Abstract
Objective:
To estimate long-term stimulant treatment associations on standardized height, weight and BMI trajectories from childhood to adulthood in the Multimodal Treatment Study of Attention-Deficit/Hyperactivity Disorder (MTA).
Method:
Of 579 children with DSM-IV ADHD-Combined Type at baseline (ages 7.0–9.9 years) and 289 classmates (local normative comparison group, LNCG), 568 and 258 respectively, were assessed 8 times over 16 years (final mean age = 24.7). Parent interview data established subgroups with self-selected Consistent (N=53, 9%), Inconsistent (N=374, 66%), and Negligible (N=141, 25%) stimulant medication use, as well as cases starting stimulants prior to MTA entry (N=211, 39%). Height and weight growth trajectories were calculated for each subgroup.
Results:
Height z-scores trajectories differed among subgroups (F=2.22, P<0.0001) and by stimulant use prior to study entry (F=2.22, P<0.001). The subgroup × assessment interaction was significant (F=2.81, P<0.0001). Paired comparisons revealed significant subgroup differences at end-point: Consistent shorter than Negligible (−0.66 z-units / −4.06 cm /1.6 inches, t=−3.17, P<0.0016), Consistent shorter than Inconsistent (−0.45 z-units / −2.74 cm / −1.08 inches, t=−2.39, P<0.0172), and the Consistent shorter than LNCG (−0.54 z-units/+3.34 cm/ 1.31 inches, t=−3.30, P<0.001). Weight z-scores initially diverged among subgroups, converged in adolescence, and then diverged again in adulthood when the Consistent outweighed the LNCG (+ 3.561 z-units / +7.47 kg / +16.46 pounds, P<0.0001).
Conclusion:
Compared with those negligibly medicated and the LNCG, 16 years of consistent stimulant treatment of children with ADHD in the MTA was associated with changes in height trajectory, a reduction of adult height, and an increase in weight and BMI.
Clinical trial registration information:
Multimodal Treatment Study of Children With Attention Deficit and Hyperactivity Disorder (MTA); https://clinicaltrials.gov/; NCT00000388
Keywords: ADHD, growth trajectory, stimulant medication, longitudinal study, adult height
INTRODUCTION
Over 45 years ago, reports1,2 suggested that children with ADHD may experience stimulant-related growth suppression. Prospective follow-up studies, reviews and consensus statements over the past 4 decades3–12 dismissed the clinical significance of this finding because initial, short-term slow-downs in height and weight growth were not associated with long-term loss of adult height. Two explanations were offered for this paradox of initial childhood growth slowing down and later normal adult height, that either growth rebound had occurred in adolescence2 or that ADHD disorder itself delayed maturation, with the childhood decelerations made up in adolescence6.
More recently, a cumulative exposure hypothesis has been introduced13. Reported cumulative exposure to stimulant medication in methylphenidate equivalents (ME) have steady increased over decades, as shown in follow-up studies of ADHD on long-term medication in the 1960s with an average exposure of 34,350 mg8, in the 1970s with an average exposure of 36,710 mg5, in the 1980s with an average exposure of 42,268 mg11, all showing no association between dose and duration of stimulants and adult height14. A small study15 conducted within the last decade reported that 22 exposed to cumulative ME doses of 117,530 mg showed a significant association with adult height depression. A wide range of cumulative ME doses also occurred over the 16-year follow-up of the Multimodal Treatment Study of ADHD (MTA Study)17,18 with the negligible, inconsistent, and consistent subgroups receiving ME doses of 2153 mg, 60 mg, 527 mg, and 117,102 mg, respectively14.
Analyses of growth rates in MTA participants (n=579) were published after the 3-year16 and again after the 16-year14 assessment point using post-hoc analyses to form naturalistic subgroups of self-selected medication usage. These analyses included a cohort of age- and sex-matched classmates, recruited after the 2-year assessment point, who served as a non-medicated Local Normative Comparison Group (LNCG, n=289 at recruitment). The 3-year analysis revealed that the subgroup-by-assessment-point interaction was significant for z height (p < .005) and z weight (p < .0001), due to the decrease of standardized growth measures (2.0 cm and 2.7kg less) in the newly medicated subgroup (n=88) compared to the not-medicated (n=65) subgroups.
The 16-year growth analysis was conducted when the MTA group was 24.8 years old14. It revealed that the entire ADHD group, including medicated and unmedicated, was 1.29 cm shorter than the LNCG non-ADHD peer group (P< 0.01, d=0.21). The sex-corrected height of medicated MTA participants (average of Consistent and Inconsistent subgroups) was significantly less than the z-height of the unmedicated youth with ADHD in the negligible subgroup (2.55 cm / 1.1 inches, P<0.005). Within the treated group, the Consistent subgroup was somewhat shorter (2.4 cm / 0.9 inches, P < 0.04), than the Inconsistently treated subgroup but significantly shorter (4.7 cm / 1.9 inches, P<0.001) than the Negligible subgroup. These findings suggested that consistent and extended use of stimulant medication may be associated with some suppression of height into the adult years.
However, these end-point analyses did not indicate whether the different levels of cumulative exposure were associated with different trajectories of growth and whether the associated growth suppression was linear across the treatment period or limited to a specific time during development.
In this paper, our hypothesis is that medication subgroup types will be associated with specific growth trajectories and with a significant interaction of two factors, Subgroup and Assessment point. We predict that the Consistently medicated subgroup trajectory will display a slower-than-average tempo of growth and a downward quadratic trajectory. Because the period of maximum growth is between 10 and 15.9 years of age, we predict that growth trajectories will be most suppressed between the 2- to 6-year assessment points, when the participants were 10.4, 11.7- and 14.9-years old, respectively.
METHOD
Participants
The NIMH MTA Study17 was originally designed as a 1.2 year randomized controlled trial (RCT) to compare the effects of pharmacological and psychosocial treatments for children (7.0–9.9 years old) with ADHD-Combined Type. Two years after baseline, 289 age- and sex-matched classmates were recruited as a local normative comparison group (LNCG). The MTA continued to follow both MTA and LNCG participants with prospective follow-up assessments 8 times from 2 to 16 years after baseline18. Mean ages for the MTA participants at baseline, 2-, 3-, 6-, 8-, 10-, 12-, 14-, and 16- year assessments were 8.4, 10.4, 11.7, 14.9, 16.8, 18.7, 21.1, 23.2 and 24.7 years, respectively.
Under local IRB auspices, informed consent was obtained from parents and assent from youth when they were below age 18. Re-consent was obtained at the age 18 to continue participation in the biannual assessments.
Growth Measures
At each assessment, trained research assistants followed a standardized protocol using stadiometers to measure height (in cm), and digital scales to measure weight (in kg) of the participants with minimum clothing (i.e., without shoes and coats). For comparison with critical studies in the literature6,7,9,10,12,13,19, the most recent growth norms provided by the US Centers for Disease Control20 were used to transform the raw scores (centimeters and kilograms) into standardized scores (z-height, z-weight, and z-BMI)23.
Self-Selected Patterns of Stimulant Medication Use
The general method of categorizing cases based on self-selected stimulant medication in the MTA has been described in detail elsewhere.14 In short, the Services for Children and Adolescents Parent Interview (SCAPI)21 was administered during assessments up to 18 years of age (at 1.2, 2, 3, 6, 8, and in some cases 10 years after baseline). Doses of other approved and available stimulants were transformed to d,l -methylphenidate equivalent (ME) doses, as detailed elsewhere14. Medication used at least 50% of days during every interval was classified as consistent usage, for some but not all intervals classified as inconsistent usage, and for no intervals as negligible use. We classified intervals with missing SCAPI information no use, and classified dropouts based on available SCAPI information up to the last observation. The selection criteria for membership in the specific self-selected subgroups in this analysis were determined from follow-up data only, and did not consider each participant’s randomized treatment group assignment during the RCT. Thus taking medication in the 14-month RCT trial did not affect the membership in the self-selected medication subgroups which were formed only after the completion of the controlled trial.
The total ME dose for each interval was estimated as the product of the days treated times the daily dose. The totals were summed for the six intervals from baseline to the 10-year assessment to estimate the cumulative ME dose from childhood through adolescence. Furthermore, we defined minimally adequate ME doses as at least 10 mg/day for at least 50% of days since the previous assessment was used to classify treatment during each assessment intervals as ‘≥minimal’ or ‘< minimal’ in order to avoid excluding low ME equivalent dosing regimens that might be effective for a few cases and regimens with medication administered only on school days24. Employing a previously published method16, sequences of intervals above or below the cutoff were used to define three long-term patterns of prospective treatment with medication from childhood through adolescence: Consistent (≥ minimally adequate ME dosing in all intervals), Inconsistent (≥ minimally adequate ME dosing in some but not all intervals), and Negligible (< minimally adequate ME dosing for all intervals).
Youth with ADHD recruited into treatment studies often are already on stimulant medication, which has been cited as a neglected factor in follow-up studies22. The MTA Study assessed whether a child started stimulants prior to entering the MTA based on a question at the baseline telephone screening (“Is medication currently being used?”), or at baseline in the SCAPI (“Has medication been used for at least 30 days in the past 3 months?”), or in the Diagnostic Interview Schedule for Children-Parent (“Has medication been used most of the time in the past 6 months?”). Prior stimulant treatment was reported for 37.15% (211/568) MTA participants, with 25.5% (36/141cases) in the Negligible subgroup, 39.0% (146/374 cases) in the Inconsistent subgroup, and 54.7% (29/53 cases) in the Consistent subgroup.
Analyses
We conducted a mixed- model multiple regression analysis (SAS Proc Mixed22) with 3 independent variables: pattern of extended use of medication based on the naturalistic subgroups and LNCG (Subgroup with 4 levels: Consistent, Inconsistent, Negligible, LNCG), prior treatment before entering the MTA (Prior with 2 levels: Yes or No), and Assessment Point (AP) variable with 10 levels: 0, 1.2, 2, 3, 6, 8, 10, 12, 14, and 16 years after baseline. Age and sex were not included as factors since they were used to create the dependent variables (z-scores). The p < 0.05 significance level was applied for tests of main effects and interactions without adjustment, since these were secondary to the previous cross-sectional end-point analyses14 and exploratory to address how end-points were attained. Based on the methodology for longitudinal analysis of z-scores for height,23 a significant Subgroup × AP interaction would support the primary hypothesis.
To unpack this interaction, paired-comparisons were performed using Least Square Mean (LSM) estimates for the subgroups at the 10 assessment points to evaluate development trends. For the LNCG, observations were not available at the baseline and the 14-month (1.2-year) assessment, so the random regression analysis was performed using only the 8 available assessments to estimate LSMs.
The paired-comparisons of subgroups at each assessment point were used to isolate medication-related and disorder-related effects as proposed by Spencer et al.6,7 We extended their logic based on the self-selected subgroups of the MTA, which provided two stimulant-treated subgroups (Consistent and Inconsistent) and two subgroups without stimulants (Negligible and LNCG). This allowed for two comparisons (Consistent vs Negligible and Inconsistent vs Negligible) to estimate possible medication-related effects, and a single comparison (Negligible vs. LNCG) to estimate the disorder-related effect of ADHD. An addition comparison (Consistent versus Inconsistent) allowed us to estimate the possible dose-response effect of ME cumulative doses.
Several sensitivity analyses were performed including a quantitative variable for time, years since baseline, instead of the assessment point variable, and doing separate analysis by removing, one at a time, patients with different baseline characteristic for race, ODD diagnosis, or those who took antipsychotics.
In the longitudinal analyses of z-scores, the differences between LSMs for each paired-comparison are expressed in standard deviation (SD) units, which is equivalent to effect size. To facilitate interpretation, effect size was transformed into metric units by multiplying by the SD obtained in previous analyses14 of raw scores adjusted for age and sex. SD units also were transformed into US/Imperial units by dividing by the scale factors, 2.54 cm/inch for height and 0.454 kg/lb for weight, as shown in Table S1, available online.
RESULTS
As shown in Table 1, the mixed-model analytic approach in this study involved 568 of the 579 original MTA participants (over 98%) and 258 of the 289 LNCG participants (over 89%). The previous end-point report on the 16-year end-point assessment14 used a different analytic method, GLM, that hused a smaller sample of 476 MTA (82.2% of those recruited) and 241 (83% of those recruited) LNCG cases. The same study14 reported strong study retention throughout childhood, adolescence and early adulthood.
TABLE 1:
Demographic Characteristics of the Subgroups With Different Medication Patterns
| Group | LNCG | ADHD | Negligible | Inconsistent | Consistent |
|---|---|---|---|---|---|
| Assessed in adulthood (n)a | 258 | 568 | 141 | 374 | 53 |
| Age at Assessment (years) | 24.4 | 24.8 | 24.9 | 24.8 | 24.9 |
| Age at baseline (years)b | 10.4 | 8.4 | 8.6 | 8.4 | 8.4 |
| Sex (% male) | 80 | 78 | 80 | 77 | 83 |
| Birth Weight (kg) | 3.4 | 3.4 | 3.4 | 3.4 | 3.3 |
| Race / Ethnicity | |||||
| (% Caucasian) | 66.4 | 62.7 | 52.7 | 64.9 | 74.3 |
| (% Black) | 11.2 | 19.4 | 27.7 | 17.1 | 14.3 |
| (% Hispanic) | 12.9 | 7.6 | 9.8 | 7.6 | 0.0 |
| (% Other) | 9.5 | 10.3 | 9.8 | 10.4 | 11.4 |
| Intelligence (IQ)c | 110 | 102 | 103 | 101 | 105 |
| Household Income($10K)c | 5.9 | 4.9 | 4.4 | 4.9 | 6.6 |
| Household Advantage (%)d | |||||
| 1 | 13 | 20 | 27 | 18 | 9 |
| 2 | 39 | 40 | 38 | 43 | 20 |
| 3 | 48 | 40 | 35 | 39 | 71 |
Note: ADHD = attention-deficit/hyperactivity disorder; LNCG = local normative comparison group
Number of participants with at least one observation of height and the paired SCAPI.
Baseline for the LNCG obtained 2 years after the baseline for the ADHD subgroups.
IQ and Income were higher in the LNCG than in ADHD group, but based on precedent (see Barkley et al, 2008 and Sibley et al, 2012), they were not included as covariates.
Composite household advantage developed by Molina et al. (2012) with three levels (1: one-parent household and no college-educated parent; 2: either two-parent household or at least one college-educated parent; 3: two-parent family and at least one college educated parent) was greater for the Consistent subgroup.
ADHD=Attention Deficit / Hyperactivity Disorder; Consistent=Subgroup that took medication at least 50% of days in all assessment intervals; Inconsistent=subgroup that took medication less than 50% of days in at least one assessment interval; LNCG=local normative Comparison Group; negligible=subgroup that took medication < 50% of days in all assessment intervals.
The average age at the time of the 16-year end-point assessment was 24.7 years (SD = 1.31) for the ADHD group and 24.4 (SD = 1.36) for the LNCG participants. Previous analyses of adult outcomes26 indicate MTA participants with and without complete data were not significantly different on most baseline demographic variables; they were ‘missing at random.’
Table 1 shows that MTA participants in different medication subgroups did not differ in demographically except for higher IQ, household advantage, and income in the Consistent subgroup. Proportions of African-American participants differed between the Negligible (34%) and the LNCG group (11%), but this did not reach significance in our sensitivity analyses. As reported previously in the 2017 end-point analyses,14 adjusting for these covariates did not change the conclusions about possible reductions in symptom severity or slow-down in standardized measures (z-scores) of growth. As these differences were not included during the design phase of the MTA when setting sample size and statistical power26, so they were not included as covariates in these analyses.
By the 16-year assessment, almost 2/3rds of MTA participants (n=374 or 65.9%) had an Inconsistent pattern of medication use; almost 1/4th (n=141 or 24.8%) had the Negligible pattern; and less than 1/10th (n=53 or 9.3%) had the Consistent pattern. Participants (211 of 568 or 37.15%) who had stimulant treatment before MTA entry formed the Prior subgroup. Of these prior-medicated cases, 146 were in the Inconsistent subgroup (constituting 42.1% of that group), 36 were in the Negligible subgroup (constituting 25.5% of that group), and 29 were in the Consistent subgroup (constituting 54.7% of that group).
Analyses of z-height:
Table 2 presents the hmixed model analyses of 4 groups over the 10 assessments revealing the main effects of Subgroup, (F=2.22, P<0.0001) and Prior medication (F=2.22, P<0.001). The Subgroup × Assessment interaction also was significant (F=2.81, <0.0001). Inspection of Table 3 (and Table S1 available online) shows that the paired-comparisons for the consistent-negligible and inconsistent-negligible subgroups had peaks at the 3 (mean age 11.4 years)- or 6-year (mean age 14.4 years) assessment points and then declined slightly over time.
TABLE 2:
Trajectories of Height, Weight, and Body Mass Index (BMI) Measures in Medication Subgroups Over Time
| ADHD Subgroups | ADHD Subgroups + LNCG | |||||||
|---|---|---|---|---|---|---|---|---|
| Effect | N-DF | D-DF | F | Pr > F | N-DF | D-DF | F | Pr > |
| a. z-Height | ||||||||
| Subgroup | 2 | 577 | 5.72 | 0.0035 | 3 | 911 | 5.82 | 0.0006 |
| Assessment | 9 | 392 | 1.58 | 0.1185 | 7 | 4057 | 8.88 | 0.0519 |
| Subgroup × A | 18 | 405 | 2.81 | <0.0001 | 21 | 4046 | 1.33 | 0.1458 |
| Prior | 1 | 590 | 4.03 | 0.0453 | ||||
| Prior × Subgroup | 2 | 577 | 0.69 | 0.5011 | ||||
| Prior × A | 9 | 392 | 1.87 | 0.0546 | ||||
| Prior × S × A | 18 | 405 | 0.32 | 0.9966 | ||||
| b. z-Weight | ||||||||
| Subgroup | 2 | 590 | 0.78 | 0.4609 | 3 | 915 | 2.22 | 0.0846 |
| Assessment | 9 | 456 | 15.45 | <0.0001 | 7 | 4043 | 24.42 | <0.0001 |
| Subgroup × A | 18 | 446 | 4.54 | <0.0001 | 21 | 4033 | 4.30 | <0.0001 |
| Prior | 1 | 604 | 3.20 | 0.0742 | ||||
| Prior × Subgroup | 2 | 590 | 0.90 | 0.4068 | ||||
| Prior × A | 9 | 456 | 1.36 | 0.2026 | ||||
| Prior × S × A | 18 | 446 | 0.76 | 0.7455 | ||||
| c. z-BMI | ||||||||
| Subgroup | 2 | 586 | 0.57 | 0.5642 | 3 | 941 | 2.16 | 0.0911 |
| Assessment | 9 | 460 | 9.49 | <0.0001 | 7 | 3977 | 9.82 | <0.0001 |
| Subgroup × A | 18 | 446 | 4.54 | <0.0001 | 21 | 3971 | 3.33 | <0.0001 |
| Prior | 1 | 601 | 0.87 | 0.3519 | ||||
| Prior × Subgroup | 2 | 586 | 0.35 | 0.7032 | ||||
| Prior × A | 9 | 460 | 1.07 | 0.3828 | ||||
| Prior × S × A | 18 | 446 | 1.05 | 0.3987 | ||||
Note: SAS Proc Mixed Model Analyses involved 4 Levels (3 attention-deficit/hyperactivity disorder [ADHD] Subgroups and local normative comparison group [LNCG]), 10 Levels for Assessment Points, 8 Levels for Assessment Points and 2 Levels for Prior. A = Assessment Point; LNCG = Local Normative Comparative Group of classmates; Prior = children with ADHD who started medication before entering the MTA Study; S or Subgroup = self-selected medication subgroups; Z-height, Z-weight, Z-BMI of standardized growth measures in standard deviations.
Table 3:
Z-Height by Medication Group Comparisons (Mixed Model Slope Estimates)
| SUBGROUP 1 | SUBGROUP 2 | AP | Estimate | SE | DF | T |
|---|---|---|---|---|---|---|
| Consistent | Inconsistent | 1.2 | −0.2211 | 0.1549 | 552 | −1.43 |
| 2 | −0.2241 | 0.1562 | 561 | −1.44 | ||
| 3 | −0.2614 | 0.1677 | 574 | −1.56 | ||
| 6 | −0.4963 | 0.1794 | 638 | −2.77b | ||
| 8 | −0.3598 | 0.1792 | 625 | −2.01a | ||
| 10 | −0.3907 | 0.1836 | 599 | −2.13a | ||
| 12 | −0.573 | 0.2206 | 547 | −2.59b | ||
| 14 | −0.4132 | 0.1935 | 557 | −2.13a | ||
| 16 | −0.4465 | 0.1868 | 587 | −2.39b | ||
| Consistent | Negligible | 1.2 | −0.5197 | 0.1759 | 554 | −2.95b |
| 2 | −0.5862 | 0.1771 | 562 | −3.31c | ||
| 3 | −0.6242 | 0.1898 | 569 | −3.29c | ||
| 6 | −0.7216 | 0.2004 | 721 | −2.75c | ||
| 8 | −0.5093 | 0.1998 | 610 | −2.55a | ||
| 10 | −0.5959 | 0.2045 | 584 | −2.91b | ||
| 12 | −0.5753 | 0.2206 | 547 | −2.59b | ||
| 14 | −0.5937 | 0.2163 | 551 | −2.75b | ||
| 16 | −0.6614 | 0.2088 | 578 | −3.17b | ||
| Inconsistent | Negligible | 1.2 | −0.2986 | 0.1139 | 556 | −2.62b |
| 2 | −0.3621 | 0.1142 | 553 | −3.17a | ||
| 3 | −0.3628 | 0.1211 | 544 | −2.99a | ||
| 6 | −0.2353 | 0.1207 | 535 | −1.87 | ||
| 8 | −0.1495 | 0.1192 | 522 | −1.25 | ||
| 10 | −0.2052 | 0.1219 | 510 | −1.68 | ||
| 12 | −0.2070 | 0.1310 | 488 | −1.58 | ||
| 14 | −0.1806 | 0.1305 | 507 | −1.38 | ||
| 16 | −0.2149 | 0.1260 | 522 | −1.71 | ||
| Local Normative Comparison Group | ||||||
| LNCG | Consistent | 2 | −0.04004 | 0.1584 | 828 | −2.53a |
| 3 | −0.4197 | 0.1668 | 824 | −2.52b | ||
| 6 | −0.5681 | 0.1754 | 889 | −3.30c | ||
| 8 | −0.4194 | 0.1698 | 891 | −2.47a | ||
| 10 | −0.4487 | 0.1743 | 878 | −2.57b | ||
| 12 | −0.4268 | 0.1780 | 847 | −2.40b | ||
| 14 | −0.4627 | 0.1798 | 840 | −2.57b | ||
| 16 | −0.4746 | 0.1746 | 855 | −2.72b | ||
| LNCG | Inconsistent | 2 | −0.1083 | 0.0789 | 781 | −1.37 |
| 3 | −0.0705 | 0.0830 | 780 | −0.85 | ||
| 6 | −0.0331 | 0.0843 | 773 | −0.39 | ||
| 8 | −0.0267 | 0.0824 | 785 | −0.32 | ||
| 10 | −0.0943 | 0.0841 | 781 | −1.12 | ||
| 12 | −0.1047 | 0.0861 | 769 | −1.22 | ||
| 14 | −0.0943 | 0.0875 | 775 | −1.08 | ||
| 16 | −0.0947 | 0.0849 | 787 | −1.40 | ||
| LNCG | Negligible | 2 | 0.2915 | 0.1097 | 807 | 2.66b |
| 3 | 0.3456 | 0.1154 | 804 | 3.00bb | ||
| 6 | 0.2240 | 0.1184 | 816 | 1.89 | ||
| 8 | 0.1527 | 0.1141 | 818 | 1.34 | ||
| 10 | 0.1383 | 0.1159 | 798 | 1.19 | ||
| 12 | 0.1300 | 0.1186 | 783 | 1.10 | ||
| 14 | 0.1220 | 0.1205 | 792 | 1.01 | ||
| 16 | 0.1420 | 0.1169 | 799 | 1.21 | ||
Note: AP = assessment point in years; DF = Degrees of Freedom; Estimate = Least Square Mean of Z-height; SE = Standard Error of the Mean; t = t-test value.
p <.05;
p <.01;
p <.001
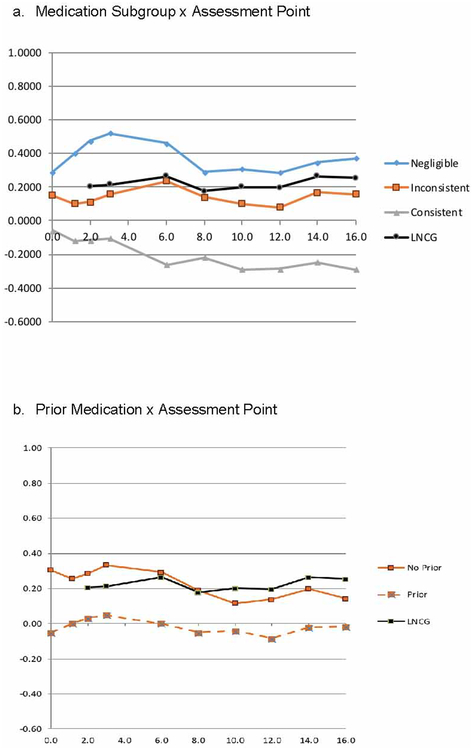
Figure 1a graphically displays z-height trajectories of the Least Square Mean (LSM) measurements for ADHD and LNCG subgroups. The z-height trajectories for the LNCG and inconsistent subgroups were flat, indicating an average tempo of growth, as would be expected for a randomly selected sample from the population. The trajectories for the Negligible and Consistent subgroups diverged significantly by the 3-year assessment (age 11.4, with a z-height difference of −0.6242, t=−3.29, P>0.0003) due to an upward height trajectory in the Negligible subgroup (z-height = +0.5207), suggesting faster-than-average growth tempo. The Consistent subgroup’s z-height decreased to reach a minimum at the 6-year assessment (age, 14.4, z-height= −0.2306), differing significantly from the negligible group’s trajectory at that time (z-height difference of −0.4963, t=−2.77, P<0.0058), suggesting medication-related growth slow down. Both subgroups’ z-height decreased in adolescence with later stabilization in adulthood.
Figure 1:
Z-Height Trajectories Note: LNCG = local normative comparison group.
The Inconsistent subgroup’s z-height trajectory remained essentially flat and significantly different from the negligible subgroup’s z-height assessments before the 3-year assessment (11.4 years old). Except for the 3-year assessment, the consistent and hinconsistent assessment points were significantly different.
The significant Prior × Assessment-point interaction (F=2.22, P<0.0001) was due to a difference between the baseline z-height of cases with and without prior stimulant medication before MTA entry (Figure1b). The group without prior medication was 0.86 inches taller at baseline and 0.78 inches taller by the final 16-year assessment. This differed by subgroup. For the Negligible and Inconsistent subgroups, the Prior effect was small and constant across assessment points until the 16-year point, when they converged, but the Consistent subgroup’s Prior effect was large at baseline and then gradually declined (Figure S3, available online). However, these patterns were not dissimilar enough and the sample sizes were not large enough for a significant 3-way interaction of Subgroup × Prior × Assessment.
Analyses of z-Weight and z-BMI:
The main effect of Assessment and the interaction of Subgroup × Assessment were significant in the analyses of z-weight and the analyses of z-BMI, as shown ;by the detailed standard scores in Table S1, available online. In contrast to the analysis of z-height, neither the main effect of Prior nor the Prior × Assessment interaction was significant in these analyses.
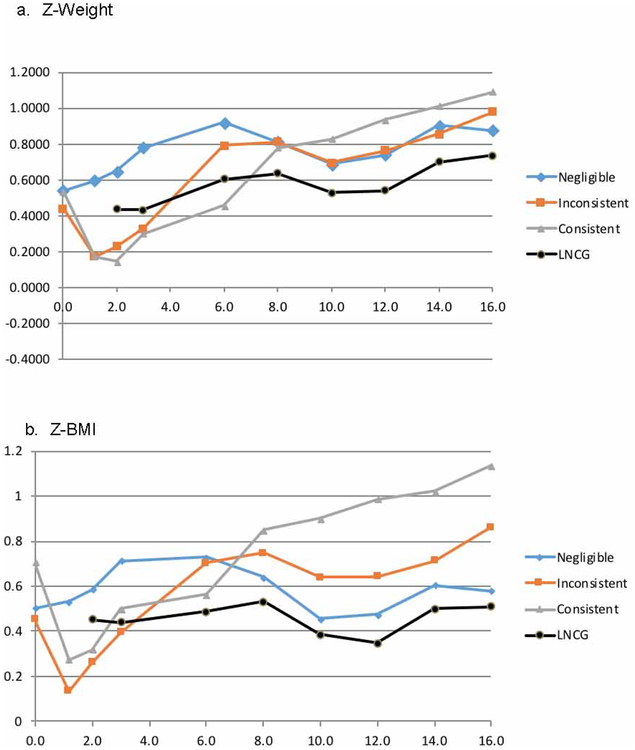
As shown in Figure 2a (for z-weight) and Figure 2b (for z-BMI), the z-scores decreased abruptly for the Consistent and Inconsistent subgroups in childhood associated with initiation of stimulant treatment, but subsequently increased rapidly in adolescence, possibly attributed to weight rebound. This resulted in convergence of the trajectories of the Consistent, Inconsistent and Negligible subgroup in adolescence. In adulthood, z-weight and z-BMI continued to increase for all 3 subgroups, in contrast to the stability of height in adulthood.
Figure 2:
Z-Weight and Z-Body Mass Index (BMI) Trajectories by Subgroup Note: LNCG = local normative comparison group.
The significant Subgroup × Assessment interactions were unpacked by using the LSM estimates for z-weight and z-BMI to form paired-comparisons. The medication-related effects revealed large weight and BMI suppression effects in childhood at the 2-year assessment when participants were 10.4 years old, but this reversed so that weight and BMI were increased in adulthood for the participants with ADHD who had been treated with stimulants.
Analyses including the LNCG:
In the analyses of z-height of the non-ADHD peers in the LNCG group, with 4 Groups and 8 Assessment Points, the main effects of Group and Assessment were significant, but the Group × Assessment interaction was not (see Table 2a, right). In the analyses of z-weight (Table 2b, right) and z-BMI (Table 2c, right) with the LNCG included, the main effects of Group were not significant, but the main effects of Assessment and the Group × Assessment interactions were significant. As shown in Figure 2, these effects were due to increases of z-weight and z-BMI from late childhood to adulthood, which were steeper in the stimulant-treated subgroups than the unmedicated (Negligible) subgroup or the LNCG.
To determine how the children with ADHD differed from their classmates at school, three additional paired-comparisons (Negligible-LNCG, Consistent-LNCG, and Inconsistent-LNCG) were calculated for the 8 assessment points, as shown in Table 3. They also are shown in more detail in Table S1a–ii, iii and iv for height; Table S1b–ii, iii, and iv for weight; and Table S1c–ii and S1ciii for BMI, available online.
The paired comparison of the Negligible and LNCG for mean z-heights showed that the negligibly-medicated ADHD group cases were significantly taller than the non-ADHD peers in the LNCG group for the 2- and 3-year assessment when the participants were 10.4 and 11.7 years old, respectively. Although not significant over the remaining assessment points, the Negligible subgroup remained taller than the LNCG. At the 16-year assessment for height, these possible dual but opposite effects were associated with less medication-associated growth suppressant effects in the Consistent-LNCG comparison (−0.53 z-units/−3.23 cm/−1.27 inch) than found in the Consistent-Negligible comparison (−0.72 z-units/−4.43 cm/−1.75 inch).
In childhood, i.e. the mean z-weight and z-BMI were lower in the consistent and inconsistent ADHD subgroups than in the LNCG. However, this reversed in adolescence and adulthood. These might have been associated with weight increases in adolescence regardless of the pattern of self-selected medication use.
Sensitivity Analyses
Sensitivity analyses for z-height used a quantitative variable for time, years since baseline, instead of the categorial variable, Assessment Point. The analyses were conducted separately for the 0- to 8-year assessment points when non-linear developmental trends were expected for slower-than-average tempo and faster-than-average tempo. As predicted, the Subgroup × Time interaction was significant, consistent with different z-height growth trajectories. On the other hand, the z-height scores between 10-year (age 18.4) and 16- year (age 24.7) assessments did not show a significant subgroup × time interaction. Details on the protocol of this sensitivity analysis are given in Supplement 2 and the outcomes of the analysis are presented in Table S2, both available online.
Sensitivity analyses were performed for z-weight, z-height and Z-BMI as detailed in Supplement 2, available online, also using the quantitative years-since-baseline. As shown in Table S2, available online, these analyses confirmed and extended the findings of subgroup differences in developmental trends due to initial suppression of weight and BMI in the stimulant-treated subgroups, followed by rebound. This produced quadratic trajectories in the analyses for the weight and BMI z-scores measured from 0- to 8-years.
Additional sensitivity analyses failed to show differences in main outcome when we removed those who were African American (Table S2b), which showed that the Negligible-LNCG z-height comparison remained significant for z-height differences at the 2 (p<0.027) and 3-year (P<0.0061) assessments. Similar analyses revealed that the findings of the main analysis did not change with and without participants who had comorbid ODD (Table S2c) or those who had taken antipsychotics (Table S2d).
DISCUSSION
To our knowledge, this is the first study investigating whether different levels of cumulative ME doses of stimulants during long-term treatment may be associated with different growth trajectories from childhood through adolescence into adulthood. The trajectory analysis expands and provides new information concerning the previously published end-point analyses of adult height in the MTA14 by identifying periods in development – mean age 11.7 years) and mean age 14.9 years) - that might be most associated with a medication-related growth suppression. Most important, the Negligible subgroup’s z-height trajectory showed a peak at the 3- and 6-year assessments (at 11.7 and 14.9 years old) followed by a decline and stability by the 8-year assessment (at 16.8 years old), suggesting faster-than-average tempo in the stimulant-untreated cases, the opposite of the Spencer et al6,7 prediction that untreated children with ADHD would experience slower-than-average tempo, smaller size in childhood, and disorder-related catch-up in adolescence.
This report has several strengths. First, the MTA sample was large and well-characterized at study entry. During the follow-up study, community-based treatment was documented during 16 years of prospective assessments14, 16, 18, 23, 25,26,27 that covered the important phases of growth in childhood and adolescence.
Second, data were expressed in standardized z scores that adjust for natural differences between growth patterns of boys and girls.
Third, parent report data were used to define the patterns of long-term stimulant treatment medication for forming naturalistic subgroups of treated and untreated cases, which helped to estimate the medication-related effects on growth trajectories separate from the disorder-related effects from having ADHD14,16.
Fourth, the mixed model analyses used here allowed cases with incomplete data to be retained using direct (full information) maximum likelihood estimation,28 which leverages all available data to improve the accuracy of model estimates and compute appropriately conservative significance tests.29 This approach allowed us to use over 90% of the MTA sample for the longitudinal analyses, compared to 82% in the previously published end-point analyses,14 and to avoid censoring data that in some other longitudinal studies reduced sample sizes.6,7,14
Fifth, this report highlights paired-comparisons with the LNCG which had been relegated to supplementary material in the previous report14. The Negligible-LNCG comparison (addressing the disorder-related effect of ADHD on growth) was significantly positive for z-height at the 2- and 3-year assessments, suggesting that individuals with ADHD recruited into the MTA were significantly taller than non-ADHD peers early in the study. While the mean z-height values remained higher in the ADHD Negligible group, this difference diminished to non-significance after the 3-year assessment. Other paired-comparisons suggested that ADHD subgroups in adulthood were heavier and had higher body mass than the LNCG, consistent with reports of an association of youth with ADHD and adult obesity30–32. These results suggest that BMI should be closely monitored in adolescents with ADHD with recommendations for exercise and healthy eating patterns. Efforts to improve weight gain in children with ADHD treated with stimulants to stimulate growth need to be re-evaluated as the children approach puberty. Increased weight gain, often used as a sign that pre-pubertal children with ADHD on stimulants have regained their normal growth patterns, may not translate to greater height gains during adolescence.
Our findings must be considered in light of the study’s limitations. First, our prospective growth assessments were uncontrolled and observational, so it is not possible to establish causal relationships between patterns of medication use and trajectories of growth. We must limit the use of these results to generating hypotheses for future testing.
Second, the LNCG non-ADHD peers were recruited 2 years after baseline when the RCT was completed, during which the MTA participants on medication had already shown changes in their growth trajectories.
Third, the loss of randomized treatment assignment 1.2 years after baseline when the MTA participants reverted to care in the community and changes in medication status occurred with self-selected starting and stopping. This removed the protection against bias afforded by a randomized controlled design, allowing underlying for the possibility that underlying differences in the baseline and post-baseline characteristics of the subgroups could bias outcomes. Lack of protection against source, selection, and retrospective recollection biases33 characterizes all open follow-up observational studies. For that reason, this manuscript does not test causal relationships, only associations. Despite this vulnerability to bias, the self-selected subgroups differed significantly on only three baseline variables (IQ and household advantages and income). Sensitivity analyses failed to show differences in main outcome when we removed those who were African American, had comorbid ODD, or those who had taken antipsychotics during the study. Additional analyses found no significant differences between the prior and non-prior medicated groups in baseline ADHD symptom severity, incidence of comorbid ODD, or in mean ME doses during the follow-up despite a higher proportion of the consistently medicated subgroup having prior medication.
Fourth, source bias was introduced when the SCAPI reports by parents – which had been used to form the medication groups -- were discontinued after the MTA participant reached 18 years of age, switching to participant self-report about medication use.
Fifth, we did not systematically collect duration and dose data on the stimulant treatment that was started before entry into the MTA in the prior group. This may have contributed to our finding that the three-way interaction of Prior × Subgroup × Assessment was not significant and supports the suggestion by Poulton and Nanan22 that prior use of medication deserves systematic assessment in future research.
Sixth, not all participants were assessed at each time point. To address this concern, we used a mixed model analysis because it does not require a height or weight measurement at every time point and provides the best statistical approximation of what those measurements might have been, had they been taken.
Seventh, stimulant treatment may have unknown interactions with the biological processes underlying growth, such as pituitary hormones, from childhood through adolescence into adult life, which have unmeasured effects of growth trajectories. This limitation could be addressed by AUXOL analytic techniques not used in this paper that employ mathematical modeling to identify effects of medication on milestones of growth (i.e. height, height velocity, age of the ‘take-off’ or ‘peak’ points of the adolescent growth spurt, or timing of puberty) and more exactly determine trajectories of growth.
Eighth, the main finding of this study -- that stimulant use over development may be associated with a possible decrease in adult height in those consistently treated -- has not been found in all prior growth studies of children with ADHD treated chronically with stimulants. However, those studies reported lower cumulative doses and did not employ our methodology of determining trajectories of growth over time.
It is reassuring that inconsistent use was associated with minimal changes in the growth trajectory and smaller decreases in final adult height than consistent use -- reassuring because inconsistent use was the most common pattern of medication in the MTA follow-up. This encourages and supports future research as to whether planned interruptions of medication such as summers off can ameliorate the stimulant-related slow-down in height acquisition.
If these data are replicated in additional studies, the decision to use stimulant medication to treat children with ADHD will require a new and ongoing risk-benefit analysis that is now in use. Practitioners will need to spell out the timing and magnitude of the effects of long-term stimulant treatment on growth, as suggested in Figure S1 of Supplement 3 (available online). Clinicians should partner with families to determine if the possible changes in adult height (up to 1.5 inches shorter) and weight and BMI (greater risks of obesity) may be outweighed by the risks of not treating the child’s ADHD. This discussion is especially important because the effect of medication prior to entry into the MTA on height trajectory suggests that treatment started early might intensify the loss of adult height if medication is taken continuously.
These concerns increase the importance for more detailed research to determine the critical periods in development when growth may be most affected by stimulant treatment. Future publications, including those that use AUXOL methods, will address how the timing and consistency of stimulant treatment might be used to help minimize the risk of medication-associated changes in growth trajectory for early starters.
Supplementary Material
Acknowledgments
This work was supported by cooperative grants and contracts from National Institute of Mental Health (NIMH) and the National Institute on Drug Abuse (NIDA) to the following: University of California-Berkeley: U01 MH50461, N01MH12009, and HHSN271200800005-C; DA-8-5550; Duke University: U01 MH50477, N01MH12012, and HHSN271200800009-C; DA-8-5554; University of California-Irvine: U01 MH50440, N01MH 12011, and HHSN271200800006-C; DA-8-5551; Research Foundation for Mental Hygiene (New York State Psychiatric Institute/Columbia University): U01 MH50467, N01 MH12007, and HHSN271200800007-C; DA-8-5552; Long Island Jewish Medical Center: U01-MH50453; New York University: N01MH 12004 and HHSN271200800004-C; DA-8-5549; University of Pittsburgh: U01 MH50467, N01 MH 12010, and HHSN 271200800008C; DA-8-5553; and McGill University: N01MH12008 and HHSN271200800003-C); DA-8-5548.
Disclosure: Dr. Greenhill has received support from the Klingenstein Third Generation Foundation and the REACH Foundation. Dr. Hechtman has received research support, served on advisory boards, and has been a speaker for Ortho Janssen, Purdue Pharma, Iron Shore, and Shire and has received book royalties from Guilford, APA, John Hopkins University Press, and Oxford University Press. Dr. Waxmonsky has received research funding from the National Institutes of Health (NIH), Supernus, and Pfizer and has served on the advisory board for Noven, Iron Shore, NLS Pharma, and Purdue Pharma. During this writing of this paper, his time was in part supported by grant MH083692. Dr. Arnold has received research funding from Curemark, Forest, Eli Lilly and Co., Neuropharm, Novartis, Noven, Shire, Young-Living, NIH, Autism Speaks, Supernus, and Roche/Genentech; has consulted with or been on advisory boards for Arbor, Gowlings, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, Roche, Seaside Therapeutics, Sigma Tau, Shire, Tris Pharma, and Waypoint; and has received travel support from Noven. Dr. Jensen has received unrestricted educational grants from Shire, Inc., has served as a consultant to Shire, Inc., and is a shareholder of an evidence-based practices consulting company (CATCH Services, LLC). Dr. Wigal has received research funding from Akili, Ironshore Pharmaceuticals, Neurolife Sciences, Neurovance, NuTec, Pfizer, Purdue, Rho, Rhodes, Shire, Sunovion, and Tris Pharma; has consulted with or been on advisory boards for Cingulate, Ironshore Pharmaceuticals, NeuroLife Sciences, NuTec, Otsuka, Pfizer, Purdue, Rho, Rhodes, Shire, Sunovion, Touchpoint, and Tris Pharma. Dr. Hanć has received travel support from MEDICE Arzneimittel Putter GmbH and Co. KG. Drs. Swanson, Molina, Hinshaw, Abikoff, Howard, and Hermussen and Ms. Stehli report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Safer D, Allen R, Barr E, Depression of growth in hyperactive children on stimulant drugs. N Engl J Med 1972; 287:217–220 [DOI] [PubMed] [Google Scholar]
- 2.Safer DJ, Allen RP, Barr E, Growth rebound after termination of stimulant drugs. J Pediatr 1975; 86:113–116 [DOI] [PubMed] [Google Scholar]
- 3.Satterfield JH, Cantwell DP, Schell A, Blaschke T, Growth of hyperactive children with methylphenidate. Arch Gen Psychiatry 1979; 36:212–217 [DOI] [PubMed] [Google Scholar]
- 4.Roche AF, Lipman RS, Overall JE, Hung W (1979), The effects of stimulant medication on the growth of hyperkinetic children. Pediatrics 1979; 63:847–850 [PubMed] [Google Scholar]
- 5.Gittelman-Klein R, Landa B, Mattes JA, Klein DF, Methylphenidate and growth in hyperactive children. Arch Gen Psychiatry 1988; 36:212–217 [DOI] [PubMed] [Google Scholar]
- 6.Spencer TJ, Biederman J, Harding M, O’Donnell D, Faraone SV, Wilens TE. Growth deficits in ADHD children revisited: evidence for disorder-associated growth delays? J Am Acad Child Adolesc Psychiatry. 1996; 35:1460–1469. [DOI] [PubMed] [Google Scholar]
- 7.Spencer T, Biederman J and Wilens T. Growth deficits in children with attention deficit hyperactivity disorder. Pediatrics 1998; 102: 501–506. [PubMed] [Google Scholar]
- 8.Kramer JR, Loney J, Ponto LB, Roberts MA, & Grossman S Predictors of adult height and weight in boys treated with methylphenidate for childhood behavior problems. Journal of the American Academy of Child and Adolescent Psychiatry, 2000; 39, 517–524. [DOI] [PubMed] [Google Scholar]
- 9.Faraone SV, Biederman J, Morley CP, Spencer TJ. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008; 47: 994–1009. [DOI] [PubMed] [Google Scholar]
- 10.Biederman J, Spencer TJ, Monuteaux MV, & Faraone SV A naturalistic 10-year prospective study of height and weight in children with attention-deficit hyperactivity disorder grown up: Sex and treatment effects. Journal of Pediatrics, 2010; 157, 635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harstad EB, Weaver AL, Katusic SK, Colligan RC, Kumar S, Chan E et al. ADHD, stimulant treatment, and growth: A longitudinal study. Pediatrics, 2014; 134, e935–e944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peyre H, Hoertel N, Cortese S, Acquaviva E, Limonsin F, & Delorme F Long-term effects of ADHD medication on adult height: Results from the NESARC. Journal of Clinical Psychiatry, 2013; 74, 1123–1125. [DOI] [PubMed] [Google Scholar]
- 13.Charach A, Figueroa M, Chen S, Ickowicz A, & Schachar R Stimulant treatment over 5 years: Effects on growth. Journal of the American Academy of Child and Adolescent Psychiatry, 2006; 45, 415–421. [DOI] [PubMed] [Google Scholar]
- 14.Swanson JM, Arnold LE, Molina BSG et al. Young adult outcomes in the follow-up of the multimodal treatment study of attention-deficit/hyperactivity disorder: symptom persistence, source discrepancy, and height suppression. J Child Psychol Psychiatry, 2018; 58: 663–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poulton AS, Melzer E, Talt PR, Garnett SP, Cowell CT, Baur LA, & Clarke S Growth and pubertal development in adolescent boys on stimulant medications for attention deficit hyperactivity disorder. The Medical Journal of Australia, 2013; 198, 29–32. [DOI] [PubMed] [Google Scholar]
- 16.Swanson JM, Elliott GR, Greenhill LL et al. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J Am Acad Child Adolesc Psychiatry. 2007; 46:1015–1025. [DOI] [PubMed] [Google Scholar]
- 17.MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Archives of General Psychiatry, 1999; 56: 1073–108. [DOI] [PubMed] [Google Scholar]
- 18.MTA Cooperative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up. Pediatrics. 2004; 113: 762–769. [DOI] [PubMed] [Google Scholar]
- 19.Biederman J, Monuteaux MC, Spencer T, Wilens TE, MacPherson HA,&Faraone SV Stimulant therapy and risk for subsequent substance use disorders in male adults withADHD: A naturalistic controlled 10-year follow-up study. American Journal of Psychiatry, 2008; 165, 597–603. [DOI] [PubMed] [Google Scholar]
- 20.Kuczmarski RJ, et al. 2000 CDC growth charts for the United States: Methods and development. National Center for Health Statistics. Vital Health Stat 2002; 11(246). [PubMed] [Google Scholar]
- 21.Jensen PS, Eaton-Hoagwood K, Roper M, et al. The services for children and adolescents-parent interview: development and performance characteristics. Journal Clinical Child and Adolescent Psychology 2004; 43:1334–44. [DOI] [PubMed] [Google Scholar]
- 22.Poulton AS and Nanan R. Prior treatment with stimulant medication: a much-neglected confounder of studies of growth in children with ADHD. J Child Adolesc Psychopharm, 2008; 18: 385–387. [DOI] [PubMed] [Google Scholar]
- 23.Institute SAS, Inc. SAS/STAT Cary, NC: 2015. [Google Scholar]
- 24.Hermanussen M Auxology: An Update. Hormone Research in Pediatrics, 2010, 74: 153–164. [DOI] [PubMed] [Google Scholar]
- 25.Jensen PS, Arnold LE, Swanson JM et al. 3-year follow-up of the NIMH MTA study.(2007). J Am Acad Child Adolesc Psychiatry 2000; 46: 988–1001. [DOI] [PubMed] [Google Scholar]
- 26.Howard AL, Stricktland NJ, Murray DW, Tamm L, Swanson JM, Hinshaw SP…Molina BSG. Progression of impairment in adolescence with attention-deficit / hyperactivity disorder through transition out of high school: Contribution of parent involvement and college attendance. Journal of Abnormal Psychology, 2016; 125: 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnold LE, Abikoff HB, Cantwell DP, Conners CK, Elliott G, Greenhill LL, Kraemer HC National institute of mental health collaborative multimodal treatment study of children with ADHD (the MTA): design challenges and choices. Arch Gen Psychiatry 1997; 54(9):865–870 [DOI] [PubMed] [Google Scholar]
- 28.Croy CD, & Novins DK (2005). Methods for addressing missing data in psychiatric and developmental research. J Am Acad Child Adolesc Psychiatry 2005; 44: 1230–1240. [DOI] [PubMed] [Google Scholar]
- 29.Kraemer HC. A source of false findings in published research studies: Adjusting for covariates. JAMA Psychiatry 2015, 72, 961–962. [DOI] [PubMed] [Google Scholar]
- 30.Cortese S, Moreira-Maia CR, St. Fleur D, Angriman M, Morcillo-Penavler C, Rohde LA, Faraone SV. Association between ADHD and Obesity: A systematic review and meta-analysis. Am J Psychiatry, 2016;173(1): 34–43. [DOI] [PubMed] [Google Scholar]
- 31.Hanc T and Cortese S. Attention-deficit/hyperactivity disorder and obsesity: A review and model of current hypotheses expalining their comorbidity. Neuroscience and Biobehavioral Review, 2018; 92: 16–28. [DOI] [PubMed] [Google Scholar]
- 32.Fliers Ellen A., Buitelaar Jan K., Maras Athanasios, Bul Kim, Esther Höhle Stephen V. Faraone, Franke Barbara, Nanda NJ Rommelse. ADHD is a risk factor for overweight and obesity in children. J Dev Behav Pediatr 2013; 34(8): 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernan M, Hernandez-Diaz S, and Robbins JM. A structural approach to selection bias. Epidemiology 2004;15: 615–625 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.