Abstract
Continuous increase in global human population and depletion of natural resources of energy posing threat to environment needs, sustainable supply of food and energy. The most ecofriendly approach ‘green technology’ has been exploited for biofertilizer preparation. Cyanobacteria are the most successful and sustained prokaryotic organism during the course of evolution. They are considered as one of the primitive life forms found on our planet. Cyanobacteria are emerging candidates for efficiently conversion of radiant energy into chemical energy. This biological system produces oxygen as a by-product. Cyanobacterial biomass can also be used for the large scale production of food, energy, biofertilizers, secondary metabolites, cosmetics and medicines. Therefore, cyanobacteria are used in ecofriendly sustainable agricultural practice for production of biomass of very high value and decreasing the level of CO2. This review article describes the methods of mass production of cyanobacterial biofertilizers and their applications in agriculture and industrial level.
Keywords: Cyanobacteria, Carrier, Crop, Biofertilizers, Technology, Contaminants, Commercial
Highlights
-
•
Cyanobacteria are emerging candidates for competently conversion of radiant energy into chemical energy.
-
•
Cyanobacteria are used in ecofriendly sustainable agricultural practice for production of biomass of very high value.
-
•
Cyanobacteria have an emerged potential as biofertilizer which are economical and environment friendly.
-
•
They are a promising source offering diverse functional foods, they are still underexplored as a natural resource.
1. Introduction
Cyanobacteria are the most abundant group of organisms on the earth. They are autotrophic and found in a diverse environment, especially in the marine and freshwater. Marine water is the richest source of nutrient for the cultivation of cyanobacteria [[1], [2], [3], [4]]. They are small and generally unicellular and often grow in large colonies. Cyanobacteria are composed of a broad range of bacteria with different shapes and sizes. They can cover 150 genera that have been identified so far. They have characteristics of the oldest fossils of about more than 3.5 billion years ago. They also have an evolutionary significance because they are responsible for present-day oxygenic environment.
Classification of cyanobacteria proposed in 1985, in which four orders of the bacteria have been identified as Chroococcales, Nostocales, Oscillatoriales and Stigonematales, and their phyla are Chroococcales, Gloeobacterales, and Pleurocapsales. Cyanobacteria are associated with the periods of origin of plants. The cyanobacteria are immensely important in determining the path of evolution and ecological changes all over the earth's history. In the late Proterozoic or the early Cambrian period, cyanobacteria began to take up residence within certain eukaryote cells, this event is called endosymbiosis, for the origin of the eukaryotes. They have the potential to fix atmospheric nitrogen, so that could be used as a biofertilizer for the cultivation of economically important crops such as rice and beans. Outer most layers of cyanobacteria are made up of distinct three types of layers such as a mucilaginous layer, cell wall, and innermost plasma membrane. The cytoplasm contains pigmented lamellae which are not organized into plastid. The pigments include chlorophylls, carotenes, xanthophylls, c-phycoerythrin and c-phycocyanin, and the last two pigments are found in blue-green algae [[5], [6], [7]].
Cyanobacteria consist of numerous organic inclusion bodies that can carry out different specialized functions. These inclusion bodies include the light-harvesting antennae, the phycobilisomes [8,9], polyphosphate bodies [10], cyanophycin granules [11], polyhydroxyalkanoate (PHA) granules [5,12,13], carboxysomes/polyhedral bodies [14], lipid bodies [11,15], thylakoid centers [16], DNA-containing regions [17] and ribosomes [18,19]. Cyanophycin granules acquire large polypeptides containing approximately the same number of the amino acids such as arginine and aspartic acid. These granules are visible large under the light microscope and store more nitrogen. Carboxysomes are also found in nitrifying bacteria and thiobacilli. Carboxysomes are polyhedral in shape about 100 nm in diameter. It is the reserve of ribulose-1,5-bisphosphate carboxylase (RuBisCo) in a paracrystalline arrangement site of CO2 fixation. Halobacterium and Thiothrix are purple and green photosynthetic bacteria; contain organic inclusion bodies such as gas vacuoles which provide buoyancy to the cyanobacteria to float over the surface. The nucleoplasm or the DNA enclosing region is present in the center of the cell and shows a fibrillar structure. There is unorganized nucleus is found in cyanobacteria and DNA is clumped without nuclear boundary and nucleolus. During the cell division, the nucleoplasmic materials dispersed throughout the cytoplasm without the participation of the spindle apparatus. Cyanobacteria consist of two important cells: (i) Heterocysts (responsible for nitrogen fixation for ammonia synthesis), and (ii) Vegetative cells (exhibit normal photosynthesis and reproductive growth).
2. Emerging roles of cyanobacteria as functional foods
Functional foods provide the necessary amounts of important nutritional compounds, such as proteins, carbohydrates, vitamins, fats, and minerals [9]. They may consist of bioactive compounds which are natural chemicals derived from plants, animals or micro-organisms and useful for human health. Cyanobacteria may be used as potential food supplements and provide nutritional, therapeutic and beneficial values. There are several characteristics which make cyanobacteria as an attractive substitute for sustainable food production: global distributions, high nutrient content, require small volumes of water for growth and development (saltwater can also be used), need lesser amounts of land which may be infertile and unsuitable for other crops, easily digestible, product constancy over an extensive pH and temperature range, etc. [[20], [21], [22]]. In the present time, cyanobacteria are available as food supplements in the market in various forms like instance capsules, tablets and liquids [23,24]. They are considered to increase the nutritive values of snack foods, pasta, candy bars or gums and beverages, and also act as a source of natural food colorants [[25], [26], [27], [28], [29], [30]].
In recent time, the most frequent cyanobacterial strain used for human nutrition value is Spirulina (Arthrospria), due to its immense protein contents and significant nutritive values [27,31,32]. According to certain statistical analysis, 1 Kg of Sprulina may substitute of 1000 Kg of diversified fruits and vegetables in terms of nutritive value. Some cyanobacterial members (for example Spirulina, Anabaena, and Nostoc) are consumed as food supplements in several countries comprising Mexico, Chile, Peru and the Philippines. Spirulina platensis is grown on a huge scale using either aqueduct ponds or sophisticated photobioreactors and promoted as flakes, powder and tablets or capsules. It comprises more than 60% proteins, broad spectrum of prophylactic and therapeutic nutrients containing beta-carotene, thiamine, riboflavin, B-complex vitamins, minerals, trace elements and many surprising bioactive compounds [[33], [34], [35], [36]].
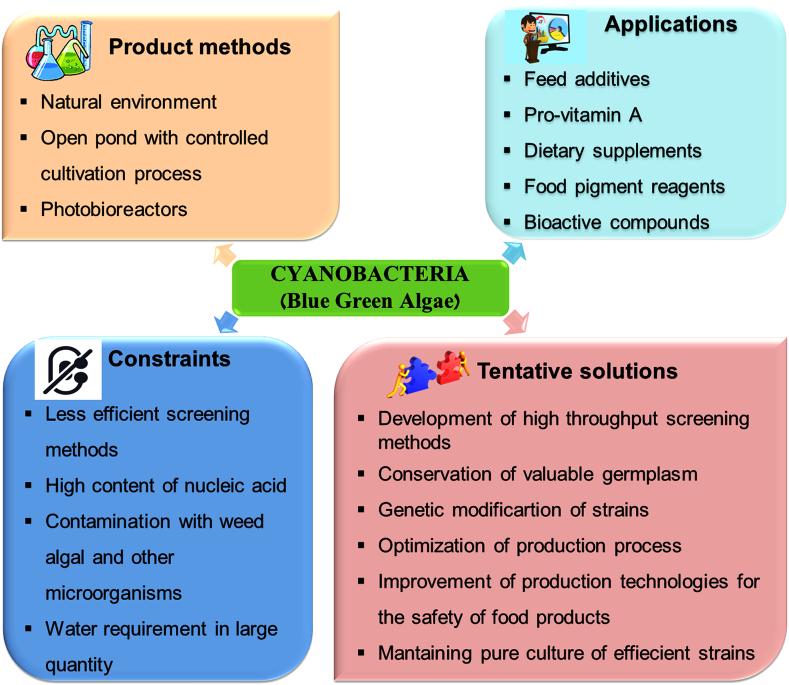
In the current scenario, it has been realized that existing requirement for wide research on functional food from cyanobacteria; several companies have introduced large scale production of cyanobacteria. The crucial steps involved for large scale productions are; culturing, harvesting, processing (drying) and packaging. At present time, more than 70 countries have commercialized products of nutritional importance which is obtained from cyanobacteria [37]. There are several commercial companies involved in the production of cyanobacteria as functional food products listed in Table 1. The demand for wide-scale production of cyanobacterial products is enhancing, but there are restrictions related to their production which essential to be determined (Fig. 1). Therefore, advancement of proficient technologies is needed to decrease the production cost along with maintenance and upgrading of product quality. Furthermore, selection of potential cyanobacterial strain should be intensive and research oriented for the collection of the valuable germplasm [[40], [41], [42]]. Moreover, limitation in relations of water constraint should be considered for possible substitutes like wastewater or seawater [40,43,44]. There are some examples of cyanobacteria such as Oscillatoria limnetica, Phormidium abronema, Oscillatoria tenuis, Lyngbya cryptovaginata, Tolypothrix tenuis [45], Spirulina platensis [46], Calothrix fusca and Gloeocapsa livida [47], Lyngbya limnetica, Scytonema bohneri, Oscillatoria acuminate, Oscillatoria calcuttensis, Oscillatoria foreaui, Spirulina pacifica and Spirulina platensis [48], which are the good source of carbohydrates and proteins. Lipids of Spirulina are cholesterol-free, which is useful for reducing blood cholesterol level, treatment of obesity, atherosclerosis, and diabetes [[49], [50], [51]]. Cyanobacteria are the source of minerals and vitamins which are good for bones, teeth, and blood. They contain vitamin A, B1, B2, B3, B6, E, H, folacin, pantothenic acid, inositol and abundant in vitamin B12 [49]. They also produce secondary metabolites which are the source of bioactive molecules including cytotoxic (41%), antitumor (13%), antiviral (4%), and antimicrobial (12%), and other compounds (18%) such as antimalarials, antimycotics, multidrug resistance reversers, herbicides, insecticides, algaecides, and immunosuppressive agents [52,53]. All the above important characteristics make cyanobacteria as an emerging and attractive alternative source for sustainable food production.
Table 1.
Some commercial companies involved in the production of cyanobacteria as functional food products [[37], [38], [39]].
| Country | Companies |
|---|---|
| USA | Earthrise Farms, Cyanotech Corporation, BioEarth Spirulina, Kalmath Valley Botanicals LLC |
| Myanmar | Myanmar Microalgae Biotechnology Project, Myanmar Spirulina Factory |
| China | Hainan DIC Microalgae, Nan Pao Resins, Fuqing King Dnarmsa Spirulina Co. Ltd, Hainan Simai Pharmacy Co. Ltd, Jiangsu Cibainian Nutrition Food Co. Ltd, Jiangxi Boyuan Spirulina Co. Ltd, Nanjing General Spirulina Developing Corporation, Bluebio Bio-Pharmaceutical Co. Ltd |
| Italy | BioEarth Spirulina |
| Germany | Green Valley Spirulina, Blue Biotech, Sanatur Spirulina |
| France | Natésis Spirulina |
| India | Ballarpur Industries, EID Parry, Zydus Cadila, Ahmedabad; Mapra Laboratories Pvt Ltd, Mumbai; Cosmic Nutracos Solutions Pvt Ltd, New Delhi; Hash Biotech Limited, Chandigarh; Sanat Products Ltd, New Delhi; Parry Neutraceuticals, Oonaiyur; Hydrolina Biotech Pvt Ltd, Chennai; Ecotech Technologies India Pvt Ltd, Mumbai; Essar Biotech, Hindupur; Miraculous Mushroom, Pune; Admark, Vijayawada; Care |
| Taiwan | Nan Pao Resins, Far East Bio-Tec Co. Ltd; Far East Microalgae Ind Co. Ltd |
| Thailand | Neotech Food, Siam Algae, Boonsom Spirulina Farm |
| UK | All Seasons Health |
| Switzerland | NaturKraftWerke Spirulina |
| Netherlands | Marcus Rohrer Spirulina |
| Monacco | Exsymol S.A.M. |
| Cuba | Genix |
| Chile | Solarium Biotechnology |
| Canada | Ocean Nutrition |
| Japan | Nippon Spirulina, Dainippon, DIC Lifetec |
| Mexico | Spirulina Mexicana |
| Australia | Panmol |
| Mongolia | Inner Mongolia Biomedical Engineering |
| Israel | Koor Foods |
Fig. 1.
Diagrammatic representation of the production methods, applications, limitations related to the production of the cyanobacterial food products and their tentative elucidations.
3. Cyanobacteria use as a biofertilizer
Present day's population is continuously increasing, and it will be reached to ~9.7 billion within 30 years. The major proportion of the population would be contributed by India (DESA UN, 2015). Increment in population has directly and indirectly dependent on the demand for contamination-free healthy food. World Health Organization has increased 50% of global food production by 2029. “Green Revolution” practices are also working for the increases in productivity of agriculture and reduce the risk of chemical-based fertilizers on human health as well as the environment. Thus, ‘green technology’ has been used by researchers for making eco-friendly environment by the exploitation of microbes. Green technology discusses several aspects of the use of cyanobacteria to improve crop productivity and soil fertility. Cyanobacteria can degrade a wide range of pollutants and perform different roles in the soil ecosystem to sustain soil fertility [54].
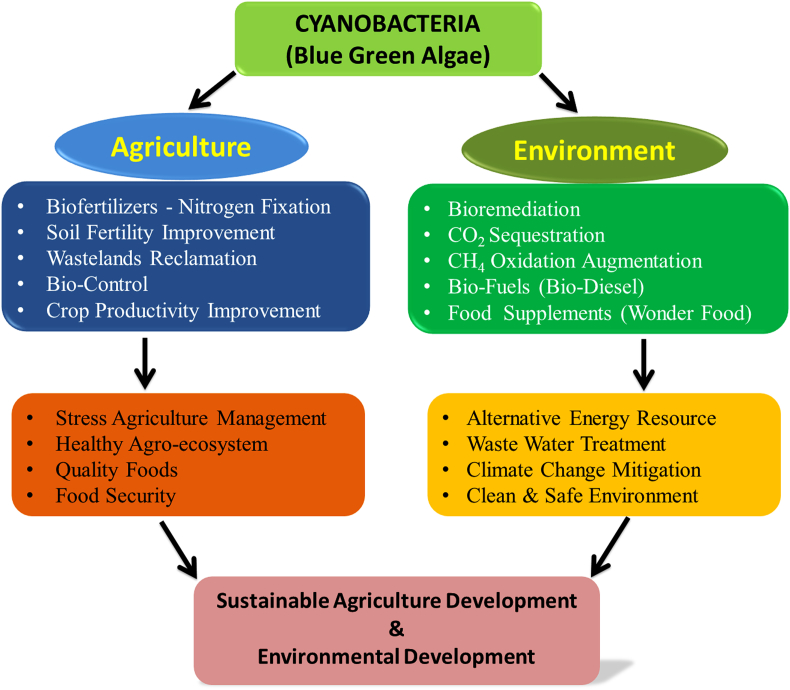
Cyanobacteria are emerging microorganism for sustainable agricultural development [24,55,56]. Fig. 2 displays a theoretical representation that exhibits the potential functions of cyanobacteria in sustainable agriculture and the environment. Diazotrophes are cyanobacteria useful for the generation of eco-friendly biofertilizers which are easily available and less costly. They can control the nitrogen deficiency in plants, improve the aeration of soil, water holding capacity and add vitamin B12 [[57], [58], [59], [60]]. The most efficient nitrogen-fixing cyanobacteria are Nostoc linkia, Anabaena variabilis, Aulosira fertilisima, Calothrix sp., Tolypothrix sp., and Scytonema sp. are present in the rice crop cultivation area [61]. Anabaena and Nostoc are surviving on the surface of soil and rocks, and fix up to 20–25 kg/ha atmospheric nitrogen. Anabaena can fix 60 kg/ha/season of nitrogen and also enriches soils with organic matter [62]. Cyanobacteria do not require a host for their growth, development, and production of valuable organic products. Azolla-Anabaena association is an example of symbiosis for nitrogen fixation and nutrient enrichment in the rice paddy field. They exhibit lysis of lignin of cell wall and released phenolic compounds which induced profuse sporulation of the organism [63]. Applications of these biofertilizers have been reported in barley, oats, tomato, radish, cotton, sugarcane, maize, chilli and lettuce [64].
Fig. 2.
A theoretical representation exhibits the potential functions of cyanobacteria in sustainable agriculture and the environment.
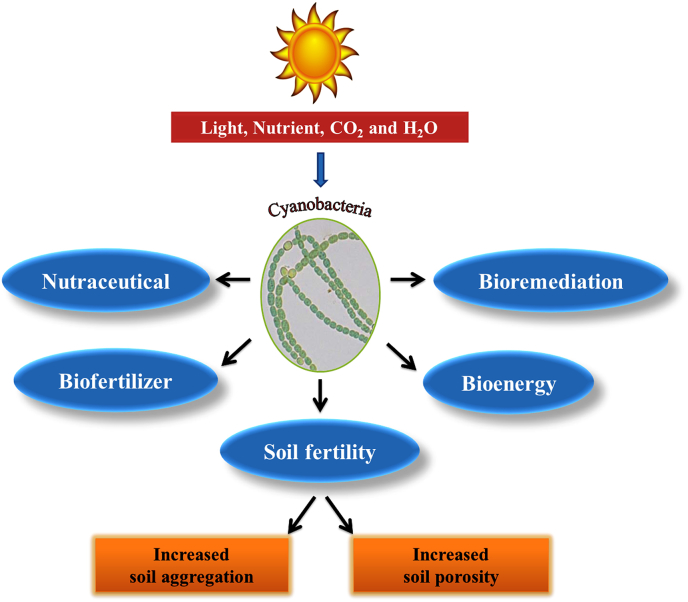
Song et al. [60] stated that cyanobacteria play a chief role in the maintenance and build-up of soil fertility, consequently yield as a natural biofertilizer (Fig. 3: Development of sustainable agricultural practices by utilization of beneficial outcomes of cyanobacterial growth). The major actions of blue-green algae include; (a) Make porous soil and produce adhesive substances. (b) Excretion of phytohormones (auxin, gibberellins, etc.), vitamins, amino acids [65,66]. (c) Improve the water holding capacity of soil through their characteristic jelly structure [65]. (d) Increase in biomass of soil after their death and decomposition [67]. (e) Decrease in soil salinity [67]. (f) Controls weeds growth [67]. (g) Availability of soil phosphate by excretion of organic acids [68]. (h) Efficient absorption of heavy metals on the microbial surface (bioremediation) [69].
Fig. 3.
Development of sustainable agricultural practices by utilization of beneficial outcomes of cyanobacterial growth.
4. Preparation of biofertilizer(s)
The pure culture of a potent strain of nitrogen-fixing cyanobacteria is grown on required agar medium on the slant. A loopful of inoculum is transferred in a 250 ml capacity conical flask containing a liquid medium. Keep the conical flak on rotary shaker or incubator for 3–7 days depending on whether they are fast-growing or slow-growing. The content of these flasks usually attain a load of 105–106 cells per ml called mother culture or starter culture. These mother cultures are further multiplied in larger flasks. The flasks are then kept on a rotary shaker for 96–120 h until the viable count per ml reaches 109–1010 cells achieved. The broths become thicker inconsistency. This broth culture with a population of 109–1010 cells per ml should not be stored more than 24 h or stored at 4 °C temperature. After that, fermenters are used for large scale production of microbial products like bio-fertilizers and bio-pesticides. A fermenter is a device in which the optimum conditions for the microbial growth and activities are established artificially. For the production of liquid biofertilizer, the broth from the fermenters directly goes to the automatic filling machine and get packed in 250 ml or 500 ml or 1 L pet bottles as per the demand of 0.5 mm thickness leaving 2/3rd space open for aeration of the bacteria. Then the bottles get sealed by automatic sealing machines. The pet bottles used for filling of microbial inoculants should be printed with the following information; Inoculants Name; Direction for Use; Crops Name; Manufacture Date; Expiry Date, etc.
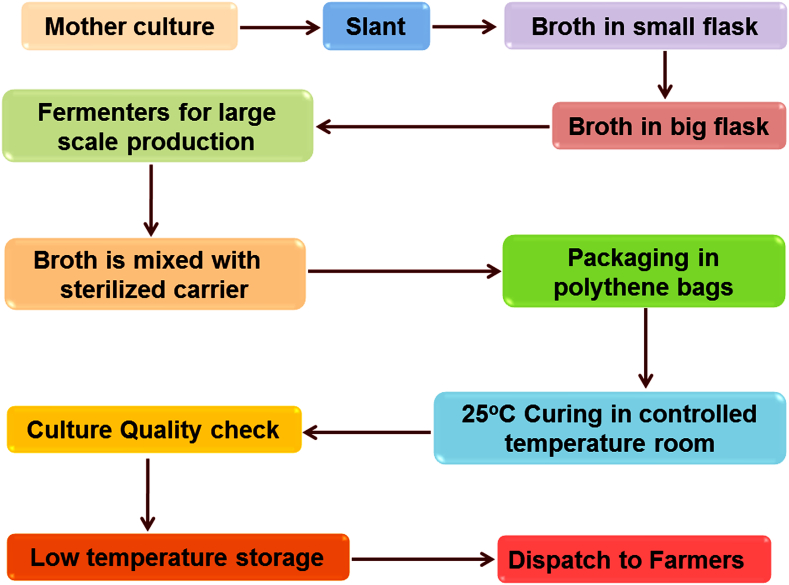
The microbial count of the inoculants has to be checked at the time of manufacturing. The viable cell count in the inoculants should be maintained as per ISI specifications. The inoculants shall be stored by the manufacturer in a cool place away from direct heat preferably at a temp of 15 °C and not exceeding 30 °C ± 2 °C for six months. For long survival of microorganisms, the bottles need to be stored below 33 °C temperature. Fig. 4 explains the preparation of biofertilizers and their steps. The biofertilizer has been carrier-based and contains 108 viable cells per gram of carrier and stored at 25–30 °C. The best suitable pH for biofertilizer preparations is at the range between 6.0 to 7.5. Biofertilizers are the mixture of the inoculants and carriers. The carriers are fine and inert, good moisture absorption capacity, free of lump forming material, easy to sterilize, economical, easily available and excellent buffering capacity. Peat is the most frequently used carrier for biofertilizer preparation but, a limited amount of peat is produced in India. Alternates of the carrier include lignite, coal, charcoal, filter mud, vermiculite, polyacrylamide, mineral soils, vegetable oils, coir waste, etc. Carrier has been processed by mining, drying, and milling. This is the expensive aspects of biofertilizer production.
Fig. 4.
Represents the mass culture of biofertilizers and their steps.
Preparation of biofertilizer has started with mining, draining and clearing off stones, roots than drying, etc. The carrier has been fined through heavy mills. 10–40 μm particle size of carrier material has been used for seed coating and carrier with particle size 500–1500 μm is used for soil implantation. The activity of carriers has to be neutralized by treating with calcium carbonate (pH 6.5–7.0). After this step, the carrier has been sterilized for use as inoculants [70], patented legume biofertilizer and marketed as Nitragin in England and the United States. Nitragin is an agar-based biofertilizer, having high mortality rate imported from the U.S.A. so, the Nitragin is replaced by a peat-based biofertilizer. In India, commercial productions of peat-based biofertilizers are started in the late 1960s at Indian Agricultural Research Institute, New Delhi. The performance of peat-based biofertilizer and ‘Nitragin’ was compared at the Indian Agricultural Research Institute, New Delhi.
Carrier material is easily handled, longer shelf life and provides higher effectiveness of biofertilizer. Among various types of biofertilizers, bacterial inoculant is one major group that includes rhizobia, nitrogen-fixing rhizobacteria, plant growth-promoting rhizobacteria, phosphate-solubilizing bacteria, etc. Various types of material are used as a carrier for seed or soil inoculation. For the preparation of seed inoculant, the carrier material is milled to a fine powder with a particle size of 10–40 μm. The properties of a good carrier material for seed inoculation are: (1) non-toxic and inert (2) good moisture content (3) good absorption capacity, (4) easy to operate and free of lump-forming materials, (5) easily to sterilized by autoclaving or gamma-irradiation, (6) available in adequate amounts, (7) inexpensive, (8) good adhesion to seeds, and (9) good pH buffering capacity and (10) non-toxic to plant, is another important property. Peat is the most frequently used carrier material for seed inoculation. Peat-based rhizobial inoculant is already used in many countries and pieces of information are available on the properties and effects of the inoculants. Carrier material with granular size 0.5–1.5 mm is generally used for soil inoculation. Granular forms of peat, perlite, charcoal or soil aggregates are suitable for the inoculation of soil. Sterilization of carrier material is essential to keep a high number of inoculant bacteria on the carrier for a long storage period. Gamma-irradiation is the most suitable way of carrier sterilization because the sterilization process makes almost no change in the physical and chemical properties of the material. Carrier material should be packed in a thin-walled polyethylene bag and then gamma-irradiated at 50 kGy (5 Mrads). Another way of carrier sterilization is autoclaving at 121 °C for 60 min.
The biofertilizer is free of contaminants and expires after one year from manufacture. Each packet of biofertilizer has contained the following information such as product name, leguminous crop, manufacture name and address, carrier type, batch or code number, manufacture date, expiry date, net quantity meant for net area and instructions of storage. Packets should be marked with ISI (BIS) certification mark. The biofertilizer has been stored at 15 °C for maintains in a viable state without multiplication. As certification arrangements are not in place at present, legislation for quality monitoring and accredited labs for testing may be needed in the future to ensure proper quality and promote these products.
5. Mass production of cyanobacterial biofertilizers
Due to the useful property of cyanobacteria and other microalgae in diverse sectors has necessitated their large-scale cultivation. Economic sustainability is the most important factor which determines the success of large scale biomass production of commercially important products. Five critical abiotic parameters are light, pH, temperature, water, carbon dioxide, and nutrient supplements (C, N, P, S, K, Fe, etc.) determine the success of the growth of cyanobacteria [[71], [72], [73], [74]]. Tremendous expertise and resources are required to control all these factors. Few cyanobacteria and microalgae such as Arthrospira, Chlorella, Haematococcus, and Dunaliella have been cultivated on a large scale as economically and commercially viable crops [75]. Commercial production of photosynthetic microorganisms can be achieved in different ways:
-
(1)
Open system cultivation using sunlight
-
(2)
Closed system cultivation using sunlight
-
(3)
Closed system cultivation using artificial light
5.1. Open system cultivation using sunlight
In open cultivation systems natural sunlight is the source of energy. Raceway or circular type shallow open ponds are used for the mass cultivation of cyanobacteria and microalgae [76,77]. There are major advantages and disadvantages compensate for the limit of the overall biomass production.
Advantage:
-
•
Solar radiation is free of cost
Disadvantage.
-
•
Contamination by algae, grazers and other microorganisms are unpreventable which compromises net productivity.
Contamination problems could be avoided for organisms requiring unique growth conditions which generally prevent the growth of other organisms, however, this strategy limits the application of open systems for cultivation of only selected organisms [78,79]. Example: Spirulina has been extensively cultivated in Mexico, USA, China, and Thailand using the open system under unique growth requirement [80,81].
5.2. Closed system cultivation using sunlight
In this system solar radiation is a source of energy [82,83]. Transparent material such as plastic or glass is used for making vessels which are placed outdoors in the natural light for illumination [84].
Advantages:
-
•
Helps in preventing contamination of grazers and competitors.
-
•
Provides a higher surface to volume ratio and cell densities obtained are often higher then in open systems.
Disadvantages:
-
•
Cost of these systems is increased significantly by the application of transparent materials [85].
-
•
Removal of oxygen produced by photosynthesis and maintenance of optimum temperature are other factors which need to be critically observed in closed systems.
There are many techniques have been developed to maintain these parameters; however, the associated costs usually, offset the cost advantage of using natural sunlight [84].
5.3. Closed system cultivation using artificial light
In this system source of radiation is artificial light [[86], [87], [88]]. Photobioreactor is used for the cultivation of various organisms. Photobioreactor is closed vessels that are similar to conventional fermenters driven by light. All empower real-time control and culture parameters are optimized using software [86,89,90]. Photobioreactors serve as an important tool for the production of high-value products such as stable-isotope-labeled biochemicals [91]. These systems are also ideal for the cultivation of genetically modified organisms. The disadvantage is the utilization of plastic or glass material for making the vessel and power consumption is costly. However, the cost can be offset by the production of high quality and quantity biomass using these systems. The mass cultivation of cyanobacteria has been done by four other techniques such as cemented tank method, shallow metal troughs method, polythene lined pit method and field method. The polythene lined method is most suitable for small and marginal farmers for the preparation of biofertilizer. In this method, small pits are prepared in the field and lined with thick polythene sheets, for example, Anabaena, Aulosira, Cylindrospermum, Gloeotrichia, Nostoc, Plectonema, Tolypothrix, etc. are used for inoculum preparation [24].
6. Potential roles of cyanobacteria in sustainable agriculture
The indiscriminate use of chemical nitrogenous fertilizers in agriculture is global concern. Sustainability considerations mandate that alternatives to nitrogen fertilizers must be urgently sought. Biological nitrogen fixation (BNF), a microbiological process that converts atmospheric nitrogen into a plant useable form, offers this alternative. Nitrogen-fixing systems offer an economically attractive and ecologically sound means of reducing external inputs and improving internal resources [92].
Production cost of inorganic nitrogen fertilizers is very high [[93], [94], [95]]. Deficiency of nitrogen in crops is fulfilled by the use of biofertilizer in a sustainable manner. Cyanobacteria are the example of biofertilizer, they can fix less than 10 kg/ha Nitrogen. Yearly approx. 10–30 kg/ha of nitrogen is fixed by dense mats of cyanobacteria [96,97].
Cyanobacteria are involved in the biogeochemical cycle of carbon, nitrogen, and oxygen [[98], [99], [100]]. They can survive in wet soils and significantly affect the nutritional status, structural stability and crop productivity [101]. During the course of evolution continuous changes occurs at the molecular level, that is required for survival under high intensity of UV radiation (280–400 nm), desiccation, fluctuation in temperature, and high salinity condition [100,102,103]. All these conditions are advantageous and provide protection from other competitors and grazers [104]. Table 2 shows the types of metabolites and their category. These cyanobacterial metabolites are important as agronomical and economically [24,94,105].
Table 2.
| Antibacterial | Antifungal | Anticancerous | Antiviral | Antimalarial |
|---|---|---|---|---|
| Abietane diterpenes, Ambiguines, Bastadin, Bis-(v-butyrolactones), Hapalindole, Didehydromirabazole, Nostocine A, Muscoride, Noscomin, Tolyporphin |
Anhydrohapaloxindole, Fischerllin, Majusculamide A–D, Tanikolide |
Acutiphycin, Ankaraholide A, Aplysiatoxin, Apratoxins, Arenastatin A, Aurilide, Bisebromoamide, Biselyngbyaside, Borophycin, Calothrixins A, B, Carmabin A, B, Caylobolide, Cocosamides B, Coibamide, C-phycocyanin, Cryptophycins, Curacin A, Hoiamide, Dragonamide C, D, Dolastatins, Diacylglycerols, Homodolastatin 16, Isomalyngamide, Kalkitoxin, Jamaicamides, Lagunamide, Largazole, Lyngbyabellin B, Majusculamide, Malevamide, Malyngamide 3, Obyanamide, Palauamide, Symplocamide A Symplostatin 3, Tasiamide, Tasipeptins, Tjipanazoles, Ulongapeptin, Veraguamides, Wewakpeptins |
Bauerines A–C, Calcium spirulan, Cyanovirin, Scytovirin, Sulfoglycolipid, Sulfolipids |
Calothrixins A, B, Carmabin A, B, Dragonamide A, B, Dragomabin, Dolastatins, Nostocarboline, Symplocamide A, Venturamide A, B |
Other applications of symbiotically associated cyanobacteria in bioremediation of affected soils or aquatic systems [[106], [107], [108]], and production of exopolysaccharides (EPS) [101]. The EPS act as a gluing agent for aggregation of soil particles, organic matter accumulation and increase in water holding capacity of the upper layer of soil [109]. PGPRs along with EPS-producing cyanobacteria may contribute to improvement and reclamation of infertility of soil [[110], [111], [112], [113], [114]].
7. Research achievements and challenges to commercialization
Cyanobacteria present a potent platform by utilizing carbon dioxide (CO2) and convert it into fuels, commodity chemicals, and value-added products using sunlight as the energy source [115]. So, they can be used as green cyanobacterial catalysts. The resultant carbon capture and utilization technologies have the potential to reduce the harmful effects of elevated CO2 levels if the technology progresses to an industrial scale. Cyanobacteria carry promising physiological processes, including light-induced hydrogen evolution by biophotolysis [116,117]. Widespread and advance research has been taken place in the relevant fields of biotechnology. Cyanobacteria may be used for food or fodder because some strains have a very high content of proteins, vitamins and other essential growth factors and vital pigments of interest can also be produced [118]. Cyanobacteria are also sources for substances of pharmaceutical interest (such as antibiotics) [119,120]. These examples are only a few of the possible applications of cyanobacteria for economic development and their utilization is among the several challenges for biotechnology for the next millennium. Despite the potential, several technological challenges need to be overcome before cyanobacteria-based processes become commercially viable [[121], [122], [123], [124], [125]].
Recent research leading to technical improvements and increased consumer demand has resulted in market expansion for cyanobacterial species and their products [[126], [127]]. However, their biotechnological potential is still not explored completely and requires exhaustive research for industrial-scale development of its approved functional food products [[128], [129], [130], [131]]. Cyanobacteria provide an ultimate mix of nutrition in the right quantities as a single food. Although they are a promising source offering diverse functional foods, they are still underexplored as a natural resource [[132], [133], [134], [135], [136], [137]].
8. Conclusion
Cyanobacteria have an emerged potential as biofertilizer. They have the ability to utilize CO2, water, and nutrients to convert solar energy into biomass. Efficient applications of cyanobacteria have been reported in agricultural practices to reduce global warming by decreasing CO2 gas. The overall study stated that cyanobacteria biomass can be utilized for improving the quality of food products, physicochemical properties of soil, controlling soil-borne diseases, added organic matter, release growth-promoting substances, solubilize the insoluble phosphates, use as nutraceuticals and also apply in pharmaceuticals. Hence, biofertilizers prepared from cyanobacteria are economical and environment-friendly.
Author contributions
MM conceived the idea of the manuscript, provided the general concept and inputs for each specific section, and drafted part of the manuscript. DC, MM, TSB, and PS have written the review after collecting literature. MM and PS both the authors edited, compiled, and finalized the draft. Finally, all the authors read and approved it for publication.
Declaration of competing interest
The authors declare that the work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
The author, MM is thankful to Mohanlal Sukhadia University, Udaipur for providing the necessary facilities during the course of study. This work was supported by University Grants Commission Startup Research Grant (UGC Faculty Research Promotion Scheme; UGC-FRPS).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2020.100737.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Pisciotta J.M., Zou Y., Baskakov I.V. Light-dependent electrogenic activity of cyanobacteria. PloS One. 2010;5(5) doi: 10.1371/journal.pone.0010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams P.J.B., Laurens L.M.L. Microalgae as biodiesel and biomass feedstock: review and analysis of the biochemistry, energetic and economics. Energy Environ. Sci. 2010;3(5):554–590. [Google Scholar]
- 3.Milledge J.J. Commercial application of microalgae other than as biofuels: a brief review. Rev. Environ. Sci. Biotechnol. 2011;10(1):31–41. [Google Scholar]
- 4.Hoekman S.K., Broch A., Robbins C., Ceniceros E., Natarajan M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012;16(1):143–169. [Google Scholar]
- 5.Koller M. Cyanobacterial polyhydroxyalkanoate production: status quo and quo vadis? Curr. Biotechnol. 2015;4(4):464–480. [Google Scholar]
- 6.Meena M., Divyanshu K., Kumar S., Swapnil P., Zehra A., Shukla V., Yadav M., Upadhyay R.S. Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon. 2019;5(12) doi: 10.1016/j.heliyon.2019.e02952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meena M., Swapnil P., Barupal T., Sharma K. A review on infectious pathogens and mode of transmission. J. Plant Pathol. Microbiol. 2019;10:472. [Google Scholar]
- 8.Bryant D.A., Guiglielmi G., Tandeau de Marsac N., Castets A., Cohen-Bazire G. The structure of cyanobacterial phycobilisomes: a model. Arch. Microbiol. 1979;123:113–127. [Google Scholar]
- 9.El-Sohaimy S.A. Functional foods and nutraceuticals – modern approach to food science. World Appl. Sci. J. 2012;20(5):691–708. [Google Scholar]
- 10.Jensen T.E. Electron microscopy of polyphosphate bodies in a blue-green alga, Nostoc pruniforme. Arch. Microbiol. 1968;62:144–152. [Google Scholar]
- 11.Pankratz H.S., Bowen C.C. Cytology of blue-green algae. I. The cells of Symploca muscorum. Int. J. Bot. 1963;50:387–399. [Google Scholar]
- 12.Jensen T.E., Sicko L.M. Fine structure of poly-β-hydroxybutyric acid granules in a blue-green alga, Chlorogloea fritschii. J. Bacteriol. 1971;106(2):683–686. doi: 10.1128/jb.106.2.683-686.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drosg B., Fritz I., Gattermayr F., Silvestrini L. Photo-autotrophic production of poly(hydroxyalkanoates) in cyanobacteria. Chem. Biochem. Eng. Q. 2015;29(2):145–156. [Google Scholar]
- 14.Jensen T.E., Bowen C.C. Organization of the centroplasm in Nostoc pruniforme. Proc. Iowa Acad. Sci. 1961;68:86–89. [Google Scholar]
- 15.Nierzwickibauer S.A., Balkwill D.L., Stevens S.E. 3-Dimensional ultrastructure of a unicellular cyanobacterium. J. Cell Biol. 1983;97:713–722. doi: 10.1083/jcb.97.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunkel D.D. Thylakoid centers: structures associated with the cyanobacterial photosynthetic membrane system. Arch. Microbiol. 1982;133:97–99. [Google Scholar]
- 17.Roberts T.M., Koths K.E. The blue-green alga Agmenellum quadruplicatum contains covalently closed DNA circles. Cell. 1976;9:551–557. doi: 10.1016/0092-8674(76)90037-4. [DOI] [PubMed] [Google Scholar]
- 18.Ris H., Singh R.N. Electron microscope studies on blue-green algae. J. Biophys. Biochem. Cytol. 1961;9:63–80. doi: 10.1083/jcb.9.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuhs G.W. Cytochemisch-elektronen mikroskopische Lokalisierung der Ribonucleinsiure und des Assimilats in Cyanophyceen. Protoplasma. 1963;56:178–187. [Google Scholar]
- 20.Meena M., Aamir M., Vikas K., Swapnil P., Upadhyay R.S. Evaluation of morpho-physiological growth parameters of tomato in response to Cd induced toxicity and characterization of metal sensitive NRAMP3 transporter protein. Environ. Exp. Bot. 2018;148:144–167. [Google Scholar]
- 21.Meena M., Swapnil P., Zehra A., Dubey M.K., Aamir M., Patel C.B., Upadhyay R.S. Virulence factors and their associated genes in microbes. In: Singh H.B., Gupta V.K., Jogaiah S., editors. New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier; 2018. pp. 181–208. [Google Scholar]
- 22.Panjiar N., Mishra S., Yadav A.N., Verma P. Functional foods from cyanobacteria: an emerging source for functional food products of pharmaceutical importance. In: Gupta V.K., Treichel H., Shapaval V.O., Antonio de Oliveira L., Tuohy M.G., editors. Microbial Functional Foods and Nutraceuticals. first ed. John Wiley & Sons Ltd; 2018. [Google Scholar]
- 23.Radmer R.J. Algal diversity and commercial algal products. Bioscience. 1996;46:263–270. [Google Scholar]
- 24.Singh J.S., Kumar A., Rai A.N., Singh D.P. Cyanobacteria: a precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016;7:529. doi: 10.3389/fmicb.2016.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rangel-Yagui C.O., Godoy Danesi E.D., Carvalho J.C.M., andSato S. Chlorophyll production from Spirulina platensis: cultivation with urea addition by fed-batch process. Bioresour. Technol. 2004;92:133–141. doi: 10.1016/j.biortech.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Bhaskar S.U., Gopalswamy G., Raghu R. A simple method for efficient extraction and purification of C-phycocyanin from Spirulina platensis Geitler. Indian J. Exp. Biol. 2005;43:277–279. [PubMed] [Google Scholar]
- 27.Soletto D., Binaghi L., Lodi A., Carvalho J.C.M., Converti A. Batch and fed-batch cultivations of Spirulina platensis using ammonium sulphate and urea as nitrogen sources. Aquaculture. 2005;243:217–224. [Google Scholar]
- 28.Meena M., Prasad V., Upadhyay R.S. Assessment of the bioweedicidal effects of Alternaria alternata metabolites against Parthenium species. Bull. Environ. Sci. Res. 2016;5(1):1–7. [Google Scholar]
- 29.Meena M., Zehra A., Dubey M.K., Aamir M., Gupta V.K., Upadhyay R.S. Comparative evaluation of biochemical changes in tomato (Lycopersicon esculentum Mill.) infected by Alternaria alternata and its toxic metabolites (TeA, AOH, and AME) Front. Plant Sci. 2016;7:1408. doi: 10.3389/fpls.2016.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meena M., Zehra A., Dubey M.K., Upadhyay R.S. Mannitol and proline accumulation in Lycopersicum esculentum during infection of Alternaria alternata and its toxins. Int. J. Biomed. Sci. Bioinform. 2016;3:64–68. [Google Scholar]
- 31.Desmorieux H., Decaen N. Convective drying of Spirulina in thin layer. J. Food Eng. 2005;66:497–503. [Google Scholar]
- 32.Meena M., Prasad V., Zehra A., Gupta V.K., Upadhyay R.S. Mannitol metabolism during pathogenic fungal–host interactions under stressed conditions. Front. Microbiol. 2015;6:1019–1026. doi: 10.3389/fmicb.2015.01019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kedar L., Kashman Y., Oren A. Mycosprine-2-glycine is the major mycosprine-like amino acid in a unicellular cyanobacterium (Euhalothece sp.) isolated from a gypsum crust in a hypersaline saltern pond. FEMS Microbiol. Lett. 2002;208:233–237. doi: 10.1111/j.1574-6968.2002.tb11087.x. [DOI] [PubMed] [Google Scholar]
- 34.Benedetti S., Benvenuti F., Pagliarani S., Francogli S., Scoglio S., Canestrari F. Antioxidant properties of a novel phycocyanin extract from the blue green alga Aphanizomenon flos-aquae. Life Sci. 2004;75:2353–2362. doi: 10.1016/j.lfs.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Subhashini J., Mahipal S.V., Reddy M.C., Mallikarjuna Reddy M., Rachamallu A., Reddanna P. Molecular mechanisms in C-phycocyanin induced apoptsis in human chronic myeloid leukemia cell line-K562. Biochem. Pharmacol. 2004;68:453–462. doi: 10.1016/j.bcp.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 36.Kulshreshtha A., Zacharia J., Jarouliya U., Bhadauriya P., Prasad G.B.K.S., Bisen P.S. Spirulina in healthcare management. Curr. Pharmaceut. Biotechnol. 2008;9:400–405. doi: 10.2174/138920108785915111. [DOI] [PubMed] [Google Scholar]
- 37.Gantar M., Svircev Z. Microalgae and cyanobacteria: food for thought. J. Phycol. 2008;44:260–268. doi: 10.1111/j.1529-8817.2008.00469.x. [DOI] [PubMed] [Google Scholar]
- 38.Chakdar H., Jadhav S.D., Dhar D.W., Pabbi S. Potential applications of blue green algae. J. Sci. Ind. Res. 2011;71(1):13–20. [Google Scholar]
- 39.Priyadarshani I., Rath B. Commercial and industrial applications of microalgae - a review. J. Algal Biomass Util. 2012;3:89–100. [Google Scholar]
- 40.Emeka U., Ndukwe G.I., Mustapha K.B., Ayo R.I. Constraints to large scale algae biomass production and utilization. J. Algal Biomass Util. 2012;3(2):14–32. [Google Scholar]
- 41.Meena M., Dubey M.K., Swapnil P., Zehra A., Singh S., Kumari P., Upadhyay R.S. The rhizosphere microbial community and methods of its analysis. In: Singh H.B., Sarma B.K., Keswani C., editors. Advancement in PGPR Research. CABI Publishers; 2017. pp. 275–295. [Google Scholar]
- 42.Meena M., Gupta S.K., Swapnil P., Zehra A., Dubey M.K., Upadhyay R.S. Alternaria toxins: potential virulence factors and genes related to pathogenesis. Front. Microbiol. 2017;8:1451. doi: 10.3389/fmicb.2017.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barupal T., Meena M., Sharma K. Inhibitory effects of leaf extract of Lawsonia inermis on Curvularia lunata and characterization of novel inhibitory compounds by GC–MS analysis. Biotechnol. Rep. 2019;23 doi: 10.1016/j.btre.2019.e00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barupal T., Chittora D., Meena M. Solid waste: characterization, assessment, monitoring, and remediation. In: Singh R.P., Prasad V., Vaish B., editors. Advances in Waste-To-Energy Technologies. Taylor & Francis/CRC Press; 2019. 01‒19. [Google Scholar]
- 45.Subramanian G., Anusha M.B., Bai A.L.G., Latha R., Sasikala J., Chandrasakaran R. Carbohydrate and protein content of five species of marine cyanobacteria from Pamban and Vadakadu (Rameswaram) coastal regions, Tamil Nadu, India. Int. J. Adv. Interdiscip. Res. 2014;1(6):18–20. [Google Scholar]
- 46.Moreira L.M., Ribeiro A.C., Duarte F.A., Morais M.G., Soares L.A.S. Spirulina platensis biomass cultivated in Southern Brazil as a source of essential minerals and other nutrients. Afr. J. Food Sci. 2013;7(12):451–455. [Google Scholar]
- 47.Rajeshwari K.R., Rajashekhar M. Biochemical composition of seven species of cyanobacteria isolated from different aquatic habitats of western ghats, southern India. Braz. Arch. Biol. Technol. 2011;54(5):849–857. [Google Scholar]
- 48.Mišurcova L., Kračmar S., Klejdus B., Vacek J. Nitrogen content, dietary fiber, and digestibility in algal food products. Czech J. Food Sci. 2010;28(1):27–35. [Google Scholar]
- 49.Shetty K., Paliyath G., Pometto A., Robert E.L. second ed. CRC Press; Boca Raton: 2006. Food Biotechnology. [Google Scholar]
- 50.Meena M., Prasad V., Upadhyay R.S. Evaluation of Alternaria alternata isolates for metabolite production isolated from different sites of Varanasi, India. J. Agric. Res. 2017;2 [Google Scholar]
- 51.Meena M., Prasad V., Upadhyay R.S. Evaluation of biochemical changes in leaves of tomato infected with Alternaria alternata and its metabolites. Vegetos. 2017;30:2. [Google Scholar]
- 52.Burja A.M., Banaigs B., Abou‐Mansour E., Burgess G., Wright P.C. Marine cyanobacteria: a prolific source of natural products. Tetrahedron. 2001;57:9347–9377. [Google Scholar]
- 53.Cardellina J.H., Moore B.S. Editorial: Richard E. Moore (1933–2007) J. Nat. Prod. 2010;73:301–302. doi: 10.1021/np100045f. [DOI] [PubMed] [Google Scholar]
- 54.Subramanian G., Uma L. Cyanobacteria in pollution control. J. Sci. Ind. Res. 1996;55:685–692. [Google Scholar]
- 55.Singh R., Parihar P., Singh M., Bajguz A., Kumar J., Singh S., Singh V.P., Prasad S.M. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: current status and future prospects. Front. Microbiol. 2017;8:515. doi: 10.3389/fmicb.2017.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh S.P., Pathak J., Sinha R.P. Cyanobacterial factories for the production of green energy and value-added products: an integrated approach for economic viability. Renew. Sustain. Energy Rev. 2017;69:578–595. [Google Scholar]
- 57.Hall D.O., Markov S.A., Watanabe Y., Rao K.K. The potential applications of cyanobacterial photosynthesis for clean technologies. Photosynth. Res. 1995;46(1-2):159–167. doi: 10.1007/BF00020426. [DOI] [PubMed] [Google Scholar]
- 58.Paumann M., Regelsberger G., Obinger C., Peschek G.A. The bioenergetic role of dioxygen and the terminal oxidase(s) in cyanobacteria. Biochim. Biophys. Acta Bioenerg. 2005;1707:231–253. doi: 10.1016/j.bbabio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 59.Malik F.R., Ahmed S., Rizki Y.M. Utilization of lignocellulosic waste for the preparation of nitrogenous biofertilizer. Pakistan J. Biol. Sci. 2001;4(10):1217–1220. [Google Scholar]
- 60.Song T., Mårtensson L., Eriksson T., Zheng W., Rasmussen U. Biodiversity and seasonal variation of the cyanobacterial assemblage in a rice paddy field in Fujian, China. FEMS Microbiol. Ecol. 2005;54(1):131–140. doi: 10.1016/j.femsec.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 61.Prasad R.C., Prasad B.N. Cyanobacteria as a source biofertilizer for sustainable agriculture in Nepal. J. Plant Sci. Bot. Orient. 2001;1:127–133. [Google Scholar]
- 62.Moore A.W. Azolla: biology and agronomic significance. Bot. Rev. 1969;35(1):17–34. [Google Scholar]
- 63.Malliga P., Uma L., Subramanian G. Lignolytic activity of the cyanobacterium Anabaena azollae ML2 and the value of coir waste as a carrier for BGA biofertilizer. Microbios. 1996;86:175–183. [Google Scholar]
- 64.Thajuddin N., Subramanian G. Cyanobacterial biodiversity and potential applications in biotechnology. Curr. Sci. 2005;89:47–57. [Google Scholar]
- 65.Roger P.A., Reynaud P.A. Microbiology of Tropical Soils and Plant Productivity. Springer; Dordrecht: 1982. Free—living blue—green algae in tropical soils; pp. 147–168. [Google Scholar]
- 66.Rodríguez A.A., Stella A.M., Storni M.M., Zulpa G., Zaccaro M.C. Effects of cyanobacterial extracellular products and gibberellic acid on salinity tolerance in Oryza sativa L. Saline Syst. 2006;2(1):7. doi: 10.1186/1746-1448-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saadatnia H., Riahi H. Cyanobacteria from paddy fields in Iran as a biofertilizer in rice plants. Plant Soil Environ. 2009;55(5):207–212. [Google Scholar]
- 68.Wilson L.T. Cyanobacteria: a potential nitrogen source in rice fields. Texas Rice. 2006;6:9–10. [Google Scholar]
- 69.Ibraheem I.B. Cyanobacteria as alternative biological conditioners for bioremediation of barren soil. Egypt. J. Phycol. 2007;8(100):99–116. [Google Scholar]
- 70.Nobbe, F., Hiltner, L., 1896. Inoculation of the Soil for Cultivating. US Patent, 570 813.
- 71.Pulz O. Photobioreactors: production systems for phototrophic microorganisms. Appl. Microbiol. Biotechnol. 2001;57:287–293. doi: 10.1007/s002530100702. [DOI] [PubMed] [Google Scholar]
- 72.Flynn K.J., Greenwell H.C., Lovitt R.W., Shields R.J. Selection for fitness at the individual or population levels: modelling effects of genetic modifications in microalgae on productivity and environmental safety. J. Theor. Biol. 2010;263:269–280. doi: 10.1016/j.jtbi.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 73.Meena M., Swapnil P., Upadhyay R.S. Isolation, characterization and toxicological potential of tenuazonic acid, alternariol and alternariol monomethyl ether produced by Alternaria species phytopathogenic on plants. Sci. Rep. 2017;7:8777. doi: 10.1038/s41598-017-09138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meena M., Swapnil P., Zehra A., Dubey M.K., Upadhyay R.S. Antagonistic assessment of Trichoderma spp. by producing volatile and non-volatile compounds against different fungal pathogens. Arch. Phytopathol. Plant Protect. 2017;50(13–14):629–648. [Google Scholar]
- 75.Rosenberg J.N., Oyler G.A., Wikinson L., Betenbaugh M.J. A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr. Opin. Biotechnol. 2008;19:430–436. doi: 10.1016/j.copbio.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 76.Cañedo J.C.G., Lizárraga G.L.L. Considerations for photobioreactor design and operation for mass cultivation of microalgae. In: Thajuddin N., Dhanasekaran D., editors. Algae-Organisms for Imminent Biotechnology. InTech Open; 2016. [Google Scholar]
- 77.Pushparaj B., Pelosi E., Tredici M.R., Pinzani E., Materassi R. An integrated culture system for outdoor production of microalgae and cyanobacteria. J. Appl. Phycol. 1997;9:113–119. [Google Scholar]
- 78.Costa J.A.V., De Morais M.G., Dalcanton F., Reichert C.C., Durante A.J. Simultaneous cultivation of Spirulina platensis and the toxigenic cyanobacteria Microcystis aeruginosa. Z. Naturforsch. 2006;61:105–110. doi: 10.1515/znc-2006-1-219. [DOI] [PubMed] [Google Scholar]
- 79.De Morais M.G., Costa J.A.V. Carbon dioxide fixation by Chlorella kessleri, C. vulgaris, Scenedesmus obliquus and Spirulina sp. cultivated in flasks and vertical tubular photobioreactors. Biotechnol. Lett. 2007;29:1349–1352. doi: 10.1007/s10529-007-9394-6. [DOI] [PubMed] [Google Scholar]
- 80.Vonshak A., editor. Physiology, Cell Biology and Biotechnology. Taylor and Francis; London: 1997. Spirulina platensis (Arthrospira) pp. 131–158. [Google Scholar]
- 81.Lee Y.K. Commercial production of microalgae in the Asia Pacific rim. J. Appl. Phycol. 1997;9:403–411. [Google Scholar]
- 82.Spektorova L., Creswell R.L., Vaughan D. Closed tubular cultivators. World Aquacult. 1997;6:39–43. [Google Scholar]
- 83.Grima E.M., Fernández F.A., Camacho F.G., Chisti Y. Photobioreactors: light regime, mass transfer, and scale up. J. Biotechnol. 1999;70:231–247. [Google Scholar]
- 84.Khatoon N., Pal R. Microalgae in biotechnological application: a commercial approach. In: Bahadur B., Rajam M.V., Sahijram L., Krishnamurthy K.V., editors. Plant Biology and Biotechnology. Springer; 2015. pp. 27–47. [Google Scholar]
- 85.Carvalho A.P., Meireles L.A., Malcata F.X. Microalgal reactors: a review of enclosed system designs and performances. Biotechnol. Prog. 2006;22:1490–1506. doi: 10.1021/bp060065r. [DOI] [PubMed] [Google Scholar]
- 86.Ratchford I.A., Fallowfield H.J. Performance of a flatplate air-lift reactor for the growth of high biomass algal culture. J. Appl. Phycol. 1992;4:1–9. [Google Scholar]
- 87.Wohlgeschaffen G.D., SubbaRao D.V., Mann K.H. Vat incubator with immersion core illumination-A new, inexpensive setup for mass phytoplankton culture. J. Appl. Phycol. 1992;4:25–29. [Google Scholar]
- 88.Lee C.G., Palsson B.O. High-density algal photobioreactors using light-emitting diodes. Biotechnol. Bioeng. 1994;44:1161–1167. doi: 10.1002/bit.260441002. [DOI] [PubMed] [Google Scholar]
- 89.Meena M., Swapnil P., Zehra A., Aamir Mohd, Dubey M.K., Upadhyay R.S. Beneficial microbes for disease suppression and plant growth promotion. In: Singh D.P., Singh H.B., Prabha R., editors. Plant-Microbe Interactions in Agro-Ecological Perspectives. Springer Nature; Singapore: 2017. pp. 395–432. [Google Scholar]
- 90.Meena M., Zehra A., Swapnil P., Dubey M.K., Patel C.B., Upadhyay R.S. Effect on lycopene, β-carotene, ascorbic acid and phenolic content in tomato fruits infected by Alternaria alternata and its toxins (TeA, AOH and AME) Arch. Phytopathol. Plant Protect. 2017;50:317–329. [Google Scholar]
- 91.Apt K.E., Behrens P.W. Commercial developments in microalgal biotechnology. J. Phycol. 1999;3:215–226. [Google Scholar]
- 92.Rashid A., Mir M.R., Hakeem K.R. Plant, Soil and Microbes. Springer; Cham: 2016. Biofertilizer use for sustainable agricultural production; pp. 163–180. [Google Scholar]
- 93.Hegde D.M., Dwivedi B.S., Sudhakara Babu S.N. Biofertilizers for cereal production in India: a review. Indian J. Agric. Sci. 1999;69(2):73–83. [Google Scholar]
- 94.Vaishampayan A., Sinha R.P., Hader D.P., Dey T., Gupta A.K., Bhan U., Rao A.L. Cyanobacterial biofertilizers in rice agriculture. Bot. Rev. 2001;67(4):453–516. [Google Scholar]
- 95.Mahanty T., Bhattacharjee S., Goswami M., Bhattacharyya P., Das B., Ghosh A., Tribedi P. Biofertilizers: a potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2017;24(4):3315–3335. doi: 10.1007/s11356-016-8104-0. [DOI] [PubMed] [Google Scholar]
- 96.Aiyer R.S., Salahudeen S., Venkataraman G.S. Long-term algalization field trial with high-yielding varieties of rice (Oryza sativa L.) Indian J. Agric. Sci. 1972;42:380–383. [Google Scholar]
- 97.Watanabe I., Espinas C.R., Berja N.S., Aligmano B.V. International Rice Research Institute; Los Bafios, Laguna, Philippines: 1977. Utilisation of the Azolla-Anabaena Complex as a Nitrogen Fertilizer for Rice. International Rice Research Paper Series No. 11, 1–15. [Google Scholar]
- 98.Karl D., Michaels A., Bergman B., Capone D., Carpenter E., Letelier R., Lipschultz F., Paerl H., Sigman D., Stal L. Dinitrogen fixation in the world's oceans. In: Boyer E.W., Howarth R.W., editors. The Nitrogen Cycle at Regional to Global Scales. Springer; Dordrecht: 2002. pp. 47–98. [Google Scholar]
- 99.Olson J.M. Photosynthesis in the archean era. Photosynth. Res. 2006;88(2):109–117. doi: 10.1007/s11120-006-9040-5. [DOI] [PubMed] [Google Scholar]
- 100.DeRuyter Y.S., Fromme P. Molecular structure of the photosynthetic apparatus. In: Herrero A., Flores E., editors. The Cyanobacteria: Molecular Biology, Genomics and Evolution. Norfolk. Caister Academic Press; 2008. pp. 217–269. [Google Scholar]
- 101.Nisha R., Kaushik A., Kaushik C.P. Effect of indigenous cyanobacterial application on structural stability and productivity of an organically poor semi-arid soil. Geoderma. 2007;138(1-2):49–56. [Google Scholar]
- 102.Gröniger A., Sinha R.P., Klisch M., Häder D.P. Photoprotective compounds in cyanobacteria, phytoplankton and macroalgae—a database. J. Photochem. Photobiol. B Biol. 2000;58(2-3):115–122. doi: 10.1016/s1011-1344(00)00112-3. [DOI] [PubMed] [Google Scholar]
- 103.Barupal T., Meena M., Sharma K. A study on preventive effects of Lawsonia inermis L. bioformulations against leaf spot disease of maize. Biocatal. Agric. Biotechnol. 2020;23:101473. [Google Scholar]
- 104.Habib M.A.B., Parvin M., Huntington T.C., Hasan M.R. Food and Agriculture Organization of the United Nations; Rome: 2008. A Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish. [Google Scholar]
- 105.Singh R.N. Indian Council of Agricultural Research; New Delhi: 1961. Role of Blue-Green Algae in Nitrogen Economy of Indian Agriculture. 1961. [Google Scholar]
- 106.Rai A.N., Söderbäck E., Bergman B. Cyanobacterium-plant symbioses. New Phytol. 2000;147(3):449–481. doi: 10.1046/j.1469-8137.2000.00720.x. [DOI] [PubMed] [Google Scholar]
- 107.Subashchandrabose S.R., Ramakrishnan B., Megharaj M., Venkateswarlu K., Naidu R. Mixotrophic cyanobacteria and microalgae as distinctive biological agents for organic pollutant degradation. Environ. Int. 2013;51:59–72. doi: 10.1016/j.envint.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 108.Hamouda R.A.E.F., Sorour N.M., Yeheia D.S. Biodegradation of crude oil by Anabaena oryzae, Chlorella kessleri and its consortium under mixotrophic conditions. Int. Biodeterior. Biodegrad. 2016;112:128–134. [Google Scholar]
- 109.Issa O.M., Le Bissonnais Y., Défarge C., Trichet J. Role of a cyanobacterial cover on structural stability of sandy soils in the Sahelian part of western Niger. Geoderma. 2001;101(3-4):15–30. [Google Scholar]
- 110.Flaibani A., Olsen Y., Painter T.J. Polysaccharides in desert reclamation: compositions of exocellular proteoglycan complexes produced by filamentous blue-green and unicellular green edaphic algae. Carbohydr. Res. 1989;190(2):235–248. [Google Scholar]
- 111.Verrecchia E., Yair A., Kidron G.J., Verrecchia K. Physical properties of the psammophile cryptogamic crust and their consequences to the water regime of sandy soils, north-western Negev Desert, Israel. J. Arid Environ. 1995;29(4):427–437. [Google Scholar]
- 112.Zulpa G., Zaccaro M.C., Boccazzi F., Parada J.L., Storni M. Bioactivity of intra and extracellular substances from cyanobacteria and lactic acid bacteria on “wood blue stain” fungi. Biol. Contr. 2003;27(3):345–348. [Google Scholar]
- 113.Paul D., Nair S. Stress adaptations in a plant growth promoting rhizobacterium (PGPR) with increasing salinity in the coastal agricultural soils. J. Basic Microbiol. 2008;48(5):378–384. doi: 10.1002/jobm.200700365. [DOI] [PubMed] [Google Scholar]
- 114.Prasanna R., Joshi M., Rana A., Shivay Y.S., Nain L. Influence of co-inoculation of bacteria-cyanobacteria on crop yield and C-N sequestration in soil under rice crop. World J. Microbiol. Biotechnol. 2012;28:1223–1235. doi: 10.1007/s11274-011-0926-9. [DOI] [PubMed] [Google Scholar]
- 115.Richmond A. Large scale microalgal culture and applications. Prog. Phycol. Res. 1990;7:269–330. [Google Scholar]
- 116.Skulberg O.M. Oscillatoialean cyanoprokaryotes and their application for algal culture technology. Arch. Hydrobiol. Suppl. 105, Algol. Stud. 1994;75:265–278. [Google Scholar]
- 117.Kumari P., Meena M., Gupta P., Dubey M.K., Nath G., Upadhyay R.S. Plant growth promoting rhizobacteria and their biopriming for growth promotion in mung bean (Vigna radiata (L.) R. Wilczek) Biocatal. Agric. Biotechnol. 2018;16:163–171. [Google Scholar]
- 118.Borowitzka M.A., Borowitzka L.J. Cambridge University Press; Cambridge: 1988. Micro-algal Biotechnology; p. 477. [Google Scholar]
- 119.Falch B.S., König G.M., Wright A.D., Sticher O., Angerhofer C.K., Pezzuto J.M., Bachmann H. Biological activities of cyanobacteria: evaluation of extracts and pure compounds. Planta Med. 1995;61:321–328. doi: 10.1055/s-2006-958092. [DOI] [PubMed] [Google Scholar]
- 120.Kumari P., Meena M., Upadhyay R.S. Characterization of plant growth promoting rhizobacteria (PGPR) isolated from the rhizosphere of Vigna radiata (mung bean) Biocatal. Agric. Biotechnol. 2018;16:155–162. [Google Scholar]
- 121.Adam M.S. The promotive effect of the cyanobacterium Nostoc muscorum on the growth of some crop plants. Acta Microbiol. Pol. 1999;2(48):163–171. [Google Scholar]
- 122.Andersen R.A., editor. Algal Culturing Techniques. Elsevier Academic press; 2005. p. IX. ASTM Int. ASTM D5373-08. [Google Scholar]
- 123.ASTM D. vol. 5. ASTM Standards; 2008. (Standard Test Method for Instrumental Determination of Carbon, Hydrogen and Nitrogen in Laboratory Samples of Coal). D5373-08. [Google Scholar]
- 124.Chaumont D. Biotechnology of algal biomass production: a review of systems for outdoor mass culture. J. Appl. Phycol. 1993;5:593–604. [Google Scholar]
- 125.El-Fouly M.M., Abdalla F.E., Shaaban M.M. vol. 21. 1992. Multipurpose large scale production of microalgae biomass in Egypt; pp. 305–314. (Proc. 1st Egypt. Etalian Sympt. Biotechnol. Assiut, Egypt). No. 23. [Google Scholar]
- 126.Flores F.G. Horizon Scientific Press; Norwich: 2008. The Cyanobacteria: Molecular Biology, Genomics, and Evolution. [Google Scholar]
- 127.Jensen T. Cell inclusions in the cyanobacteria. Ann. N. Y. Acad. Sci. 2006;435:279–282. [Google Scholar]
- 128.Kulik M.M. The potential for using cyanobacteria (blue-green algae) and algae in the biological control of plant pathogenic bacteria and fungi. Eur. J. Plant Pathol. 1995;101(6):585–599. [Google Scholar]
- 129.Meena M., Samal S. Alternaria host-specific (HSTs) toxins: an overview of chemical characterization, target sites, regulation and their toxic effects. Toxicol. Rep. 2019;6:745–758. doi: 10.1016/j.toxrep.2019.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Meena M., Swapnil P. Regulation of WRKY genes in plant defense with beneficial fungus Trichoderma: current perspectives and future prospects. Arch. Phytopathol. Plant Protect. 2019;52(1–2):1–17. [Google Scholar]
- 131.Meena M., Zehra A. Tomato: a model plant to study plant-pathogen interactions. Food Sci. Nutr. Technol. 2019;4(1) [Google Scholar]
- 132.Oswald W.J. Large scale algal culture systems (engineering aspects) In: Borowitzka M.A., Borowitzka L.J., editors. Microalgal Biotechnology. Cambridge University Press; Cambridge: 1988. pp. 357–410. [Google Scholar]
- 133.Prasanna R., Sharma B.K., Sharma R.K., Kaushik B.D. Standardization of growth parameters and formulation of medium for cyanobacterial biofertilizer strains. Indian J. Microbiol. 1998;38(4):211–215. [Google Scholar]
- 134.Prasanna R., Sharma E., Sharma P., Kumar A., Kumar R., Gupta V., Pal R.K., Shivay Y.S., Nain L. Soil fertility and establishment potential of inoculated cyanobacteria in rice crop grown under non-flooded conditions. Paddy Water Environ. 2013;11:175–183. [Google Scholar]
- 135.Roger P.A., Ladha J.K. Biological Nitrogen Fixation for Sustainable Agriculture. Springer; Dordrecht: 1992. Biological N2 fixation in wetland rice fields: estimation and contribution to nitrogen balance; pp. 41–55. [Google Scholar]
- 136.Sahu D., Priyadarshani I., Rath B. Cyanobacteria–as potential biofertilizer. CIB Tech. J. Microbiol. 2012;1:20–26. [Google Scholar]
- 137.Shaaban M.M. Nutritional status and growth of maize plants as affected by green microalgae as soil additives. J. Biol. Sci. 2001;6:475–479. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.