Abstract
An 11 month old Caucasian male presented with swelling of the right side of the nose and buccal vestibule of unknown duration. Radiographic images revealed a well-circumscribed, hypodense soft tissue mass with a peripheral hyperdense circumference involving the anterior aspect of the right maxilla. The lesion extended from the inferior aspect of the orbital rim superiorly to the maxillary bone inferiorly without invading any nearby structures. The lesion was completely resected via combined extraoral and intraoral approach. The clinical, radiographic, histologic and immunohistochemical features of a sinonasal myxoma in an infant are discussed.
Keywords: Myxoma, Sinonasal, Infant, Pediatric, Beta-catenin, Radiology
History
An 11 month old Caucasian male with right sided paranasal swelling that had increased in size over several weeks, and was suspected to be of odontogenic origin, was referred to the Oral and Maxillofacial Surgery Department by Otorhinolaryngology. Clinical examination revealed a non-tender, firm fullness in the right lateral nasal area with mild intraoral swelling of the right maxillary vestibule. The nose was somewhat elevated and the skin slightly stretched. The patient was healthy, had no other symptoms, and no history of trauma or co-morbid conditions.
Radiographic Features
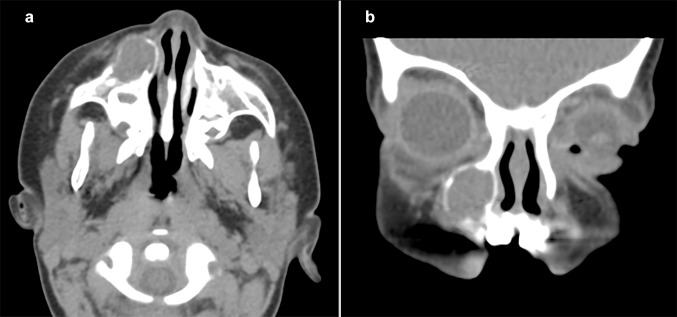
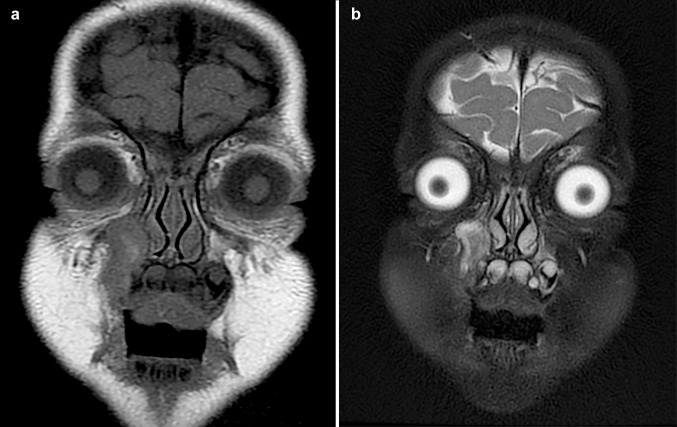
The initial computerized tomography (CT) scan (Fig. 1) revealed a well-circumscribed, hypodense intra-osseous mass with a mostly intact bony periphery in the anterior aspect of the right maxilla. The lesion extended from the inferior aspect of the orbital rim superiorly to the alveolar process inferiorly without invading any nearby structures. The orbital floor (superior maxillary sinus wall) and the medial sinus wall were intact. The lesion abutted the right orbital floor and nasolacrimal duct and appeared to be related to the root of the right maxillary canine tooth. An anterior dehiscence was present with a focal area of soft tissue prominence. The initial impression was that of an expansile lesion of the right maxilla, suggestive of dental origin. Based on these findings, the patient underwent a transoral excisional biopsy. After the histologic diagnosis was established, magnetic resonance imaging (MR) was performed 6 days post-biopsy and compared to the initial CT scan. The MR (Fig. 2) revealed a T1 isointense and T2 bright expansile soft tissue mass in the right maxillary bone at the level of a right primary maxillary tooth root. The mass demonstrated peripheral enhancement with a small non-enhancing central area. Complete opacification of the right maxillary sinus, similar to the initial CT, was noted. The MR was not definitive as to whether the findings were of inflammatory, post-surgical origin or represented a residual soft tissue lesion. After presentation at a collaborative multidisciplinary conference, it was determined that the lesion be completely excised. Follow-up MR at 5, 36, and 48 months post-resection demonstrated no evidence of residual or recurrent lesional tissue.
Fig. 1.
Axial (a) and coronal (b) views on CT scans demonstrate a well-circumscribed, expansile, hypodense soft tissue mass with a peripheral hyperdense circumference in the right maxilla, anterior to the right maxillary sinus. a Encroachment of the anterior wall of the right maxillary sinus and right lateral nasal wall without invasion of nearby structures. b The lesion extends from the inferior aspect of the orbital rim superiorly to the maxillary bone inferiorly
Fig. 2.
Axial view on MR displaying a T1 isointense and b T2 fat saturated bright expansile soft tissue mass in the right maxilla demonstrating peripheral enhancement with a small, central non-enhancing area
Treatment
The patient underwent a transoral excisional biopsy of the right maxillary sinus lesion including extraction of the right primary maxillary canine. At the time of biopsy, the aggressive nature of the lesion was noted with erosion through the maxilla and nasal bone, without penetrating the mucosa, and extension to the inferior orbit. Complete resection of the residual lesion was performed 6 weeks after initial surgery through combined right inferior orbitotomy (transconjunctival approach) and Caldwell–Luc approach. Crawford tubes were placed at the time of surgery to prevent damage to the nasolacrimal duct. Repair of the orbital floor defect was performed with absorbable implant. At 6 weeks post-resection surgery, a temporal right lower lid entropion with surgical scar in the inferior fornix was noted but the patient was asymptomatic. Two months later the Crawford tubes were removed early due to displacement. Three months after the resection surgery, the entropion had spontaneously improved and there was no evidence of lacrimal outflow dysfunction. At the 5 months post-resection visit, the patient had excellent orbital and facial symmetry with no residual entropion; it resolved spontaneously without surgical intervention. There appeared to be no evidence of lacrimal outflow insufficiency or tumor recurrence by MR. The intraoral incision was well-healed. The patient has been monitored by specialty and primary care services for over 48 months and no evidence of recurrence has been identified.
Diagnosis
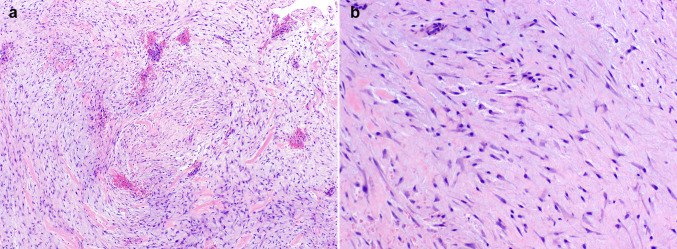
Histologic evaluation of the hematoxylin and eosin stained specimens (transoral excisional biopsy and complete resection) revealed a moderately cellular proliferation of haphazardly arranged, spindled and stellate cells embedded in a fibromyxoid background with slit-like vascular spaces and extravasated erythrocytes. The neoplastic cells had small, hyperchromatic nuclei without significant pleomorphism or atypia and long cytoplasmic processes. Occasional mitotic figures and bands of dense, keloidal-type collagen were present in the lesion (Fig. 3). No necrosis or odontogenic epithelial rests were identified. The tumor was partially encompassed by a rim of reactive bone representing expanded osseous cortex. The lesional spindle cells were weakly immunoreactive for smooth muscle actin (SMA) and Ki-67 demonstrated a low proliferation index. There was strong, uniform nuclear staining with β-catenin (Fig. 4). The margins of the initial excisional biopsy were positive for lesional tissue with little or no adherent normal tissue. Evaluation of the resection specimen revealed negative margins. The clinical and cytomorphologic features supported classification as a sinonasal myxoma (SNM).
Fig. 3.
a, b The lesion demonstrates a moderately cellular proliferation of haphazardly arranged spindled and stellate mesenchymal cells in a fibromyxoid background. The neoplastic cells exhibit elongated nuclei without significant pleomorphism or atypia (H&E, × 40, × 200 respectively)
Fig. 4.
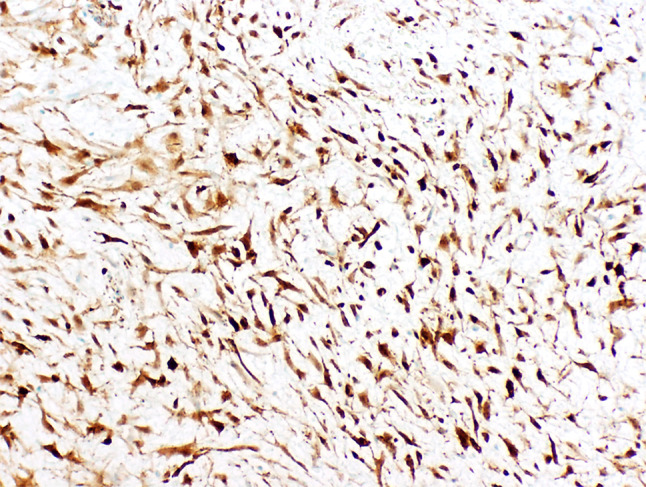
Strong positive nuclear reactivity with β-catenin (× 200)
Discussion
Myxomas of the head and neck, first described by Virchow in 1871, can be derived from soft tissue or from the facial skeleton [1]. Those arising in the facial skeleton most commonly present in the mandible, followed by the maxilla, are of odontogenic origin [2] and termed odontogenic myxomas. True extragnathic osseous myxomas are extremely rare; McClure and Dahlin identified only 3 out of 5000 bone tumors [3]. Head and neck myxomas involving the maxillary sinus are also rarely encountered and thought to be of osseous origin [4]. They are not categorized as a separate entity by the World Health Organization WHO. Most studies of odontogenic myxomas suggest a female predominance [2, 5–8]. They present in patients ranging in age from 1 to 73 years, but predominate in the second to fourth decades (75%), and only occasionally occur (7.3%) in patients below 10 years of age [2, 7]. Myxomas presenting in patients younger than 24 months old are almost exclusively seen in the sinonasal complex and/or maxilla [9].
The SNM is a rare, benign mesenchymal neoplasm of uncertain histogenesis and etiology that has not been well characterized. It is generally considered a non-odontogenic tumor arising from the sinonasal region [10, 11]. It has been described as a slow growing, expansile, locally destructive, and infiltrative lesion of potentially aggressive behavior with the ability to erode bone [4, 12]. Although often identified in the literature as an odontogenic myxoma and not currently regarded as a distinct entity by the WHO, published reports of SNM [8–10, 12–34] demonstrate remarkably consistent presentations, including age, location, and other clinical features, which are inconsistent with odontogenic myxomas. Table 1 summarizes 37 published cases, including the current case, of SNM found in English language literature since 1951. The average age at presentation was 15.4 months (2.5–36) with a 1.2:1 male:female ratio. The characteristic clinical presentation is a painless swelling of the affected area, usually the nasolabial or paranasal region. Despite varied descriptors, this classic presentation is reflected in all cases listed in Table 1. Common symptoms include nasal congestion, epistaxis and nasal obstruction. When the tumor extends to adjacent structures, it may cause pain, discomfort, malocclusion and/or diplopia.
Table 1.
Summary of SNM in children 3 years of age or younger (1951–2018)
| Study | Age (months)/gender | Clinical presentation | Location | Follow-up period (post-operative months) | Recurrence (post-operative months) |
|---|---|---|---|---|---|
| Current case | 11/M | Right paranasal and intraoral swelling of the right maxillary vestibule | Anterior right maxilla from inferior orbital rim to the maxillary alveolar process | 48 | NED |
| Hansen et al. [34] | 36/M | Right nasolabial fold | Maxilla and floor of right orbit | 60 | NED |
| Kelly et al. [33] | 5/M | Right nasolabial fold and nasolacrimal duct obstruction | Right nasolacrimal mass and eroding left nasomaxillary groove and floor of right orbit | 30 | NED |
| Zainine et al. [32] | 2.5/NS | Left nasal obstruction and rhinorrhea | Left ethmoid-nasal mass with intra-orbital extension | 30 | NED |
| Kadlub et al. [31] | 18/F | Paranasal, red overlying skin | Maxilla, orbital rim and nasal cavity | 72 | NED |
| 23/F | Paranasal | Maxilla | 48 | NED | |
| 21/F | Paranasal | Maxilla and orbital rim | 18 | 8 or 14* | |
| 14/M | Paranasal, epiphora | Maxilla and orbital rim | 12 | 8 or 14* | |
| Chen et al. [30] | 15/F | Right nasolabial fold | Right maxillary sinus with disruption of anterior maxillary wall and medial orbital floor | 27 | NED |
| Kansy et al. [29] | 12/NS | Left paranasal | Inferior, anterior, medial wall of maxillary sinus and left infraorbital rim | 24 | NED |
| 11/NS | Left paranasal, infection left tear duct and eye | Left maxillary sinus and left nasal cavity to left medial canthus and floor of orbit | 96 | NED | |
| Ríos y Valles-Valles et al. [28] | 11/F | Left medial canthus | Anterior aspect of maxillary sinus | 48 | NED |
| Safadi et al. [9] | 20/M | Left nasolabial mass | Premaxilla, anterior wall of maxillary sinus adjacent to nasal bone and inferior orbital wall | 12 | NED |
| Iatrou et al. [10] | 12/M | Left nasobuccal groove | Anterior maxilla to ipsilateral orbit, protruding to left nasal cavity and anterior ethmoid cells | 42 | NED |
| King et al. [27] | 18/M | Left nasomaxillary groove | Erosion/obliteration of maxillary vestibule and maxillary sinus with encroachment of lateral nasal wall and orbital floor | 18 | NED |
| 17/M | Left anterior maxila | Anterior medial aspect of left maxillary bone with expansion and obliteration of maxillary bone | 24 | NED | |
| Boussault et al. [26] | 14/M | Right lateral nose | Expansile mass with medial displacement of lateral nasal wall extending up medial canthus and down the nasolabial fold; involving anterior wall right maxillary sinus reaching teeth roots, compressing right maxillary sinus cavity, nasal cavity, lacrimal duct without invasion; pressure erosion anterior wall maxillary antrum | NS | NED |
| Prasannan et al. [12] | 20/F | Right infraorbital mass pushing nose to left side | Right maxillary sinus extending into anterior subcutaneous soft tissues, inferior aspect of right orbit, right nasal cavity and right ethmoidal air cells | 8 | NED |
| Simon et al. [8] | 3/NS | Rapid aggressive growth | Unknown | NS | NS |
| Rotenberg et al. [25] | 13/F | Left nasolabial groove | Anterior maxilla, nasomaxillary mass eroding into maxillary sinus | 48 | NED |
| 16/M | Left nasolabial fold | Left maxilla infiltrating nasal antrum and orbital rim | 84 | NED | |
| 18/F | Left maxillary mass | Invading maxillary sinus, nasal cavity, tooth sockets and infraorbital rim | 168 | NED | |
| Wachter et al. [24] | 13/M | Left nasolabial fold mass | Mass in left anterior maxillary sinus with mild encroachment upon left lateral nasal wall | 24 | NED |
| 19/F | Right nasofacial groove mass | Expansile mass right anterior maxilla, from above the root of the canine to inferior orbital rim | 24 | NED | |
| Fenton et al. [23] | 17/M | Left lateral nose extending up medial canthus | Anterior maxillary antrum encroaching antrum and nasal septum | 16 | NED |
| Brewis et al. [22] | 13/M | Left nasomaxillary groove | Anterior of left maxillary antrum extending to inferior aspect of maxilla | 4 | NED |
| Caleffi et al. [21] | 24/M | Right alar groove, nasolabial region | Connected to anterior wall of maxillary sinus | 12 | NED |
| Heffner [20] | 12/M | Left paranasal cheek swelling | Anterior maxillary sinus wall extending to anterior turbinate | NS | NS |
| 24/M | Left cheek swelling near nose | Anterior maxillary sinus involving anterior sinus wall | 144 | NED | |
| Ang et al. [19] | 13/M | Left nasal swelling encroaching nasal cavity | Anteromedial angle of maxillary antrum involving upper alveolus | 10 | NED |
| 14/F | Right side of nose | Arising from maxilla, extending outwards, destroying orbital floor | 89 | 5 | |
| Hayes et al. [18] | 12/F | Mass lateral right nares obliterating nasolabial crease | Right anterior maxillary sinus wall | 3 | NED |
| James et al. [17] | 11/F | Left lateral nose swelling | Anterior of left maxillary antrum encroaching the anterior sinus wall, bulging through the lateral wall of the nose, encroaching on the nasal septum and enveloping the nasolacrimal duct | 84^ | NED |
| Smith et al. [16] | 15/F | Swelling right side of nose overlying the ascending process of right maxilla | Nasal mass in right upper nasal cavity destroying nasal septum and extending into right maxilla; upon recurrence, eroded medial anterior walls of maxillary sinus extending into orbital rim and upper nasal cavity | 13 | 5 |
| Harris et al. [15] | 12/F | Lump right lateral nose | Medioinferior aspect of right orbit, extending into ethmoid sinus | 12 | NED |
| Fu et al. [14] | 15/F | Left nasofacial cleft | Left anterior antral wall, lateral to nasolacrimal canal projecting anteriorly into subcutaneous tissues and posteriorly into maxillary sinus | 108 | NED |
| Greenfield et al. [13] | 24/M | Facial asymmetry | Filling maxillary antrum | 3 | NED |
| Average (range) | 15.4 (2.5–36) | 43 (3–168) | 8 (5–14) |
NED No evidence of disease, NS not specified
*Specific case and recurrence time was not specified
^NED at 8 months in original article, NED at 84 months in Ang et al. [19]
Radiographic imaging for both traditional odontogenic myxomas and SNM demonstrate a well-defined, expansile, unilocular or multilocular radiolucent lesion that may or may not infiltrate adjacent tissue. CT and MR studies of SNM exhibit well-defined borders, usually typified by a peripheral hyperintense signal. In contrast, with these advanced imaging modalities, up to 50% of traditional odontogenic myxomas show poor, diffuse or infiltrative radiographic border definition [2, 9]. On gross examination, myxomas are gray–white, nodular, glistening masses of variable consistency, ranging from gelatinous to firm, without a true capsule. The amount of collagen influences the consistency of the lesion and may be demonstrated by prominent white bands that become visible during sectioning [1, 2, 24].
Histologically, odontogenic myxomas and SNM are similar, consisting of a non-encapsulated, loose proliferation of spindled, stellate or round cells with small, hyperchromatic nuclei and thin cytoplasmic projections in a fibromyxoid to mucinous background stroma. Pleomorphism, mitotic figures and necrosis are usually absent [2, 4]. Immunohistochemistry may not be a particularly helpful diagnostic adjunct when evaluating SNM. This is reflected in the case reports and series of infantile SNM since 2000, which all describe positivity for vimentin and negative staining for S100, cytokeratins, BCL2, Alk-1, other neural and muscle markers [9, 10, 12, 22, 23, 25, 26, 28–31, 33]. SMA reactivity, when reported, was focal and weak to negative in SNM [10, 23, 25, 28–31], as well in our described case. Two studies have shown that most, but not all, odontogenic myxomas show reactivity for alpha-smooth muscle actin [35, 36]. β-Catenin reactivity in SNM has not been well studied with one reported example of cytoplasmic staining, but staining in this case and one unpublished case suggests the need for further investigation [29]. It is possible that SNM belongs in the spectrum of β-catenin driven mesenchymal lesions affecting the head and neck such as angiofibroma and cranial fasciitis. This also supports the contention of a non-odontogenic origin of SNM, as odontogenic myxomas are not known to express β-catenin. SNM have a consistently low Ki67 demonstrating a low proliferative index [5], as supported in our case and other previously published reports [9, 26, 29]. Traditional odontogenic myxomas rarely contain odontogenic epithelial rests (approximately 5%) [2]. Existing case reports of SNM have not reported the presence of odontogenic epithelial rests in their histologic descriptions and as previously discussed, no cytokeratin positivity has been noted in SNM. The clinical, radiographic and microscopic differential diagnosis of SNM includes, but is not limited to sinonasal inflammatory polyp, fibrous dysplasia, odontogenic cysts and tumors (ameloblastoma, odontogenic fibroma, odontogenic keratocyst), fibroblastic tumors (fibromatosis, myofibroma, inflammatory myofibroblastic tumor) and pediatric sarcomas (particularly rhabdomyosarcoma). Care must be taken when interpreting biopsy specimens. Reported cases of infantile SNM have been initially diagnosed on biopsy as fibrous dysplasia [32], nodular fasciitis [17, 31], infantile fibromatosis [19], pseudosarcomatous fasciitis and rhabdomyosarcoma [25].
The treatment of choice for an infantile SNM appears to be surgical resection via local excision along with conservative ostectomy [4, 12], although some authors have recommended adjuvant chemotherapy [27] or radical resection with margins [10, 12]. As noted in Table 1, 36 additional literature cases of infantile SNM were identified. Of these, 34 cases reported an average of 43 months of follow-up, with four recurrences at an average of 8 months post-surgery [16, 19, 31]. This 11% recurrence rate compares with an approximate 25% recurrence rate for true odontogenic myxomas [2]. The true recurrence rate for infantile SNM is likely much lower if complete removal is accomplished at initial surgery. Smith et al. [16] reported an initial removal without any normal margin, which was subsequently curetted and rongeured in the second excision with no repeat recurrence 8 months later. Kadlub et al. [31] also believe that “incomplete tumor removal” contributed to their two reported recurrences and support conservative surgical excision as treatment of choice. There is no evidence that SNM are responsive to radiation or chemotherapy, undergo malignant transformation, or metastasize. The distinctive clinical and radiographic features, along with the microscopic and immunophenotypic findings warrant recognition of SNM as a specific entity.
Compliance with Ethical Standards
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of retrospective case report, formal consent is not required. The tumor tissue included in the manuscript was obtained as part of the standard of care for the patient and retrospectively collected for the case report.
Footnotes
Disclaimer
The views expressed in this manuscript are those of the author and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government. I certify that all individuals who qualify as authors have been listed; each has participated in the conception and design of this work, the writing of the document, and the approval of the submission of this version; that the document represents valid work; that if we used information derived from another source, we obtained all necessary approvals to use it and made appropriate acknowledgements in the document; and that each takes public responsibility for it. We are military Service Members. This work was prepared as part of our official duties. Title 17 U.S.C. 105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military Service Member or employee of the United States Government as part of that person’s official duties.
References
- 1.Moore B, Wine T, Burkey B, et al. Sphenoid sinus myxoma: case report and literature review. Ochsner J. 2008;8(4):166–171. [PMC free article] [PubMed] [Google Scholar]
- 2.Odell EW, Adebiyi K, et al. Odontogenic myxoma/myxofibroma. In: El-Naggar AK, Chan JKC, Grandis JR, et al., editors. World Health Organization Classification of head and neck tumors. 4. Lyon: International Agency for Research on Cancer; 2017. pp. 229–230. [Google Scholar]
- 3.McClure DK, Dahlin DC. Myxoma of bone. Report of three cases. Mayo Clin Proc. 1977;52(4):249–253. [PubMed] [Google Scholar]
- 4.Wenig B. Sinonasal myxoma and fibromyxoma. Atlas of head and neck pathology. 3. Philadelphia: Elsevier; 2016. pp. 118–122. [Google Scholar]
- 5.Martinez-Mata G, Mosqueda-Taylor A, Carlos-Bregni R, et al. Odontogenic myxoma: clinico-pathological, immunohistochemical and ultrastructural findings of a multicentric series. Oral Oncol. 2008;44:601–607. doi: 10.1016/j.oraloncology.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Noffke CE, Raubenheimer EJ, Chabikuli NJ, et al. Odontogenic myxoma: review of the literature and report of 30 cases from South Africa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:101–109. doi: 10.1016/j.tripleo.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 7.Kaffe I, Naor H, Buchner A. Clinical and radiological features of odontogenic myxoma of the jaws. Dentomaxillofac Radiol. 1997;26:299–303. doi: 10.1038/sj.dmfr.4600261. [DOI] [PubMed] [Google Scholar]
- 8.Simon ENM, Merkx MAW, Vuhahula E, et al. Odontogenic myxoma: a clinicopathological study of 33 cases. Int J Oral Maxillofac Surg. 2004;33:333–337. doi: 10.1016/j.ijom.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Safadi A, Fliss D, Issakov J, et al. Infantile sinonasal myxoma: a unique variant of maxillofacial myxoma. J Oral Maxillofac Surg. 2011;69:553–558. doi: 10.1016/j.joms.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Iatrou I, Lygidakis N, Leventis M, et al. Sinonasal myxoma in an infant. J Craniofac Surg. 2010;21(5):1649–1651. doi: 10.1097/SCS.0b013e3181ef680a. [DOI] [PubMed] [Google Scholar]
- 11.Slater LJ. Letter to the editor: infantile lateral nasal myxomas—is it odontogenic? J Oral Maxillofac Surg. 2004;62:391. doi: 10.1016/j.joms.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Prassanan L, Warren L, Herzog CE, et al. Sinonasal myxoma: a pediatric case. J Pediatr Hematol Oncol. 2005;27(2):90–92. doi: 10.1097/01.mph.0000153443.36193.de. [DOI] [PubMed] [Google Scholar]
- 13.Greenfield SS, Friedman O. Myxoma of maxillary sinus. NY State J Med. 1951;51:1319–1320. [PubMed] [Google Scholar]
- 14.Fu YS, Perzin KH. Non-Epithelial tumors of the nasal cavity, paranasal sinuses and nasopharynx: a clinicopathologic study. Cancer. 1977;39:195–203. doi: 10.1002/1097-0142(197701)39:1<195::AID-CNCR2820390131>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Harris RJ, Garrow E, Spinnato G. Myxoma of the maxilla: report of case. J Oral Surg. 1977;35:70–73. [PubMed] [Google Scholar]
- 16.Smith GA, Konrad HR, Canalis RF. Chilhood myxomas of the head and neck. J Otolaryngol. 1977;6(5):423–430. [PubMed] [Google Scholar]
- 17.James DR, Lucas VS. Maxillary myxoma in a child of 11 months: a case report. J Craniofac Maxillofac Surg. 1987;15:42–44. doi: 10.1016/S1010-5182(87)80014-8. [DOI] [PubMed] [Google Scholar]
- 18.Hayes DK, Madsen JM, Simpson R, Jarchow RC. Myxomas of the maxilla in infants and children. Otolaryngol Head Neck Surg. 1991;105:464–468. doi: 10.1177/019459989110500319. [DOI] [PubMed] [Google Scholar]
- 19.Ang HK, Ramani P, Michaels L. Myxoma of the maxillary antrum in children. Histopathology. 1993;23:361–365. doi: 10.1111/j.1365-2559.1993.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 20.Heffner DK. Sinonasal myxomas and fibromyxomas in children. Ear Nose Throat J. 1993;72(5):365–369. doi: 10.1177/014556139307200514. [DOI] [PubMed] [Google Scholar]
- 21.Caleffi E, Toshi S, Bocchi A. Myxoma of the maxilla: surgical treatment of an unusual case in a child. Plast Reconstr Surg. 1994;93:1272–1274. doi: 10.1097/00006534-199405000-00027. [DOI] [PubMed] [Google Scholar]
- 22.Brewis C, Roberts DN, Malone M, et al. Maxillary myxoma: a rare midfacial mass in a child. Int J Pediatr Otorhinolaryngol. 2000;56:207–209. doi: 10.1016/S0165-5876(00)00413-4. [DOI] [PubMed] [Google Scholar]
- 23.Fenton S, Slootweg P, Dunnebier E, et al. Odontogenic myxoma in a 17-month-old child: a case report. J Oral Maxillofac Surg. 2003;61:734–736. doi: 10.1053/joms.2003.50121. [DOI] [PubMed] [Google Scholar]
- 24.Wachter BJ, Steinberg MJ, Darrow DH, et al. Odontogenic myxoma of the maxilla: a report of two pediatric cases. Int J Pediatr Otorhinolaryngol. 2003;67:389–393. doi: 10.1016/S0165-5876(02)00349-X. [DOI] [PubMed] [Google Scholar]
- 25.Rotenberg BW, Daniel SJ, Nish IA, et al. Myxomatous lesions of the maxilla in children: a case series and review of management. Int J Pediatr Otorhinolaryngol. 2004;68:1251–1256. doi: 10.1016/j.ijporl.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Boussault P, Boralevi F, Raux-Rakotomalala F, et al. Odontogenic myxoma: a diagnosis to add to the list of facial tumours in infants. J Eur Acad Dermatol Venereol. 2006;20:864. doi: 10.1111/j.1468-3083.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 27.King TJ, Lewis J, Orvidas L, et al. Pediatric maxillary odontogenic myxoma: a report of 2 cases and review of management. J Oral Maxillofac Surg. 2008;66:1057–1062. doi: 10.1016/j.joms.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Ríos y Valles-Valles D, Vera-Torres AM, Rodríguez-Martínez HA, et al. Case report: periocular myxoma in a child. Case Rep Ophthalmol Med. 2012;2012:1–4. doi: 10.1155/2012/273526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kansy K, Juergens P, Krol Z, et al. Odontogenic myxoma: diagnostic and therapeutic challenges in paediatric and adult patients—a case series and review of the literature. J Craniomaxillofac Surg. 2012;40:271–276. doi: 10.1016/j.jcms.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Chen HH, Streubel S-O, Durarairaj VD. Odontogenic myxoma with orbital involvement. Opthal Plast Reconstr Surg. 2013;29(2):e47–e49. doi: 10.1097/IOP.0b013e31826a2358. [DOI] [PubMed] [Google Scholar]
- 31.Kadlub N, Mbou VB, Leboulanger N, et al. Infant odontogenic myxoma: a specific entity. J Craniomaxillofac Surg. 2014;42:2082–2086. doi: 10.1016/j.jcms.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Zainine R, Mizouni H, El Korbi A, et al. Case report: maxillary bone myxoma. Eur Ann Otorhinolaryngol. 2014;131:257–259. doi: 10.1016/j.anorl.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Kelly TG, Hong S, Jarzembowski J, et al. Infant with nasolacrimal sinonasal myxoma: diffusion with MR features. Radiol Case Rep. 2015;10(2):1–4. doi: 10.2484/rcr.v10i2.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen TS, Danielsson LI, Fast S, et al. Odontogenic myxoma involving the orbit in a 3-year-old-boy: removal, reconstruction and review of the literature. BMJ Case Rep. 2016 doi: 10.1136/bcr-2015-212465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lombardi T, Lock C, Samson J, et al. S100, α-smooth muscle actin and cytokeratin 19 immunohistochemistry in odontogenic and soft tissue myxomas. J Clin Pathol. 1995;48:759–762. doi: 10.1136/jcp.48.8.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kouhsoltani M, Halimi M, Jabbari G. Immunohistochemical evaluation of myofibroblast density in odontogenic cysts and tumors. J Dent Res Dent Clin Dent Prospects. 2016;10(1):37–42. doi: 10.15171/joddd.2016.006. [DOI] [PMC free article] [PubMed] [Google Scholar]