Highlights
-
•
PI3K/AKT pathway alterations are frequently seen in ovarian cancer, providing rationale for targeted AKT inhibition.
-
•
AKT inhibitor MK-2206 in platinum resistant high grade serous ovarian cancer was notable for dermatologic toxicity.
-
•
Best response of stable disease was seen, with one patient experiencing a prolonged SD of 19 weeks.
Keywords: MK-2206, AKT inhibitor, PI3K/AKT pathway, PTEN, Platinum resistant ovarian cancer, Serous ovarian carcinoma
Abstract
Platinum-resistant, recurrent, high grade epithelial ovarian carcinoma remains challenging to treat. Chemotherapy produces limited responses with modest survival benefits in the treatment of recurrent disease. In this context, targeted therapies may improve upon conventional therapies. PI3K/AKT pathway alterations are frequently found in several cancer types, including ovarian cancer, and thus AKT inhibition is a rational targeted therapy. Here we report the results of an abbreviated trial of AKT inhibitor MK-2206 in platinum resistant high grade serous ovarian, fallopian tube, and primary peritoneal cancer with PTEN loss.
1. Introduction
Ovarian, fallopian tube, and primary peritoneal high grade serous carcinomas are morphologically similar, may derive from a common fallopian tube precursor, and given similar clinical behaviors are treated in the same manner. In the platinum-resistant setting, these high grade serous carcinomas (HGSC) are characterized by diminishing responses to additional lines of chemotherapy and a poor prognosis. This has placed a premium on the discovery and evaluation of new agents, particularly those targeting key growth and metastasis regulatory processes. Phosphatidylinositol-3-kinase (PI3K) is well documented to play important roles in regulating cell growth, survival, and metabolism. Dysregulation of PI3K, its downstream effectors, and its negative regulators are commonly implicated in solid tumor pathogenesis in multiple tumor lineages. PI3K activates downstream kinases AKT and mTORC1, while being negatively regulated by PTEN. Overactivation of the PI3K/AKT pathway can occur through alterations of PI3K itself, such as by mutations in PIK3CA or PIK3R1, through AKT upregulation or loss of PTEN. Ovarian cancer (EOC) is one tumor type in which alterations in PI3K/AKT signaling are present: 68% exhibit activated AKT (Altomare and Testa, 2005), 13% of high-grade EOC are PIK3CA-amplified, 8% have activating PIK3CA mutations (Forbes et al., 2017), and 18% are AKT2-amplified (Nakayama et al., 2006). Mutations have also been characterized in PIK3R1 and AKT1 (De Marco et al., 2013), as has loss of inhibitory phosphatases PTEN and INPP4B (Gewinner et al., 2009). Given the importance of the PI3K/AKT pathway and the multiple possible targets for signal modulation, there has been marked interest in developing selective inhibitors of this pathway.
In a Phase I trial of the pan-PI3K inhibitor pictilisib (GDC-0941), a heavily pretreated platinum-refractory EOC patient harboring PIK3CA amplification and PTEN loss achieved a partial response (Sarker et al., 2015), suggesting there was clinical utility in targeting this pathway. MK-2206 was developed as the first allosteric inhibitor of AKT, with greatest potency against AKT1 and AKT2, and lower potency against AKT3. It demonstrated single-agent anti-proliferative activity, as well as activity in combination with other agents, across multiple breast, ovarian, lung, and prostate cancer cell lines. MK-2206 showed preclinical synergistic anti-tumor activity when combined with docetaxel, erlotinib, and carboplatin in various human tumor xenograft models (Hirai et al., 2010), leading to a Phase 1 trial of MK-2206 in advanced solid tumors that included 2 EOC patients (Yap et al., 2011). Interim results showed that the EOC patients achieved serologic CA125 responses (Tolcher et al., 2009). The best response, however, was seen in a patient with pancreatic adenocarcinoma with PTEN loss by IHC, who achieved a 23% tumor reduction and remained on treatment for 24 weeks. The most frequent DLT was reversible grade 3 to 4 erythematous rash (n = 8, 24%). The RP2D was established as 60 mg of MK-2206 on alternating days. Results from these trials suggested clinical efficacy of MK-2206, particularly in those with molecular alterations affecting the PI3K/AKT pathway, providing a logical basis in which to evaluate MK-2206 in EOC.
2. Patients & methods
We designed and conducted an open-label Phase II study of MK-2206 in patients with platinum-resistant high grade serous ovarian, fallopian tube, or primary peritoneal cancer from April 2011 through November 2012. The primary objective was to assess the objective response rate (ORR). Secondary endpoints included safety, tolerability, progression free survival (PFS), and overall survival (OS). Institutional review board approval was obtained. Each patient provided signed informed consent before study enrollment.
2.1. Patient population
Participants were required to have histologically confirmed high grade serous ovarian, fallopian tube, or primary peritoneal carcinoma, with evidence of PTEN loss by immunohistochemistry, or evidence of a PIK3CA or AKT mutation per a CLIA-certified assay. Additional eligibility included recurrence within 6 months of the last platinum-containing regimen, ECOG performance status of 0 or 1, and measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. Participants were limited to two prior lines of therapy in the recurrence setting, including biologic and targeted therapies, but excluding hormonal therapies and prior use of an AKT/PI3K pathway inhibitor.
Participants were required to have recovered from prior treatment-related toxicities to grade 1 or better. Adequate bone marrow and organ function were required, including ANC ≥ 1500/µL, platelets ≥ 100,000 µL, and hemoglobin ≥ 8 g/dL. Exclusion criteria included chemotherapy or radiation within 4 weeks prior to study entry. Due to the risk of MK-2206-associated hyperglycemia, diabetic participants were excluded if glycemic control was inadequate, defined as a fasting serum glucose of >130 mg/dL or HgbA1c > 7.5 mg/dL, or required the use of non-oral glycemic medications. Additionally, patients were excluded if they had severe or uncontrolled comorbidities, or evidence of other malignancies within the previous 5 years, excepting carcinoma-in-situ of the breast or cervix and basal or squamous cell carcinomas of the skin.
2.2. Treatment plan & safety assessment
MK-2206 was administered orally on days 1, 8, 15, and 22 of a 28-day cycle, starting at 200 mg (dose level 0). Toxicity was assessed using the National Cancer Institute Common Terminal Criteria for Adverse Events version 4.0. Dose reduction occurred for any non-hematologic Grade 2 AE lasting >7 days despite support, any non-hematologic Grade 3 AE except hyperglycemia, and second occurrence of neutropenia (ANC < 1000/µL), thrombocytopenia (<75,000/µL), or anemia (<8 g/dL). Dose reduction levels included 135 mg (dose level −1) and 90 mg (dose level −2) given weekly. Patients were treated on an outpatient basis and remained on study until disease progression, voluntary withdrawal, or drug-related toxicity. Patients were followed for up to 3 years after removal from protocol therapy or until death. The primary endpoint was determination of the ORR. Tumor assessment by CT or MRI was repeated every 2 cycles.
2.3. Study design
The trial was designed using a Simon Two-Stage Optimum Design with an ORR ≥ 20% considered to be of interest (alternative hypothesis) and ORR ≤ 5% not of further interest (null hypothesis). Using these parameters, ten patients were planned for enrollment in the first stage. If at least one response was seen, an additional 19 patients would be enrolled in the second stage. A maximum enrollment of 29 evaluable patients was planned, and if 4 or more patients demonstrated a response, then the null hypothesis could be rejected in favor of the alternative hypothesis (with α = 0.05 and β = 0.20).
3. Results
3.1. Patients
Of 61 patients screened between April 19, 2011 and November 8, 2012, fifty-eight were tested for PTEN loss by IHC, 13 of whom showed PTEN loss. Of these, a total of 6 patients ultimately enrolled, 5 of whom received treatment on protocol. The 7 patients with PTEN loss who did not start treatment on protocol are tabulated in Supplemental Table 1. One patient enrolled but did not receive protocol therapy. No patients screened for this trial had predetermined PIK3CA or AKT mutations as assessed by CLIA-certified assay. Patient baseline and disease characteristics are detailed in Table 1. All were Caucasian with a median age of 65 years. There were three patients with a diagnosis of epithelial ovarian carcinoma, two with primary peritoneal carcinoma, and one with fallopian tube carcinoma. All patients had high grade papillary serous histology. The study was closed early due to poor accrual.
Table 1.
Patient demographics & characteristics.
| Demography | N (%) |
|---|---|
| Race | |
| White | 6 (100%) |
| Ethnicity | |
| Non-Hispanic | 6 (100%) |
| Stage at diagnosis | |
| III | 3 (50%) |
| IV | 3 (50%) |
| Primary Site | |
| Epithelial ovarian | 3 (50%) |
| Fallopian tube | 1 (17%) |
| Primary peritoneal | 2 (33%) |
| Histology | |
| Papillary serous | 6 (100%) |
| Differentiation Grade | |
| High Grade | 6 (100%) |
| Age (in years), Median (Range) | 65 (52, 74) |
3.2. Safety
Therapy was well tolerated with the exception of dermatologic toxicity, with all patients experiencing rash. Four of the 5 evaluable patients (80%) experienced grade 3 maculo-papular rash, and the remaining 1 patient (20%) experienced grade 2 acneiform rash. One patient with grade 3 maculo-papular rash concurrently developed grade 2 palmar-plantar erythrodysesthesia. The next most common treatment-related AEs (TRAEs) were grade 1 hyperglycemia in 2 patients (40%) and grade 1 oral mucositis in 2 patients (40%). The remaining TRAEs were all grade 1 and are further detailed in Table 2.
Table 2.
Treatment-related adverse events.
| Adverse Event | Grade |
|||||
|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
||||
| N | % | N | % | N | % | |
| Eye disorders | ||||||
| Conjunctivitis | 1 | 20% | . | . | . | . |
| Gastrointestinal disorders | ||||||
| Abdominal pain | 1 | 20% | . | . | . | . |
| Cheilitis | 1 | 20% | . | . | . | . |
| Diarrhea | 1 | 20% | . | . | . | . |
| Mucositis, oral | 2 | 40% | . | . | . | . |
| Nausea | 1 | 20% | . | . | . | . |
| General disorders | ||||||
| Fatigue | 1 | 20% | . | . | . | . |
| Metabolism and nutrition disorders | ||||||
| Increased urea | 1 | 20% | . | . | . | . |
| Hyperglycemia | 2 | 40% | . | . | . | . |
| Nervous system disorders | ||||||
| Dysgeusia | 1 | 20% | . | . | . | . |
| Respiratory, thoracic and mediastinal disorders | ||||||
| Cough | 1 | 20% | . | . | . | . |
| Throat tightening | 1 | 20% | . | . | . | . |
| Wheezing | 1 | 20% | . | . | . | . |
| Skin and subcutaneous tissue disorders | ||||||
| Palmar-plantar erythrodysesthesia syndrome | . | . | 1 | 20% | . | . |
| Pruritus | 1 | 20% | . | . | . | . |
| Rash acneiform | . | . | 1 | 20% | . | . |
| Rash maculo-papular | . | . | . | . | 4 | 80% |
3.3. Clinical activity
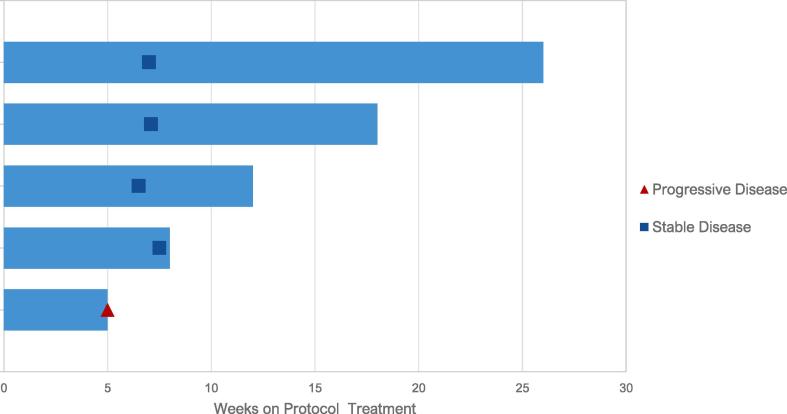
Patients received a median of 3 cycles of treatment. Best response was stable disease in 4 of 5 evaluable patients (Fig. 1). One patient had stable disease for 19 weeks, and one patient had stable disease for 10.8 weeks. One patient was removed from protocol due to unacceptable toxicity; the remaining patients were removed at time of progression. Treatment histories are detailed in Supplemental Table S2. No objective responses were observed in the 5 treated patients. Secondary endpoint of median PFS was estimated at 19 weeks, and median OS was estimated at 48 weeks. (Supplemental Figs. S1 & S2).
Fig. 1.
Swimmer plot of responses.
4. Discussion
Targeted therapies for ovarian cancer remain an area of significant interest. The rationale for the use of AKT inhibition in HGSOC was based on evidence demonstrating frequent alterations in the PI3K/AKT pathway in this disease type. Demonstration of IHC-confirmed PTEN loss, intended to enrich this population for responders to AKT inhibition, contributed to poor accrual, suggesting that this method of screening is not feasible as an eligibility criterion in this population. Although the results are inconclusive due to poor accrual and early trial termination, our preliminary findings suggest that single-agent MK-2206 was not clinically effective at the dose administered in this population of highly selected patients with PI3K/AKT pathway alterations. No patients had an objective response, though one patient experienced a prolonged SD of 19 weeks. Patients were removed from trial due to progressive disease (n = 4) or unacceptable toxicity (n = 1).
Dermatologic toxicity was seen in all evaluable patients, occurring at grade levels 2 and 3. The high rate of dermatologic toxicity seen in this trial reflects that of other trials evaluating MK-2206, including in breast cancer (Kalinsky et al., 2018, Yap et al., 2014), renal cell carcinoma (Jonasch et al., 2017), and gastric/GEJ carcinoma (Ramanathan et al., 2015) and occurring despite prednisone pre-medication (Ma et al., 2017).
In this trial, clinical activity of single-agent MK-2206 showed a best response of stable disease in four patients. The absence of response in a population selected for susceptibility to inhibition of this pathway suggest that there may be bypass mechanisms of resistance, such as through ERK/MAPK signaling or MEK overexpression, that circumvent MK-2206 monotherapy. Alternatively, MK-2206 at the dose delivered may not have fully inhibited the AKT pathway. Additionally, loss of PTEN by IHC, theoretically suggesting uninhibited PI3K/AKT signaling, may not capture alterations in PI3K or AKT that could reduce response to an AKT inhibitor. A best response of SD is comparable to other trials evaluating single-agent MK-2206 (Ahn et al., 2015, Yap et al., 2014, Jonasch et al., 2017, Ramanathan et al., 2015). Intriguingly, a trial in renal cell carcinoma demonstrated that while the majority of MK-2206-treated patients had progressive disease (n = 13, 44.8%), there was 1 CR (3.4%) and 3 PRs (10.3%) (Jonasch et al., 2017). Genomic analysis of responding patients did not reveal activating mutations in the PI3K pathway nor shared mutations amongst the responders. Additionally, a combination trial using neoadjuvant MK-2206 did not demonstrate additional apoptosis on resection specimens and no pathologic CRs occurred (Ma et al., 2017), providing a hypothesis for cytostatic but not cytocidal activity to correlate with best response of SD.
In conclusion, MK-2206 in high grade serous ovarian cancer was well tolerated apart from dermatologic toxicity, however was without evidence of objective response in this small cohort of patients. Insofar as one patient experienced prolonged SD (19 weeks), there may be a subset of patients with HGSOC who will derive benefit from MK-2206. At the time of this trial, next-generation sequencing was not routinely available, and the ability to reproducibly identify patients with known ovarian cancer PI3K/AKT pathway alterations such as PIK3CA or AKT amplification was not available. Further investigation and correlative studies are therefore needed to better determine whether response to PI3K/AKT-directed agents may be present in a molecularly-selected subset.
5. Contributions
JFL, CA, RLC, UAM, LCC, GBM, and AD contributed to trial conception and design.
JFL, EKL and ZTW contributed to the analysis and interpretation of data.
MSH contributed to acquisition of data.
JC provided administrative support and study supervision.
All authors contributed to the writing and review of the manuscript.
6. Declaration of Competing Interest & Financial Support
This work was funded by a Stand Up to Cancer Dream Team Translational Research Grant, a Program of the Entertainment Industry Foundation (SU2C-AACR-DT0209). Research grants are administered by the American Association for Cancer Research, the scientific partner of SU2C. The study was sponsored by the National Cancer Institute under the Cancer Therapy Evaluation Program.
GBM is an advisory board member with AstraZeneca, ImmunoMET, Ionis, Nuevolution, PDX bio, Signalchem, Symphogen, and Tarveda, has stock options with Catena Pharmaceuticals, ImmunoMet, SignalChem, Spindle Top Ventures and Tarveda, travel support from Chrysallis Bio, and has licensed technology to Nanostring and Myriad Genetics. GBM is supported by a kind gift from the Miriam and Sheldon Adelson Medical Research Foundation, the Ovarian Cancer Research Foundation, The Breast Cancer Research Foundation, Prospect Creek Foundation, The Komen Foundation SAC110052, and NCI grants: CA217685, CA217842, and CA098258.
LCC owns equity in, receives compensation from, and serves on the scientific advisory board of Agios Pharmaceuticals. He is also a founder of and receives laboratory support from Petra Pharmaceuticals and receives compensation for being on the scientific advisory board of Petra Pharmaceuticals. No drugs from these companies were involved in this study. LCC reports support through the National Cancer Institute of the National Institutes of Health under Award Number R35CA197588, and through a Stand Up To Cancer Colorectal Cancer Dream Team Translational Research Grant (Grant number: SU2C-AACR-DT22-17). Additionally he reports personal fees and other from Cell Signaling Technology and Sanofi USA, grants and personal fees from Pfizer, personal fees from Novo Nordisk, other from Volastra, EIP Pharma, and Faeth, outside the submitted work. In addition, Dr. Cantley has a patent US8883438 licensed to Agios Pharmaceuticals, a patent US8715665 licensed to BIDMC, a patent US5532167 licensed to BIDMC, a patent US8552050 licensed to BIDMC, a patent US6004757 licensed to BIDMC, a patent US9493813 licensed to BIDMC, a patent US9745631 licensed to BIDMC, a patent US9119858 licensed to GENESYS RESEARCH INSTITUTE, a patent US10138479 licensed to BIDMC, a patent US6462173 licensed to CLEAR-COAT HOLDING, a patent US8877791 licensed to BIDMC, a patent US20190010144A1 licensed to Cornell University, California Institute of Biological Research, a patent US20170073760A1 pending to BIDMC, and a patent US20190136238A1 pending to BIDMC.
CA reports personal fees from Tesaro, Immunogen, Eisai/Merck, and Mateon Therapeutics, grants and personal fees from Clovis, and grants from Genentech, AbbVie, and Astra Zeneca, outside the submitted work.
RLC reports grants from the NIH, Gateway Foundation, and V-Foundation during the conduct of this study. He reports grants and personal fees from AstraZeneca, Clovis, Genmab, Roche/Genentech, and Janssen outside of the submitted work. He reports grants from Merck outside the submitted work. He reports personal fees from Tesaro, Medivation, Gamamab, Agenus, Regeneron, and OncoQuest outside the submitted work.
UAM reports personal fees from Astrazeneca, Myriad Genetics, Clovis, Merck, Eli Lilly, Mersana, Geneos, Fuji Film, 2X Oncology, Cerulean, Immunogen, and Novartis outside the submitted work.
JFL reports advisory board participation for AstraZeneca, Tesaro, Inc, Mersana Therapeutics, Clovis Oncology, Merck, and Genentech outside the submitted work. She reports participation as an Institutional PI on industry-sponsored trials from Genentech/Roche, AstraZeneca, Boston Biomedical, Atara Biotherapeutics, Bristol-Myers Squibb, Agenus, CytomX Therapeutics, Regeneron Pharmaceuticals, Tesaro, Clovis Oncology, Aravive Biologics, Vigeo Therapeutics, Acetylon Pharmaceuticals, and Arch Oncology.
The remaining authors have no disclosures.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2020.100546.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Altomare D.A., Testa J.R. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- Forbes S.A., Beare D., Boutselakis H., Bamford S., Bindal N., Tate J., Cole C.G., Ward S., Dawson E., Ponting L., Stefancsik R., Harsha B., Kok C.Y., Jia M., Jubb H., Sondka Z., Thompson S., De T., Campbell P.J. COSMIC: somatic cancer genetics at high-resolution. Nucleic Acids Res. 2017;45:D777–D783. doi: 10.1093/nar/gkw1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K., Nakayama N., Kurman R.J., Cope L., Pohl G., Samuels Y., Velculescu V.E., Wang T.-L., Shih I.-M. Sequence mutations and amplification of PIK3CA and AKT2 genes in purified ovarian serous neoplasms. Cancer Biol. Ther. 2006;5:779–785. doi: 10.4161/cbt.5.7.2751. [DOI] [PubMed] [Google Scholar]
- De Marco C., Rinaldo N., Bruni P., Malzoni C., Zullo F., Fabiani F., Losito S., Scrima M., Marino F.Z., Franco R., Quintiero A., Agosti V., Viglietto G. Multiple genetic alterations within the PI3K pathway are responsible for AKT activation in patients with ovarian carcinoma. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewinner C., Wang Z.C., Richardson A., Teruya-Feldstein J., Etemadmoghadam D., Bowtell D., Barretina J., Lin W.M., Rameh L., Salmena L., Pandolfi P.P., Cantley L.C. Evidence that inositol polyphosphate 4-phosphatase type II Is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16:115–125. doi: 10.1016/j.ccr.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker D., Ang J.E., Baird R., Kristeleit R., Shah K., Moreno V., Clarke P.A., Raynaud F.I., Levy G., Ware J.A., Mazina K., Lin R., Wu J., Fredrickson J., Spoerke J.M., Lackner M.R., Yan Y., Friedman L.S., Kaye S.B., Derynck M.K., Workman P., de Bono J.S. First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I Phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin. Cancer Res. 2015;21:77–86. doi: 10.1158/1078-0432.CCR-14-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H., Sootome H., Nakatsuru Y., Miyama K., Taguchi S., Tsujioka K., Ueno Y., Hatch H., Majumder P.K., Pan B.-S., Kotani H. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol. Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- Yap T.A., Yan L., Patnaik A., Fearen I., Olmos D., Papadopoulos K., Baird R.D., Delgado L., Taylor A., Lupinacci L., Riisnaes R., Pope L.L., Heaton S.P., Thomas G., Garrett M.D., Sullivan D.M., de Bono J.S., Tolcher A.W. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J. Clin. Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- Tolcher A.W., Yap T.A., Fearen I., Taylor A., Carpenter C., Brunetto A.T., Beeram M., Papadopoulos K., Yan L., de Bono J. A phase I study of MK-2206, an oral potent allosteric Akt inhibitor (Akti), in patients (pts) with advanced solid tumor (ST) J. Clin. Oncol. 2009;27:3503. [Google Scholar]
- Kalinsky K., Sparano J.A., Zhong X., Andreopoulou E., Taback B., Wiechmann L., Feldman S.M., Ananthakrishnan P., Ahmad A., Cremers S., Sireci A.N., Cross J.R., Marks D.K., Mundi P., Connolly E., Crew K.D., Maurer M.A., Hibshoosh H., Lee S., Hershman D.L. Pre-surgical trial of the AKT inhibitor MK-2206 in patients with operable invasive breast cancer: a New York Cancer Consortium trial. Clin. Transl. Oncol. 2018;20:1474–1483. doi: 10.1007/s12094-018-1888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap T.A., Yan L., Patnaik A., Tunariu N., Biondo A., Fearen I., Papadopoulos K.P., Olmos D., Baird R., Delgado L., Tetteh E., Beckman R.A., Lupinacci L., Riisnaes R., Decordova S., Heaton S.P., Swales K., deSouza N.M., Leach M.O., Garrett M.D., Sullivan D.M., de Bono J.S., Tolcher A.W. Interrogating two schedules of the AKT inhibitor MK-2206 in patients with advanced solid tumors incorporating novel pharmacodynamic and functional imaging biomarkers. Clin. Cancer Res. 2014;20:5672–5685. doi: 10.1158/1078-0432.CCR-14-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasch E., Hasanov E., Corn P.G., Moss T., Shaw K.R., Stovall S., Marcott V., Gan B., Bird S., Wang X., Do K.A., Altamirano P.F., Zurita A.J., Doyle L.A., Lara P.N., Tannir N.M., Tannir N.M. A randomized phase 2 study of MK-2206 versus everolimus in refractory renal cell carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017;28:804–808. doi: 10.1093/annonc/mdw676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan R.K., McDonough S.L., Kennecke H.F., Iqbal S., Baranda J.C., Seery T.E., Lim H.J., Hezel A.F., Vaccaro G.M., Blanke C.D. Phase 2 study of MK-2206, an allosteric inhibitor of AKT, as second-line therapy for advanced gastric and gastroesophageal junction cancer: a SWOG cooperative group trial (S1005) Cancer. 2015;121:2193–2197. doi: 10.1002/cncr.29363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.X., Suman V., Goetz M.P., Northfelt D., Burkard M.E., Ademuyiwa F., Naughton M., Margenthaler J., Aft R., Gray R., Tevaarwerk A., Wilke L., Haddad T., Moynihan T., Loprinzi C., Hieken T., Barnell E.K., Skidmore Z.L., Feng Y.-Y., Krysiak K., Hoog J., Guo Z., Nehring L., Wisinski K.B., Mardis E., Hagemann I.S., Vij K., Sanati S., Al-Kateb H., Griffith O.L., Griffith M., Doyle L., Erlichman C., Ellis M.J. A phase II trial of neoadjuvant MK-2206, an AKT inhibitor, with anastrozole in clinical stage II or III PIK3CA -mutant ER-positive and HER2-negative breast cancer. Clin. Cancer Res. 2017;23:6823–6832. doi: 10.1158/1078-0432.CCR-17-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn D.H., Li J., Wei L., Doyle A., Marshall J.L., Schaaf L.J., Phelps M.A., Villalona-Calero M.A., Bekaii-Saab T. Results of an abbreviated phase-II study with the Akt Inhibitor MK-2206 in Patients with Advanced. Biliary Cancer., Sci. Rep. 2015;5:12122. doi: 10.1038/srep12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.