wall thickness of each TEVG during the four-week implantation was normalized to the average wall thickness of TEVGs 1 week post-implantation. (E) Representative stress-strain plots of tissue rings sectioned from pre-implant or explanted hiPSC-TEVGs. (F) Mechanical parameters (maximum modulus, ultimate tensile stress, and failure strain) were compared between the tissue rings sectioned from pre-implant or explanted hiPSC-TEVGs (Two-tailed paired Student’s T-test). (G-J) Histological analysis (H&E staining [G], Masson’s trichrome staining [H], Alizarin Red staining [I], and EVG staining [J]) of explanted hiPSC-TEVGs (TEVG2). Scale bar: 100 μm. (K-S) Immunostaining of sections of hiPSC-TEVGs. The sections were stained for VSMC markers α-SMA (K, K’, K”), CNN1 (L, L’, L”), and MYH11 (M, M’, M”), and fluorescence intensity (gray value) of α-SMA, CNN1, and MYH11 per HLA-A-positive cell from 3 representative sections (>80 cells/section) in each hiPSC-TEVG was quantified (N). Cell proliferation marker KI67 was stained (O), and percentage of KI67-positive cells in HLA-A-positive cells (>80 cells/section) in each hiPSC-TEVG was quantified (P). Additionally, sections were stained for macrophage marker CD68 (Q) (positive cells indicated by white arrow heads) and endothelial cell markers CD31 (R) and vWF (S). The human specific surface antigen (HLA-A) was stained to indicate the human cells in TEVGs (K-M). The white dashed line indicates the boundary between the human cells and host rat cells (K, L, M, and O). DNA was counterstained by DAPI. Asterisk always indicates the lumen of the graft. Scale bar: 100 μm. All data was presented as Mean ± S.E.M; n≥3; N.S: not significant.
Implantation of hiPSC-TEVGs into Nude Rats
Six hiPSC-TEVGs were then implanted, as an interpositional graft, into the abdominal aorta of 6 nude rats (Figure 4A). All rats were followed for 30 days, and the morphometry of implanted hiPSC-TEVGs (mid-graft inner diameter, mid-graft outer diameter, and length) were assessed weekly via ultrasonography (Figure 4A). All implanted grafts were patent when harvested, and no sign of rupture or aberrant deformation was observed in any of the explants (Figure 4B), potentially due to the high mechanical strength of the grafts (Figure 3E-F). No sign of teratoma formation was observed in any animals. Ultrasonography revealed that the grafts did not present any evident dilation, elongation, or wall thickening (Figure 4C-D). Limited thrombosis was observed at distal ends of some TEVGs, possibly due to the lack of an endothelial lining prior to graft implantation in this proof-of-principle study, and/or the potential intraluminal turbulence of blood flow. These results indicated that the hiPSC-TEVGs were able to withstand aortic blood pressure during the period of evaluation.
Next, we examined the mechanical properties of the explanted hiPSC-TEVGs by cutting them into tissue rings and deriving stress-strain plots. As a reference, tissue rings made from the same hiPSC-TEVG prior to implantation were also measured for comparison. The mechanical properties, including maximum modulus, ultimate tensile stress, and failure strain of tissue rings derived from hiPSC-TEVGs pre- and post-implantation, appeared to be statistically comparable (Figure 4E-F). Tissue rings derived from native porcine coronary arteries were used as a control (Figure S3K). The porcine arterial rings showed a higher failure strain, and a trend towards an enhanced ultimate tensile stress and a reduced maximum modulus compared with the hiPSC-TEVG counterparts (Figure S3L-O), potentially due to more effective ELN and collagen deposition in the native porcine vessels. We also evaluated the contractile function of the tissue rings sectioned from hiPSC-TEVGs in the presence of the vasoconstrictor carbachol (Figure S3P). No statistically significant difference was detected between the contractility of pre-implant and explanted hiPSC-TEVGs (Figure S3Q). There appeared to be a trend of decreased contractility in the explanted tissue rings (p=0.13), possibly due to suboptimal luminal lining and remodeling. In summary, these results suggest that hiPSC-TEVGs maintained mechanical strength and contractile function after implantation.
Histological analysis revealed that explanted grafts remained cellularized (Figure 4G), that extracellular collagen was abundantly deposited (Figure 4H), and there was no apparent calcification in the media of the grafts (Figure 4I). Mature ELN fibers were not detected (Figure 4J), similar to previously reported TEVGs derived from hiPSC-VSMCs or primary VSMCs (Gui et al., 2016; Niklason et al., 1999). VSMC markers were readily observed in human cells (positive for human HLA-A staining, above the white dashed line in Figure 4K-M and Figure S3R) within the grafts and were expressed in hiPSC-VSMCs in explanted TEVGs (Figure 4N) at levels comparable to hiPSC-VSMCs in pre-implant grafts (Figure 3N). A minimal number of human cells in the grafts were positive for proliferation marker KI67 (Figure 4O-P), suggesting that hiPSC-derived cells within the vessel walls were primarily in a quiescent state, which was similar to cells in the human native HUAs (Figure S3I-J). In addition, only very limited numbers of CD68+ cells were observed near the luminal side, suggesting minimal or no presence of macrophages in the grafts (Figure 4Q). Notably, some portions of the luminal surface-displayed coverage by endothelial cells from the host, revealed by CD31 and von Willebrand factor (vWF) staining (Figure 4R-S). In summary, these data suggest that hiPSC-derived cells in the engineered vessel preserved their mature VSMC phenotype following in vivo implantation.
Discussion
The current study represents the most mechanically robust derivation of TEVGs from hiPSC-VSMCs in the field, with properties approaching those of native vessels used for arterial bypass. This advancement is based on a routine production (~180 million) of functional VSMCs from self-renewable hiPSCs, an optimized TEVG culture medium, and a beneficial biophysical training regimen of hiPSC-TEVGs in a bioreactor. Importantly, hiPSC-TEVGs showed excellent patency without radial dilation or longitudinal elongation and effectively maintained both mechanical and contractile function 4 weeks after aortic implantation in rats. This study has thus established the foundation for large-scale manufacture of mechanically robust hiPSC-TEVGs as an efficacious therapy (Figure 1B-C).
Additionally, this technology could integrate immunocompatible, HLA-engineered universal hiPSC lines (Deuse et al., 2019; Gornalusse et al., 2017; Xu et al., 2019) in the future for allogeneic graft implantation as a treatment option for any patient in need (Figure 1B). We derived VSMCs from hiPSCs with enhanced immunocompatibility, in which both HLA-A and -B alleles and HLA-class II are disrupted (Figure S4A) (Xu et al., 2019). Such hiPSC-VSMCs expressed VSMC and ECM markers (Figure S4B), showed contraction in response to carbachol (Figure S4C-D), and formed vascular tissue on PGA scaffolds with effective collagen production (Figure S4E-J), suggesting the potential feasibility of vascular tissue engineering using HLA-edited, immunocompatible hiPSCs.
There are limitations and challenges remaining to be addressed in this study. The mechanisms by which TGF-β1 modulates the expression of VSMC and glucose metabolism markers via the SMAD-dependent or SMAD-independent pathway in conjunction with Myocardin/MRTF/SRF signaling (Bernard et al., 2015; Shi and Chen, 2016), as well as the correlation between metabolic activity and cellular contractility in hiPSC-VSMCs, awaits in-depth investigation. Future endeavors will also be made to understand how removal of FGF2 and EGF in the “primed” medium may relieve their antagonizing effect on TGF-β1 in regulation of VSMC marker expression.
In addition, similar to previously engineered vessels (Dahl et al., 2011; Niklason et al., 1999), hiPSC-TEVGs did not contain mature ELN fibers, expected to be imperative for an effective treatment for patients with peripheral and coronary arterial diseases. In a longer-term in vivo, 60-day rat aortic implantation of hiPSC-TEVG, although mature ELN fibers were not observed in the medial layers, limited extracellular, discontinuous ELN fibers were detected via EVG staining (green arrows in Figure S4O). Future endeavors are warranted to perform long-term hiPSC-TEVG implantation for ELN detection. It has been shown that hiPSC-VSMCs seeded onto 3D electrospun scaffolds in a quiescent medium containing 0.5% FBS and 1 ng/ml TGF-β1 under laminar shear stress resulted in promising ELN fiber formation (Eoh et al., 2017). Further optimization is warranted to develop an optimized medium with balanced cell proliferation and ELN deposition. ELN expression in hiPSC-TEVGs could be enhanced by retinoic acid, insulin-like growth factor-1, or a nitric oxide donor (Sugitani et al., 2001; Watson et al., 2008; Wolfe et al., 1993). Moreover, ELN assembly could be reinforced through the treatment of BMP4 or LTBP4 via enhancing the deposition of essential ECM proteins that interact with and stabilize ELN (Noda et al., 2013; Tojais et al., 2017). In addition, enhancement of ELN cross-linking could be achieved by augmenting lysyl oxidase function (Smith-Mungo and Kagan, 1998; Sugitani et al., 2001).
To further enhance the biomechanical strength of hiPSC-TEVGs to approach that of the human internal thoracic artery (rupture pressure: 3196 ± 1264 mmHg; suture retention: 138 ± 50 g) (Dahl et al., 2011), optimization of pulsatile stretching from current 3% strain up to 5% will be explored. Moreover, oxygenation of the TEVG medium will be enhanced to promote cellular growth and ECM production. Additionally, endothelialization of hiPSC-TEVGs is needed to enhance their vascular function in future endeavors. In summary, the derivation of hiPSC-TEVGs with robust mechanical strength in this study will move the vessel engineering field forward and set the stage for innovative therapies for patients with cardiovascular diseases.
STAR Methods
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Yibing Qyang (yibing.qyang@yale.edu).
All unique/stable reagents generated in this study are available from the Lead Contact, Dr. Yibing Qyang, with a completed Materials Transfer Agreement.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Wildtype Human Induced Pluripotent Stem Cells
Human cell populations were derived using protocols approved by Yale University Human Investigation Committee. The previously established wildtype human induced pluripotent stem cell (hiPSC) line Y6 was employed in our research (Dash et al., 2016; Gui et al., 2016). Y6 hiPSCs were originally produced through reprogramming fibroblasts derived from discarded female neonatal skin tissue, under Yale Institutional Review Board approval, using Sendai viral particles that encode human OCT4, KLF4, SOX2, and c-MYC genes (ThermoFisher). To maintain the pluripotency, hiPSCs were expanded in mTeSR1 medium (STEMCELL Technologies) on Growth Factor Reduced (GFR)-Matrigel (Corning)-coated plates under feeder-free conditions at 37°C and were passaged every 5–7 days by Ethylenediaminetetraacetic acid (EDTA; ThermoFisher) treatment.
HLA-C-retained (CIITA-knockout) Human Induced Pluripotent Stem Cells
The human leukocyte antigen-C (HLA-C) retained (CIITA-knockout) hiPSC line (named as 585A1 hiPSCs in previously published study) was established via reprogramming peripheral blood mononuclear cells isolated from male donor by episomal vectors as previously described (Xu et al., 2019). These HLA-C-retained hiPSCs were expanded in mTeSR1 medium on GFR-Matrigel-coated plates under feeder-free conditions at 37°C and were passaged every 5–7 days by EDTA treatment.
Human Vascular Smooth Muscle Cells
Primary human vascular smooth muscle cells (VSMCs) derived from the aorta of male donors were purchased from Lonza. The primary human VSMCs were expanded in Smooth Muscle Growth Medium (SmGM-2; Lonza) on 0.1% (w/v) gelatin (Sigma-Aldrich)-treated culture dishes at 37°C and were passaged upon reach ing 80% confluency by 0.05% trypsin-EDTA (ThermoFisher) treatment. To further induce the maturation phenotype of primary VSMCs for characterization of marker expression and contractile functions, primary VSMCs grown in SmGM-2 medium were subcultured in the VSMC maturation medium (Dulbecco’s Modified Eagle Medium [DMEM; high glucose, ThermoFisher] with 1% [v/v] fetal bovine serum [Fetal Bovine Serum, FBS; Gemini], 2 mM L-glutamine [ThermoFisher], 1% [v/v] non-essential amino acid [NEAA; ThermoFisher], 1% [v/v] penicillin/streptomycin [pen/strep; ThermoFisher], 0.012 mM 2-mercaptoethanol [β-ME; ThermoFisher] and 1 ng/ml TGF-β1 [Peprotech]) for seven days.
Human Umbilical Vein Endothelial Cells
Human umbilical vein endothelial cells (HUVECs) derived from the umbilical cords of female donors were purchased from Lonza. The HUVECs were expanded in Endothelial Growth Medium (EGM-2; Lonza) on gelatin-treated culture dishes at 37°C and were passaged upon reaching 80% confluency by 0.05% trypsin-EDTA treatment.
Animal Use
NIH-Foxn1rnu nude rats were obtained from Charles River Laboratories. All experiments were performed on male nude rats 10 weeks of age, weighing about 300 g. All procedures involving animal subjects were performed under the approval of the Institutional Animal Care and Use Committee (IACUC) of the Yale School of Medicine. All animal care complied with the NIH Guidelines for the Care and Use of Laboratory Animals.
Human Umbilical Arteries
Human umbilical cords (deidentified) were obtained from Yale–New Haven Hospital (New Haven, CT), delivered at 4°C, and processed im mediately after delivery. Human umbilical arteries (HUAs) were then isolated from the umbilical cords (20–30 cm in length) within 30 minutes via sharp dissection in a sterile manner. A pair of Metzenbaum scissors were used to remove the Wharton’s jelly surrounding the HUAs. The newly isolated HUAs were then gently washed with Dulbecco’s Phosphate-Buffered Saline (PBS; ThermoFisher) containing penicillin 100U/mL and streptomycin 100 μg/mL (ThermoFisher) to remove blood clots and were immediately used for either biomechanical analysis or were subjected to histological analysis.
Porcine Coronary Arteries
The porcine coronary arteries were isolated from Yorkshire pigs (male, three-month-old) in 20 minutes after euthanization via Veterinary Clinical Services from Yale Animal Resources Center. Coronary arteries were immediately transferred to PBS containing penicillin 100U/mL and streptomycin 100 μg/mL (ThermoFisher) at 4°C. The adherent connective tissue and fat tissue were removed in a sterile manner, and the segments of arteries with inner diameters of approximately 3 mm were cut into vessel rings with 1–2 mm in length for evaluation of mechanical strength. The vessel rings from coronary arteries of three Yorkshire pigs were used to mechanical strength evaluation.
METHOD DETAILS
Generation of VSMCs from hiPSCs
The hiPSC-VSMCs were obtained via an embryoid body (EB)-based approach (Dash et al., 2016; Gui et al., 2016), with significant modifications (Figure S1A). Briefly, hiPSCs were expanded until 80% confluency and treated with 0.5 mM EDTA (ThermoFisher) for 3 minutes at 37°C on Day 0. The dissociated cells (3 wells of a 6-well plate) were resuspended in mTeSR1 medium supplemented with 1:100 (v/v) GFR-Matrigel and 5 μM ROCK inhibitor (Y-27632; Millipore) and transferred to a 6-well low attachment plate (3 wells; Corning) for 24 hours, which allowed the formation of EBs with uniform size. The mTeSR1 medium (hiPSC self-renewal medium) was gradually mixed with EB medium (DMEM high glucose [ThermoFisher] supplemented with 10% FBS, 2 mM L-glutamine, 1% (v/v) NEAA, 1% (v/v) pen/strep, and 0.012 mM β-ME) in 2:1 (volume) on Day 1, 1:1 on Day 2 and 1:2 on Day 3, respectively. From Day 4 to Day 5, EBs were cultured with EB medium in suspension. EBs were then collected and seeded on a gelatin-coated culture dish for six days with EB medium. To induce VSMC lineage, the adherent EB-derived cells were dissociated by 0.05% trypsin-EDTA, re-seeded at 20,000 cells/cm2 on a GFR-Matrigel-coated dish, and cultured in SmGM-2 medium until cells reached 80% confluence. This stage typically took 7–10 days, and these proliferative hiPSC-derived VSMCs were termed hiPSC-VSMCs-P. In order to expand hiPSC-VSMCs-P for vascular graft engineering, hiPSC-VSMCs-P were passaged to GFR-Matrigel-coated plates or flasks and cultured in expansion medium (DMEM supplemented with 10% [v/v] FBS, 2 mM L-glutamine, 1% [v/v] NEAA, 1 mM sodium pyruvate [ThermoFisher] and 1% [v/v] pen/strep) to “prime” the cells for an additional 1 or 2 passages. This led to the generation of hiPSC-VSMCs with effective proliferative potential while also expressing an enhanced level of VSMC markers and extracellular matrix proteins collagen I and III. The derivation of hiPSC-VSMCs for tissue engineered vascular graft (TEVG) generation typically took around four weeks.
To further induce the maturation phenotype of hiPSC-VSMCs (hiPSC-VSMCs-M) for characterization of marker expression and contractile functions, hiPSC-VSMCs-P grown in SmGM-2 medium were subcultured in the VSMC maturation medium (DMEM with 1% FBS, 1% [v/v] NEAA, 2 mM L-glutamine, 0.012 mM β-ME and 1 ng/ml TGF-β1) for seven days.
The lineage specific hiPSC-VSMCs (including hiPSC-VSMCs with embryonic origin of neuroectoderm, lateral plate mesoderm, or paraxial mesoderm) were derived following the chemically-defined method as previously reported (Cheung et al., 2012). The lineage specific hiPSC-VSMCs were subcultured in expansion medium (DMEM supplemented with 10% [v/v] FBS, 2 mM L-glutamine, 1% [v/v] NEAA, 1 mM sodium pyruvate and 1% [v/v] pen/strep) along with those hiPSC-VSMCs derived from our newly optimized EB-based method for comparison of proliferative capacity.
Immunostaining of Cultured Cells
Cells were washed with PBS and fixed in 4% paraformaldehyde (PFA; Electron Microscopy Sciences) for 10 minutes at room temperature (RT). The cells were then blocked in 10% normal goat serum (NGS; ThermoFisher) in PBST buffer (PBS with 0.3% Triton X-100 [Sigma-Aldrich]) for 30 minutes at RT. Subsequently, cells were incubated with the primary antibody in 1% NGS in PBST at 4°C overnight. Cells were washed again with PBS, incubated with secondary antibody (1:1000 in 1% NGS in PBST) for one hour at RT and washed with PBS again. Filamentous actin was stained with phalloidin (ThermoFisher) and the nuclei were counterstained with DAPI (ThermoFisher). All antibodies are listed in the Key Resource Table. Immunostained samples were analyzed using a fluorescent microscope (Leica). Fluorescence intensity (gray scale) of the markers in immunostained cells were analyzed with the ImageJ software and expressed relatively to cell number. Percentage of cells positive for the markers was quantified by using ImageJ software.
Quantitative Reverse Transcription PCR
Cells or engineered vascular tissues were subjected to RNA extraction and a quantitative reverse transcription PCR (qRT-PCR) assay to evaluate the gene expression of markers of interest. RNA extraction and purification were completed using the TRIzol RNA Isolation Kit (ThermoFisher), following the manufacturer’s instructions. Subsequently, total RNA was subjected to reverse transcription using an iScript cDNA synthesis Kit (Bio-rad). The primer sequences of the genes used in qRT-PCR are listed in Supplementary Table S1. qRT-PCR was performed using Bio-Rad IQ SYBR green supermix. Expression of genes of interest was normalized to that of human GAPDH. Three biological replicates were used for the analysis of each gene expression.
Cell Contractility Assay
hiPSC-VSMCs or human primary VSMCs cultured in either SmGM-2 medium, maturation medium (1% [v/v] FBS with 1 ng/ml TGF-β1 [Peprotech]), or TEVG medium (DMEM medium supplemented with 20% [v/v] FBS, 50 μg/mL ascorbic acid [Sigma-Aldrich], 50 μg/mL proline [Sigma-Aldrich], 20 μg/mL alanine [Sigma-Aldrich], 50 μg/mL glycine [Sigma-Aldrich], 3 ng/mL CuSO4 [Sigma-Aldrich], 0.13 U/mL human insulin [Sigma-Aldrich], 100 U/mL Penicillin G [Sigma-Aldrich] and 1 ng/mL TGF-β1 [Peprotech]) were treated with 1 mM carbachol (Abcam) or PBS (vehicle control) as a control for 20 minutes. Cell surface areas were recorded at the beginning and the end of the treatment. The changes of surface area were evaluated with the ImageJ software. Three independent batches of hiPSC-VSMCs or primary VSMCs were used in the contractility assay, and the changes in surface area of 10 randomly selected cells in each batch were recorded and analyzed, respectively.
MTT Assay
hiPSC-VSMCs derived from EB-based or chemically defined approach were seeded at 20,000 cells/well density into GFR-Matrigel-coated 96-well plates with expansion medium and cultured for three days. Cell proliferation was measured as a function of metabolic activity using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) (Sigma-Aldrich) on Day 4. MTT at 0.5 mg/ml was added into the medium of each well and incubated with the cells at 37°C for 2 hours, follo wed by cell solubilization with DMSO (AmericanBIO) for 15 minutes. Absorbance was measured at 540 nm using the Synergy 2 multi-mode plate reader (BioTek). Three biological replicates were completed for evaluation of each cell group.
Culturing hiPSC-VSMCs on 5 mm x 5 mm PGA Scaffolds
The engineered vascular tissues were developed by seeding “primed” hiPSC-VSMCs onto PGA scaffolds, to evaluate the effect of growth factor components (TGF-β1 and PDGF-BB) in the TEVG medium (Figure 2A). Nonwoven-PGA polymer mesh (0.3 mm x 150 mg/cc, 20 cm x 30 cm sheet, Biofelt) was cut into small-size meshes (5 mm x 5 mm squares). The PGA squares were treated with 1.0 N NaOH (Sigma-Aldrich) for 1 minute, rinsed extensively with distilled water, sterilized with 70% ethanol, and air-dried overnight in a sterile manner. On the following day, PGA meshes were coated with 0.1% gelatin at 37°C for 1 hour, air-dried, and transferred into the 24-well low attachment dish (Corning).
“Primed” hiPSC-VSMCs cultured in the expansion medium (DMEM supplemented with 10% [v/v] FBS, 2 mM L-glutamine, 1% [v/v] NEAA, 1 mM sodium pyruvate [ThermoFisher] and 1% [v/v] pen/strep) were harvested, and 40 μL of the expansion medium containing 0.4 million hiPSC-VSMCs were dropped onto the PGA mesh and incubated at 37°C and 5% CO2 for one hour. The wells were then filled with 1 mL of expansion medium, and cells were cultured overnight at 37°C. On the following day, t he medium was changed to the TEVG medium (DMEM medium supplemented with 20% [v/v] FBS, 50 μg/mL ascorbic acid [Sigma-Aldrich], 50 μg/mL proline [Sigma-Aldrich], 20 μg/mL alanine [Sigma-Aldrich], 50 μg/mL glycine [Sigma-Aldrich], 3 ng/mL CuSO4 [Sigma-Aldrich], 0.13 U/ml human insulin [Sigma-Aldrich] and 100 U/ml Penicillin G [Sigma-Aldrich]), supplemented with one of the following: 1) both human TGF-β1 (1 ng/ml, Peprotech) and human PDGF-BB (10 ng/ml, R&D system) (T/P), 2) TGF-β1 only (T/-), 3) PDGF-BB only (-/P), or 4) no growth factor (−/−). Medium was changed every other day. The tissues were cultured for 21 days and then harvested for both histological analysis and hydroxyproline assay.
Tissue Immunohistochemistry and Histology
Tissue samples were fixed in 4% PFA for three hours at RT and incubated in 15% (w/v) sucrose (Sigma-Aldrich) in PBS at 4°C for 16 hours. Subsequently, the fixed tissues were embedded in Tissue-Tek Optimal Cutting Temperature (OCT) Compound (Sakura Finetek) to develop the frozen blocks. Frozen blocks were sectioned at 5 μm intervals using cryostat (Leica CM1950) and the sections were stored at −80°C.
For immunostaining, slides were submerged in PBS for 10 minutes. Tissue sections were then incubated with PBST containing 10% NGS for 30 minutes at RT, and incubated with primary antibody in PBST containing 1% NGS at 4°C overnight in a humidified chamber. On the second day, sections were washed with PBS, incubated with secondary antibody (1:1000 in PBST with 1% NGS) for one hour at RT and washed with PBS again. Nuclei were counterstained with DAPI. Fluorescence intensity (gray scale) of positiveness of the markers in the total immunostained cells or HLA-A-positive cells were analyzed by using ImageJ software and expressed relatively to cell number. Percentage of positiveness of the markers in the HLA-A-positive cells were analyzed by using ImageJ software.
For hematoxylin and eosin (H&E) staining, Masson’s Trichrome staining, Alizarin Red staining and elastic Verhoeff-Van Gieson staining (EVG staining), tissue sections were processed by Yale Pathology Tissue Services based on standard protocols.
TUNEL Assay
Tissue sections were processed according to the instructions from the TUNEL staining kit (Roche). DNA was counterstained by DAPI (ThermoFisher). Three biological replicates were completed for each group for statistical analysis of the percentage of TUNEL-positive cells. All immunofluorescence, histology and TUNEL staining micrographs were captured under an inverted microscope (Nikon Eclipse 80i).
Hydroxyproline Assay
The collagen weight of the vascular tissues or hiPSC-VSMCs cultured in Uniflex 6-well culture plates (Flexcell International Corporation) were determined by measuring the levels of hydroxyproline following the instruction of the hydroxyproline assay kit (Sigma-Aldrich). Collagen weight was then calculated based on the estimation that collagen contains approximately 10.0% hydroxyproline by weight (Dijkman et al., 2012; Piez and Likins, 1960). Three biological replicates were completed for each group.
Cell Culture with Pulsatile Uniaxial Stretch
The “primed” hiPSC-VSMCs expanded in DMEM containing 10% FBS, 2 mM L-glutamine, 1% NEAA, 1 mM sodium pyruvate and 1% pen/strep (or named as Gen 2 hiPSC-VSMCs-10% FBS in Figure S1) were seeded at 20,000/cm2 onto the Uniflex 6-well culture plates coated with 0.5% (w/v) gelatin (Flexcell International Corporation) and cultured in the presence of the T/- or T/P TEVG medium for 48-hours. The plates were then either transferred to a FlexCell FX-6000T™ Tension System (Flexcell International Corporation) for uniaxial, pulsatile stretching (2.5% strain, 2.75 Hz, sinusoidal waveform, mimicking the stretching regimen for generating TEVG using human primary VSMCs [(Dahl et al., 2011)]), or maintained under static culture for an additional 48-hours. Next, 1 mL medium samples were immediately collected from each well to test the glucose consumption. Additionally, the central rectangular areas of the wells (stretched or static samples) were cut off with a scalpel, and immediately subjected to the subsequent experiments, including the qRT-PCR, immunostaining, hydroxyproline assay, and evaluation of cellular ATP concentration.
Evaluation of Glucose in Media
The medium samples prior to and after the hiPSC-VSMC culture in the presence or absence of a 48-hour stretching period via a FlexCell FX-6000T™ Tension System were collected. The amounts of glucose in media were next evaluated using GlucCell glucose monitoring system and glucose test strips (CESCO). The cell numbers in each experimental group were determined by cell counting. To calculate the glucose consumption rates, the changes of amount of glucose during the 48-hour culture period were normalized to the average cell numbers and the duration of culture of each experimental group (2 days). Three biological replicates were completed for evaluation of each experimental group.
Evaluation of Cellular ATP Concentration
To test the cellular ATP concentrations of hiPSC-VSMCs with or without 48-hour stretching via using FlexCell FX-6000T™ Tension System, the total ATP amounts were measured by using the colorimetric ATP assay kit (Abcam) according to the instruction. The cell numbers in each experimental group were determined by cell counting using a separated well of hiPSC-VSMCs in the same batch of culture under the same culture condition, and the total ATP amount was normalized to the average cell numbers to derive the cellular ATP concentration of each sample. Three biological replicates were completed for evaluation of each experimental group.
Generation of hiPSC-TEVGs
Small-caliber tissue engineered vascular grafts were derived by culturing hiPSC-VSMCs on PGA scaffolds as described previously (Dahl et al., 2011; Gui et al., 2016; Niklason et al., 1999). Fifteen million hiPSC-VSMCs were seeded onto tubular, 0.1% gelatin-coated PGA scaffolds which were sewn around silicon tubing (3.2 mm outer diameter; Saint-Gobain) and mounted inside sterilized glass bioreactors (Figure 3A). Vessels were cultured in 10% FBS expansion medium at 37°C, 5% CO2 for 24 hours. The expansion medium was then replaced by the optimized TEVG medium (DMEM medium supplemented with 20% FBS, 50 μg/mL ascorbic acid, 50 μg/mL proline, 20 μg/mL alanine, 50 μg/mL glycine, 3 ng/mL CuSO4, 100 U/ml Penicillin G, 0.13U/ml insulin and 1ng/ml TGF-β1). Bioreactor medium (75%) was refreshed every two days. The engineered vessels were cultured without mechanical stimuli for the first week. Thereafter, pulsatile radial stress from a peristaltic pump (Cole-Parmer) was added incrementally, and the frequency of the mechanical stimuli was adjusted via the rotating speed of the peristaltic pump. The strain induced by the pulsatile radial stress started from 0.5% and was progressively increased to the desired maximum strain at the end of week 4 according to the experiment design. To generate the hiPSC-TEVGs with optimal mechanical strength, 3% of maximum strain was applied, and maintained at the maximum (3%) during the following 4 weeks of culture. By the end of week 8, hiPSC-TEVGs were harvested under sterile conditions for implantation and further analysis.
Implantation of hiPSC-TEVG into Nude Rat
hiPSC-TEVG segments were implanted as abdominal aorta interpositional grafts into 10-week-old, male NIH-Foxn1rnu nude rats (around 300 grams) (Charles River Laboratories). Nude rats were anesthetized with isoflurane, and subsequently opened under standard sterile conditions via a midline abdominal incision leaving the infrarenal abdominal aorta exposed. The cross-clamps were then applied to aid the removal of an aortic segment between the renal artery and the iliac artery. A hiPSC-TEVG segment (7–10 mm in length) was next implanted into the aorta in the “end-to-end” manner using a 10–0 monofilament nylon suture. After confirmation of blood flow and hemostasis following removal of the clamp, the wound area was closed. The animals were left to recover from surgery and maintained for 30 days or 60 days after surgery.
Ultrasonographical Assessment of Grafts
Nude rats were examined using a Vevo 770® Micro-ultrasound System (Visual-Sonics, Toronto, Canada) equipped with the RMV-704 scanhead (spatial resolution 40 mm) to determine patency and morphometry of the implanted hiPSC-TEVGs. The inner diameters and outer diameters of the grafts at the midpoint were measured from both transverse and longitudinal axis ultrasound images. The lengths of the grafts were measured from longitudinal axis ultrasound images. The average wall thickness of the midpoint of the implanted grafts was calculated as half of the difference between the outer and inner diameters. For TEVG 1–6, the implanted hiPSC-TEVGs were ultrasonographically analyzed on Day 7, Day 14, Day 21 and Day 28 post-implantation and explanted for histological analysis on Day 30. For TEVG 7, the graft was ultrasonographically analyzed on Day 7, Day 14, Day 21, Day 28, Day 35, Day 42, Day 49 and Day 56 post-implantation and explanted for histological analysis on Day 60.
Mechanical Evaluation of hiPSC-TEVGs
The suture retention strength and rupture pressure of hiPSC-TEVGs were determined as previously described (Gui et al., 2016), and HUAs were utilized as a control. Suture retention strength was evaluated by adding weights on a loop of 6–0 Prolene suture (Ethicon) threaded through one side of the TEVG wall, 2 mm from the end, with force applied axially to the graft. The weights were augmented in 10 g to 20 g increments until failure. Four biological replicates were completed for either hiPSC-TEVG or HUA. To measure rupture pressure, vessel segments of 1–1.5 cm long were connected to a flow system coupled with a pressure transducer. PBS was injected into the flow system until vessel rupture. Four biological replicates were completed for either hiPSC-TEVG or HUA.
The maximum modulus, maximum tensile stress and failure strain of pre-implanted and explanted hiPSC-TEVGs were analyzed using an Instron 5960 microtester (Instron) equipped with a 10 N load cell as previously described (Luo et al., 2017). The porcine coronary artery segments with inner diameters of approximately 3 mm were employed as control. The hiPSC-TEVGs or porcine coronary arteries were cut into vessel rings with 1–2 mm in length. The vessel rings were mounted between two stainless steel pins, with one anchored to actuator and the other to the load cell. Vessel rings were next cyclically pre-stretched for three cycles to 10% strain and then increasingly stretched until failure to determine the ultimate tensile strength. Tissue stress was quantified by normalizing tensile force to total cross-sectional area (A =2 * π * r2; supposing ring cross section to be circular, r is half of the ring thickness, and cross-sectional area of the ring is multiplied by two to include both sides of the ring), and then the maximum stress, failure strain, maximum modulus of the rings were calculated and plotted. Three independent batches of pre-implanted hiPSC-TEVGs, explanted hiPSC-TEVGs or porcine coronary arteries were subjected to mechanical property evaluation and analyzed.
Contractility Evaluation of hiPSC-TEVGs
The contractility of the hiPSC-TEVGs was measured as previously described (Luo et al., 2017). Briefly, the vessel rings (1–2 mm in length) of pre-implanted or explanted hiPSC-TEVGs 30 days after implantation were sectioned and transferred into a temperature-controlled perfusion bath as shown in Figure S3P. Vessel rings were hooked between two motorized micromanipulators, which kept the ring suspended between an anchoring point and a force transducer (KG7, SI Heidelberg). When measuring the force, rings were placed in freshly bubbled Tyrode’s solution (NaCl 140 mM, KCl 5.4 mM, MgCl2 1 mM, HEPES 25 mM, glucose 10 mM and CaCl2 1.8 mM, pH 7.3, all from Sigma-Aldrich) at 37°C. Force were measured at the original length, and the manipulators were then moved apart 1.5 mm to evaluate the second reference force prior to stimulation of agonist. The vessel rings were kept untreated for one minute to achieve a baseline value for force change, and then carbachol solution was immediately added to the bath at 1 mM to induce contraction of the ring. The force measurements of vessel rings were recorded for 30 minutes using a Customized Matlab software. The ultimate changes in tension (Pa) were quantified by normalizing the force by the cross-sectional area. The areas of cross-section were derived using optical coherence tomography (Ganymede-II- HR, Thorlabs). An index of refraction of 1.38 was employed for each vessel ring. Images were taken, and their cross-sectional area was quantified by using the NIH Image J software and averaged to calculate the overall cross-sectional area of the vessel rings. Three independent batches of pre-implanted hiPSC-TEVGs or explanted hiPSCs were subjected to contractility tests and analyzed.
QUANTIFICATION AND STATISTICAL ANALYSES
All graphic illustrations and statistical analyses were completed using GraphPad Prism 6. One-way or two-way ANOVA followed by Tukey post-hoc test was applied for comparison among multiple groups when appropriate. Two-tailed Student’s T-test was used to determine the significance of difference between the controls and the experimental groups. A p-value lower than 0.05 was considered significant. Numerical data were reported in format of mean ± S.E.M from at least three or more independent experiments. The sample size (n) for each analysis stands for the number of biological replicates and can be found in the figure legends. The statistical details of each experiment can also be found in figure legends and related results.
Supplementary Material
Highlights.
Functional VSMCs could be efficiently generated on a large scale from hiPSCs.
Optimized biochemical and biophysical conditions were used to generate hiPSC-TEVGs.
hiPSC-TEVGs presented mechanical strength comparable to that of saphenous veins.
hiPSC-TEVGs maintained patency and mechanical function following rat implantation.
Acknowledgements
This work was supported by NIH 1R01HL116705, CRMRF 12-SCB-YALE-06, 15-RMB-YALE-08 (Y.Q.). Work was also supported by AHA 19POST34450100 (J.L.) and NIH F31-HL143924 and T32-GM0007324 (M.W.E.). We thank Christopher Anderson, Luke Batty, Tristan Driscoll and Minghao Chen for their support.
Footnotes
Declaration of Interests
L.E.N. is a founder and shareholder in Humacyte, Inc. Humacyte produces engineered blood vessels from allogeneic smooth muscle cells for vascular surgery. L.E.N.’s spouse has equity in Humacyte, and L.E.N. serves on Humacyte’s Board of Directors. L.E.N. is an inventor on patents that are licensed to Humacyte and that produce royalties for L.E.N. Humacyte neither funded current studies, nor influenced the conduct, description or interpretation of the findings in this report. The authors declare a patent filed related to this work.
DATA AND CODE AVAILABILITY
Main data have been listed in the primary figures and supplemental information. All the original data and images are available from the Lead Contact upon request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akoh JA, and Patel N (2010). Infection of hemodialysis arteriovenous grafts. J Vasc Access 11, 155–158. [DOI] [PubMed] [Google Scholar]
- Atchison L, Zhang H, Cao K, and Truskey GA (2017). A Tissue Engineered Blood Vessel Model of Hutchinson-Gilford Progeria Syndrome Using Human iPSC-derived Smooth Muscle Cells. Sci Rep 7, 8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai VK, Mistriotis P, Loh YH, Daley GQ, and Andreadis ST (2012). Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates. Cardiovasc Res 96, 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Logsdon NJ, Ravi S, Xie N, Persons BP, Rangarajan S, Zmijewski JW, Mitra K, Liu G, Darley-Usmar VM, et al. (2015). Metabolic Reprogramming Is Required for Myofibroblast Contractility and Differentiation. J Biol Chem 290, 25427–25438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PY, Qin L, Li G, Tellides G, and Simons M (2016). Fibroblast growth factor (FGF) signaling regulates transforming growth factor beta (TGFbeta)-dependent smooth muscle cell phenotype modulation. Sci Rep 6, 33407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C, Bernardo AS, Trotter MW, Pedersen RA, and Sinha S (2012). Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol 30, 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte MS (2013). Critical appraisal of surgical revascularization for critical limb ischemia. J Vasc Surg 57, 8S–13S. [DOI] [PubMed] [Google Scholar]
- Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT, et al. (2011). Readily available tissue-engineered vascular grafts. Sci Transl Med 3, 68 ra 69. [DOI] [PubMed] [Google Scholar]
- Dash BC, Levi K, Schwan J, Luo J, Bartulos O, Wu H, Qiu C, Yi T, Ren Y, Campbell S, et al. (2016). Tissue-Engineered Vascular Rings from Human iPSC-Derived Smooth Muscle Cells. Stem Cell Reports 7, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuse T, Hu X, Gravina A, Wang D, Tediashvili G, De C, Thayer WO, Wahl A, Garcia JV, Reichenspurner H, et al. (2019). Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol 37, 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkman PE, Driessen-Mol A, Frese L, Hoerstrup SP, and Baaijens FP (2012). Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno- and homografts. Biomaterials 33, 4545–4554. [DOI] [PubMed] [Google Scholar]
- Elliott MB, Ginn B, Fukunishi T, Bedja D, Suresh A, Chen T, Inoue T, Dietz HC, Santhanam L, Mao HQ, et al. (2019). Regenerative and durable small-diameter graft as an arterial conduit. Proc Natl Acad Sci U S A 116, 12710–12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eoh JH, Shen N, Burke JA, Hinderer S, Xia Z, Schenke-Layland K, and Gerecht S (2017). Enhanced elastin synthesis and maturation in human vascular smooth muscle tissue derived from induced-pluripotent stem cells. Acta Biomater 52, 49–59. [DOI] [PubMed] [Google Scholar]
- Gornalusse GG, Hirata RK, Funk SE, Riolobos L, Lopes VS, Manske G, Prunkard D, Colunga AG, Hanafi LA, Clegg DO, et al. (2017). HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat Biotechnol 35, 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui L, Dash BC, Luo J, Qin L, Zhao L, Yamamoto K, Hashimoto T, Wu H, Dardik A, Tellides G, et al. (2016). Implantable tissue-engineered blood vessels from human induced pluripotent stem cells. Biomaterials 102, 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Nikolovski J, Bonadio J, and Mooney DJ (1999). Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat Biotechnol 17, 979–983. [DOI] [PubMed] [Google Scholar]
- Lawson JH, Glickman MH, Ilzecki M, Jakimowicz T, Jaroszynski A, Peden EK, Pilgrim AJ, Prichard HL, Guziewicz M, Przywara S, et al. (2016). Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet 387, 2026–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Qin L, Kural MH, Schwan J, Li X, Bartulos O, Cong XQ, Ren Y, Gui L, Li G, et al. (2017). Vascular smooth muscle cells derived from inbred swine induced pluripotent stem cells for vascular tissue engineering. Biomaterials 147, 116–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden RL, Lipkowitz GS, Browne BJ, and Kurbanov A (2005). A comparison of cryopreserved vein allografts and prosthetic grafts for hemodialysis access. Ann Vasc Surg 19, 686–691. [DOI] [PubMed] [Google Scholar]
- McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, Zagalski K, Fiorillo A, Avila H, Manglano X, et al. (2009). Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet 373, 1440–1446. [DOI] [PubMed] [Google Scholar]
- Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, and Langer R (1999). Functional arteries grown in vitro. Science 284, 489–493. [DOI] [PubMed] [Google Scholar]
- Noda K, Dabovic B, Takagi K, Inoue T, Horiguchi M, Hirai M, Fujikawa Y, Akama TO, Kusumoto K, Zilberberg L, et al. (2013). Latent TGF-beta binding protein 4 promotes elastic fiber assembly by interacting with fibulin-5. Proc Natl Acad Sci U S A 110, 2852–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch C, Challet-Meylan L, Thoma EC, Urich E, Heckel T, O’Sullivan JF, Grainger SJ, Kapp FG, Sun L, Christensen K, et al. (2015). Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nature cell biology 17, 994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piez K, and Likins RC (1960). The nature of collagen In Calcification in biological systems A symposium presented at the Washington meeting of the American Association for the Advancement of Science, December 29, 1958, Sognnaes RF, ed. (American Association for the Advancement of Science; ), pp. 411–420. [Google Scholar]
- Poh M, Boyer M, Solan A, Dahl SL, Pedrotty D, Banik SS, McKee JA, Klinger RY, Counter CM, and Niklason LE (2005). Blood vessels engineered from human cells. Lancet 365, 2122–2124. [DOI] [PubMed] [Google Scholar]
- Quint C, Arief M, Muto A, Dardik A, and Niklason LE (2012). Allogeneic human tissue-engineered blood vessel. J Vasc Surg 55, 790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumberger W, Thie M, Rauterberg J, and Robenek H (1991). Collagen synthesis in cultured aortic smooth muscle cells. Modulation by collagen lattice culture, transforming growth factor-beta 1, and epidermal growth factor. Arterioscler Thromb 11, 1660–1666. [DOI] [PubMed] [Google Scholar]
- Shi N, and Chen SY (2016). Smooth Muscle Cell Differentiation: Model Systems, Regulatory Mechanisms, and Vascular Diseases. J Cell Physiol 231, 777–787. [DOI] [PubMed] [Google Scholar]
- Smith-Mungo LI, and Kagan HM (1998). Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol 16, 387–398. [DOI] [PubMed] [Google Scholar]
- Solan A, Dahl SL, and Niklason LE (2009). Effects of mechanical stretch on collagen and cross-linking in engineered blood vessels. Cell Transplant 18, 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HG, Rumma RT, Ozaki CK, Edelman ER, and Chen CS (2018). Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell 22, 340–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinetti G, Kraenkel N, Emanueli C, and Madeddu P (2008). Diabetes and vessel wall remodelling: from mechanistic insights to regenerative therapies. Cardiovasc Res 78, 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugitani H, Wachi H, Tajima S, and Seyama Y (2001). Nitric oxide stimulates elastin expression in chick aortic smooth muscle cells. Biol Pharm Bull 24, 461–464. [DOI] [PubMed] [Google Scholar]
- Syedain ZH, Graham ML, Dunn TB, O’Brien T, Johnson SL, Schumacher RJ, and Tranquillo RT (2017). A completely biological “off-the-shelf” arteriovenous graft that recellularizes in baboons. Sci Transl Med 9, eaan4209. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, and Yamanaka S (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. [DOI] [PubMed] [Google Scholar]
- Tojais NF, Cao A, Lai YJ, Wang L, Chen PI, Alcazar MAA, de Jesus Perez VA, Hopper RK, Rhodes CJ, Bill MA, et al. (2017). Codependence of Bone Morphogenetic Protein Receptor 2 and Transforming Growth Factor-beta in Elastic Fiber Assembly and Its Perturbation in Pulmonary Arterial Hypertension. Arterioscler Thromb Vasc Biol 37, 1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hu J, Jiao J, Liu Z, Zhou Z, Zhao C, Chang LJ, Chen YE, Ma PX, and Yang B (2014). Engineering vascular tissue with functional smooth muscle cells derived from human iPS cells and nanofibrous scaffolds. Biomaterials 35, 8960–8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RE, Long SP, Bowden JJ, Bastrilles JY, Barton SP, and Griffiths CE (2008). Repair of photoaged dermal matrix by topical application of a cosmetic ‘antiageing’ product. Br J Dermatol 158, 472–477. [DOI] [PubMed] [Google Scholar]
- Wolfe BL, Rich CB, Goud HD, Terpstra AJ, Bashir M, Rosenbloom J, Sonenshein GE, and Foster JA (1993). Insulin-like growth factor-I regulates transcription of the elastin gene. J Biol Chem 268, 12418–12426. [PubMed] [Google Scholar]
- Wu W, Allen RA, and Wang Y (2012). Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat Med 18, 1148–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wang B, Ono M, Kagita A, Fujii K, Sasakawa N, Ueda T, Gee P, Nishikawa M, Nomura M, et al. (2019). Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates iPSCs with Enhanced Immune Compatibility. Cell Stem Cell 24, 566–578 e567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


