Abstract
The majority of older patients who develop heart failure (HF), particularly older women, have a preserved left ventricular ejection fraction (HFpEF). Patients with HFpEF have severe symptoms of exercise intolerance, poor quality‐of‐life, frequent hospitalizations, and increased mortality. The prevalence of HFpEF is increasing and its prognosis is worsening. However, despite its importance, our understanding of the pathophysiology of HFpEF is incomplete, and drug development has proved immensely challenging. Currently, there are no universally accepted therapies that alter the clinical course of HFpEF. Originally viewed as a disorder due solely to abnormalities in left ventricular (LV) diastolic function, our understanding has evolved such that HFpEF is now understood as a systemic syndrome, involving multiple organ systems, likely triggered by inflammation and with an important contribution of aging, lifestyle factors, genetic predisposition, and multiple‐comorbidities, features that are typical of a geriatric syndrome. HFpEF is usually progressive due to complex mechanisms of systemic and cardiac adaptation that vary over time, particularly with aging. In this review, we examine evolving data regarding HFpEF that may help explain past challenges and provide future directions to care patients with this highly prevalent, heterogeneous clinical syndrome.
Keywords: aging, geriatric syndrome, heart failure, preserved ejection fraction, therapy
1. INTRODUCTION
Heart failure (HF) with preserved ejection fraction (HFpEF) is the most common form of HF in patients older than 65 years and represents >50% of prevalent HF cases in community.1 In the highest age decile, (≥90 years old), nearly all patients with HF have preserved EF. HFpEF is associated with high morbidity and mortality. After HF hospitalization, the 5‐year survival of HFpEF is a dismal 35%, worse than many cancers.2 The risk of death in patients with HFpEF increases with increasing comorbidity burden.3 Even after adjustment for comorbid conditions, mortality rates associated with HFpEF are higher than in general population age‐matched controls.4 Patients with HFpEF have similarly high rehospitalization rates as patients with HF with reduced EF (HFrEF).5 In patients hospitalized with HFpEF, 20% are readmitted within 30 days of hospital discharge and >50% within 1 year.6 Quality of life in HFpEF is as poor as or worse than HFrEF and is associated with physical activity levels that are as suppressed as those observed in patients with moderate‐to‐severe chronic obstructive pulmonary disease (COPD).7 Despite this, there are currently few effective therapies for HFpEF, as most approved therapies for HFrEF have been demonstrated to be ineffective for HFpEF, suggesting major differences in fundamental pathophysiology and therapeutic targets in HFpEF compared to HFrEF. We review relatively recent data that have enhanced our understanding of this complex disorder and that may lead to improved care of patients with this highly prevalent disorder.
2. CASE STUDY
A 79‐year‐old woman with long‐standing hypertension, obesity, and type II diabetes presents with shortness of breath on exertion that began 6 months earlier and has since gradually worsened and interferes with daily activities. She denies exertional chest pain. While she is able to shop in the local supermarket, carrying her packages home has become increasingly difficult. She desires to return to her previously active life. Current medications include amlodipine 10 mg daily, metformin 1000 mg daily, Lisinopril 20 mg daily, atorvastatin 20 mg daily. On exam, her blood pressure (BP) is 160/80 mm Hg, heart rate (HR) is 78/minutes, body mass index (BMI) 36 kg/m2. She also has peripheral edema; increased jugular venous distention elevated 10 cm above the right atrium. An electrocardiogram did not demonstrate ischemic changes. Her baseline echocardiogram showed mild left ventricular (LV) hypertrophy with an EF of 55% and right ventricular (RV) systolic pressure of 50 mm Hg. During a stress echocardiogram, she exercised for only 3 minutes on a modified Bruce protocol, stopping for extreme shortness of breath. Her resting BP was 160/70 mm Hg and her HR was 76 bpm. At peak exercise, her BP was 196/90 mmHg, with a peak HR of 105 bpm. Echocardiographic images at the end of exercise demonstrated augmentation of contractility of all walls without significant mitral regurgitation. What can be done to improve her symptoms and quality of life?
2.1. Making the HFpEF diagnosis: Challenges
Diagnosing HF in older adults poses specific challenges; false‐positive clinical diagnoses are not uncommon.6 The most common symptoms of HFpEF are exertional dyspnea. However, symptoms of reduced exercise tolerance are common in the older adults and have been shown to reflect normal physiological changes related to aging or could be related to non‐cardiac etiologies. Furthermore, the diagnosis of HF in the older patients may be difficult due to the presence of co‐morbidities, some of which can mimic HF signs and further confound the diagnosis of HF.8 In addition, older patients with HFpEF may not present with “classic” HF symptoms and may instead have very subtle clinical presentations. Up to one‐third of HFpEF outpatients may have a B‐type natriuretic peptide (BNP) level that is below the typical diagnostic thresholds.8 This can challenge the common practice of using BNP to make HF diagnosis. In addition, limited predictive capabilities of the echocardiographic variables for diagnosis of diastolic dysfunction further puzzle the clinical picture. There is also no universally agreed upon definition to define HFpEF. The American College of Cardiology/American Heart Association (ACC/AHA) consensus states that the diagnosis of HFpEF is based on typical symptoms and signs of HF in a patient with a normal range LVEF, and no significant valvular abnormalities by echocardiography and no other obvious precipitating factors for HF.9 By contrast, the European Society of Cardiology (ESC) requires diastolic dysfunction for the diagnosis of HFpEF, along with symptoms and signs of HF and normal or mildly abnormal LV function.10
2.2. Aging: A model for HFpEF as a true geriatric syndrome
Cardiac aging is known to affect many, if not all, of the pathophysiological components present in HFpEF. Specific alterations in structural and function in aging, such as ventricular vascular stiffening, vascular dysfunction, impaired [Ca2+]i regulation, decreased β‐adrenergic reserve, and physical deconditioning, have been identified as important contributing causes for HFpEF.11 Aging is also associated with a decline in a variety of neural, hormonal, and environmental trophic signals; this can leads to loss of muscle mass and mass‐specific strength, characteristic changes in body composition, including decreases in lean body mass and muscle strength, and increases in adiposity which increase vulnerability for sarcopenic obesity.12 In addition, older adults hospitalized with a primary diagnosis of HF often have multiple non‐cardiac comorbidities (5.5 on average) and high proportions are frail.13 The adverse impacts of aging, frailty and comorbidities on functional capacity and clinical outcomes are cumulative and synergistic.14 Indeed, approximately 85% of elderly HFpEF patients are overweight or obese, and the HFpEF epidemic has largely paralleled the obesity epidemic.15 Furthermore, aging and obesity are well established risk factors for both HFpEF. Along with comorbidities, aging may initiate and/or aggravate chronic systemic inflammation that may affect myocardial remodeling and dysfunction in HFpEF through a signaling cascade, which begins with coronary microvascular endothelial dysfunction as shown in Figure 1.3, 17
Figure 1.
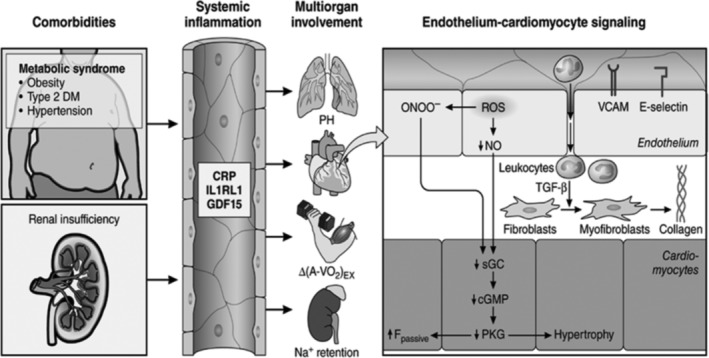
Systemic and myocardial signaling in heart failure (HF) with preserved ejection fraction (HFpEF). Comorbidities induce systemic inflammation, evident from elevated plasma levels of inflammatory biomarkers, such as soluble interleukin 1 receptor‐like 1 (IL1RL1), C‐reactive protein (CRP), and growth differentiation factor 15 (GDF15). Chronic inflammation affects the lungs, myocardium, skeletal muscle, and kidneys leading to diverse HFpEF phenotypes with variable involvement of pulmonary hypertension (PH), myocardial remodeling, deficient skeletal muscle oxygen extraction (ΔA‐Vo2) during exercise (Ex), and renal Na + retention. Myocardial remodeling and dysfunction begin with coronary endothelial microvascular inflammation manifest from endothelial expression of adhesion molecules, such as vascular cell adhesion molecule (VCAM) and E‐Selectin. Expression of adhesion molecules attracts infiltrating leukocytes secreting transforming growth factor β (TGF‐β), which converts fibroblasts to myofibroblasts with enhanced interstitial collagen deposition. Endothelial inflammation also results in the presence of reactive oxygen species (ROS), reduced nitric oxide (NO) bioavailability, and production of peroxynitrite (ONOO–). This reduces soluble guanylate cyclase (sGC) activity, cyclic guanosine monophosphate (cGMP) content, and the favorable effects of protein kinase G (PKG) on cardiomyocyte stiffness and hypertrophy. HFpEF indicates heart failure with preserved ejection fraction. (Reproduced with permission from Reference 16)
2.3. Key knowledge gap
Is HFpEF is a single entity or comprised of several different diseases?
Are there inflammatory biomarkers that may help to diagnose HFpEF and better understand its pathophysiology?
2.4. Do any meds improve outcomes in HFpEF?
Tables 1 and 2 shows non‐pharmacological and pharmacological clinical trials that were positive in HFpEF on their primary endpoints. Not shown here are the trials that were negative or neutral, which are far greater in number. However, unlike HFrEF, there are currently no disease‐modifying agents available for HFpEF that improve clinical outcomes. Indeed recently, Sacubitril‐valsartan did not result in a significantly lower rate of total hospitalizations for HF and death among patients with HFpEF.44 This leads to the question: Why have most pharmacological therapies to date not shown clear benefit in HFpEF? To date, pharmacologic interventions applied in HFpEF have been principally based upon the assumption of underlying, severe neurohormonal abnormalities. However, neurohormonal derangements appear more limited in breadth and severity in HFpEF than in HFrEF. Furthermore, diagnosis of HFpEF is challenging due to the lack of a single objective marker that defines the syndrome, such as a reduced LVEF in HFrEF and the high frequency of comorbidities that may mimic or accompany the HFpEF syndrome. In addition, exercise intolerance, the cardinal manifestation of HF regardless of EF, has a complex pathophysiology and is rarely explained by a single process. Furthermore, most HFpEF studies have only measured diastolic function at rest rather than during exercise where symptoms become manifest.45, 46 So far, clinical trials generally enrolled “all comers” with clinical syndrome of HF and objective evidence of preserved LVEF. However, evolving evidence indicates that HFpEF is a much more complex disorder than originally thought, influenced by aging processes as explained before, likely systemic in nature, involving many organs and organ systems in addition to the heart, and also involving abnormalities in vascular and skeletal muscle function as well, and likely has multiple phenotypes. These issues and concepts have generally not been addressed in trial designs to date. Given such a multi‐factorial, complex milieu, it is not surprising that drugs and interventions aimed primarily at a central hemodynamics repeatedly failed to strongly impact overall outcomes in HFpEF. As discussed in more detail below, lifestyle modifications (exercise and diet) have been more consistently successful, likely due to addressing HFpEF as a systemic syndrome, and by addressing peripheral, non‐cardiac factors that appear more mutable than cardiac factors.
Table 1.
Non‐pharmacological interventions that were positive in HFpEF on their primary endpoints
| Intervention first author/trial (Ref. #) | HFpEF patient type | Outcomes |
|---|---|---|
| Calorie restriction exercise training | Exercise capacity and QOL | |
| SECRET‐1/Kitzman et al18 (n = 100) | Ambulatory, stable, obese HF patients (body mass index of 39) with NYHA classes II‐III symptoms (aged 67 ± 5 years, 41% female) | Robust increase in exercise capacity. QOL scores was improved, and benefit was greatest for calorie restriction |
| Exercise training | Exercise capacity and QOL | |
| PARIS/Kitzman et al19 (n = 53) | Ambulatory, stable HF patients with NYHA classes II‐III symptoms (aged 70 ± 6 years, 87% female) | Improved peak and submaximal exercise capacity |
| PARIS‐II/Kitzman et al20 (n = 63) | Ambulatory, stable HF patients with NYHA classes II‐III symptoms (aged 70 ± 7 years, 76% female) | Improved peak VO2 and 6MWD |
| Ex‐DHF trial21 (n = 64) | Ambulatory, symptomatic NYHA II/III symptoms, ECHO‐DD (aged 65 ± 7 years, 56% female) | Improved exercise capacity and QOL scores |
| Smart et al22 (n = 25) | Ambulatory, well‐compensated HF (aged 64 ± 8 years, 48% female) | Improved peak VO2 |
| Fu et al23 (n = 30) | NYHA class II/III HF with episodes of acute pulmonary edema (aged 61 ± 3 years, 33% female) | Improved Peak VO2, diastolic function with reduction of the E/e′ ratio and QOL scores |
| Gary et al24 (n = 32) | NYHA class II/III diastolic HF, h/o ECHO –DD or diastolic HF (aged 67 ± 11 years, all females) | Improved 6MWD, QOL and depression scores |
| Angadi et al25 (n = 9) | NYHA class II/III HF, ECHO‐DD (aged 69 ± 6 years, 11% female) | Improved peak VO2 and diastolic dysfunction |
| Alves et al,26 (n = 31) | Admission with clinical signs of HF (aged 63 ± 11 years, 29% female) | Improved exercise tolerance, cardiac systolic (LVEF)and diastolic function (E/e′) |
| CardioMEMs sensor | Hospitalization for HF | |
| CHAMPION27 (n = 119, had LVEF ≥40% | NYHA class III symptoms, hospitalization for HF in last 12 months (aged 62 ± 13 years, 29%female) | Significant and large reduction in hospitalization for patients with NYHA class III HF |
| Transcatheter interatrial shunt device | LV filling pressure | |
| REDUCE LAP‐HF28 (n = 68) | NYHA class II/IV symptoms, PCWP at rest >15 mm of Hg and during exercise >25 mm of Hg measured invasively, (aged 69 ± 8 years, 61% female) | Reduced PCWP during exercise |
| REDUCE LAP –HF29 (n = 44) | NYHA class II/IV, PCWP at rest >15 mm Hg and during exercise >25 mm Hg measured invasively, LVEF >40% (aged 70 ± 9 years,50% female) | Reduced PCWP during exercise |
| Adaptive servo‐ventilation | Moderate to severe sleep disorder breathing | NYHA class, LV diastolic function, CV hospitalization |
| Yoshihisa et al30 (n = 36) | NYHA class II‐IV HF, stable clinical status, with moderate to severe sleep disorder breathing (age ± 16 years, 11%female) | Improved NYHA class, LA volume, BNP, ECHO‐DD, Proportion of patients had less CV events or hospitalization for HF |
| CAT HF31 (n = 126) | Hospitalized HF, BNP ≥300 pg/mL, with moderate to severe sleep apnea, 24 (19%) had HFpEF (aged 61 ± 14 years, 26% female) | The risk of the primary composite endpoint was reduced by 62% Composite global rank score (hierarchy of death, CV hospitalizations, and percent changes in 6MWD) |
Abbreviations: A‐VO2 Diff, arterial‐venous oxygen difference; CV, cardiovascular; DD, diastolic dysfunction; ECHO, echocardiographicaly assessed; E, mitral early diastolic velocity; e′, mitral annular velocity; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; LA, left atrium; LV, left ventricle; MWD, minute walk distance; n, number of participants; NYHA, New York Heart Association; QOL, quality of life; PCWP, pulmonary capillary wedge pressure; VO2, oxygen consumption; B‐type natriuretic peptide.
Table 2.
Pharmacological interventions that were positive in HFpEF on their primary endpoints
| Intervention first author/trial (Ref.#) | HFpEF patient type | Primary outcomes |
|---|---|---|
| ACE‐I/ARB | CV death/HF admissions | |
| CHARM‐Preserved32/Candesartan (n = 3023)a | NYHA classes II‐IV HF with prior cardiac hospitalization (aged 67 ± 11 years, 40% female) | Fewer HF admissions |
| The PEP‐CHF33/Perindopril (n = 852)a | Diagnosis of HF and treated with diuretics and an ECHO‐DD. Prior cardiac hospitalization within 6 months. (aged 76 ± 5 years, 55% female) | Fewer HF admissions, improved symptoms and exercise capacity |
| Aldosterone antagonists | HF admission, LV remodeling and LV filling pressure | |
| TOPCAT34/Spironolactone (n = 3445)a | Patients had h/o HF hospitalization within previous 12 months and elevated BNP within 60 days before randomization. (aged 69 years [median], 52% female) | Modest decline HF hospitalization |
| Aldo‐DHF35/Spironolactone (n = 422) | Ambulatory patients/NYHA class II‐III symptoms, ECHO‐DD and normal or near‐normal BNP levels. (aged 67 ± 8 years, 52% female) | LV remodeling, neurohumoral activation were improved |
| Kosmala,et al36/Spironolactone (n = 150) | NYHA class II/III, ECHO‐DD, and baseline increased exercise E/e′ ratio. (aged 67 ± 9 years; 85% female) | Improvement in exercise capacity. Reduction in exercise‐induced ECHO measure of increased LV filling pressure |
| Inorganic nitrates | Exercise capacity, Biventricular filling and pulmonary pressure | |
| Borlaug et al37/Inhaled sodium nitrite (n = 26) | Elevated PCWP at rest (>15 mmHg) and with exercise (≥25 mmHg). (aged 70 ± 9 years, 54% female) | Acute administration reduced biventricular filling pressures and PAP at rest and during exercise |
| Kitzman et al38/ Beet root juice (n = 20 | Ambulatory HF patients with NYHA classes II‐III (aged 69 ± 7 years of age) | Improved submaximal Endurance |
| Zamani P et al39/NO3‐rich beetroot juice (n = 17) | Symptomatic HF, ECHO‐DD, elevated NT‐pro‐BNP or PCWP >12 mmHg on prior cardiac catheterization. (aged 66 ± 9, 12% female) | Improved peak VO2 in subjects with HFpEF by significant reduction in systemic vascular resistance |
| Phosphodiesterase‐5 inhibitor | PAP and RV function | |
| Guazzi, et al40, 41/Sildenafil (n = 44) |
HF signs and symptoms, ECHO‐DD, invasively measured PASP >40 mmHg. (aged 72 years [median], 20% female) |
Improvement in PAP, RV function and dimension, LV ventricular relaxation and distensibility |
| Vericiguat (soluble guanylate cyclase stimulator)a | Change in NT–proBNP and LA volume index | |
| SOCRATES‐PRESERVED42 (n = 477) | 73 ± 10, 48% female, NYHA class II‐IV, LVEF ≥ 45%, increased BNP ≥ 100 pg/mL or NT‐proBNP levels ≥ 300 pg/ML, ECHO evidence of DD, LVEF ≥ 45%. | Improvements in quality of life |
| LCZ696(ARNI)b | NT‐proBNP | |
| (Sacubitril/valsartan) | ||
| PARAMOUNT43/(n = 301) | NYHA class II‐III HF, NT‐pro BNP > 400 pg/nL and be on a diuretic therapy. (aged 71 ± 9 years, 57% female) | Significant reduction in NT‐proBNP |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; ARNI , angiotensin receptor‐neprilysin; BNP, B‐type natriuretic peptide; DD, diastolic dysfunction; E, Mitral early diastolic velocity; e′, mitral annular velocity; ECHO , echocardiographicaly assessed; HFpEF, heart failure with preserved ejection fraction; HF, heart failure; LVEF, left ventricular ejection fraction; LA, left atrium; LV, left ventricle; n, number of participants; NT pro BNP; N terminal, B‐type natriuretic peptide; NYHA , New York Heart Association; PCWP, pulmonary capillary wedge pressure; PAP , pulmonary artery pressure; RV , right ventricle.
Neutral on composite primary outcome.
Except this trial, among all other trials study drug was compared with placebo. In this trial, comparison made between study drug and valsartan.
2.5. Key knowledge gap
Was the lack of definite benefits in pharmacological trials to date caused by a flawed study designs or by ineffective study interventions?
Should future HFpEF trials include broader group of subjects or individual subpopulations?
2.6. Prevent and delay the progression of HFpEF
Table 3 summarizes the practical approaches to managing HFpEF. In older patients, multi‐level strategies and interventions aimed at improving adherence to guidelines and tailoring therapy could be the key to improving outcome, and to reducing costs related to HF‐related re‐admissions. An important component of treating a patient with HFpEF is treating the contributing factors and comorbidities that are frequently present and significantly impact the clinical course, such as obesity, hypertension, coronary artery disease, diabetes, COPD, anemia, chronic kidney disease, and sleep‐disordered breathing.9 Several hypertension trials, including the systolic BP intervention trial (SPRINT), have shown a reduction in incident HF with treatment of hypertension, although these trials did not differentiate between incident HFpEF and HFrEF.47, 48, 49 Considering the age distribution in these trials and the age‐dependent relative incidence of HFpEF, control of hypertension may be the single most important prevention strategy for HFpEF. In SPRINT, both HFpEF and HFrEF incident cases were significantly reduced, including specifically in older patients ≥75 years old.50 The BP goals in the ACC/AHA HF guideline are similar to those in the general population, with the exception that the 2017 ACC/AHA HF guideline update recommends the lower systolic BP target of 130 mm Hg.9, 48, 51 ACC/AHA HF guidelines support the use of beta‐blockers, angiotensin‐converting enzyme inhibitors (ACEI), and angiotensin receptor blockers (ARBs) for hypertension (IIa recommendation) and ARBs and aldosterone antagonists receive a relatively weak recommendation (class IIb, level of evidence B) as reasonable to consider for decreasing hospitalizations in HFpEF. Metabolic risk factors: HFpEF patients demonstrate a high prevalence of obesity and diabetes. Increased adiposity promotes inflammation, hypertension, insulin resistance, and dyslipidemia and impairs cardiac, arterial, skeletal muscle, and physical function,7, 52, 53 all of which are common in HFpEF and contribute to its pathophysiology.54 Recently, studies in type 2 diabetes patients showed reduced risk of HF hospitalization in patients receiving either empagliflozin or dapagliflozin, which are novel sodium‐glucose cotransporter 2 inhibitors,55, 56 Coronary Artery Disease (CAD) Patients with HFpEF and symptoms and signs of ischemia are treated with standard therapy including beta‐blockers and calcium channel blockers.57 Patients with epicardial CAD may require complete coronary revascularization by percutaneous coronary intervention or coronary artery bypass graft surgery.57 However, retrospective data suggest that clinically evident, acute coronary ischemia may not be the key trigger for acute decompensation in HFpEF, that the EF does not decline during an acute episode,58 and that revascularizing epicardial coronary stenoses has little effect on preventing the recurrence of acute HFpEF.59 Atrial fibrillation (AF) prevalence has been increasing due to an aging general population and increased longevity. AF in HFpEF associated with impaired LV systolic, diastolic function and functional reserve, larger left atria (LA) with poor LA function, RV dysfunction, more severe neurohumoral activation, and impaired exercise tolerance.60, 61 Tachycardia is also deleterious by shortening the time of diastole that may impair adequate diastolic filling. For these reasons, restoration and maintenance of sinus rhythm are preferred when AF occurs in patients with HFpEF. To restore sinus rhythm, cardioversion is recommended because catheter ablation of AF had limited long‐term success in HFpEF.62 If cardioversion is unsuccessful, rate control and permanent anticoagulation become mandatory.57 Anemia is more prevalent in HFpEF than in HFrEF patients and associated with increased risk of HF hospitalization and overall mortality.63 The 2017 ACC/AHA HF management update included a class IIb recommendation for iron replacement therapy in appropriately selected patients, although HFpEF patients have not been included in the cited trials.9 Treatment of anemia with erythropoietin analogs received a class III recommendation (no benefit).9
Table 3.
Practical management of heart failure with preserved ejection fraction
|
|
|
|
|
|
|
|
|
|
Abbreviations: AF, atrial fibrillation; HF, heart failure.
2.7. Key knowledge gap
Is rate control alone or rhythm control the best strategy for treatment in HFpEF patients?
What is the best way to manage comorbidities in HFpEF patients?
2.8. Lifestyle interventions in HFpEF
Recent data support the beneficial impacts of lifestyle modification, including weight reduction, dietary and nutrient consumption, physical activity, and cardiorespiratory fitness on HF risk. In a pooled analysis of 51 000 participants from the Women's Health Initiative, Multiethnic Study of Atherosclerosis, and Cardiovascular Health Study cohorts, the risk for incident HFpEF increased in a dose‐dependent manner as BMI increased and leisure‐time physical activity declined.45 Recently, Kitzman et al showed that among older obese patients with chronic, stable HFpEF, intentional weight loss via calorie restriction (CR) diet significantly improved exercise capacity to a degree similar to and was additive to exercise training (ET).18 In addition, CR but not exercise significantly improved the HF specific quality of life measures (Figure 2, Table 1).18 Even though, a recent meta‐analysis of randomized trials among older patients without HF indicates that CR is associated with a 15% reduction in total mortality,64 because of the reported “HF obesity paradox,” further studies are needed to determine role of CR in older patients with HFpEF.42
Figure 2.
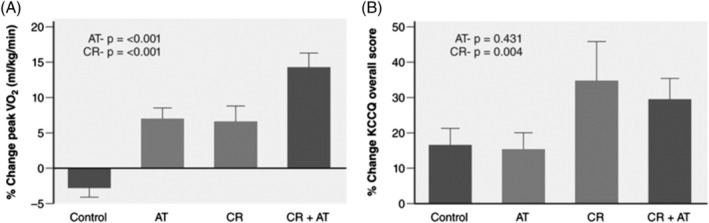
Effects of a 20‐week caloric restriction diet on exercise capacity and quality of life in heart failure (HF) with preserved ejection fraction (HFpEF). The graph displays percent changes ± SEs at the 20‐week follow‐up relative to baseline by randomized group for peak VO2 (mL·kg–1·min–1, A) and quality of life scores, P‐values represent effects for AT and CR. AT indicates aerobic exercise training; and CR, caloric restriction diet. (Reproduced with permission from Reference 16)
In 2010, Kitzman et al reported the first randomized controlled trial evaluating ET as a treatment for HFpEF, showing substantial improvement in cardiorespiratory fitness with training (Table 1). 19 Since that time, a number of other studies have substantiated this benefit and demonstrated favorable effects on quality of life (Table 1). Existing data suggest that the majority of ET‐related improvement in exercise capacity may be related to microvascular and/or skeletal muscle adaptations that increase diffusive oxygen transport and/or utilization by the active muscles.65, 66 A supervised maximal exercise test with monitoring for ischemia should be performed before HFpEF patients beginning an exercise program. Exercise protocols used in clinical trials primarily included aerobic‐type activities, such as walking, stationary cycling, or rowing. After supervised setting with direct supervision and monitoring, depending on individual progress, patients may be able to be transitioned to a home exercise maintenance training program. Randomized exercise intervention trials also showed that ET appeared safe in older, deconditioned HFpEF patients (Table 1), although the trials were not large enough or designed to definitively address the question of safety. For the same reasons, the potential impact of ET in HFpEF on clinical events, including hospitalizations and death, is unknown. Recently, the REHAB‐HF, prospective, multicenter pilot trial which successfully randomized 27 patients ≥60 years of age hospitalized with acute decompensated HF (both HFrEF and HFpEF) showed that a novel, tailored, progressive, multidomain physical rehabilitation is feasible in older patients with acute decompensated HF who have high rates of frailty and comorbidities and has the potential to improve physical function and reduce rehospitalization rates.67 A larger trial is underway to verify these findings.
2.9. Exercise prescription
A supervised maximal exercise test with monitoring for ischemia should be performed before HFpEF patients beginning an ET program. The ET program for stable HFpEF patients should consist of continuous large muscle mass moderate intensity endurance exercise performed for 20 to 60 minutes per session, 3 to 5 days per week. The exercise is usually performed on a bicycle or treadmill. The duration and frequency of effort should be up titrated before intensity is increased. Once patients demonstrate a tolerance of aerobic training levels, resistance training activities should be considered. It is recommended to initiate ET in a structured, supervised, center‐based program. This can be either in‐hospital or in a specialized facility, as long as close supervision are available. After supervised setting, depending on individual progress, patients usually should be able to be transitioned to a home exercise maintenance training program. Ideally, a patient‐tailored ET program is prescribed instead of a “one size fits all” approach especially in older patients with HFpEF. In addition, to increase long‐term adherence to ET, the patient's preferences should be taken into account.
ACC/AHA guidelines recommend moderate, regular physical activity for all HF patients, which seem reasonable. However, in the absence of data regarding effect of ET on clinical events, the Centers for Medicare & Medicaid Services does not reimburse for cardiac rehabilitation in either acute or chronic HFpEF patients, in contrast to its policy for chronic (but not acute) HFrEF.
2.10. Key knowledge gap
What is the most effective and safe exercise prescription for older HFpEF patient?
2.11. Treatment of congestion
In the CHAMPION trial (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III HF Patients trial), clinical management guided by physician knowledge of central hemodynamics significantly reduced HF hospitalization (Table 1).27, 68 This finding was confirmed in more recent analyses of Medicare beneficiaries.67 The CARDIOMEMS device is a wireless, implanted pulmonary artery pressure monitor implanted in the distal PA during right heart catheterization. Patients transmit hemodynamic data daily using a wireless RF transmitter.
Given that rises in left atrial (LA) pressure and pulmonary venous congestion are shown to herald HF events in patients with HFpEF, creating a controlled left‐to‐right interatrial shunt to allow LA decompression could be a rational non‐pharmacological strategy for alleviating symptoms. The Reduce Elevated LA Pressure in Patients with HF (REDUCE LAP‐HF) study is a multicenter, prospective, non‐randomized, single‐arm phase 1 study designed to assess the safety and performance of the device in patients with HFpEF with NYHA II‐IV despite optimal medical or device therapy and demonstrated reductions in LA pressure during exercise with improvements in functional capacity and health‐related quality of life scores 6 and 12 months after implantation of this device (Table 2).69 Recently, REDUCE LAP‐HF I, a phase 2 randomized parallel‐groups, and blinded multi‐center sham‐controlled trial published short‐term results (Table 2).29
2.12. Key knowledge gap
Can we do remote hemodynamic monitoring effectively in older patients?
What are the best metrics for determining when a patient is adequately decongested during an acute decompensated HF hospitalization?
Thinking like a Geriatrician—“Sometimes the disease is not the most important focus—maintaining the patient's function is” as said by Dr. Covinsky.
In older patients hospitalized primarily for HF, many factors outside the heart such as advanced age, globally reduced organ system reserve capacity, physical frailty, impaired cognition, and comorbidities strongly influence outcomes.70 In addition, the hospital environment—with immobilization, fasting, sleep deprivation, and disorientation—can dramatically worsen physical frailty with rapid, severe loss of muscle mass and function.70 When older HF patients are thought to be ready for discharge, careful attention should focus on their multiple comorbidities, globally reduced organ reserve, severe physical deconditioning, and cognitive dysfunction to prevent the “post‐hospitalization syndrome,” consisting of high rates of rehospitalization, mortality, and nursing home admission, prolonged physical disability, poor quality of life, and high health care costs.71 Thus, it is important to treat not just the disease and but the whole patient particularly in the older population. Improved care of complex older patients with HFpEF is dependent on a new model of collaboration and teamwork between primary care provider, geriatrician, and cardiologist, with timely access to palliative care to accommodate the fundamental heterogeneity of aging and the patient's choices. In addition, cardio‐geriatric clinics are designed to meet the needs of older patients with HF and their caregivers by providing comprehensive care focusing on improving quality of life and functional independency. If managed adequately in a multidisciplinary ambulatory care setting, we can potentially prevent most of the unnecessary hospital readmissions. To improve care delivery and minimize the need for emergency visit and rehospitalization, development of ambulatory services with effective chronic disease management and integrated care programs are needed urgently.
2.13. Key knowledge gap
What are the optimal disease management strategies/programs and transitional care for older hospitalized HFpEF patients?
How can we best implement interdisciplinary approaches to treat this unique and rapidly growing population?
2.14. HFpEF management based on clinical phenotypes
A key evolving concept in HFpEF therapy is that the disorder is highly heterogeneous and manifestations can vary markedly from patient to patient even within a specific HFpEF patient population.72 More recently, Shah et al proposed a matrix combining predisposition phenotypes with clinical presentation phenotypes as a starting point to guide clinical care in HFpEF (Figure 3).7 The approach starts with general treatment recommendations beneficial to the majority of HFpEF patients because they address the presentation phenotypes of lung congestion and the most common phenotype of overweight/obesity, present in >80% of HFpEF patients. Subsequently, supplementary recommendations are suggested for additional phenotypes, such as metabolic/obesity, arterial hypertension, renal dysfunction, and coronary artery disease. Additional clinical presentation phenotypes are suggested in whom specific therapeutic interventions could be meaningful like chronotropic incompetence, pulmonary hypertension, skeletal muscle weakness, and AF. This phenotype‐specific approach may prove a valuable advance.
Figure 3.
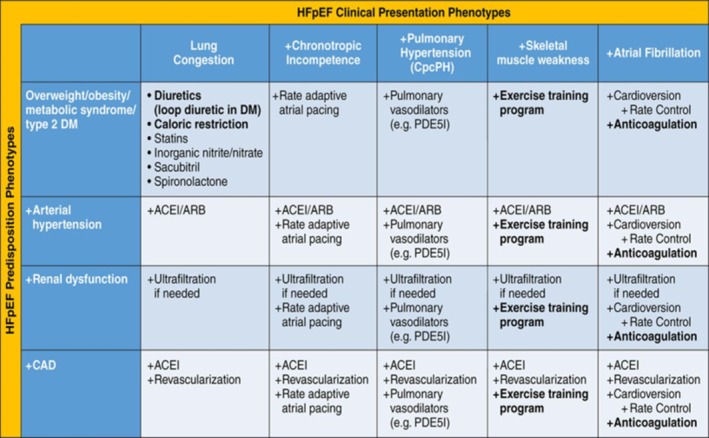
Phenotype‐specific heart failure (HF) with preserved ejection fraction (HFpEF) treatment strategy using a matrix of predisposition phenotypes and clinical presentation phenotypes. (Reproduced with permission from Reference 73)
2.15. HFpEF management based on biologic phenotypes
An understanding of which are the causes and which are the downstream effects, may allow the HFpEF syndrome to be concentrated into distinct diagnoses based on the underlying biology. From this, specific interventions can follow, targeting individuals identified on the basis of their biological phenotype. This concept was recently expanded upon by Lewis et al,74 suggesting that determining the underlying pathobiologic disease mechanism which ultimately leads to specific clinical phenotypes can drive discernment of “biological phenotype” and integrate with the clinical” phenotypes” thereby further enhancing the potential gains from individualized approaches on the basis of enhanced phenotypic categorization. Biological heterogeneity has potentially compromised in prior HFpEF trials, thus HFpEF now needs “to get personal.”
2.16. Key knowledge gap
Should HFpEF diagnosis and management be clinically or mechanistically based?
2.17. Proposals for the future: Clues to be remembered
(a) Diastolic dysfunction by itself is not enough to establish HFpEF. (b) HFpEF is not simply a disease of aging nor does it occur only in females. (c) HFpEF has significant phenotypic and etiologic heterogeneity. (d) Due to its heterogeneity, a “one‐size‐fits‐all” strategy is unlikely to work in HFpEF. (e) HFpEF is associated with multiple comorbidities. (f) HFpEF is a multi‐system disease, with the heart being a major component but with others providing major contributions.72 (g) To date, two strategies that have been shown most definitively to be beneficial for improving clinically meaningful outcomes in HFpEF, ET and CR and both of these interventions have broad, pleotropic, systemic, and anti‐inflammatory effects, as well as favorable effects on multiple organ systems, including on arterial, cardiac, and skeletal muscle. (h) Clinical trials defining optimal management for comorbidities have by and large excluded HFpEF patients. In addition, much broader research into myocardial and non‐myocardial abnormalities at a tissue level in carefully phenotyped HFpEF subgroups is very much needed.
2.18. Case conclusion
Our 79‐year‐old woman should be placed on diuretics at the lowest effective dose for symptomatic relief. She should have a home scale and weigh herself daily. We should provide instruction for steps to take diuretics based on weight changes. Her BP was not well controlled and lisinopril to be adjusted to keep SBP < 130 mm of Hg. She should be advised regarding dietary compliance. She should be encouraged to exercise daily. She should be provided comprehensive HF disease management, including education, diet, exercise therapy, and close follow‐up. Ideally as described earlier, she is recommended to participate in a structured, supervised ET program; however, lack of CMS coverage can be a major barrier to formal cardiac rehab in older HFpEF patients.
3. CONCLUSIONS
HFpEF is the most common form of HF in the community, its prevalence is increasing, and prognosis has not improved or even worsened. It is nearly unique to older adults and is a true geriatric syndrome. Despite a moderate number of clinical trials, therapeutic successes to date have been few, and clinical management is largely empiric. An evolving paradigm suggests that, like other geriatric syndromes, HFpEF is complex and multifactorial, probably systemic, and clinically heterogeneous and has a multifactorial pathophysiology, underlying age‐related changes, frequent multiple chronic comorbidities and multiorgan involvement. Understanding the relationship between HFpEF and aging may help with understanding the biology of HFpEF more generally. In addition, machine learning techniques suggested by Shah et al, when applied to large phenotyped (both biological and clinical) datasets in combination with clinical outcomes may help to improve our understanding of how biological phenotypes integrate with clinical phenotypes. Efforts are underway to utilize these concepts to identify novel therapeutic targets, improve the design of future clinical trials, and to develop effective clinical management algorithms. Finally, the complexities of these patients demand an approach that is more holistic by addressing not only direct HF‐related conditions but also optimal management of geriatric syndromes, focusing on quality of life.
CONFLICT OF INTEREST
Dr. Kitzman declares the following relationships: Consultant for Abbvie, Bayer, Merck, Medtronic, AstraZeneca, Merck, Corvia Medical, and Actavis, research grant funding from Novartis, Bayer, and AstraZeneca, and stock ownership in Gilead Sciences and Relypsa. Dr. Upadhya has received research funding from Novarits and Corvia.
ACKNOWLEDGMENTS
Supported in part by the following research grant awards from the National Institutes of Health: R01AG045551 and R01AG18915. Also supported in part by the Kermit Glenn Phillips II Chair in Cardiovascular Medicine at Wake Forest School of Medicine (DWK); the Claude D. Pepper Older Americans Independence Center NIH Grants P30AG021332 (DWK) and P30AG028716 (AMP); the OAIC Pepper National Coordinating Center NIH Grant U24 AG05964; and the Wake Forest Clinical and Translational Science Award, NIH Grant UL1TR001420.
Upadhya B, Kitzman DW. Heart failure with preserved ejection fraction: New approaches to diagnosis and management. Clin Cardiol. 2020;43:145–155. 10.1002/clc.23321
Funding information national institue of health, Grant/Award Numbers: R01AG045551, R01AG18915
REFERENCES
- 1. Kitzman DW, Gardin JM, Gottdiener JS, et al. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS research group. Cardiovascular health study. Am J Cardiol. 2001;87:413‐419. [DOI] [PubMed] [Google Scholar]
- 2. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251‐259. [DOI] [PubMed] [Google Scholar]
- 3. Paulus W, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and Remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263‐271. [DOI] [PubMed] [Google Scholar]
- 4. Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. 2008;1:91‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE‐HF registry. J Am Coll Cardiol. 2007;50:768‐777. [DOI] [PubMed] [Google Scholar]
- 6. Cheng RK, Cox M, Neely ML, et al. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J. 2014;168:721‐730. [DOI] [PubMed] [Google Scholar]
- 7. Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype‐specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134:73‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anjan VY, Loftus TM, Burke MA, et al. Prevalence, clinical phenotype, and outcomes associated with Normal B‐type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:870‐876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the Management of Heart Failure. J Am Coll Cardiol. 2017;70:776‐803. [DOI] [PubMed] [Google Scholar]
- 10. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the european society of cardiology. Developed in collaboration with the heart failure association (HFA) of the ESC. Eur Heart J. 2012;33:1787‐1847. [DOI] [PubMed] [Google Scholar]
- 11. Rich MW, Kitzman DW. Heart failure in octogenarians: a fundamentally different disease. Am J Geriatr Cardiol. 2000;9:97‐104.11416547 [Google Scholar]
- 12. Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW. Sarcopenic obesity and the pathogenesis of exercise intolerance in heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2015;12:205‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murad K, Kitzman D. Frailty and multiple comorbidities in the elderly patient with heart failure: implications for management. Heart Fail Rev. 2011;17:581‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kitzman DW, Shah SJ. The HFpEF obesity phenotype: the elephant in the room. J Am Coll Cardiol. 2016;68:200‐203. [DOI] [PubMed] [Google Scholar]
- 16. Upadhya B, Pisani B, Kitzman DW. Evolution of a geriatric syndrome: pathophysiology and treatment of heart failure with preserved ejection fraction. J Am Geriatr Soc. 2017;65(11):2431‐2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Franssen C, Chen S, Unger A, et al. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail. 2015;4:312‐324. [DOI] [PubMed] [Google Scholar]
- 18. Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomised clinical trial. Jama. 2016;315:36‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kitzman D, Brubaker P, Morgan T, Stewart K, Little W. Exercise training in older patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2010;3:659‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kitzman DW, Brubaker PH, Herrington DM, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single‐blind trial. J Am Coll Cardiol. 2013;62:584‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Edelmann F, Gelbrich G, Dungen H, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the ex‐DHF (exercise training in diastolic heart failure) pilot study. J Am Coll Cardiol. 2011;58:1780‐1791. [DOI] [PubMed] [Google Scholar]
- 22. Smart NA, Haluska B, Jeffriess L, Leung D. Exercise training in heart failure with preserved systolic function: a randomized controlled trial of the effects on cardiac function and functional capacity. Congest Heart Fail. 2012;18:295‐301. [DOI] [PubMed] [Google Scholar]
- 23. Fu TC, Yang NI, Wang CH, et al. Aerobic interval training elicits different hemodynamic adaptations between heart failure patients with preserved and reduced ejection fraction. Am J Phys Med Rehabil. 2016;95:15‐27. [DOI] [PubMed] [Google Scholar]
- 24. Gary RA, Sueta CA, Dougherty M, et al. Home‐based exercise improves functional performance and quality of life in women with diastolic heart failure. Heart Lung. 2004;33:210‐218. [DOI] [PubMed] [Google Scholar]
- 25. Angadi SS, Mookadam F, Lee CD, Tucker WJ, Haykowsky MJ, Gaesser GA. High‐intensity interval training vs. moderate‐intensity continuous exercise training in heart failure with preserved ejection fraction: a pilot study. J Appl Physiol. 2014;95:15‐27. [DOI] [PubMed] [Google Scholar]
- 26. Alves AJ, Ribeiro F, Goldhammer E, et al. Exercise training improves diastolic function in heart failure patients. Med Sci Sports Exerc. 2012;44:776‐785. [DOI] [PubMed] [Google Scholar]
- 27. Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658‐666. [DOI] [PubMed] [Google Scholar]
- 28. Hasenfuss G, Hayward C, Burkhoff D, et al. A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP‐HF): a multicentre, open‐label, single‐arm, phase 1 trial. Lancet. 2016;387:1298‐1304. [DOI] [PubMed] [Google Scholar]
- 29. Feldman T, Mauri L, Kahwash R, et al. A Transcatheter InterAtrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP‐HF I): a phase 2, randomized, sham‐controlled trial. Circulation. 2017;137:364‐375. [DOI] [PubMed] [Google Scholar]
- 30. Yoshihisa A, Suzuki S, Yamaki T, et al. Impact of adaptive servo‐ventilation on cardiovascular function and prognosis in heart failure patients with preserved left ventricular ejection fraction and sleep‐disordered breathing. Eur J Heart Fail. 2013;15:543‐550. [DOI] [PubMed] [Google Scholar]
- 31. O'Connor CM, Whellan DJ, Fuizat M, et al. Cardiovascular outcomes with minute ventilation ‐ targeted adaptive servo‐ventilation therapy in heart failure. J Am Coll Cardiol. 2017;69:1577‐1587. [DOI] [PubMed] [Google Scholar]
- 32. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐preserved trial. Lancet. 2003;362:777‐781. [DOI] [PubMed] [Google Scholar]
- 33. Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP‐CHF) study. Eur Heart J. 2006;27:2338‐2345. [DOI] [PubMed] [Google Scholar]
- 34. Pitt B, Pfeffer M, Assmann S, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383‐1392. [DOI] [PubMed] [Google Scholar]
- 35. Edelmann F, Aldo‐DHF Investigators . Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the aldo‐dhf randomized controlled trial. JAMA. 2013;309:781‐791. [DOI] [PubMed] [Google Scholar]
- 36. Kosmala W, Rojek A, Przewlocka‐Kosmala M, Wright L, Mysiak A, Marwick TH. Effect of aldosterone antagonism on exercise tolerance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;68:1823‐1834. [DOI] [PubMed] [Google Scholar]
- 37. Borlaug BA, Melenovsky V, Koepp KE. Inhaled sodium nitrite improves rest and exercise hemodynamics in heart failure with preserved ejection fraction. Circ Res. 2016;119:880‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eggebeen J, Kim‐Shapiro DB, Haykowsky MJ, et al. One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart Faliure and preserved ejection fraction. JACC Heart Fail. 2015;4:428‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zamani P, Rawat D, Shiva‐Kumar P, et al. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase‐5 inhibition in a 1‐year study. Circulation. 2011;124:164‐174. [DOI] [PubMed] [Google Scholar]
- 41. Guazzi M, Bandera F, Forfia P. Sildenafil and exercise capacity in heart failure. Jama. 2013;310:432. [DOI] [PubMed] [Google Scholar]
- 42. Haass M, Kitzman DW, Anand IS, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction/clinical perspective. Circ Heart Fail. 2011;4:324‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Solomon S, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double‐blind randomised controlled trial. The Lancet. 2012;380:1387‐1395. [DOI] [PubMed] [Google Scholar]
- 44. Solomon SD, McMurray JJV, Anand IS, et al. AngiotensinΓÇôNeprilysin inhibition in heart failure with preserved ejection fraction. New Eng J Med. 2019;381:1609‐1620. [DOI] [PubMed] [Google Scholar]
- 45. Borlaug BA, Olson TP, Lam CSP, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Borlaug BA. Exercise haemodynamics and outcome in patients with dyspnoea. Eur Heart J. 2014;35:3085‐3087. [DOI] [PubMed] [Google Scholar]
- 47. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2002;288:2981‐2997. [DOI] [PubMed] [Google Scholar]
- 48. Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kostis J, Davis BR, Cutler JA, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA. 1997;278:212‐216. [PubMed] [Google Scholar]
- 50. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged >/=75 years: a randomized clinical trial. JAMA. 2016;315:2673‐2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Upadhya B, Rocco M, Lewis CE, et al. Effect of intensive blood pressure treatment on heart failure events in the systolic blood pressure reduction intervention trial. Circ Heart Fail. 2017;10:e003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Haykowsky M, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Normandin E, Houston DK, Nicklas BJ. Caloric restriction for treatment of geriatric obesity: do the benefits outweigh the risks? Curr Opin Cardiol. 2015;4:143‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sharma K, Kass D. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res. 2014;115:79‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fitchett D, Inzucchi SE, Cannon CP, et al. Empagliflozin reduced mortality and hospitalization for heart failure across the Spectrum of cardiovascular risk in the EMPA‐REG OUTCOME trial. Circulation. 2019;139:1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Furtado RHM, Bonaca MP, Raz I, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes and prior myocardial infarction: a sub‐analysis from DECLARE TIMI‐58 trial. Circulation. 2019;139:2516–2527. [DOI] [PubMed] [Google Scholar]
- 57. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart‐failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013;62:e147‐e239. [DOI] [PubMed] [Google Scholar]
- 58. Gandhi SK, Powers JC, Nomeir AM, et al. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med. 2001;344:17‐22. [DOI] [PubMed] [Google Scholar]
- 59. Kramer K, Kirkman P, Kitzman DW, Little WC. Flash pulmonary edema: association with hypertension, reocurrence despite coronary revascularization. Am Heart J. 2000;140:451‐455. [DOI] [PubMed] [Google Scholar]
- 60. Zakeri R, Borlaug BA, McNulty SE, et al. Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail. 2014;7:123‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lam CS, Rienstra M, Tay WT, et al. Atrial fibrillation in heart failure with preserved ejection fraction: association with exercise capacity, left ventricular filling pressures, natriuretic peptides, and left atrial volume. JACC Heart Fail. 2016;5:92‐98. [DOI] [PubMed] [Google Scholar]
- 62. Machino‐Ohtsuka T, Seo Y, Ishizu T, et al. Efficacy, safety, and outcomes of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;62:1857‐1865. [DOI] [PubMed] [Google Scholar]
- 63. Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Anemia and heart failure: a community study. Am J Med. 2008;121:726‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kritchevsky SB, Beavers KM, Miller ME, et al. Intentional weight loss and all‐cause mortality: a meta‐analysis of randomized clinical trials. PLoS One. 2015;10:e0121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pandey A, Parashar A, Kumbhani DJ, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta‐analysis of randomized control trials. Circ Heart Fail. 2015;8:33‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Reeves GR, Whellan DJ, O'Connor CM, et al. A novel rehabilitation intervention for older patients with acute decompensated heart failure: the REHAB‐HF pilot study. JACC Heart Fail. 2016;5:359‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Adamson PB, Abraham WT, Bourge RC, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:935‐944. [DOI] [PubMed] [Google Scholar]
- 69. Kaye DM, Hasenfub G, Neuzil P, et al. One‐year outcomes after Transcatheter insertion of an interatrial shunt device for the Management of Heart Failure with Preserved Ejection Fraction. Circ Heart Fail. 2016;9:e003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization‐associated disability: "she was probably able to ambulate, but I'm not sure". Jama. 2011;306:1782‐1793. [DOI] [PubMed] [Google Scholar]
- 71. Krumholz HM. Post‐hospital syndrome: an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shah AM, Solomon SD. Phenotypic and pathophysiological heterogeneity in heart failure with preserved ejection fraction. Eur Heart J. 2012;33:1716‐1717. [DOI] [PubMed] [Google Scholar]
- 73. Upadhya B, Haykowsky MJ, Kitzman DW. Therapy for heart failure with preserved ejection fraction: current status, unique challenges, and future directions. Heart Fail Rev. 2018;23(5):609‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lewis GA, Schelbert EB, Williams SG, et al. Biological phenotypes of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2017;70:2186‐2200. [DOI] [PubMed] [Google Scholar]


