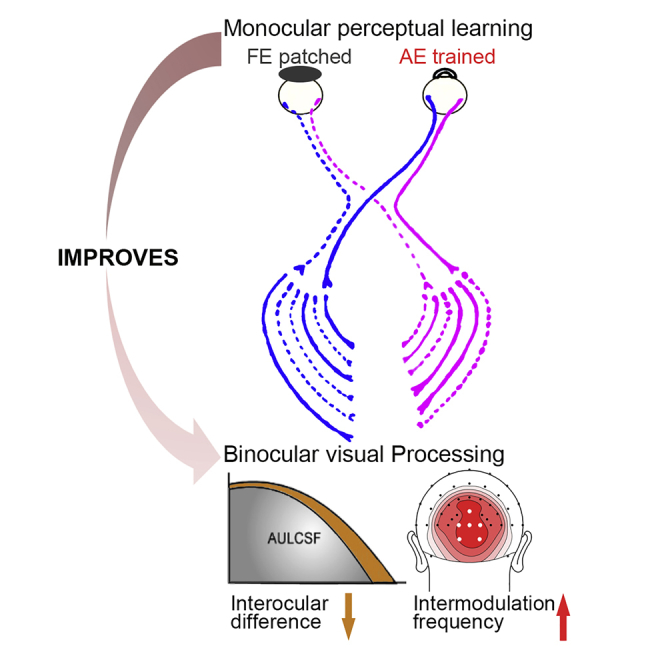
Summary
Re-establishing normal binocular visual processing is the key to amblyopia recovery beyond the critical period of visual development. Here, by combining perceptual learning, behavioral testing, and steady-state visually evoked potentials (SSVEPs), we examined how monocular perceptual learning in the amblyopic eye could change binocular visual processing in the adolescent and adult amblyopic visual system. We found that training reduced the interocular difference between amblyopic and fellow eyes and increased the amplitude of a binocular SSVEP component, with a significant negative correlation between the two measures. Our results demonstrate that training in the amblyopic eye primarily improves binocular rather than monocular visual processing in the amblyopic visual system, suggesting that behavioral training could potentially address key neural deficits in adolescent and adult amblyopia.
Subject Areas: Biological Sciences, Behavioral Neuroscience, Sensory Neuroscience
Graphical Abstract
Highlights
-
•
PL reduced the interocular difference
-
•
PL increased the amplitude of a binocular SSVEP component
-
•
Training in the amblyopic eye improves binocular visual processing
Biological Sciences; Behavioral Neuroscience; Sensory Neuroscience
Introduction
Amblyopia is the most common developmental neuro-visual condition and affects approximately 2%–5% of the world population (Holmes and Clarke, 2006). It is mostly a cortical disorder resulting from the formation of abnormal binocular visual inputs during early postnatal development due to strabismus, large refractive errors, or form-deprivation (Holmes and Clarke, 2006, Hubel and Wiesel, 1964). In animal models, amblyopia is often associated with the abnormal development of ocular dominance columns (Hubel and Wiesel, 1964). In vision clinics, patients with amblyopia exhibit impaired spatial and binocular vision (Holmes and Clarke, 2006). Studies have shown that both monocular and binocular deficits are important predictors of amblyopic visual functions (Hess and Thompson, 2015, Kiorpes, 2006, Kiorpes, 2016, McKee et al., 2003). Re-establishing normal binocular visual processing in the amblyopic visual system is the key to amblyopia recovery (Hess and Thompson, 2015, Hubel and Wiesel, 1964, McKee et al., 2003).
In current clinical practice, children with amblyopia are treated by monocularly patching or penalizing the non-amblyopic eye, whereas adolescents and adults with amblyopia are not treated (Holmes and Clarke, 2006). However, a large number of recent studies have shown that monocular perceptual learning in the amblyopic eye could improve visual functions in adolescents and adults with amblyopia (Dosher and Lu, 2017, Hess and Thompson, 2015, Huang et al., 2008, Levi and Li, 2009, Lu et al., 2005, Polat, 2009, Polat et al., 2004, Sagi, 2011, Sasaki et al., 2010, Watanabe and Sasaki, 2015, Zhou et al., 2006). In this study, we ask the following question: How does monocular perceptual learning in the amblyopic eye change binocular visual processing in the amblyopic visual system? We combined perceptual learning, behavioral testing, and steady-state visually evoked potentials (SSVEPs) to address this question.
SSVEPs are often used to tag neural responses to visual stimuli at specific temporal frequencies (Norcia et al., 2015). The technique has been widely used to investigate neural responses during binocular rivalry (Katyal et al., 2016, Regan and Regan, 1988, Zhang et al., 2011). In response to dichoptically presented visual stimuli flickering at two different temporal frequencies (f1, f2), SSVEP components presented at fundamental (f1, f2) and harmonic frequencies (mf1, nf2) are associated with monocular visual processing and SSVEP components at the intermodulation frequencies m f1 ± n f2 are associated with binocular visual processing (Baitch and Levi, 1988, Regan and Regan, 1989, Zhang et al., 2011). Here, we used the amplitudes of the intermodulation components of SSVEP to evaluate changes in binocular visual processing following perceptual learning in anisometropic amblyopia. We hypothesized that perceptual learning would reduce the interocular difference between amblyopic and fellow eyes and that this reduction would be associated with higher amplitudes of intermodulation SSVEP components.
Results
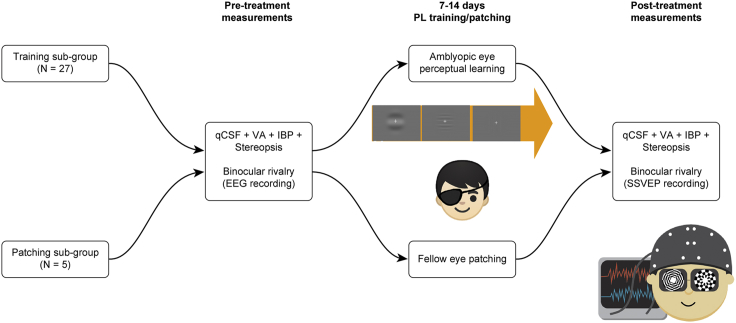
A total of forty-six patients with anisometropic amblyopia and twelve subjects with normal vision participated in this study. Twenty-seven of the amblyopic subjects were trained in a monocular two-alternative-forced-choice (2AFC) identification task at the cutoff spatial frequency in the amblyopic eye (Huang et al., 2008), and five of these subjects received patching treatment (see Supplemental Information for details). We recorded SSVEPs while the subjects viewed binocular rivalry stimuli consisting of a pair of incompatible circular checkerboard patterns flickering at two different temporal frequencies. The SSVEPs were recorded for all subjects at baseline and for those in the treatment groups after treatment (Figure 1). To gauge the impact of perceptual learning on amblyopic vision, a number of visual functions, including monocular visual acuity (VA), monocular contrast sensitivity function (CSF), interocular balance point (IBP) in binocular phase combination, and stereopsis (Hou et al., 2010, Huang et al., 2008, McKee et al., 2003), were also assessed before and after treatment (Figure 1).
Figure 1.
Experimental Procedure
Subjects in the treatment groups were either trained in a monocular 2AFC identification task in the amblyopic eye or received patching treatment in the fellow eye. Before and after treatment, we measured monocular visual acuity, monocular contrast sensitivity function (Hou et al., 2010), interocular balance point in binocular phase combination (Ding and Sperling, 2006), stereopsis, and SSVEPs while the subjects viewed flickering binocular rivalry stimuli. See also Table S1 for clinical details.
SSVEPs and Behavioral Measurements at Baseline
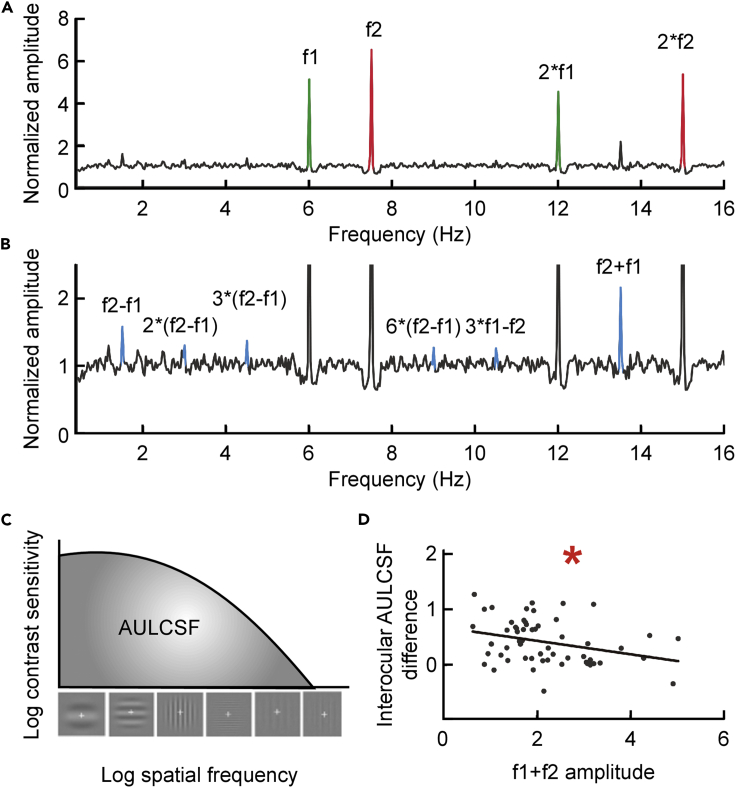
We first evaluated SSVEPs in all the subjects at baseline (see Supplemental Information for details). The SSVEPs exhibited robust monocular responses at the two fundamental (f1, f2) and second harmonic flicker frequencies (2f1, 2f2) (Mf1 = 5.122 ± 0.417, t57 = 9.889, p < 0.001; Mf2 = 6.535 ± 0.573, t57 = 9.661, p < 0.001; M2∗f1 = 4.538 ± 0.424, t57 = 3.659, p = 0.001; M2∗f2 = 5.367 ± 0.450, t57 = 5.301, p < 0.001) (Figure 2A). The SSVEPs also exhibited significant binocular responses at a series of intermodulation frequencies (Cunningham et al., 2017, Liu-Shuang et al., 2014, Rossion et al., 2012), with the clearest response recorded at f1+f2 (Mf1+f2 = 2.1589 ± 0.133, t57 = 8.711, p < 0.001) (Figure 2B). We further assessed the correlation between SSVEP responses and behavioral measures of monocular and binocular visual functions. For the amblyopic subjects, only the amplitude of the 2∗f2 component was negatively correlated with the cutoff spatial frequency of the amblyopic eye, cutoffAE (r = −0.276, p = 0.036); none of the other correlations between monocular behavioral measures in amblyopic and fellow eyes (VA, AULCSF [Area Under the Log CSF, see Figure 2C for diagram]) and the amplitudes of f1, 2∗f1, f2, or 2∗f2 was significant (all p > 0.064). Across all the subjects at baseline, the amplitude of the f1+f2 component was negatively correlated with the interocular AULCSF difference (r = −0.312, p = 0.017; Figure 2D). None of the other correlations between binocular behavioral measures (interocular visual acuity difference, IBP, stereopsis) and amplitudes of SSVEP intermodulation components was significant (all p > 0.067). We therefore focused on the amplitude of the f1+f2 component in subsequent analyses.
Figure 2.
Illustration of the SSVEP Components, AULCSF, and the Correlation between the Amplitude of the f1+f2 Component and the Interocular AULCSF Difference at Baseline
(A) The average baseline SSVEP spectrum across all 58 subjects. The fundamental and second harmonic components are highlighted (Red: f2 and 2*f2 components are associated with the amblyopic eye; Green: f1 and 2*f1 components are associated with the fellow eye).
(B) An enlarged version of (A) with blue-highlighted SSVEP intermodulation components (f2-f1, 2*f2-2*f1, 3*f2-3*f1, 6*f2-6*f1, 3f1-f2, and f1+f2).
(C) A schematic diagram of AULCSF.
(D) A scatterplot of the interocular AULCSF difference versus the amplitude of the f1+f2 SSVEP component across all subjects at baseline and result of correlation analysis. An asterisk * indicates a significance level of p < 0.05.
See also Figure S1 for scalp topography.
Effects of Perceptual Learning
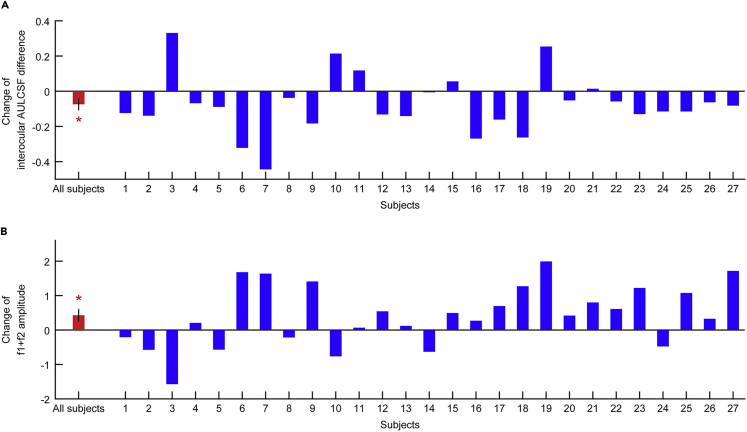
We then examined the effects of perceptual learning. A two-way ANOVA with eye (fellow eye and amblyopic eye) and training (pre-training and post-training) factors showed a significant main effect of eye (F1,26 = 76.332, p < 0.001, partial η2 = .746), a significant main effect of training (F1,26 = 17.455, p < 0.001, partial η2 = 0.402), and a significant interaction between the two factors (F1,26 = 5.271, p = 0.030, partial η2 = .169). Consistent with previous findings (Hess and Thompson, 2015, Huang et al., 2008, Levi and Li, 2009, Levi and Polat, 1996, Zhou et al., 2006), perceptual learning significantly improved the AULCSF of the amblyopic eye (Mdiff = 0.130 ± 0.023, t26 = 5.713, p < 0.001), reduced the interocular AULCSF difference (Mdiff = -0.074 ± 0.033, t26 = −2.292, p = 0.030; Figure 3A), but had no significant effect on the AULCSF of the fellow eye (Mdiff = 0.056 ± 0.032, t26 = 1.772, p = 0.088). It also improved the cutoff spatial frequency and visual acuity of the amblyopic eye as well as stereopsis (Table 1).
Figure 3.
Effects of Perceptual Learning
(A) Effects of perceptual learning on the interocular AULCSF difference.
(B) Effects of perceptual learning on the amplitude of the f1+f2 SSVEP component. One-sample t test for the change of interocular AULCSF difference or the amplitude of the f1+f2 SSVEP component. Data are represented as mean ± SEM. An asterisk * indicates a significance level of p < 0.05.
Table 1.
Mean Values of SSVEPs at Target Frequencies and Behavioral Measures in Amblyopic Subjects before and after Training
| Pre-training (Mean ± SE) | Post-training (Mean ± SE) | PL Change (Mean ± SE) | t-Value | p Value | |
|---|---|---|---|---|---|
| SSVEP-Normalized Value | |||||
| Fellow eye | |||||
| f1 | 4.875 ± 0.665 | 4.459 ± 0.710 | −0.416 ± 0.380 | −1.096 | 0.283 |
| 2*f1 | 4.869 ± 0.704 | 4.117 ± 0.578 | −0.753 ± 0.422 | −1.782 | 0.086 |
| Amblyopic eye | |||||
| f2 | 5.495 ± 0.820 | 6.139 ± 0.781 | 0.644 ± 0.656 | 0.982 | 0.335 |
| 2*f2 | 5.223 ± 0.653 | 4.724 ± 0.585 | −0.499 ± 0.642 | −0.777 | 0.444 |
| Interocular | |||||
| f2-f1 | 1.397 ± 0.139 | 1.090 ± 0.101 | −0.307 ± 0.172 | −1.786 | 0.086 |
| 2*f2-2*f1 | 1.236 ± 0.106 | 1.040 ± 0.115 | −0.197 ± 0.159 | −1.236 | 0.227 |
| 3*f2-3*f1 | 1.296 ± 0.145 | 1.243 ± 0.103 | −0.054 ± 0.185 | −0.292 | 0.773 |
| 6*f2-*6*f1 | 1.327 ± 0.147 | 1.206 ± 0.099 | −0.120 ± 0.138 | −0.874 | 0.390 |
| 3*f1-f2 | 1.148 ± 0.097 | 1.162 ± 0.138 | 0.014 ± 0.140 | 0.098 | 0.923 |
| f1+f2 | 2.025 ± 0.153 | 2.453 ± 0.181 | 0.428 ± 0.171 | 2.495 | 0.019* |
| Behavioral Measurements | |||||
| Fellow eye | |||||
| AULCSFFE | 1.442 ± 0.047 | 1.498 ± 0.035 | 0.056 ± 0.032 | 1.772 | 0.088 |
| CutoffFE | 1.391 ± 0.026 | 1.384 ± 0.025 | −0.007 ± 0.016 | −0.453 | 0.654 |
| VAFE | −0.049 ± 0.019 | −0.073 ± 0.020 | −0.024 ± 0.008 | −3.008 | 0.006* |
| Amblyopic eye | |||||
| AULCSFAE | 0.814 ± 0.072 | 0.944 ± 0.065 | 0.130 ± 0.023 | 5.713 | <0.001** |
| CutoffAE | 0.954 ± 0.048 | 1.020 ± 0.046 | 0.066 ± 0.019 | 3.530 | 0.002* |
| VAAE | 0.473 ± 0.062 | 0.341 ± 0.048 | −0.132 ± 0.021 | −6.311 | <0.001** |
| Interocular and binocular metrics | |||||
| Interocular AULCSF difference | 0.628 ± 0.072 | 0.554 ± 0.070 | −0.074 ± 0.033 | −2.292 | 0.030* |
| Interocular cutoff difference | 0.437 ± 0.054 | 0.364 ± 0.049 | −0.073 ± 0.026 | −2.836 | 0.009* |
| VA interocular difference | −0.522 ± 0.061 | −0.414 ± 0.046 | 0.108 ± 0.024 | 4.628 | <0.001** |
| Interocular balance point | 0.445 ± 0.060 | 0.471 ± 0.067 | 0.026 ± 0.045 | −0.598 | 0.557 |
| Stereopsis | 0.003 ± 0.001 | 0.005 ± 0.001 | 0.002 ± 0.001 | 2.239 | 0.034* |
Note: f1 = 6 Hz, f2 = 7.5 Hz. Only 20 subjects completed the binocular phase combination task. A single asterisk indicates a significance level of p < 0.05. Two asterisks indicate a significance level of p < 0.001.
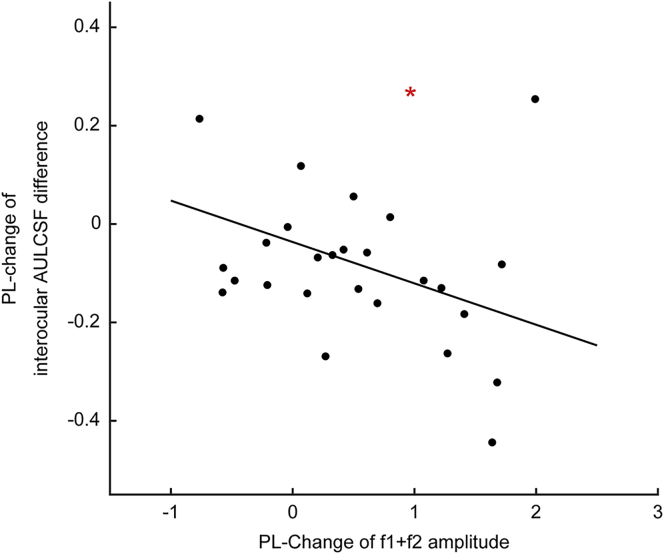
Perceptual learning had no significant effect on the SSVEP components associated with monocular processing in the amblyopic (f2, 2f2) and fellow (f1, 2f1) (all p > 0.08) eyes. However, it did increase the amplitude of the f1+f2 SSVEP component in 19 of the 27 amblyopic subjects, producing a significant effect across all subjects (Mpre = 2.025 ± 0.153, Mpost=2.453 ± 0.181, Mdiff=0.428 ± 0.171, t26= 2.495, p = 0.019) (Figure 3B). Most interestingly, we found that there was a significant negative correlation between reductions in the interocular AULCSF differences and increases in the amplitude of the f1+f2 SSVEP component following perceptual learning (r = −0.436, p = 0.023; Figure 4). This significant correlation held true even after we controlled for changes in SSVEP components at the fundamental and second harmonic frequencies (f1, f2, 2∗f1, 2∗f2) in a multivariable regression analysis (β = −0.481, p = 0.024). In addition, we also found that there was a significant correlation between changes in the stereopsis and increases in the amplitude of the f1+f2 SSVEP component (r = 0.387, p = 0.046; β = 0.430, p = 0.046 in the multivariable regression analysis controlling for f1, f2, 2∗f1, 2∗f2). There was no significant correlation between changes in any monocular behavioral measure and changes in SSVEP components associated with monocular processing (f1, 2∗f1, f2, and 2∗f2; all p > 0.050).
Figure 4.
A Scatterplot of Changes in the Interocular AULCSF Difference and the Amplitude of the f1+f2 SSVEP Component and Result of Correlation Analysis
Asterisk indicates a significance level of p < 0.05.
In addition to the pre-/post-training assessments, subjects also performed a monocular 2AFC identification task during the training period. Focusing on the first and last days of training, we found that perceptual learning significantly improved the contrast threshold (Mstart = 2.208 ± 0.494, Mend=3.183 ± 1.032, Mdiff = 0.967 ± 0.995, t26 = 4.287, p < 0.001), and the improvement was significantly correlated with the increase of f2 amplitude (r = 0.415, p = 0.031). However, the correlation became only marginally significant when we used multi-variate regression to control for other SSVEP components (f1, 2∗f1, 2∗f2, f1+f2) (β = 0.364, p = 0.096).
Control for the Influence of Patching
To control for the influence of patching during the training procedure, five additional patients with anisometropic amblyopia completed 10–13 days of patching treatment. The only difference between the patching and perceptual learning groups was that patching was applied instead of training. A two-way ANOVA with group (training and patching) and treatment (pre-treatment and post-treatment) factors showed a significant interaction effect for AULCSF of AE (F1,30 = 4.875, p = 0.035, partial η2 = 0.140) (main effect of group factor: F1,30 = 1.092, p = 0.304, partial η2 = 0.035; main effect of treatment factor: F1,30 = 4.501, p = 0.042, partial η2 = 0.130). Further analysis showed a significant AULCSF treatment effect in the training group (F1,30 = 29.99, p < 0.001) but no significant AULCSF changes before and after patching in the control group (F1,30 < 0.005, p = 0.963). No significant interaction was found for other electrophysiological or behavioral assessments.
Discussion
As a neuro-visual condition resulting from abnormal binocular visual experience during development, amblyopia can only be successfully treated by restoring normal binocular visual processing. In this study, we show that monocular perceptual learning in the amblyopic eye reduced the interocular difference between the amblyopic and fellow eyes and increased the amplitude of a binocular SSVEP component in adults with anisometropic amblyopia; furthermore, there was a significant negative correlation between the two. These results suggest that monocular perceptual learning in the amblyopic eye could improve binocular visual processing in the amblyopic visual system.
A large number of recent studies have shown that extensive perceptual learning in the amblyopic eye can improve monocular and binocular visual functions (Hess and Thompson, 2015, Huang et al., 2008, Levi and Li, 2009, Li et al., 2013, Polat, 2009, Polat et al., 2004, Zhou et al., 2006). The current study is the first to demonstrate the effects of monocular perceptual learning on amblyopic binocular visual processing using SSVEPs. By measuring the intermodulation f1+f2 component of SSVEP before and after perceptual learning, we were able to demonstrate that the change in the amplitude of the component was correlated with behavioral improvements that have been reported in many previous psychophysical studies (Huang et al., 2008, Levi and Polat, 1996, Li et al., 2013, Lu et al., 2005, Zhou et al., 2006). We also did not observe reliable correlation between the behavioral improvements that followed perceptual learning and the changes in the amplitudes of monocular SSVEP components. Collectively, our results suggest that monocular perceptual learning in the amblyopic eye to a large extent improved binocular rather than monocular visual processing in the amblyopic visual system. This is consistent with previous reports showing that monocular perceptual learning in the amblyopic eye led to improved vision in both amblyopic and fellow eyes (Hess and Thompson, 2015, Huang et al., 2008, Levi and Li, 2009, Polat, 2009).
We adopted four behavioral measures in this study: monocular visual acuity, monocular contrast sensitivity function, interocular balance point, and stereopsis. Visual acuity measures the limit of spatial resolution in high contrast, whereas the contrast sensitivity function is a more comprehensive assessment of spatial vision (Pelli and Bex, 2013). The interocular balance point in phase combination is largely an assessment of interocular inhibition in supra-threshold contrast (Huang et al., 2009). Stereopsis is a popular clinical measure of binocular function in amblyopia. Here, we found that the interocular difference in AULCSF and stereopsis but not the interocular balance point and interocular visual acuity difference was most correlated with the SSVEP intermodulation components. We speculate that interocular phase combination and visual acuity may reflect both inhibitory and excitatory processes in binocular processing (Hess and Jenkins, 1980, Hess and Malin, 2003) and could not be evaluated with the SSVEP measures used in this study.
SSVEP studies using binocular rivalry paradigms have shown a non-linear relationship between the intermodulation frequencies and binocular visual processing (Baitch and Levi, 1988, Regan and Regan, 1989, Zhang et al., 2011), although it remains unclear whether the relationship reflects binocular competition or integration (Gordon et al., 2019, Tong et al., 2006). In this study, we found that increased f1+f2 amplitude was correlated with decreased interocular AULCSF difference and increased stereopsis. Note that the decrease of interocular AULCSF difference and the increase of stereopsis both indicated improvement of binocular balance. The results suggest that the observed increase of f1+f2 amplitude in the binocular conflict paradigm might be related to improved binocular integration. On the other hand, perceptual learning improved binocular balance in the amblyopic visual system and may lead to better inter-ocular conflict resolution. Additional studies are necessary to evaluate this.
Huang et al. (2008) and Hou et al. (2011) showed that, for adults with amblyopia, perceptual learning in contrast detection at the cutoff spatial frequency can transfer to a wide range of spatial frequencies and to motion detection and discrimination in a wide range of temporal frequencies. These results suggest that the amblyopic visual system may possess more plasticity than the normal visual system. Our results are in line with those previous results. Using the same cutoff spatial frequency training paradigm, Huang et al. (2009) found that perceptual learning improved contrast sensitivity and visual acuity in the amblyopic visual system via a combination of internal additive noise reduction and external noise exclusion. Xu et al. (2006) and Huang et al. (2007) found that both increased additive noise and mismatched perceptual template underlay performance deficits in the amblyopic visual system, although the degree of perceptual template mismatch increased with the spatial frequency of the test stimuli. That perceptual learning reduced internal noise and improved external noise exclusion suggests that the training scheme can address both mechanisms underlying amblyopic deficits. Performance improvements in high external noise conditions are potentially related to improved forward and backward masking, whereas improved performance in all the external noise conditions may be related to improved temporal integration in the amblyopic visual system.
Limitations of the Study
Our control experiment with patching only showed that mere repetition of the pre-/post-training assessments did not produce improved behavioral performance or improved f1+f2 amplitude. We note that the control group had only five subjects, which may limit our statistical power in observing patching effects. In addition, it also is possible that the observed training effects in the current study were due to the influences of both training and patching. Therefore, the effects of patching were not entirely ruled out in this study. Further investigations with more subject and only training (no patching) are necessary.
Conclusions
In summary, by combining perceptual learning, behavioral testing, and SSVEP, we found that monocular perceptual learning in the amblyopic eye improved binocular visual processing in the amblyopic visual system. These results suggest that it is possible to use behavioral training to address a key issue in amblyopia treatment, that is, the recovery of binocular processing.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This research was supported by a grant from the National Natural Science Foundation of China (81770954) to J.L., grants from the National Natural Science Foundation of China (31371129) and the Research Project of Sun Yat-sen University (26000-31620003) to X.W., and a grant from the National Eye Institute (EY021553) to Z.-L.L..
Author Contributions
J.L. and X.W. designed the research; L.G., S.D., L.F., and Z.C. performed the research; L.G. analyzed the data; and Z.-L.L., J.L., and X.W. wrote the manuscript.
Declaration of Interests
Zhong-Lin Lu: Commercial Relationship(s), Adaptive Sensory Technology: Code I (Personal Financial Interest), Adaptive Sensory Technology: Code P (Patent). All remaining authors declare no conflicting interests.
Published: February 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100875.
Contributor Information
Xiang Wu, Email: wuxiang3@mail.sysu.edu.cn.
Jinrong Li, Email: lijingr3@mail.sysu.edu.cn.
Supplemental Information
Note: M, male; F, female; AE, amblyopic eye; FE, fellow eye; OD, right eye; OS, left eye; Aniso, anisometropic amblyopia; Mixed, mix of anisometropic and strabismic amblyopia.
References
- Baitch L.W., Levi D.M. Evidence for nonlinear binocular interactions in human visual cortex. Vision Res. 1988;28:1139–1143. doi: 10.1016/0042-6989(88)90140-x. [DOI] [PubMed] [Google Scholar]
- Cunningham D.G.M., Baker D.H., Peirce J.W. Measuring nonlinear signal combination using EEG. J. Vis. 2017;17:10. doi: 10.1167/17.5.10. [DOI] [PubMed] [Google Scholar]
- Ding J., Sperling G. A gain-control theory of binocular combination. Proc. Natl. Acad. Sci. U S A. 2006;103:1141–1146. doi: 10.1073/pnas.0509629103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher B.A., Lu Z.-L. Visual perceptual learning and models. Annu. Rev. Vis. Sci. 2017;3:343–363. doi: 10.1146/annurev-vision-102016-061249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon N., Hohwy J., Davidson M.J., van Boxtel J.J.A., Tsuchiya N. From intermodulation components to visual perception and cognition-a review. NeuroImage. 2019;199:480–494. doi: 10.1016/j.neuroimage.2019.06.008. [DOI] [PubMed] [Google Scholar]
- Hess R.F., Jenkins S. Amblyopia cannot be explained by considering only detection thresholds. Perception. 1980;9:569–576. doi: 10.1068/p090569. [DOI] [PubMed] [Google Scholar]
- Hess R.F., Malin S.A. Threshold vision in amblyopia: orientation and phase. Invest. Opthalmol. Vis. Sci. 2003;44:4762. doi: 10.1167/iovs.03-0259. [DOI] [PubMed] [Google Scholar]
- Hess R.F., Thompson B. Amblyopia and the binocular approach to its therapy. Vision Res. 2015;114:4–16. doi: 10.1016/j.visres.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Holmes J.M., Clarke M.P. Amblyopia. Lancet. 2006;367:1343–1351. doi: 10.1016/S0140-6736(06)68581-4. [DOI] [PubMed] [Google Scholar]
- Hou F., Huang C.-B., Lesmes L., Feng L.-X., Tao L., Zhou Y.-F., Lu Z.-L. qCSF in clinical application: efficient characterization and classification of contrast sensitivity functions in amblyopia. Invest. Ophthalmol. Vis. Sci. 2010;51:5365–5377. doi: 10.1167/iovs.10-5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou F., Huang C., Tao L., Feng L., Zhou Y., Lu Z.-L. Training in contrast detection improves motion perception of sinewave gratings in amblyopia. Invest. Opthalmol. Vis. Sci. 2011;52:6501. doi: 10.1167/iovs.11-7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Tao L., Zhou Y., Lu Z.-L. Treated amblyopes remain deficient in spatial vision: A contrast sensitivity and external noise study. Vision Res. 2007;47:22–34. doi: 10.1016/j.visres.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Huang C.-B., Zhou Y., Lu Z.-L. Broad bandwidth of perceptual learning in the visual system of adults with anisometropic amblyopia. Proc. Natl. Acad. Sci. U S A. 2008;105:4068–4073. doi: 10.1073/pnas.0800824105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.-B., Zhou J., Lu Z.L., Feng L., Zhou Y. Binocular combination in anisometropic amblyopia. J. Vis. 2009;9:17. doi: 10.1167/9.3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D.H., Wiesel T.N. Effects of monocular deprivation in kittens. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1964;248:492–497. doi: 10.1007/BF00348878. [DOI] [PubMed] [Google Scholar]
- Katyal S., Engel S.A., He B., He S. Neurons that detect interocular conflict during binocular rivalry revealed with EEG. J. Vis. 2016;16:18. doi: 10.1167/16.3.18. [DOI] [PubMed] [Google Scholar]
- Kiorpes L. Visual processing in amblyopia: animal studies. Strabismus. 2006;14:3–10. doi: 10.1080/09273970500536193. [DOI] [PubMed] [Google Scholar]
- Kiorpes L. The puzzle of visual development: behavior and neural limits. J. Neurosci. 2016;36:11384–11393. doi: 10.1523/JNEUROSCI.2937-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi D.M., Li R.W. Perceptual learning as a potential treatment for amblyopia: a mini-review. Vision Res. 2009;49:2535–2549. doi: 10.1016/j.visres.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi D.M., Polat U. Neural plasticity in adults with amblyopia. Proc. Natl. Acad. Sci. U S A. 1996;93:6830–6834. doi: 10.1073/pnas.93.13.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Thompson B., Deng D., Chan L.Y., Yu M., Hess R.F. Dichoptic training enables the adult amblyopic brain to learn. Curr. Biol. 2013;23:R308–R309. doi: 10.1016/j.cub.2013.01.059. [DOI] [PubMed] [Google Scholar]
- Liu-Shuang J., Norcia A.M., Rossion B. An objective index of individual face discrimination in the right occipito-temporal cortex by means of fast periodic oddball stimulation. Neuropsychologia. 2014;52:57–72. doi: 10.1016/j.neuropsychologia.2013.10.022. [DOI] [PubMed] [Google Scholar]
- Lu Z.-L., Chu W., Dosher B.A., Lee S. Independent perceptual learning in monocular and binocular motion systems. Proc. Natl. Acad. Sci. U S A. 2005;102:5624–5629. doi: 10.1073/pnas.0501387102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee S.P., Levi D.M., Movshon J.A. The pattern of visual deficits in amblyopia. J. Vis. 2003;3:5. doi: 10.1167/3.5.5. [DOI] [PubMed] [Google Scholar]
- Norcia A.M., Appelbaum L.G., Ales J.M., Cottereau B.R., Rossion B. The steady-state visual evoked potential in vision research: a review. J. Vis. 2015;15:4. doi: 10.1167/15.6.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli D.G., Bex P. Measuring contrast sensitivity. Vision Res. 2013;90:10–14. doi: 10.1016/j.visres.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U. Making perceptual learning practical to improve visual functions. Vision Res. 2009;49:2566–2573. doi: 10.1016/j.visres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Polat U., Ma-Naim T., Belkin M., Sagi D. Improving vision in adult amblyopia by perceptual learning. Proc. Natl. Acad. Sci. U S A. 2004;101:6692–6697. doi: 10.1073/pnas.0401200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan M.P., Regan D. A frequency domain technique for characterizing nonlinearities in biological systems. J. Theor. Biol. 1988;133:293–317. [Google Scholar]
- Regan M.P., Regan D. Objective investigation of visual function using a nondestructive zoom-FFT technique for evoked potential analysis. Can. J. Neurol. Sci. 1989;16:168–179. doi: 10.1017/s0317167100028845. [DOI] [PubMed] [Google Scholar]
- Rossion B., Prieto E.A., Boremanse A., Kuefner D., Van Belle G. A steady-state visual evoked potential approach to individual face perception: effect of inversion, contrast-reversal and temporal dynamics. NeuroImage. 2012;63:1585–1600. doi: 10.1016/j.neuroimage.2012.08.033. [DOI] [PubMed] [Google Scholar]
- Sagi D. Perceptual learning in vision research. Vision Res. 2011;51:1552–1566. doi: 10.1016/j.visres.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Nanez J.E., Watanabe T. Advances in visual perceptual learning and plasticity. Nat. Rev. Neurosci. 2010;11:53–60. doi: 10.1038/nrn2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong F., Meng M., Blake R. Neural bases of binocular rivalry. Trends Cogn. Sci. 2006;10:502–511. doi: 10.1016/j.tics.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Sasaki Y. Perceptual learning: toward a comprehensive theory. Annu. Rev. Psychol. 2015;66:197–221. doi: 10.1146/annurev-psych-010814-015214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Lu Z.-L., Qiu Z., Zhou Y. Identify mechanisms of amblyopia in Gabor orientation identification with external noise. Vision Res. 2006;46:3748–3760. doi: 10.1016/j.visres.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Zhang P., Jamison K., Engel S., He B., He S. Binocular rivalry requires visual attention. Neuron. 2011;71:362–369. doi: 10.1016/j.neuron.2011.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Huang C.-B., Xu P., Tao L., Qiu Z., Li X., Lu Z.-L. Perceptual learning improves contrast sensitivity and visual acuity in adults with anisometropic amblyopia. Vision Res. 2006;46:739–750. doi: 10.1016/j.visres.2005.07.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note: M, male; F, female; AE, amblyopic eye; FE, fellow eye; OD, right eye; OS, left eye; Aniso, anisometropic amblyopia; Mixed, mix of anisometropic and strabismic amblyopia.