Abstract
The knowledge about a potential in vivo uptake and subsequent toxicological effects of aluminum (Al), especially in the nanoparticulate form, is still limited. This paper focuses on a three day oral gavage study with three different Al species in Sprague Dawley rats. The Al amount was investigated in major organs in order to determine the oral bioavailability and distribution. Al-containing nanoparticles (NMs composed of Al0 and aluminum oxide (Al2O3)) were administered at three different concentrations and soluble aluminum chloride (AlCl3·6H2O) was used as a reference control at one concentration. A microwave assisted acid digestion approach followed by inductively coupled plasma mass spectrometry (ICP-MS) analysis was developed to analyse the Al burden of individual organs. Special attention was paid on how the sample matrix affected the calibration procedure. After 3 days exposure, AlCl3·6H2O treated animals showed high Al levels in liver and intestine, while upon treatment with Al0 NMs significant amounts of Al were detected only in the latter. In contrast, following Al2O3 NMs treatment, Al was detected in all investigated organs with particular high concentrations in the spleen. A rapid absorption and systemic distribution of all three Al forms tested were found after 3-day oral exposure. The identified differences between Al0 and Al2O3 NMs point out that both, particle shape and surface composition could be key factors for Al biodistribution and accumulation.
Subject terms: Metals, Nanoparticles, Analytical chemistry
Introduction
Aluminum is one of the most abundant elements on earth and is widely used in many different consumer product applications due to its unique characteristics. As an ubiquitous element, Al occurs in natural sources, e.g. food and drinking water, as well as in food additives, packaging and kitchenware1,2. Besides industrial, also agricultural, medical and consumer product uses are known for synthetic alumina, mixed Al silicate and Al oxide NMs. Especially composite materials containing Al show favourable properties when being used as packaging materials for the protection of food against humidity and oxidation and are more and more common3–6. Moreover, various Al salts are used in food as additives, e.g. as stabilizers, pH regulators and anti-caking agents7,8. As a consequence of modern life style Al-containing materials and substances are of high abundance in the human environment. In our study we could show de novo synthesis of Al-containing nanomaterials from ions after induction of a pH shift9. Based on this and because of the detection and quantification of Al in biological media is difficult, one should assume at least partial particle concentration in the body after oral uptake of Al. This raises questions about the potential hazard of particulate Al species.
Adverse effects of Al have been repeatedly discussed in the past. Neurodegenerative diseases such as Alzheimer’s10,11 as well as certain bone diseases12–14 and dialysis dementia15,16 have been attributed to Al exposure. Although inhalation and dermal uptake of Al may occur, ingestion is mainly responsible for Al organ burden with regard to the public population17. Consequently, a tolerable weekly intake (TWI) of 1 mg/kg body weight was implemented by the European Food Safety Authority (EFSA), while a provisional tolerable weekly intake (PTWI) of 2 mg/kg body weight has been set by the Joint FAO/WHO Expert Committee on Food Additives (JECFA). However, both values do not distinguish between ionic and nanoparticulate Al species18,19, despite the increasing use of Al-containing NMs20. Al NMs may change the impact on human health due to their unique physico-chemical properties, e.g. their size, high specific surface area, and high reactivity21–24. Hence, reliable information on oral uptake and the resulting organ burden of Al-containing NMs is needed. In a recent in vitro study, the influence of an artificial digestion process on two different Al NMs and one salt species was examined9. This process led to the de novo formation of nanoparticles in the artificial intestinal juice for the two NM species and also for the Al salt9. In vivo studies in rats showed that ionic Al is systemically distributed throughout the organs after oral uptake. High concentrations were found in kidneys, liver, spleen, bones and brain. Al is also highly persistent with half-life times up to 150 days, for example, in the brain25–28. On the contrary, little is known about the distribution of particulate Al species, and possible differences between individual Al species.
In this study, we compared Al0 and Al2O3 NMs with an ionic Al species. Oral uptake was investigated by measuring Al concentrations in duodenum and colon samples. Systemic exposure was estimated through analyses of blood, liver, kidney and spleen samples. We carefully examined matrix suppression effects since they may affect the Al levels detected by ICP-MS29. For this purpose, a calibration for each organ was compared with the standard aqueous calibration method. Our approach highlights the advantages of matrix-matching in ICP-MS based in vivo studies. A reduction of the number of animals needed for a robust analysis combined with low limits of detection were achieved, while at the same time, matrix effects were taken into account.
Results
Characterization of Al and Al2O3 NMs
Both, Al0 and Al2O3 NMs have been characterized elsewhere (Table 1)30. In brief, the core particle diameter was confirmed to be between 2–50 nm for the nearly spherical Al0 NMs by transmission electron microscopy (TEM). Al2O3 NMs are more rod-shaped with a width mostly around 10 nm and a length that varies between 20–50 nm as determined by TEM. The size distribution showed a broader variance for Al0 than for Al2O3 NMs. Dynamic light scattering (DLS) determined the hydrodynamic diameter in dispersion of 0.05% BSA. For Al0 NMs, a hydrodynamic diameter of 250 nm was detected, while Al2O3 NMs showed a smaller diameter of 180 nm. X-ray diffraction (XRD) and TEM in an element resolved mode demonstrated the difference of the Al0 and Al2O3 NMs composition and especially their surface. While Al0 NMs had a core-shell structure with Al core and a 2–5 nm oxygen shell, the Al2O3 NMs were homogeneously oxidized. An overview about the main characterization data is given in Table 1. An artificial digestion approach showed a decreased solubility coupled to de novo formation of Al-containing particles in the nanoscale range occurring in the intestinal fluid compared to the stomach9.
Table 1.
Characterization data for Al0 and Al2O3 NM. Modified from30.
| Methods | Al0 NM | Al2O3 NM |
|---|---|---|
| TEM | Primary particle size and shape: 2–50 nm, nearly spherical | Primary particle size and shape: 10 × 20–50 nm, grain-like shape |
| EELS-TEM | Core-shell structure, thin (2–5 nm) oxide layer | Fully oxidized particle |
| XRD | Aluminum surface; partially oxidized | Fully oxidized surface |
| SAXS | Particle radius: >10 nm | Primary particle radius: 7.1 nm Aggregates’ radius: >10 nm |
| SP-ICP-MS | Primary particle size: 54–80 nm | Primary particle size: 50–80 nm |
| ICP-MS | Ion release: 0.2–0.5% (in 0.05% BSA) | Ion release: 0.2–0.4% (in 0.05% BSA) |
| ToF-SIMS | Particle-amino acid agglomerates | Particle-amino acid agglomerates; polyoxo-aluminates |
| IBM, atom number % | Impurities: P (1%); biocorona: S (5%), protein; Ca3(PO4)2 coating: P (3%) | Impurities: S (0.2%); biocorona: S (1%), Ca3(PO4)2 coating: P (1%) |
Matrix-matching
After an in-house validation of the analytical method for digestion, quantification and evaluation of the Al content in the investigated organs, an ANOVA and PCA analysis based on the different treatment groups were performed (see Methods section for details). This step was conducted to exclude the possibility that animals did not correspond to the variation in the overall population. The results of ANOVA and PCA (see Supplementary Table S1 and Supplementary Fig. S1) showed no significant difference among the animals of the different treatment groups.
In order to minimize measurement uncertainties and standard deviation for the quantification, a matrix calibration was carried out for each organ investigated. The calibration points quantified in the different matrices were highly correlated to the data points calculated from the regression curve of each organ (Fig. 1 and Table 2). For each tested organ a calibration linearity between 0.9960 and 0.9997 was achieved.
Figure 1.
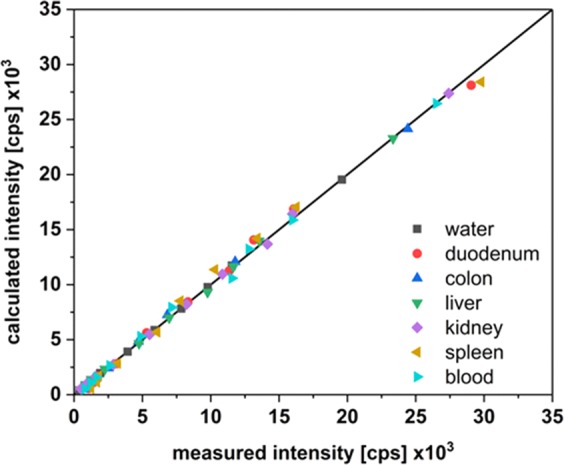
Regression analysis of matrix calibration data. The measured intensities were plotted versus the calculated intensities which were obtained from the regression functions.
Table 2.
Analytical figure of merit. The different matrices (water, duodenum, colon, liver, kidney, spleen and blood) were evaluated based on the quality parameters of the calibration (linearity, sensitivity, limit of detection (LOD), limit of quantification (LOQ), recovery). F-Test was carried out to compare the variance between water and matrix calibration sensitivities.
| Matrix | Linearity (r²) | Sensitivity [cps (ng g−1)−1] | LOD [µg g−1] organ | LOQ [µg g−1] organ | F-Test* | Recovery [%] |
|---|---|---|---|---|---|---|
| water | 0.9994 | 390.68 | 0.035 | 0.042 | 1 | 100 |
| duodenum | 0.9979 | 526.35 | 0.070 | 0.115 | 0.13 | 135 |
| colon | 0.9995 | 483.10 | 0.466 | 1.394 | 0.56 | 92 |
| liver | 0.9996 | 465.66 | 0.024 | 0.049 | 0.44 | 96 |
| kidney | 0.9997 | 568.27 | 0.032 | 0.082 | 0.16 | 122 |
| spleen | 0.9959 | 547.35 | 0.039 | 0.082 | 0.13 | 96 |
| blood | 0.9983 | 528.93 | 0.008 | 0.015 | 0.20 | 97 |
*F-Test: water/matrix; F ≥ 0.99 for using water calibration only.
The F-Test compares the variances of both water and matrix calibration sensitivities. This revealed significant differences between the calibration in water and in the several matrices. It is therefore not possible to use the water calibration without correction factors calculated from the matrix calibration. In order to avoid the preparation of matrix calibration for each day of measurement a daily response factor (DRF) was used. The DRF accounts for day to day ICP-MS sensitivity variations as well as for matrix effects. After the introduction of the DRF, all sensitivities of the matrix calibrations could be correlated to the water calibration of each day to calculate the recovery rate. The intensities for Al in water are considerably higher than those of each matrix used. Thus, the values for recovery depend on the matrix (see Supplementary Fig. S2). These results pointed out the necessity for a matrix-matched calibration, which led to reliable results. The sample intensities were corrected by subtracting the value of averaged microwave blanks (see Eq. (1) in the methods section). In order to avoid performing a matrix calibration curve each day, a matrix calibration was done on one day and the resulting intensities of the samples were corrected using a DRF (see Eq. (2) in the methods section). We defined the sensitivity of the ICP-MS device as the slope of the calibration curve (Table 2). Therefore, the sensitivity obtained using a water calibration on the day when the samples were analysed was divided by the sensitivity of the water calibration from the day of the matrix calibration (see Eq. (3) in the methods section). Additionally, the recovery of the microwave-assisted digestion was calculated. Considering a factor taking into account these corrections, it was possible to calculate the concentration of Al burden per gram of tissue in a highly robust manner (see Eqs. (4–6) in the methods section). All obtained values were above the LOQ (Fig. 2). Assuming that the data are not equally distributed, through median and average comparison (Table 3), the Wilcoxon sign test was used for statistical analysis of non-normal distributed dependent groups. For each dose group, the distribution of Al concentrations in the organs was examined. The significance levels were corrected according to Holm-Šidàk and an unspecific pairwise comparison was carried out31. For almost all comparisons, a significant difference between dose groups and the Al species as well as to the control group at the significance level of 0.05 (one-side) could be observed.
Figure 2.
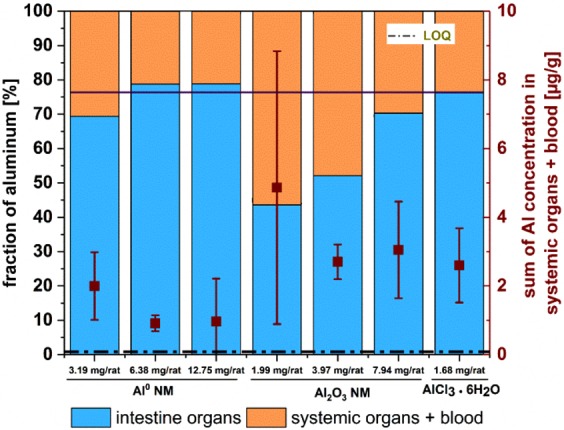
Overall fraction of Al in investigated organs. The intestine (duodenum and colon; blue) and the systemic organs (liver, kidney, spleen) and blood (orange) values [%] are given. The black bar indicates the reference value found for ionic AlCl3·6H2O fraction [%]. Brown squares indicate the median of the sum of Al concentration in systemic organs and blood [µg/g] and the vertical bars indicate the interquartile ranges.
Table 3.
Comparison of median and mean for determination of skewness for doses per kg body weight. “All organs” refers to all examined organs.
| Dose | ∑ all organs | ∑ Liver, Kidney, Spleen | ||||
|---|---|---|---|---|---|---|
| Median µg/g | Mean µg/g | Skewness | Median µg/g | Mean µg/g | Skewness | |
| Al0 6.25 mg/kg | 8.16 | 10.21 | right skew | 2.46 | 3.29 | right skew |
| Al0 12.5 mg/kg | 3.85 | 4.88 | right skew | 0.72 | 1.30 | right skew |
| Al0 25 mg/kg | 5.79 | 9.56 | right skew | 1.22 | 2.54 | right skew |
| Al2O3 6.25 mg/kg | 11.16 | 25.83 | right skew | 5.01 | 6.78 | right skew |
| Al2O3 12.5 mg/kg | 10.08 | 16.09 | right skew | 4.81 | 5.84 | right skew |
| Al2O3 25 mg/kg | 15.37 | 19.80 | right skew | 4.58 | 5.84 | right skew |
| AlCl3·6H2O 25 mg/kg | 9.38 | 14.23 | right skew | 2.33 | 6.85 | right skew |
Uptake evaluation
In this study, AlCl3·6H2O is regarded as a reference substance to determine the uptake of Al ions. According to the mass balance in all investigated organs, ca. 25% of the ionic AlCl3·6H2O did pass the intestinal barrier (Fig. 2). A similar behaviour was observed for the Al0 NMs at all three concentrations tested although the total Al content administered with Al0 NMs was 1.9–7.6-fold higher when compared to AlCl3·6H2O. If the overall uptake of Al was comparable for the Al0 NM and the salt species, in contrast, a higher overall Al uptake could be observed in animal tissues treated with Al2O3 NMs (Fig. 2). It is worth noting that the accumulation of Al in the intestine (washed duodenum and colon) and in systemic organs differed significantly. In contrast to Al0 treatment, a concentration-dependent increase of intestinal Al was detected after Al2O3 administration.
Indeed, in the intestine, a dose-dependent linear increase of the Al fraction was recorded. Figure 2 depicts the fractions of Al found in intestine and systemic organs investigated in this study. The sum of both Al fractions was normalized to 100%.
After administration of Al0 NMs, the lowest dose of 3.19 mg/rat resulted in the highest concentrations in intestine, both duodenum and colon with concentrations in the range of 1–2 µg/g and of 2–3.5 µg/g respectively (Fig. 3A).
Figure 3.
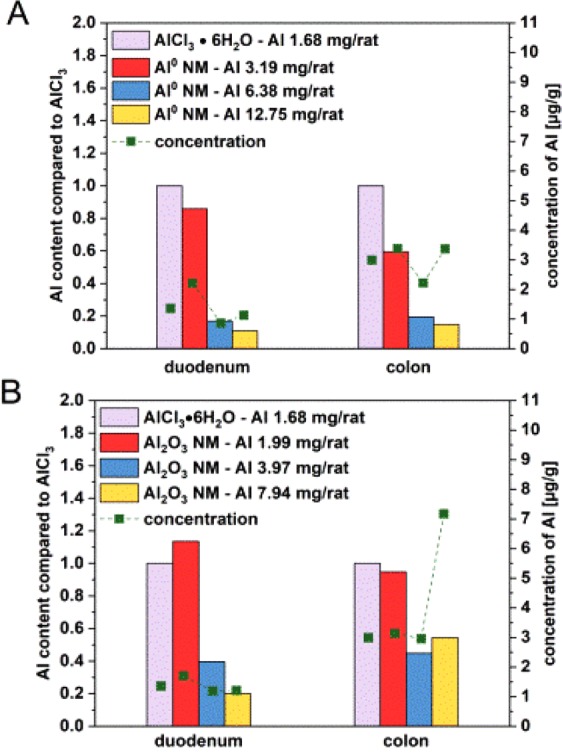
Al concentrations in duodenum and colon after 3 day oral treatment. The organs were collected 3 hours after the last administration of Al0 NMs (A) and Al2O3 NMs (B). Each bar was normalized on the concentration obtained with AlCl3·6H2O at 1.7 mg/rat corrected by the corresponding Al content of AlCl3·6H2O and the applied NM. Green squares display the Al concentration which was found in the organ [µg/g].
The Al uptake was calculated based on the mass fraction of Al in the compound. The Al concentration was then median normalized to the values found in samples with a 1.68 mg/rat AlCl3·6H2O exposure (see Eq. (7) in the methods section, for further details see Supplementary Tables S2 and S3). An overview about all investigated organs with the Al concentration in µg/g for all eight treatment groups is given in Supplementary Fig. S3. The application of Al0 NM at the lowest dose resulted in the highest Al concentration in the duodenum. Normalized to the Al concentration found in the same organs following exposure to AlCl3·6H2O, only 85% were detected. A less prominent concentration was determined relatively to the AlCl3·6H2O for the mid (about 17%) and the high dose (about 11%). A comparable effect was observed in the colon, where the relative amount was about 60% for the lowest dose, 20% for the mid and about 15% for the highest dose (Fig. 3A). Comparing Al0 NMs and Al2O3 NMs, more Al could be detected in the duodenum after Al2O3 exposure. However, Al2O3 NMs contain only about 62% of the Al content than Al0 NMs. While no dose dependency was observed in the duodenum, the Al concentration increased dose dependently in the colon. A maximum concentration of 7.7 µg/g organ was observed at the highest dose level (Fig. 3B). Normalized to an ionic AlCl3·6H2O dose of 1.68 mg Al/rat, the treatment with Al2O3 NMs resulted in an Al content of 20–113% in the duodenum and of 45–95% in the colon (Fig. 3B).
After crossing the intestinal barrier, the different Al species were distributed to systemic organs via blood. At the lowest dose of Al0 NM, Al was detected to a large extent in liver and blood (Fig. 4A), and in the two intestinal sections (Fig. 3A). Compared to AlCl3·6H2O, a lower amount of Al was detected in liver (0.07–0.33-fold of the amount detected for AlCl3·6H2O), while a 8.98–41.45-fold increased uptake was measured in blood for mid and low doses of Al0 NMs (Fig. 4A).
Figure 4.
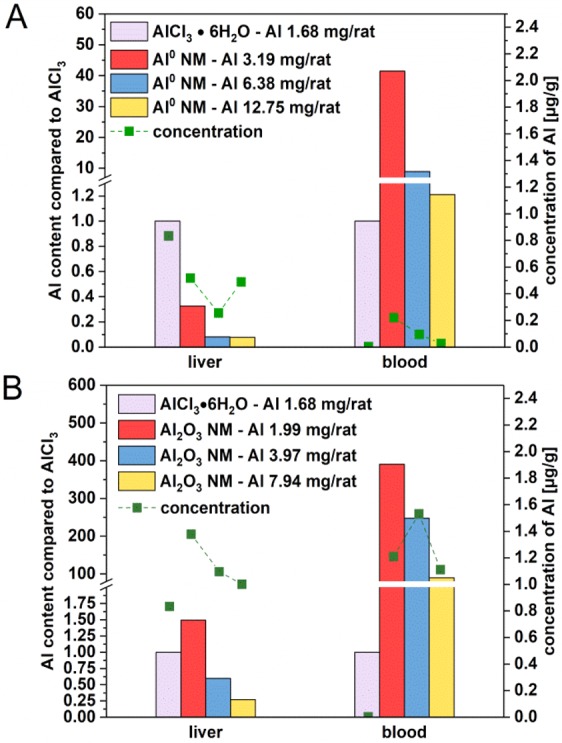
Accumulation of Al in liver and blood after 3 day oral treatment. All organs were collected 3 hours after the last administration of Al0 NMs (A) and Al2O3 NMs (B). Each bar was normalized on the concentration of 1.7 mg Al/rat. Values were corrected by the corresponding Al content of AlCl3·6H2O and the applied NM. Green squares display the Al concentration which was found in the organ in [µg/g].
A similar concentration-dependent behaviour (in respect to the amount given) was observed for Al2O3 NMs: the lowest dose showed the highest difference in comparison to the median normalized and fraction-corrected concentration of AlCl3·6H2O. However, the concentration dependency showed a linear decrease (Fig. 4B). Compared to the corresponding values in AlCl3·6H2O treated animals, the Al content varied between 1.49-fold for the lowest and 0.27-fold for the highest dose (liver). In blood, an almost 390.92-fold increase for the lowest dose and a 89.92-fold increase for the highest dose were observed in comparison to animals treated with AlCl3·6H2O (Fig. 4B). Al concentrations in liver and blood were found between 1.07 and 1.64 µg/g for Al2O3 NMs treated rats, compared to a range between 0.03 and 0.52 µg/g for Al0 NM treated rats. When comparing Al0 and Al2O3 NMs, the latter one is found up to ten times higher in the blood (Fig. 4).
Since liver is the first organ after intestinal uptake, a significant accumulation of Al was expected. Therefore, we normalized the liver uptake as 100% to show the different uptake and accumulation patterns of Al0 and Al2O3 NMs at various concentrations. With the low and medium Al0 NM doses, a relatively high concentration of Al was found in the kidney with concentrations of 0.65 and 0.23 µg/g respectively. However, with Al0 NMs, the maximum Al concentration (1.02 µg/g) was found in the spleen with the lowest treatment dose (Fig. 5A). Al2O3 NMs accumulated three times higher in the liver than Al0 NMs. Following to administration of Al2O3 NMs, the Al levels detected in the liver (1.07 to 1.48 µg/g) are the highest among the other systemic organs (Fig. 5B).
Figure 5.
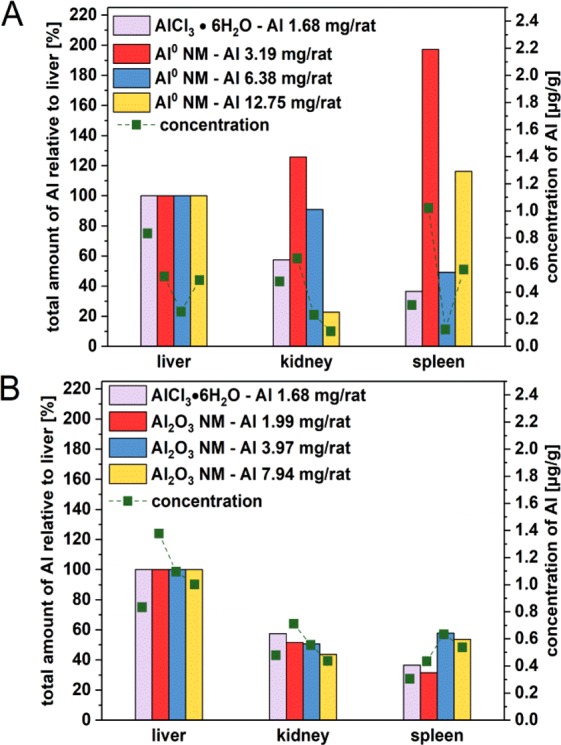
Comparison of the Al content of liver, kidney and spleen after 3 day oral treatment. The organs were collected 3 hours after the last administration of Al0 NMs (A) and Al2O3 NMs (B). Each bar was normalized on the uptake of the liver in the corresponding treatment group. Green squares display the Al concentration which was found in the respective organ in [µg/g].
Discussion
In the current study we compared the oral bioavailability and distribution of ionic and nanoparticulate Al0 and Al2O3 in rats following a 3-day oral treatment. ICP-MS was used to quantify the Al concentration in duodenum, colon, blood, liver, kidney and spleen to evaluate the biodistribution patterns of the three Al species. Some challenges were faced for the quantification of Al amounts. First, the ubiquitous presence of Al in the tissues hinders the detection of Al that originates from the treatment. The background levels of Al were shown to vary from organ to organ (see Supplementary Fig. S3). For this purpose, a robust calibration methodology was developed where matrix effects were taken into account. Our matrix calibration data clearly indicated the need for matrix correction (see Supplementary Fig. S2). In fact, the calibration in water showed a higher sensitivity compared to all calibrations in matrices. The matrix affects the analyte sensitivity due to signal suppression or enhancement by dissolved salts. Another possible interference especially in high matrix load samples is the deposition of solid matrix on the ICP-MS apertures32. After setting up the matrix calibration method, we were able to detect very low concentrations of Al in the tissue samples above the background levels (see Table 2 and Supplementary Fig. S3). However, as daily matrix calibration requires an analytical effort and the recurrent use of animal tissues, the DRF, an established approach in chromatography, was adapted to this study. This approach not only compensates for daily measurement fluctuations but also adjusts the sensitivity of the calibration in water by the calibration with matrix in order to take matrix effects into account. The DRF methodology contributes to achieve the high sensitivity required for a reliable detection of low Al levels by ICP-MS. Consequently, we recommend always testing the influence of sample matrix on the analyte detection. If, as demonstrated in this study, analyte suppression is observed, matrix calibration in combination with the DRF approach is advised for sample analysis.
Following oral administration by gavage with Al0 NM, Al2O3 NM and ionic aluminum, Al levels were detected in all investigated organs and specific differences were highlighted between Al0 and Al2O3 NMs compared to ionic AlCl3·6H2O. These findings are in contrast to a previous inhalation study where, despite high lung burdens of AlOOH NMs, no significant systemic organ burden (brain, liver, and kidney) could be detected33. This highlights the importance of the oral uptake route for the overall Al burden in humans.
After gavage the distribution of Al2O3 NM between intestine and systemic organs showed significant differences (Fig. 2), while distribution of Al0 NM was rather comparable to the ionic AlCl3·6H2O. Therefore, NMs with the same metallic component may show different accumulation behaviour in living organisms. We observed a dose-dependent decrease of Al levels in systemic organs with increasing concentrations of Al2O3 NM suggesting an impairment of the intestinal barrier function with increasing dose and subsequent accumulation in the intestine. An explanation could be that the agglomeration state of Al2O3 NMs was higher with increasing concentrations. Above a certain size, agglomerates may be less prone to cross the gut-blood barrier. Therefore not only the composition of the NM, but also its agglomeration state may be regarded as a key parameter for oral uptake assessment34,35. The results in our study showed that the rod-shaped Al2O3 NMs were present in higher concentrations compared to the spherical Al0 NMs in the organs investigated. These findings were in good accordance with the results of uptake investigations of polystyrene NMs on Caco-2 cells36. However, this assumption is not always confirmed37. Al0 NMs might be more easily taken up but also may be excreted faster, whereas the rod shaped structure of Al2O3 NMs prevents a fast elimination out of the animal. A similar difference between spherical and rod-shaped NMs was also observed for SiO2 NM in rats, of which spherical particles were taken up to a higher amount after 2 and 24 h. After 7 days, the rod-shaped particles showed the highest concentrations in the organs38. Furthermore, the surface composition might also influence the biokinetic behaviour of the NMs39. For Al0 NM, a core-shell structure with an Al core and a thin Al oxide layer (2–5 nm) due to passivation with oxygen in the manufacturing process has been confirmed by XRD and element-specific TEM. Al0 NMs were shown to contain 85% Al (Table 1). In contrast, Al2O3 is fully oxidized, with a rather homogenous distribution of Al and oxygen on its surface. Recently we showed that the protein corona formed upon contact with cell culture media is less complex for Al0 NM compared to Al2O3 NM40. The different surface properties of NMs facilitate interactions with the surrounding environment, thus the NM, thus influencing bioavailability and tissue retention of the NM30. Our results highlight that physico-chemical characteristics including composition, shape, aggregation state and surface composition could affect the kinetics of NMs. As shown in an artificial digestion experiment in vitro, AlCl3·6H2O formed efficiently Al-containing salts and particles in the nano range in the artificial small intestine environment. These newly formed nanoparticles which originated from AlCl3·6H2O, showed a low solubility comparable to Al0 NM9. This observation may explain the almost identical uptake behaviour of Al0 NM and AlCl3·6H2O in our study. In vitro results from intestinal culture models incubated with the different Al species also showed low uptake rates and no major differences between Al0 and Al2O3 NMs and AlCl3·6H2O accumulation within the cells40. In our study, although the distribution pattern was similar between the two NMs, but the Al concentrations differed significantly. We clearly observed that the Al concentrations found in the organs are not correlated to the total amount of Al given by gavage. In fact, although with the lowest amount of Al in the administered dose, AlCl3·6H2O was clearly taken up to a higher degree in comparison to NMs (1.9–7.6-fold higher depending on the tissue). The increased uptake of AlCl3·6H2O might be due to a higher permeability of the intestinal barrier for ions versus particles. However, this was not observed during in vitro studies40. Nevertheless, compared to the larger agglomerates originating from Al0 and Al2O3 NMs, ions are expected to cross the intestinal barrier more easily. It is worth to mention that the application of Al2O3 NM with a lower mass fraction of Al than Al0 NM, led to a higher accumulation of Al in the organs.
Al0 and Al2O3 NM accumulation between the small and the large intestine differed significantly from each other with a higher concentration found in the colon. The difference of pH between duodenum and colon may impact the agglomeration behaviour of the particles. Moreover, the longer retention time in the colon could also favour their uptake leading to an increased Al amount in the colon9.
After passage of the gut –blood barrier, Al was identified in the blood where it possibly is distributed to the systemic organs through the blood flow. Previous work suggested that ionic Al is able to associate with transferrin and other plasma proteins as well as with low molecular weight fractions (citrate, hydroxide)1. In our study the blood concentration of AlCl3·6H2O was relatively low compared to the 1.2-fold higher burden found for the highest dose of Al0 NMs. The measured value for Al2O3 NMs was even 100 times higher than AlCl3·6H2O (Fig. 4B). Based on the low solubility of Al0 and Al2O3 NMs determined earlier9, these results suggests that particulate Al was also transported by blood but probably was better retained in the blood by the formation of a specific protein corona. Especially low molecular weight fractions can circulate between tissue and blood. As AlCl3·6H2O could potentially interact with low molecular weight proteins, it might reach liver and other organs with a higher efficiency (Fig. 5A, B). In contrast, as shown by protein corona data40, Al0 NMs and especially Al2O3 NMs might preferentially interact with higher molecular weight plasma proteins, then limiting their access into tissues and possibly enhancing Al half-life in blood. A few studies investigated the bioavailability and biodistribution of Al-containing NMs after oral treatment in mice. Our results after only three days exposure to Al2O3 NMs (Fig. 5B) are in good accordance with the biodistribution pattern in liver, kidney and spleen reported after 13 weeks41. Another animal study suggested that Al2O3 NMs was systemically available since liver and kidney impairments were observed after oral uptake42.
In conclusion, our in vivo study showed a significant uptake of Al from both, Al0 and Al2O3 NMs, following a 3-day oral gavage treatment. Rapid absorption and systemic distribution of Al for all three different forms tested is concluded. Nevertheless, some differences between Al0 and Al2O3 NMs were observed assuming that agglomerate shape and surface composition may play an important role in particle accumulation. In contrast to in vitro results from the literature Al2O3 NM accumulates to a higher extent in comparison to Al0 NM in both, intestine and systemic organs. Our findings identify challenges in the extrapolation of NM accumulation, even if the used materials possess a similar chemical composition. An interesting difference to ionic Al was the long retention time of Al levels in blood with Al2O3 NMs. The low dose study design challenged conventional ICP-MS approaches. In order to increase the sensitivity and robustness of the technique we conducted a matrix calibration in combination with a DRF, which showed superior performance in terms of sensitivity compared to the standard water calibration method. The applied method also eliminated the influence of day to day variation on the calibration. This method enabled the reliable quantification of all Al organ burdens even in the low µg/g range. Our study successfully showed that a respective matrix matched calibration can easily improve the data quality for the evaluation of low dose studies. The approach of statistical evaluation presented here utilizes a DRF to enable robust data analysis in order to obtain resilient data for risk assessment.
Methods
Materials
Al0 NMs (mean diameter 18 nm (TEM), 99.9%) and Al2O3 NMs (mean diameter 20 nm (TEM), 99+%) were purchased from Ionic Liquids Technologies GmbH (IoLiTec), Heilbronn, Germany. AlCl3·6H2O and bovine serum albumin (BSA) were bought from Sigma Aldrich. All other chemicals used in this study were reagent grade.
Animals and experimental design
All experiments were in accordance with the ethical recommendations of the Directive 2010/63/EU of the European Parliament and were validated by the Anses ethical committee (COMETH). This study was performed to investigate the biodistribution of Al nanomaterials and served as a preliminary dose-finding study for genotoxicity testing, where the doses administered should represent the maximum tolerated doses according to regulatory guidelines. In fact, according to the OECD guidelines 474 and 489 for the micronucleus and comet assays respectively, it is clearly required that the highest dose should be the maximum tolerated dose for a few repeated administrations and that 2 other lower doses must additionally be tested. The experimental design was chosen to fulfil all the above-mentioned criteria.
Male Sprague-Dawley rats (8–10 weeks old, around 200 g) were purchased from Janvier (Saint Berthevin, France). Rats were housed in conventional cages and had free access to water and food. Temperature and humidity were constant with a light/dark cycle of 12 h/12 h. The animals were treated after at least 5 days of acclimatization. Animals (5 per group) were randomly assigned to one of eight groups (including vehicle controls).
Animals were treated by gavage (9.76 ml/kg) at 0 h, 24 h, and 45 h and sacrificed 3 h after the last administration. Al0 and Al2O3 NMs were given at 6, 12.5 and 25 mg/kg body weight (bw), and AlCl3·6H2O at 25 mg/kg bw in ultra-pure water (UPW) with 0.05% BSA according to the NanoGenoTox protocol and as previously described30. UPW with 0.05% BSA was used as negative control.
Tissue collection and sample preparation
Animals were anesthetized with an intraperitoneal sublethal dose of pentobarbital (60 mg/kg). The following samples were collected: blood, liver, spleen, kidney, duodenum, and colon. The intestinal sections were washed and thoroughly cleaned. A portion of each organ (always a similar part irrespective of treatment) was weighed and rapidly frozen in liquid nitrogen. For the blood samples always 1 ml blood were taken and rapidly frozen in liquid nitrogen. The samples were kept at −80 °C until further processing.
For matrix calibration, a sample of each organ from the controls was spiked with 25 µg/ml of ionic Al. All samples were then digested using a microwave-assisted acid digestion with 69% HNO3 and 30% H2O2 for 30 min at 200°C and 160 bars. The samples were then diluted to a concentration of 500 ng/g. This solution served as stock for the calibration concentrations (1–50 ng/g). All samples were analysed in triplicate.
Data analysis
The PCA and the one-way ANOVA was carried out in Origin 9.1 (OriginLab Corporation, USA). Microsoft Excel (2016) was used for all other calculations including the F-Test, used for correlation of matrix specific calibrations. The organ weights in combination with the treatment groups were used as input vectors for PCA and ANOVA.
ICP-MS measurements
Measurements were performed at a quadrupole ICP mass spectrometer (XSeries 2, Thermo Fisher Scientific GmbH, Dreieich, Germany) equipped with a PFA ST Nebulizer, a quartz cyclonic spray chamber and a 2.5 mm quartz injector (all from Thermo Fisher Scientific) using the following isotopes: 27Al and, as an internal standard, 103Rh. Daily calibrations were performed using ionic standards of Al in a 3.5% HNO3 solution ranging from 2 to 500 μg/L. Rh as internal standard was added to each sample. The gas flow for the cool gas and the auxiliary gas were set to 14 L/min, and 0.65 L/min respectively. The sample flow rate was 0.4 mL/min. All isotopes were analysed using the collision cell technique at 5 mL/min collision gas flow (93% He and 7% H2).
Equations
The sample intensities were corrected by subtracting the number of averaged microwave blanks (≤10) :
| 1 |
To determine the , the sensitivity on each day was divided by the sensitivity of the matrix adjusted calibration of that day:
| 2 |
DRF was then used to correct the intensity values:
| 3 |
Dividing Eq. 1 by Eq. 2 considering the Al sensitivity in the corresponding matrix, the used organ mass and the recovery after the microwave digestion results in the Al organ burden per gram organ:
| 4 |
LOD and LOQ were determined using the blank value method43. The data of the control group (5 animals) were used for the determination of the lowest detectable signal. The intensities obtained were converted into concentrations using the matrix calibrations (Table 2). The data for the different groups did not follow a normal distribution. Therefore, median and interquartile ranges (IQR) of the control group for each organ were used to calculate the LOD and LOQ. A 1.5 × IQR corresponds to a standard deviation of 3σ:
| 5 |
| 6 |
Al concentrations were normalized on the AlCl3·6H2O concentration (Figs. 3 and 4), corrected by the mass fraction of AlCl3·6H2O per rat and the corresponding NM species :
| 7 |
Therefore, the term “Al concentration” refers to the concentration of Al measured by ICP-MS. The median normalized Al concentration is only used for subsequent evaluation of Al organ burdens.
Supplementary information
Acknowledgements
The French-German bilateral project SolNanoTOX was funded by the German Research Foundation (DFG, Project ID: DFG (FKZ LA 3411/1-1 and LA 1177/9-1) and the French National Research Agency (ANR, Project ID: ANR-13-IS10-0005). The BfR provided intramural support SFP1322-642 for F.L.K. and A.L.
Author contributions
V.F., J.T., H.J., P.L. and A.L. designed and supervised the study. B.K., N.D. and P.J. planned the experiments. P.J. performed the in vivo study. B.K., F.L.K. and N.D. analysed the ICP-MS samples. F.L.K., B.K. and D.R. evaluated the data and drafted the manuscript. D.R. designed the figures with contributions from B.K. and F.L.K. V.F., P.L., J.T., H.J. and A.L. revised and finalized the manuscript. All authors reviewed and approved the final manuscript.
Data availability
The ICP-MS data sets can be obtained by the authors upon individual request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Benjamin C. Krause, Fabian L. Kriegel and Daniel Rosenkranz.
Supplementary information
is available for this paper at 10.1038/s41598-020-59710-z.
References
- 1.Yokel RA, McNamara PJ. Aluminium toxicokinetics: an updated minireview. Pharmacol. Toxicol. 2001;88(4):159–67. doi: 10.1034/j.1600-0773.2001.d01-98.x. [DOI] [PubMed] [Google Scholar]
- 2.Tietz T, et al. Aggregated aluminium exposure: risk assessment for the general population. Archives of Toxicology. 2019;93(12):3503–3521. doi: 10.1007/s00204-019-02599-z. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, et al. Aluminium in food and daily dietary intake assessment from 15 food groups in Zhejiang Province, China. Food Additives & Contaminants: Part B. 2016;9(2):73–78. doi: 10.1080/19393210.2015.1135193. [DOI] [PubMed] [Google Scholar]
- 4.Stahl T, et al. Migration of aluminum from food contact materials to food—a health risk for consumers? Part I of III: exposure to aluminum, release of aluminum, tolerable weekly intake (TWI), toxicological effects of aluminum, study design, and methods. Environmental Sciences Europe. 2017;29(1):19. doi: 10.1186/s12302-017-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta VK, Agarwal S, Saleh TA. Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. Journal of Hazardous Materials. 2011;185(1):17–23. doi: 10.1016/j.jhazmat.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 6.Seo J, et al. Hierarchical and Multifunctional Three-Dimensional Network of Carbon Nanotubes for Microfluidic Applications. Advanced Materials. 2012;24(15):1975–1979. doi: 10.1002/adma.201104958. [DOI] [PubMed] [Google Scholar]
- 7.Saiyed SM, Yokel RA. Aluminium content of some foods and food products in the USA, with aluminium food additives. Food Additives and Contaminants. 2005;22(3):234–244. doi: 10.1080/02652030500073584. [DOI] [PubMed] [Google Scholar]
- 8.Asimakopoulos, A. G. et al. Colour Additives for Foods and Beverages, ed. Scotter, M. United Kingdom: Elsevier Science & Technology. 260 (2015).
- 9.Sieg H, et al. Impact of an Artificial Digestion Procedure on Aluminum-Containing Nanomaterials. Langmuir. 2017;33(40):10726–10735. doi: 10.1021/acs.langmuir.7b02729. [DOI] [PubMed] [Google Scholar]
- 10.Crapper DR, Krishnan SS, Dalton AJ. Brain Aluminum Distribution in Alzheimers Disease and Experimental Neurofibrillary Degeneration. Science. 1973;180(4085):511–513. doi: 10.1126/science.180.4085.511. [DOI] [PubMed] [Google Scholar]
- 11.Lukiw, W. J. et al. Aluminum in neurological disease - a 36 year multicenter study. J Alzheimers Dis Parkinsonism, 8(6) (2019). [DOI] [PMC free article] [PubMed]
- 12.Bushinsky DA. Bone disease in moderate renal failure: Cause, nature, and prevention. Annual Review of Medicine. 1997;48:167–176. doi: 10.1146/annurev.med.48.1.167. [DOI] [PubMed] [Google Scholar]
- 13.Crapper McLachlan DR, et al. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet. 1991;337(8753):1304–8. doi: 10.1016/0140-6736(91)92978-B. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez J, Mandalunis PM. A Review of Metal Exposure and Its Effects on Bone Health. J. Toxicol. 2018;2018:4854152. doi: 10.1155/2018/4854152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunea G, et al. Role of Aluminum in Dialysis Dementia. Annals of Internal Medicine. 1978;88(4):502–504. doi: 10.7326/0003-4819-88-4-502. [DOI] [PubMed] [Google Scholar]
- 16.Seidowsky A, et al. Aluminic intoxication in chronic hemodialysis. A diagnosis rarely evoked nowadays. Clinical case and review of the literature. Nephrologie & Therapeutique. 2018;14(1):35–41. doi: 10.1016/j.nephro.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Krewski D, et al. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. Journal of Toxicology and Environmental Health-Part B-Critical Reviews. 2007;10:1–269. doi: 10.1080/10937400701597766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.JECFA, Evaluation of certain food additives and contaminants. World Health Organization. p. 7–17 (2011). [PubMed]
- 19.EFSA Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials on a request from European commission on Safety of aluminium from dietary intake. EFSA Journal. 2008;6(7):1–34. doi: 10.2903/j.efsa.2008.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laux P, et al. Nanomaterials: certain aspects of application, risk assessment and risk communication. Archives of Toxicology. 2018;92(1):121–141. doi: 10.1007/s00204-017-2144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moores A, Goettmann F. The plasmon band in noble metal nanoparticles: an introduction to theory and applications. New Journal of Chemistry. 2006;30(8):1121–1132. doi: 10.1039/b604038c. [DOI] [Google Scholar]
- 22.Kelly KL, et al. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. Journal of Physical Chemistry B. 2003;107(3):668–677. doi: 10.1021/jp026731y. [DOI] [Google Scholar]
- 23.Brown DM, et al. Size-dependent proinflammatory effects of ultrafine polystyrene particles: A role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicology and Applied Pharmacology. 2001;175(3):191–199. doi: 10.1006/taap.2001.9240. [DOI] [PubMed] [Google Scholar]
- 24.Hussain S, et al. Oxidative stress and proinflammatory effects of carbon black and titanium dioxide nanoparticles: Role of particle surface area and internalized amount. Toxicology. 2009;260(1–3):142–149. doi: 10.1016/j.tox.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Greger JL, Chang MM, Macneil GG. Tissue Turnover of Aluminum and Ga-67 - Effect of Iron Status. Proceedings of the Society for Experimental Biology and Medicine. 1994;207(1):89–96. doi: 10.3181/00379727-207-43796. [DOI] [PubMed] [Google Scholar]
- 26.Greger JL, Radzanowski GM. Tissue Aluminum Distribution in Growing, Mature and Aging Rats - Relationship to Changes in Gut, Kidney and Bone Metabolism. Food and Chemical Toxicology. 1995;33(10):867–875. doi: 10.1016/0278-6915(95)00059-B. [DOI] [PubMed] [Google Scholar]
- 27.Yokel RA, et al. Entry, half-life, and desferrioxamine-accelerated clearance of brain aluminum after a single Al-26 exposure. Toxicological Sciences. 2001;64(1):77–82. doi: 10.1093/toxsci/64.1.77. [DOI] [PubMed] [Google Scholar]
- 28.Jouhanneau P, et al. Gastrointestinal absorption, tissue retention, and urinary excretion of dietary aluminum in rats determined by using Al-26. Clinical Chemistry. 1997;43(6):1023–1028. doi: 10.1093/clinchem/43.6.1023. [DOI] [PubMed] [Google Scholar]
- 29.Gerotto M, et al. Interference Effects and Their Control in ICP-MS Analysis of Serum and Saline Solutions. Microchemical Journal. 1995;51(1):73–87. doi: 10.1006/mchj.1995.1011. [DOI] [Google Scholar]
- 30.Krause B, et al. Characterization of aluminum, aluminum oxide and titanium dioxide nanomaterials using a combination of methods for particle surface and size analysis. Rsc Advances. 2018;8(26):14377–14388. doi: 10.1039/C8RA00205C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics. 1979;6(2):65–70. [Google Scholar]
- 32.Chen, X. Matrix effects in inductively coupled plasma mass spectrometry. (1995).
- 33.Pauluhn J. Pulmonary Toxicity and Fate of Agglomerated 10 and 40 nm Aluminum Oxyhydroxides following 4-Week Inhalation Exposure of Rats: Toxic Effects are Determined by Agglomerated, not Primary Particle Size. Toxicological Sciences. 2009;109(1):152–167. doi: 10.1093/toxsci/kfp046. [DOI] [PubMed] [Google Scholar]
- 34.des Rieux A, et al. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. Journal of Controlled Release. 2006;116(1):1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Lundquist P, Artursson P. Oral absorption of peptides and nanoparticles across the human intestine: Opportunities, limitations and studies in human tissues. Advanced Drug Delivery Reviews. 2016;106:256–276. doi: 10.1016/j.addr.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Banerjee A, et al. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. Journal of Controlled Release. 2016;238:176–185. doi: 10.1016/j.jconrel.2016.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foroozandeh, P. & Aziz, A. A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Research Letters, 13 (2018). [DOI] [PMC free article] [PubMed]
- 38.Zhao, Y.T. et al. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Scientific Reports7 (2017). [DOI] [PMC free article] [PubMed]
- 39.Fadeel B, et al. Keeping it real: The importance of material characterization in nanotoxicology. Biochemical and Biophysical Research Communications. 2015;468(3):498–503. doi: 10.1016/j.bbrc.2015.06.178. [DOI] [PubMed] [Google Scholar]
- 40.Sieg H, et al. Uptake and molecular impact of aluminum-containing nanomaterials on human intestinal caco-2 cells. Nanotoxicology. 2018;12(9):992–1013. doi: 10.1080/17435390.2018.1504999. [DOI] [PubMed] [Google Scholar]
- 41.Park EJ, et al. A 13-week repeated-dose oral toxicity and bioaccumulation of aluminum oxide nanoparticles in mice. Archives of Toxicology. 2015;89(3):371–379. doi: 10.1007/s00204-014-1256-0. [DOI] [PubMed] [Google Scholar]
- 42.Prabhakar PV, et al. Oxidative stress induced by aluminum oxide nanomaterials after acute oral treatment in Wistar rats. J. Appl. Toxicol. 2012;32(6):436–45. doi: 10.1002/jat.1775. [DOI] [PubMed] [Google Scholar]
- 43.Harrington JM, et al. Validation of a Metallomics Analysis of Placenta Tissue by Inductively-Coupled Plasma Mass Spectrometry. Biological Trace Element Research. 2016;169(2):164–173. doi: 10.1007/s12011-015-0431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The ICP-MS data sets can be obtained by the authors upon individual request.


