Abstract
In experimental placebo and nocebo studies, neutral control treatments are often administered for comparison with active treatments, but are of little interest, as, on average, they result in little change. Yet, when considered at an individual level, they fluctuate between baseline and subsequent measurements and may reveal important information about participants’ placebo/nocebo responding tendencies.
In a paradigm involving application of creams paired with positive, negative and neutral expectations, some subjects rated identical stimuli in the neutral condition as more painful while others as less painful after treatment with inert cream. We divided subjects into two groups based on the median split in these pre-post responses in the neutral control condition, and investigated 1) fMRI signal differences (post minus pre) between the two groups in neutral condition, and 2) seed-based resting state connectivity of the bilateral amygdala, known to be involved in emotional self-regulation, as well as ambiguous stimulus processing and aversive learning.
The results suggested that subjects who rated the same pain stimuli after treatment with explicitly neutral cream as more painful showed stronger fMRI activation of the amygdala during the experiment and had higher connectivity between the left amygdala and the striatum at rest. Neutral pre-post changes predicted behavioral placebo/nocebo response in this and 2 independent datasets. These findings suggest that measuring pre-post change in the neutral control condition might provide important information about subjects’ individual differences in placebo/nocebo response.
Keywords: pain, placebo, nocebo, functional magnetic resonance imaging (fMRI), resting state fMRI
Introduction
Placebos serve as a control in testing new drugs to disentangle the effect of active treatment and non-specific components of treatment (Benedetti et al., 2010; Kong and Benedetti, 2014; Walach et al., 2005). Previous studies documented significant individual differences/variability in the ability to modulate pain and placebo/nocebo responses, linking them to personality traits (Pecina et al., 2013), genes (Schmahl et al., 2012), brain activity during pain anticipation (Palermo et al., 2015; Wager et al., 2011), resting state brain functioning (Hashmi et al., 2014; Tu et al., 2019), or a combination thereof (Yu et al., 2014). There is still no consensus, thus the search for new reliable predictors of placebo and nocebo response continues.
If placebo and nocebo represent inherent self-healing/harming ability, an unbiased test of an individual’s placebo/nocebo responding tendency would assess behavior in a situation where neither placebo nor nocebo response is induced or expected. In experimental placebo/nocebo studies, control treatments not paired with any expectation are used as ‘no effect’ controls to quantify specific expectancy or conditioning effects (Kong et al., 2006b; Scott et al., 2008). In other words, while placebo/nocebo conditions induce positive/negative expectations through verbal instruction or conditioning, in a ‘neutral’ or ‘control’ condition, investigators attempt to create neither positive nor negative associations. Participants are explicitly told that the treatment has no effect, and no changes in pain perception should be expected. Typically, these control conditions are of little interest, as they either represent a baseline or in experimental designs that measure pre-post treatment effects should result in no difference between the two. However, considered at an individual level, pre-post responses (e.g., pain rating changes or fMRI signal changes) to identical experimental pain in a control condition tend to be non-zero. They possibly reflect an individual’s pain self-regulation ability and thus may be useful for identifying placebo and nocebo responders.
In a previous placebo/nocebo study (Freeman et al., 2015), we found not only significant placebo/nocebo effects, but also a significant association between the pre-post treatment pain rating changes in the control condition and the placebo and nocebo conditions. In addition, we reported a complex pre-post fMRI activation pattern in the control condition, involving the amygdala, hippocampus and the insula, regions typically observed during aversive learning (Buchel et al., 1998; McHugh et al., 2014; Ploghaus et al., 2000). We hypothesized that despite averaging to zero at a group level, pre-post pain ratings in the neutral condition at individual level exhibit positive or negative changes that may predict placebo and nocebo effects.
To test this hypothesis, in this study, we investigated an association between pre-post changes in pain ratings in the neutral condition and placebo/nocebo responses, replicating behavioral results in two independent data sets. Then, to understand whether these individual differences in pre-post changes in pain ratings in the neutral condition have an underlying biological substrate, we 1) explored pre-post treatment fMRI activation differences between those who showed pain rating increase after treatment and those who showed pain rating decrease after treatment; 2) investigated resting state functional connectivity of the amygdala, a key region involved in pain modulation, conditioning learning, appetitive/reward and aversive processing, and importantly, ambiguity resolution (Madarasz et al., 2016; Neta et al., 2013; Seymour et al., 2005), with a special role of the amygdala-striatal circuitry (Cador et al., 1989; Fernando et al., 2013; Li et al., 2011; Phelps and LeDoux, 2005; Wanigasekera et al., 2012; Watanabe et al., 2013; Zhang et al., 2016). Note that the amygdala ROI was chosen based on these previous studies a priori and independently of the fMRI activation results reported in (Freeman et al., 2015) due to its role in ambiguity resolution. We hypothesized that a critical feature of our test session was that all stimuli in all conditions were identical (exactly the same temperature for all conditions, but different expectations). Therefore, ambiguity of the stimuli in the test phase may be crucial to individual differences in pain processing, with the amygdala being a prime candidate for examining these differences with neuroimaging.
Methods
All study procedures were approved by the Institutional Review Board at Massachusetts General Hospital. All enrolled subjects provided written informed consent before the study began and were debriefed at the end of the study with an option to remove their data from the study if they had any concerns due to the inherent need for deception in the experimental paradigm. All subjects allowed their data to be used.
Subjects
Twenty-four healthy right-handed subjects completed the study. Exclusion criteria for this study were ongoing or past major medical, neurological, or psychiatric illnesses; pregnancy, breast feeding, menopause, and/or irregular menstrual cycles; a history of substance abuse or dependence; a history of impaired urinary elimination; use of psychotropic drugs within the past year; claustrophobia; head trauma; or any other contraindications to MRI. After enrollment, three subjects withdrew from the study, one due to discomfort with the heat pain and two due to scheduling conflicts. Eleven subjects were excluded after Session 1 or 2, seven due to the inability to reliably distinguish between high and low pain intensities, and four due to equipment malfunctions. Data from all 24 subjects who completed Session 3 were included in the analysis.
Experimental Design
The full details of the experimental design are described in our previous publication using this dataset (Freeman et al., 2015). Briefly, the study involved 1) a training session, 2) a conditioning session, and 3) a scan session, each separated by 2–14 days. In all sessions, we delivered calibrated heat pain stimuli to the right volar forearm of each subject using a Pathway Medoc (Contact Heat-Evoked Potential Stimulator, Medoc LTD Advanced Medical Systems, Rimat Yishai, Israel). All stimuli initiated at a baseline temperature of 32°C and subsequently increased to a given target temperature. Each stimulus lasted 12 seconds, including a ramp up from baseline (2.5 seconds) to the target temperature (7 seconds) and a ramp down to baseline (2.5 seconds).
In the training session, subjects were familiarized with the heat pain stimuli and the Gracely Scales (0–20) (Gracely and Dubner, 1987) that they would use to rate their pain throughout the study. Heat pain was calibrated to determine mild, moderate and strong pain levels. For pain calibration we administered one or two ascending sequences consisting of stimuli that got progressively more painful over the course of the sequence, followed by one or two sequences consisting of three mild (rated as 5–6 out of 20), three moderate (rated as 10–11 out of 20) and three strong (rated as 14–15 out of 20) pain stimuli presented in random order. Finally, we administered one or two sequences consisting of six identical moderate heat pain stimuli. Each sequence was administered to a separate region within the grid on the forearm. For all sessions, a 3×3 grid comprised of 2×2 cm regions on the right volar forearm of the subject was drawn as shown in Figure 1.
Figure 1.
Experimental procedure. During the Conditioning session held outside of the scanner, a 3×3 grid was drawn on the right inner arm of each participant and 9 moderate heat pain sequences were applied. Subsequently 3 identical inert creams labeled Capsaicin, Lidocaine and Neutral were applied on each grid row. Following the 20-minute waiting period, the heat pain intensity was surreptitiously lowered in the Lidocaine labeled squares; raised in the Capsaicin labeled squares and left unchanged in the Neutral squares. During the test session held in the scanner, first 9 moderate heat pain sequences were applied on each square. Then 3 creams were applied in the same way as during conditioning. Subjects were informed that the two sessions were identical. However, decreased and increased levels of heat were applied only on the first of the 3 Lidocaine- and Capsaicin- labeled squares, for the other 2 the same medium pain level was applied as during the pre-cream phase and the Neutral cream squares. Throughout all sessions subjects rated pain intensity on the 0–20 Gracely Scale.
Session 2 was a behavioral conditioning session. This session involved an expectancy manipulation paradigm as used in our previous studies (Gollub et al., 2018; Kong et al., 2006b, 2008, 2009). The subjects were informed that the aim of the study was to investigate the analgesic effect of Lidocaine cream and the hyperalgesic effect of Capsaicin cream. We told subjects that we would apply three creams (Lidocaine, Capsaicin, and a neutral moisturizing cream) to different regions of their right volar forearm and test their response to heat pain stimuli both before and after the application of the creams (Figure 1). In reality, three samples of one inert moisturizing cream, each dyed a different color, were used. One sampling was dyed light blue and labeled “Lidocaine,” one was dyed pink and labeled “Capsaicin,” and one was left white and labeled “Neutral.” Nine heat pain sequences (one sequence per square on the grid) were administered, each about 6 minutes in duration and including 6 identical heat pain stimuli at the temperature that elicited a moderate (10–11 out of 20) rating as determined in the previous session. Then we applied one cream to each row (set of 3 adjacent squares) on the grid and counterbalanced the order of cream application across subjects. Note that we used all 6 possible orders (capsaicin-neutral-lidocaine, neutral-capsaicin-lidocaine etc.). The subjects were told to wait 15–20 minutes for the creams to take effect and to identify any allergic reactions. Following the waiting period after cream application, we conducted the experimental manipulation. We informed subjects that they would be receiving 9 heat pain stimuli sequences comprised of stimuli at temperatures identical to those they had received prior to cream application. In reality, we surreptitiously lowered the heat to temperatures that elicited mild pain ratings in the “Lidocaine” squares, and raised the temperatures to elicit strong pain ratings in the “Capsaicin” squares. Identical moderate intensity stimuli were administered to the neutral squares (Eippert et al., 2009; Scott et al., 2008). Only subjects who could distinguish between the pre- and post-treatment stimuli on the “Lidocaine” and “Capsaicin” regions, as indicated by average pain ratings, were permitted to continue with the study.
Session 3 took place in the MRI scanner. We informed subjects that the proceedings of Session 3 would be identical to those of Session 2. In reality, Session 3 was designed to test the placebo and nocebo effects evoked by the expectancy manipulation in Session 2. Thus, the process of Session 3 was identical to that of Session 2, with the exception that decreased and increased levels of heat in the Lidocaine and Capsaicin conditions were applied only on the first of the 3 Lidocaine and Capsaicin squares to reinforce the expectation. The temperature was changed to moderate (identical for all squares and the same as the neutral control during the conditioning) on the final 6 regions marked on the volar forearm. The pre- and post-treatment changes in subjective pain ratings and fMRI signal changes evoked by the post-treatment identical moderate pain stimuli serve as the primary outcomes of this study (Figure 1).
During scanning, subjects were instructed to focus on a small black fixation cross in the center of a screen in front of them. The fixation cross turned red to cue the onset and duration of each heat pain stimulus (12 s) and then turned black during the variable inter-stimulus interval duration (4, 6, or 8 s). After administration of each stimulus, we displayed the Gracely Sensory Scale on the screen (8 s) and subjects used a button press device controlling a pointer to indicate their subjective ratings. Participants underwent a resting state scan prior to pain manipulation.
Behavioral Analysis
The main analysis of behavioral results is reported in (Freeman et al., 2015). We here focused on individual differences in the neutral (pre-post) responses. First, we performed a regression between the placebo (Lidocaine post minus pre) and neutral cream post minus pre changes, as well as the nocebo (Capsaicin post minus pre) and neutral cream post minus pre changes. In addition, we split subjects into 2 groups based on the median split – those whose pain ratings increased compared to baseline (‘More pain’ group) and those whose pain ratings decreased compared to baseline (‘Less pain’ group). In order to investigate the relationship between the neutral pain responses and anxiety, we performed a regression with State-Trait Anxiety Inventory (STAI) scores (trait component) and the neutral cream post minus pre changes. We also performed regressions with neutral pain and STAI scores with placebo and nocebo effects.
fMRI data acquisition
Brain imaging was performed with a three-axis gradient head coil in a 3-Tesla Siemens TIM Trio MRI System equipped for echo-planar imaging. Thirty axial slices (4 mm thick with 1 mm skip) parallel to the anterior and posterior commissure covering the whole brain were acquired with 2000 ms repetition time, 40 ms echo time, 90° flip angle, and 3.13 × 3.13mm in-plane spatial resolution. We also collected a high-resolution 3D MPRAGE sequence for anatomic localization.
We performed preprocessing and statistical analyses using SPM12 software (Wellcome Department of Cognitive Neurology, London, UK). Preprocessing included motion correction, normalization to MNI stereotactic space, and spatial smoothing with an 8 mm Gaussian kernel. We calculated a GLM (general linear model) design matrix for each subject, including all 18 pain functional runs (1 run before and 1 run after treatment on each of the 9 “Lidocaine”, “Capsaicin” and Neutral sites, see Figure 1), modeling each pain stimulus and rating scale as events. We used this to generate the following contrast maps: 1) all pre-treatment pain functional runs; 2) comparisons of before and after treatment (post minus pre) on “Lidocaine,” “Capsaicin,” and Neutral sites when identical pain stimuli were applied; and 3) contrasts comparing post minus pre differences in response to identical pain stimuli among each different condition (“Lidocaine,” “Capsaicin,” and Neutral) separately. Full fMRI analysis is reported in our previous publication. Here we only focus on one contrast - post minus pre contrast on Neutral sites (part of point 2 above). Using this contrast, we first performed a one-sample t-test on all subjects’ data showing activation and deactivation (post minus pre) during the Neutral condition. We also performed a 2-sample t-test to detect any differences between the ‘More pain’ and ‘Less pain’ groups identified based on the median split in the neutral pain ratings change. For all analyses significance level was set at peak-level p<0.005, cluster-corrected at p<0.05 family wise error (FWE) for the whole-brain or with small-volume corrected (SVC) peak level p<0.005 (FWE) for the specific a priori defined ROIs. All coordinates were reported in MNI coordinates, as used by SPM.
For SVC we used brain regions implicated in reward processing, anxiety, and pain regulation as described in (Freeman et al., 2015) but limited to the areas that were relevant for the Neutral cue analysis from our previous study, namely the hippocampus, amygdala, and insula defined using Harvard-Oxford Cortical and Subcortical Structural Atlas (Desikan et al., 2006). The full details of the Neutral cue analysis of the original experimental paradigm are described in (Freeman et al., 2015), here we only reproduce it for completeness. The focus of the current work is on the group differences (‘More pain’ vs. ‘Less pain’) in fMRI activation and the relevance of bilateral amygdalae ROIs based on previous literature (Cador et al., 1989; Fernando et al., 2013; Li et al., 2011; Phelps and LeDoux, 2005; Wanigasekera et al., 2012; Watanabe et al., 2013; Zhang et al., 2016).
Resting state data acquisition and analysis
For the resting state analysis, scans were acquired with the following parameters: TR=3000 ms, TE=30 ms, flip angle of 85 degrees, the field of view of 216 mm2, 3×3×3 mm in-plane spatial resolution. The preprocessing of resting state images was done using SPM 12 software (http://www.fil.ion.ucl.ac.uk/spm) implemented in a MATLAB suite (Mathworks, Inc, Natick, Massachusetts). It included head motion correction, co-registration to subjects’ structural images, segmentation, normalization, linear detrending and smoothing with a 6 mm kernel.
Functional connectivity analysis was carried out with the CONN toolbox (www.nitrc.org/projects/conn) (Whitfield-Gabrieli and Nieto-Castanon, 2012). Time courses from the components associated with white matter and cerebrospinal fluid (CSF) were regressed out of whole-brain gray matter activity, 12 motion regressors (6 realignment parameters and first derivatives) were used to control for correlations during movement. Data were filtered between 0.008 and 0.09 Hz, global brain signal was not subtracted.
Seed-to-voxel connectivity analysis was performed using left and right amygdalae as seeds, based on their relevance for pain, placebo/nocebo, anxiety, as well as ambiguous stimuli processing (Davis and Whalen, 2001; Scott et al., 2008; Veinante et al., 2013; Wager and Atlas, 2015; Whalen, 1999). Significance level was set at p<0.005, k=20, cluster-corrected at p<0.05 FWE.
Replication of the association between neutral pre-post differences and placebo/nocebo effects in other datasets
In order to investigate whether the correlations between placebo/nocebo responses and the neutral pain inter-sessional differences in ratings are robust, we conducted Pearson correlations in 2 additional datasets – one with a placebo (Kong et al., 2006a) and the other with a nocebo manipulation (Kong et al., 2008) involving sham acupuncture (rather than cream) as treatment. Both of these studies had pre-post sessions with and without acupuncture treatment. In both studies sham acupuncture was delivered using a validated device (Streitberger and Kleinhenz, 1998) that retracts the needle into its casing when pressed on the skin. The heat manipulation in these two studies was similar to the current study. The full methods and behavioral results of these previous studies are published elsewhere. We here only focused on the correlations between the ratings pre-post placebo acupuncture and pre-post (no treatment) control (N=16), and pre-post nocebo acupuncture and pre-post control (N=13).
Results
Behavioral results
On average the change between post and pre- application of neutral cream was close to zero (M=−0.32, SE=±0.43), as expected in a control condition. Subjects were explicitly told that the cream is inert and that pre- and post-treatment heat stimuli are identical in the neutral condition, both of which were true. Nevertheless, we observed variability in subjects’ pain ratings, namely, some subjects rated identical stimuli as more painful and others as less painful in post- compared to pre-treatment (Figure 2A). We divided subjects into 2 equally sized groups (N=12 (6 female) in each) based on their neutral pre- and post-treatment pain ratings, Table S1. The groups did not differ in gender (equal number of male/female) or age (p=0.6, 2-tailed independent samples t-test). We observed that subjects reporting ‘More pain’ also had greater nocebo effects (p=0.01, 2-tailed independent samples t-test, Cohen’s d 1.06), whereas subjects reporting ‘Less pain’ had greater placebo effects (Figure 2B) (p=0.05, 2-tailed independent samples t-test, Cohen’s d 0.84). Importantly, neutral changes correlated with both placebo [beta = 0.69, T(22)=3.13, p=0.005 (2-tailed), Cohen’s d 1.33] and nocebo [beta = 0.49, T(22)=3.02, p=0.006, Cohen’s d 1.29)] effects (Figure 3A and 3B).
Figure 2.
Behavioral results. Pain ratings by condition (Lidocaine, Neutral, Capsaicin) showing average conditioning results (post > pre) and individual response variability. Average ratings grouped by median split – light colors – ‘Less pain’; dark colors – ‘More pain’.
Figure 3.
Correlations. A. A correlation between the Neutral (post>pre) changes and the placebo effect (Lidocaine labeled post > pre), showing a significant association. B. A correlation between the Neutral (post>pre) changes and the nocebo effect (Capsaicin labeled post > pre), showing a significant association. C. A correlation between the State-Trait-Anxiety-Inventory (STAI) and the Neutral (post>pre) changes, showing a significant association. D. A correlation between the STAI and the nocebo effect, showing no significant relationship. E. A correlation between the left amygdala-striatum connectivity and the STAI scores, not showing any significant association. F. A correlation between the left amygdala-striatum connectivity significantly associated with the nocebo effect.
Acknowledging that the order in which participants experienced the different conditions could have influenced their responses (Adamczyk et al., 2019; André-Obadia et al., 2011), we additionally tested for order effects. The order of conditions was randomized across subjects, with 6 possible randomizations. A linear regression did not reveal any significant effects of randomization on neutral responses. The distribution of the different orders across the “More pain” and “Less pain” groups was similar.
In addition, we investigated the neutral pre-post ratings from Session 2, to understand whether the effect we observed is related to perceptual fluctuations between sessions or is specific to processing ambiguous stimuli. In Session 2, participants experienced different levels of pain in different conditions, while in Session 3, all stimuli were identical and therefore ambiguous. We found that neutral response (post>pre) did not significantly differ between Session 2 and Session 3 (p=0.48), yet there were no significant differences between the “More pain” and “Less pain” groups (p=0.49) in neutral responses in Session 2. Finally, Session 2 neutral responses did not correlate with either neutral, placebo or nocebo response in Session 3. This suggests that the fluctuations in neutral responses found in Session 3 are not merely reflecting test-retest reliability in perception but are rather related to the tendency to experience more or less pain (placebo/nocebo) in the presence of ambiguity of the perceptual stimulus.
While differentiating between placebo/nocebo responders and non-responders has been clinically useful (Kaptchuk et al., 2008; Niklson et al., 2006; Seo et al., 2013), identifying people who are more / less prone to having non-specific treatment effects in clinical trials, the distinction is quite simplistic. To further test the reliability of the finding that neutral condition pain ratings are associated with placebo/nocebo responses, we re-analyzed behavioral data from two of our previous studies on placebo and nocebo effects of sham acupuncture, treating placebo/nocebo as a continuum by using correlations rather than group division. The results of the Pearson correlations in the placebo acupuncture manipulation study (N=16) (Kong et al., 2006a) showed a significant correlation between pre-post control and pre-post placebo effects, r=0.58, p=0.018. The results in the nocebo acupuncture manipulation study (N=13) (Kong et al., 2008) showed a significant correlation between the pre-post control and pre-post nocebo effects, r = 0.77, p=0.002.
fMRI activation results
Although average behavioral change in the neutral condition ratings between post and pre sessions were close to zero, the results of the one-sample t-test across all subjects on the post > pre fMRI activations during the neutral pain condition revealed signal increases in a number of brain regions, including the insula, hippocampus and amygdala (Table 1).
Table 1.
Neutral cream (post vs. pre) fMRI activation (one-sample t-test).
| Brain region | Hemi-sphere | MNI coordinates | k | T | Z | peak p(unc) | p(FEW-corr) | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Post > Pre | |||||||||
| Posterior insula | L | −62 | −16 | 44 | 2392 | 4.77 | 3.88 | <0.001 | <0.001 cluster-level |
| −42 | −16 | −2 | 3.17 | 3.66 | 0.002 | ||||
| −38 | −16 | 8 | 3.00 | 3.63 | 0.003 | ||||
| Precuneus | L/R | 22 | −48 | 36 | 1479 | 4.11 | 3.53 | <0.001 | <0.001 Cluster-level |
| −24 | −36 | 66 | 3.85 | 3.35 | |||||
| −4 | −34 | 54 | 3.81 | 3.32 | |||||
| Hippocampus | R | 34 | 2 | −40 | 647 | 4.79 | 3.95 | 0.002 | 0.044 cluster-level |
| 48 | −10 | −32 | 4.73 | 3.92 | |||||
| 50 | 24 | −28 | 4.35 | 3.68 | |||||
| Hippocampus | L | −26 | −8 | −28 | 109 | 4.66 | 3.87 | <0.001 | 0.008 SVC |
| Amygdala | R | 14 | 0 | −18 | 25 | 4.17 | 3.56 | <0.001 | 0.009 SVC |
| Amygdala | L | −24 | −4 | −28 | 64 | 3.96 | 3.42 | <0.001 | 0.014 SVC |
| −32 | −6 | −22 | 3.84 | 3.34 | <0.001 | 0.018 SVC | |||
| Pre > Post | |||||||||
| None | |||||||||
When the ‘More pain’ and ‘Less pain’ neutral change groups were compared directly for this contrast, a significant difference in the right amygdala was observed [p<0.005, k=20, p=0.023 FWE-SVC, T=3.75, Z=3.26, k=31, (MNI: 22, −2, −24)] (Figure 4). More amygdala activation was observed in subjects reporting an increase in pain after the application of neutral cream. This result was further validated when pain ratings were treated as a continuous variable rather than a group contrast and a regression analysis was applied [p<0.005, k=20, p=0.025, FWE-SVC, T = 3.7, (MNI 22, −2, −24)].
Figure 4.
Between group differences in fMRI signal. Whole-brain analysis for the contrast Neutral cream (post > pre), p<0.005, k=20, p=0.023 SVC (right amygdala, Harvard-Oxford Atlas), T=3.75, Z=3.26, k=31, (MNI: 22, −2, −24).
Resting state connectivity results
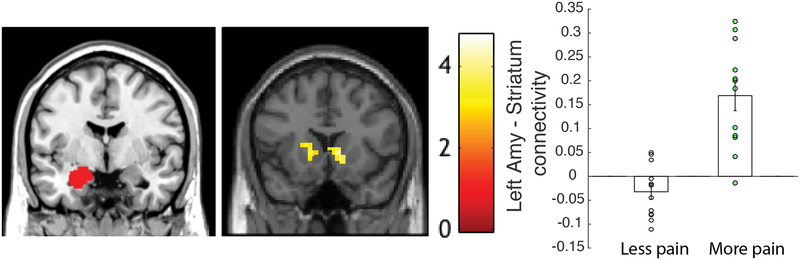
We further investigated differences in groups by exploring the connectivity between the bilateral amygdalae. Significantly greater left amygdala connectivity (Figure 5) with bilateral striatum, including the caudate, putamen and nucleus accumbens was observed in the ‘More pain’ group compared to the ‘Less pain’ group, p<0.005, cluster-corrected at p=0.012, k=465, (MNI: 0, 14, 0; 10, 10, 0; 12, 16, −6). We further assessed left amygdala connectivity using regression analysis with neutral rating differences as a continuous variable and confirmed the bilateral striatum result (height threshold p<0.01, k=638, cluster-corrected at FWE p=0.02 (MNI: 4 ,−4, 12)). No significant connectivity differences were observed with the right amygdala as a seed.
Figure 5.
Resting state. Left amygdala ROI seed (left), cluster in the striatum (right) showing significant group differences, p<0.005, cluster-corrected at p=0.012, k=465, (MNI: 0, 14, 0; 10, 10, 0; 12, 16, −6). Connectivity values by group – Less pain vs. More pain.
Anxiety and neutral pre-post fluctuations
Since amygdalae appeared as the main source of activation (right amygdala) and resting state (left amygdala) differences between neutral change groups, we investigated whether anxiety alone could explain these group differences. Trait anxiety was strongly associated with neutral pain changes [beta = 1.61, T(22)=3.65, p=0.001 (2-tailed)] (Figure 3C), suggesting that individuals with higher anxiety felt more pain post cream application, showing a nocebo response. Behavioral anxiety scores, however, did not correlate with nocebo response (Figure 3D). By contrast, left amygdala - striatum resting state connectivity values identified in the group difference analysis and extracted for each subject significantly were associated with the nocebo response [beta = 5.94, T(22)=2.1, p=0.047 (2-tailed)] (Figure 3F), although no significant relationship was identified with anxiety for the resting state marker (Figure 3E).
Discussion
In this study, we investigated individual differences in response to identical heat pain before and after application of an inert cream with neutral instructions not inducing positive or negative expectations. Observing heterogeneity of individual responses, we were able to divide subjects into 2 groups, which we contrasted in both fMRI activation during the heat manipulation on the part of the arm where the neutral cream was applied and resting state connectivity analyses. We observed that the ‘More pain’ group had increased right amygdala fMRI activation in response to identical pain stimuli as well as increased resting state connectivity of the left-amygdala and striatum, compared to the ‘Less pain’ group. These activation patterns being similar to the anxiety response, we observed that the neutral pain pre-post changes were associated with anxiety. Anxiety, however, was not a good predictor of placebo and nocebo responses. While behavioral changes correlated with both placebo and nocebo responses and the left-amygdala – striatum connectivity was significantly associated with nocebo responses, there were no significant associations between anxiety and either placebo or nocebo. The association between neutral pre-post pain changes and placebo and nocebo responses was further confirmed in 2 independent behavioral datasets using sham acupuncture to elicit placebo and nocebo responses respectively. We conclude that focusing on the individual differences in the neutral control responses could be important not only for the understanding of ambiguous neutral pain processing but also predicting placebo/nocebo responses. While we were able to observe the relationship between pre-post neutral responses and placebo/nocebo effects in three separate datasets, they all had fairly small sample sizes and were not designed to specifically investigate the neutral responses. Future studies with larger samples are needed to fully understand individual variability in neutral conditioning.
Our results could be accounted for either by short-term changes in the perception of pain at the time of fMRI testing or could reflect a more stable pain self-regulation mechanism grounded in specific brain network and personal trait differences. We discuss both possibilities below.
One of the main purposes of adding a neutral control in a placebo/nocebo paradigm is to contrast positive and negative conditions with one in which nothing changes. Ideally, there should be no change in neutral pain perception in this scenario. However, if subjects feel more pain in the neutral condition post cream application, i.e., the neutral condition feels more like nocebo, it might intensify the effect of placebo. Conversely, if subjects feel less pain in the neutral condition, so that the neutral cream feels like placebo, the effect of the actual placebo might be reduced thus increasing the nocebo effect. This is similar to the order effects previously observed in other studies (Adamczyk et al., 2019; André-Obadia et al., 2011). However, this was not the case in our results – subjects who reported more pain on neutral trials also reported more pain in the nocebo condition. In addition, no specific effects of the order were identified, suggesting that neutral pre-post responses are not different due to the preceding effect of placebo or nocebo.
Another potential explanation is that subjects used neutral pain as an anchor (zero) to gauge the distribution of placebo and nocebo pain ratings. This is quite compatible with the observed results, as there was a graded response in both groups, with a shifted mean pain ratings for all conditions. In other words, if participants experienced higher pain on the neutral condition, they would increase pain ratings on all the other conditions, resulting in higher nocebo (post>pre) and no placebo (as the ratings would be higher post application, compared to pre-application) or reduced placebo if subjects’ analgesic expectation is sufficiently strong. Note, however, that while there were intercept differences, in that higher ratings in the neutral condition were associated with higher pain ratings, the effect sizes of placebo and nocebo were significantly different. Namely, participants experiencing more pain in the neutral condition had bigger nocebo effects and smaller placebo effects. The right amygdala activation difference between groups during the neutral cream sessions would also be consistent with this explanation. In particular, the right amygdala in experimental pain has been associated with increased pain sensation (Neugebauer, 2015; Simons et al., 2014). In addition, neutral pain here could reflect the different pain baselines across different individuals. Few studies identify individual pain baseline in placebo/nocebo manipulations, yet we hypothesize that variability of baseline pain perception and expectations can be crucial for shaping placebo/nocebo responses.
It should be noted, however, that the actual level of heat pain, once calibrated, was kept constant throughout the whole experiment, so the physical pre-post differences in heat are unlikely, although, one cannot completely rule out sensitivity changes between fMRI runs. If the changes between the ‘More pain’ and ‘Less pain’ groups were due solely to physical differences in pain levels during the fMRI test session, no correlations would be expected with any of the more trait variables, such as subjects’ personality characteristics or resting state MRI scans recorded in the absence of pain. The neutral pain ratings, however, also correlated with anxiety levels and had a resting state marker – increased amygdala-striatum connectivity that differed between the groups. Therefore, differences between the groups suggest that they could reflect not only experimental session-relevant processing of pain but also the influence of trait anxiety on identical pain processing or even some pain-regulation strategies.
The link between anxiety and pain has been underlined in previous studies (Amanzio et al., 2013) and is often related to the amygdala (Stein et al., 2007). If anxiety increased neutral pain perception post cream application, it would render more anxious individuals more sensitive to pain and result in enhanced nocebo responses, even if the same stimulus was expected. However, the anxiety measure alone could not reliably correlate with the nocebo response, suggesting the relationship between anxiety and nocebo is more complex. This finding is also consistent with the results of another study showing that although anxiety plays a role in individual differences in pain-related fear, it does not significantly correlate with pain-fear brain correlates (Ochsner et al., 2006).
The neutral pre-post pain changes significantly correlated with both placebo and nocebo effects in this study. Moreover, we replicated the association between the neutral-placebo and neutral-nocebo conditions in two independent studies that used pre-post placebo or nocebo sham acupuncture manipulation (similar to the placebo/nocebo cream manipulation) and measured the control pre-post changes (comparable to the neutral cream condition), where no differences were expected. These additional studies had only either placebo or nocebo manipulations, avoiding the potentially complicated relationship between placebo, nocebo and the neutral conditions. Yet, control pre-post changes were significantly associated with the pre-post changes following the sham acupuncture manipulation in both of these separate placebo and nocebo studies, further supporting the idea that neutral pre-post pain fluctuations could be used for independently predicting placebo and nocebo responses.
Another explanation could be that the amygdala activation in the current study had nothing to do with pain and only appeared to reflect differences between the two groups on the anxiety factor. However, the resting state left amygdala – striatum connectivity was not significantly associated with anxiety, whereas it did correlate with the nocebo effect, underlining the role of the amygdala in processing both emotion and sensation (Veinante et al., 2013) and especially in aversive learning (Cador et al., 1989; Li et al., 2011; Watanabe et al., 2013). It is therefore possible that neutral pre-post changes rather reflect individual differences in ability to self-modulate pain responses in that they might be affected by emotion and anxiety but are more specific to pain processing.
The amygdala has long been known to be involved in Pavlovian conditioning, aversive learning and pain, however, there is growing understanding that these behaviors are not mediated only by the amygdala, but rather by a complex network involving numerous interactions with the cortico-striatal circuitry (Fernando et al., 2013). The specific role of the amygdala-striatal circuitry has been identified in several recent studies that suggest potential interpretations of our findings (Cador et al., 1989; Fernando et al., 2013; Li et al., 2011; Phelps and LeDoux, 2005; Wanigasekera et al., 2012; Watanabe et al., 2013; Zhang et al., 2016). For example, learned associability and prediction error have been found to correlate with fMRI brain responses in amygdala-striatal regions during the classic aversive (fear) learning, suggesting the role of the amygdala-striatal system in learning preparatory responses (Zhang et al., 2016). Amygdala-striatum interactions have also been found important for the modulation of reward prediction errors by emotion (Watanabe et al., 2013), which would explain individual differences in placebo/nocebo learning modulated by differences in emotional self-regulation abilities. Finally, key structures of the reward circuitry have been identified as central to the expression of behavioral opioid analgesia (Wanigasekera et al., 2012) having a direct relationship with pain perception and placebo response. The findings of our resting state connectivity analysis further highlight the importance of amygdala-striatum connection for placebo/nocebo response.
Neutral conditions in studies that involve both placebo and nocebo manipulation are also ambiguous. The amygdala has been shown to be sensitive to ambiguity (De Gelder et al., 2014; Neta et al., 2013); for example, more amygdala activation was observed in response to fearful (inherently more ambiguous) compared to angry faces (Davis and Whalen, 2001; Kapp et al., 1992; Whalen, 1999). As subjects in our study did not receive clear verbal suggestion as to what the pain levels during the neutral cream fMRI runs would be, amygdala activation could be the result of more effortful ambiguous stimulus processing. This was further supported by the analysis of Session 2 neutral responses, which were not different between groups and did not predict placebo/nocebo responses, given that pain stimuli in Session 2 were very distinct (unambiguous).
Finally, although subjects might not have formed very clear expectations of higher or lower pain during the neutral condition, they were explicitly told that the pain they would experience between the sessions would be identical and that the cream would be inert. Yet, some subjects rated pre-post pain as very different, experiencing more or less pain. This yields further support to the previous finding that experimentally induced expectations (or an expectation of a difference in our case) remain strong even when the truth about the manipulation is revealed. For example, in a study, in which experimenters subjected participants to a long session of conditioning and revealed to the subjects that they never received a real active drug, placebo effects persevered (Schafer et al., 2015). Only the long (4 days) but not the short (1 day) conditioning produced this result. In our study, spanning several days, induced placebo and nocebo changes could have also induced unintentional neutral pain changes.
Limitations
The main limitation of the current study is that the relationship between neutral pre-post ratings and placebo/nocebo was not examined a priori in the design but is a re-analysis of a previous study and that the sample size is limited. This impacted some of our analysis choices. For example, the grouping of subjects based on median split is somewhat arbitrary and does not take into account that some of the subjects in each group exhibited change of less than 0.5, suggestive of the more ‘stable’ rating that could in principle form a separate group, in addition to the “More pain” or “Less pain” grouping discussed here. Yet, we here were limited by the sample size and opted for only comparing 2 groups for fMRI analyses, based on the median split, which is a standard and unbiased approach to grouping, in the absence of an a priori hypothesis as to what would constitute a ‘no change’ cut-off in pre-post ratings. Future studies that are designed to rigorously examine the question can compare ‘stable’ raters (showing no or little pre-post difference) and “More pain” and “Less pain” groups as reported here.
While the replication of the correlation between neutral and placebo/nocebo responses in 3 studies here suggests this effect is somewhat independent of the experimental design/setting, with our data we cannot address the question of whether it is only reproducible during the experimental manipulation involving placebo/nocebo or represents a more stable trait of pain regulation in ambiguity.
Conclusions
We observed that pre-post changes in the neutral condition may reflect individual differences in pain self-modulation that affect placebo and nocebo responses. Our finding suggests an important role of individual differences and associated brain network organization in estimating placebo/nocebo effects. Specifically, amygdala activity during the presentation of stimuli and intrinsic connectivity of the amygdala and striatum may act as promising neuroimaging targets for placebo/nocebo prediction.
Supplementary Material
Acknowledgements
This work was supported by R01AT006364, R01AT008563, R21AT008707, R61AT009310, and P01AT006663 from NIH/NCCIH. NE was supported by the Australian Research Council DE180100893.
Footnotes
Conflict of interest
J.K. has a disclosure to report (holding equity in a startup company (MNT) and a pending patent to develop new neuromodulation device), but declares no conflict of interest.
References
- Adamczyk WM, Wiercioch-Kuzianik K, Bajcar EA, Bąbel P (2019). Rewarded placebo analgesia: A new mechanism of placebo effects based on operant conditioning. Eur J Pain (United Kingdom) 23, 923–935. [DOI] [PubMed] [Google Scholar]
- Amanzio M, Benedetti F, Porro C.a, Palermo S, Cauda F (2013). Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum Brain Mapp 34, 738–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André-Obadia N, Magnin M, Garcia-Larrea L (2011). On the importance of placebo timing in rTMS studies for pain relief. Pain 152, 1233–1237. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Carlino E, Pollo A (2010). How Placebos Change the Patient’s Brain. Neuropsychopharmacology 36, 339–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ (1998). Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron 20, 947–957. [DOI] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ (1989). Involvement of the amygdala in stimulus-reward associations: Interaction with the ventral striatum. Neuroscience 30, 77–86. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ (2001). The amygdala: vigilance and emotion. Mol Psychiatry 6, 13–34. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. [DOI] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C, Buchel C (2009). Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63, 533–543. [DOI] [PubMed] [Google Scholar]
- Fernando ABP, Murray JE, Milton AL (2013). The amygdala: securing pleasure and avoiding pain. Front Behav Neurosci 7, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S, Yu R, Egorova N, Chen X, Kirsch I, Claggett B, Kaptchuk TJ, Gollub RL, Kong J (2015). Distinct neural representations of placebo and nocebo effects. Neuroimage 112, 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gelder B, Terburg D, Morgan B, Hortensius R, Stein DJ, van Honk J (2014). The role of human basolateral amygdala in ambiguous social threat perception. Cortex 52, 28–34. [DOI] [PubMed] [Google Scholar]
- Gollub RL, Kirsch I, Maleki N, Wasan AD, Edwards RR, Tu Y, Kaptchuk TJ, Kong J (2018). A Functional Neuroimaging Study of Expectancy Effects on Pain Response in Patients With Knee Osteoarthritis. J Pain 19, 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracely RH, Dubner R (1987). Reliability and validity of verbal descriptor scales of painfulness. Pain 29, 175–185. [DOI] [PubMed] [Google Scholar]
- Hashmi JA, Kong J, Spaeth R, Khan S, Kaptchuk TJ, Gollub RL (2014). Functional network architecture predicts psychologically mediated analgesia related to treatment in chronic knee pain patients. J Neurosci 34, 3924–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp BS, Whalen PJ, Supple WF, Pascoe JP (1992). Amygdaloid contributions to conditioned arousal and sensory information processing In The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction., Aggleton JP, ed. (New York: Wiley-Liss; ), pp. 229–254. [Google Scholar]
- Kaptchuk TJ, Kelley JM, Deykin A, Wayne PM, Lasagna LC, Epstein IO, Kirsch I, Wechsler ME (2008). Do “placebo responders” exist? Contemp Clin Trials 29, 587–595. [DOI] [PubMed] [Google Scholar]
- Kong J, Benedetti F (2014). Placebo and nocebo effects: an introduction to psychological and biological mechanisms In Handbook of Experimental Pharmacology, Benedetti P, Enck E, Frisaldi M, and Schedlowski M, eds. (Germany: Springer; ), pp. 3–15. [DOI] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Polich G, Kirsch I, Laviolette P, Vangel M, Rosen B, Kaptchuk TJ (2008). A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. J Neurosci 28, 13354–13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ (2006a). Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci 26, 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Kaptchuk TJ, Polich G, Kirsch I, Vangel M, Zyloney C, Rosen B, Gollub RL (2009). An fMRI study on the interaction and dissociation between expectation of pain relief and acupuncture treatment. Neuroimage 47, 1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, Gollub RL (2006b). Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp 27, 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Schiller D, Schoenbaum G, Phelps EA, Daw ND (2011). Differential roles of human striatum and amygdala in associative learning. Nat Neurosci 14, 1250–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madarasz TJ, Diaz-Mataix L, Akhand O, Ycu EA, LeDoux JE, Johansen JP (2016). Evaluation of ambiguous associations in the amygdala by learning the structure of the environment. Nat Neurosci 19, 965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh SB, Barkus C, Huber A, Capitão L, Lima J, Lowry JP, Bannerman DM (2014). Aversive prediction error signals in the amygdala. J Neurosci 34, 9024–9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, Kelley WM, Whalen PJ (2013). Neural responses to ambiguity involve domain-general and domain-specific emotion processing systems. J Cogn Neurosci 25, 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V (2015). Pain Control. In Handb Exp Pharmacol, pp. 285–301. [Google Scholar]
- Niklson I, Edrich P, Verdru P (2006). Identifying baseline characteristics of placebo responders versus nonresponders in randomized double-blind trials of refractory partial-onset seizures. Epileptic Disord 8, 37–44. [PubMed] [Google Scholar]
- Ochsner KN, Ludlow DH, Knierim K, Hanelin J, Ramachandran T, Glover GC, Mackey SC (2006). Neural correlates of individual differences in pain-related fear and anxiety. Pain 120, 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo S, Benedetti F, Costa T, Amanzio M (2015). Pain anticipation: An activation likelihood estimation meta-analysis of brain imaging studies. Hum Brain Mapp 36, 1648–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina M, Azhar H, Love TM, Lu T, Fredrickson BL, Stohler CS, Zubieta J-K (2013). Personality trait predictors of placebo analgesia and neurobiological correlates. Neuropsychopharmacology 38, 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48, 175–187. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Clare S, Gati JS, Rawlins JN, Matthews PM (2000). Learning about pain: the neural substrate of the prediction error for aversive events. Proc Natl Acad Sci U S A 97, 9281–9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer SM, Colloca L, Ph D, Wager TD, Ph D (2015). Conditioned placebo analgesia persists when subjects know they are receiving a placebo. J Pain 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahl C, Ludäscher P, Greffrath W, Kraus A, Valerius G, Schulze TG, Treutlein J, Rietschel M, Smolka MN, Bohus M (2012). COMT val158met polymorphism and neural pain processing. PLoS One 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe R.a, Zubieta J-KK (2008). Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry 65, 220–231. [DOI] [PubMed] [Google Scholar]
- Seo JS, Jamieson K, Cosgrove V, Gwizdowski IS, Yang H, Sheehan DV, McElroy SL, Suppes T (2013). Characteristics of responders and non-responders to risperidone monotherapy or placebo in co-occurring bipolar disorder and anxiety disorder. Eur Psychiatry 28, 190–196. [DOI] [PubMed] [Google Scholar]
- Seymour B, O’Doherty JP, Koltzenburg M, Wiech K, Frackowiak R, Friston K, Dolan R (2005). Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat Neurosci 8, 1234–1240. [DOI] [PubMed] [Google Scholar]
- Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D (2014). The human amygdala and pain: Evidence from neuroimaging. Hum Brain Mapp 35, 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP (2007). Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry 164, 318–327. [DOI] [PubMed] [Google Scholar]
- Streitberger K, Kleinhenz J (1998). Introducing a placebo needle into acupuncture research. Lancet 352, 364–365. [DOI] [PubMed] [Google Scholar]
- Tu Y, Park J, Ahlfors SP, Khan S, Egorova N, Lang C (2019). NeuroImage A neural mechanism of direct and observational conditioning for placebo and nocebo responses. Neuroimage 184, 954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Yalcin I, Barrot M (2013). The amygdala between sensation and affect: a role in pain. J Mol Psychiatry 1, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Atlas LY (2015). The neuroscience of placebo effects: Connecting context, learning and health. Nat Rev Neurosci 16, 403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Leotti LA, Rilling JK (2011). Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci 31, 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walach H, Sadaghiani C, Dehm C, Bierman D (2005). The therapeutic effect of clinical trials: understanding placebo response rates in clinical trials--a secondary analysis. BMC Med Res Methodol 5, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanigasekera V, Lee MC, Rogers R, Kong Y, Leknes S, Andersson J, Tracey I (2012). Baseline reward circuitry activity and trait reward responsiveness predict expression of opioid analgesia in healthy subjects. Proc Natl Acad Sci U S A 109, 17705–17710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Sakagami M, Haruno M (2013). Reward Prediction Error Signal Enhanced by Striatum-Amygdala Interaction Explains the Acceleration of Probabilistic Reward Learning by Emotion. J Neurosci 33, 4487–4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ (1999). Fear, vigilance and ambiguity: Initial neuroimaging studies of the human amygdala. Curr Direct Psychol Sci In Press. [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2, 125–141. [DOI] [PubMed] [Google Scholar]
- Yu R, Gollub RL, Vangel M, Kaptchuk T, Smoller JW, Kong J (2014). Placebo analgesia and reward processing: Integrating genetics, personality, and intrinsic brain activity. Hum Brain Mapp 35, 4583–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Mano H, Ganesh G, Robbins T, Seymour B (2016). Dissociable Learning Processes Underlie Human Pain Conditioning. Curr Biol 26, 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.