Abstract
Our understanding of adipocytic tumors classification and diagnosis continues to evolve. We present a brief review and updated summary of selected adipocytic tumors involving the head and neck region. For the practicing pathologist, knowledge of these established and emerging entities is critical for the correct pathologic diagnosis and treatment of the patient.
Keywords: Spindle cell lipoma, Pleomorphic lipoma, Liposarcoma, Anisometric cell lipoma, Dysplastic lipoma, Atypical spindle cell lipomatous tumor
Spindle Cell/Pleomorphic Lipoma
Although spindle cell lipoma and pleomorphic lipoma were originally described as two separate entities [1, 2], these lesions represent a spectrum of the same entity, given the considerable overlap in clinical, morphologic, immunophenotypic, and genetic features. In several previous studies, spindle cell/pleomorphic lipomas were also included within a group called “atypical lipoma” [3]. However, this term could cause significant confusion with a completely unrelated group of adipocytic tumors, such as atypical lipomatous tumor/well-differentiated liposarcoma (ALT/WDL).
Spindle cell/pleomorphic lipoma typically presents as a solitary, painless subcutaneous mass located in the upper back or the posterior aspect of the neck. The salivary gland, oral cavity, orbit, face and maxillofacial region are rare, but well-documented sites [4–6]. Found predominantly in males (the male-to-female ratio is 10:1), the peak incidence is between the fourth and the fifth decade. Very rarely, these lesions are multiple and some, even less frequently, familial [7]. Spindle cell and pleomorphic lipomas are entirely benign that rarely recur. Excision is curative.
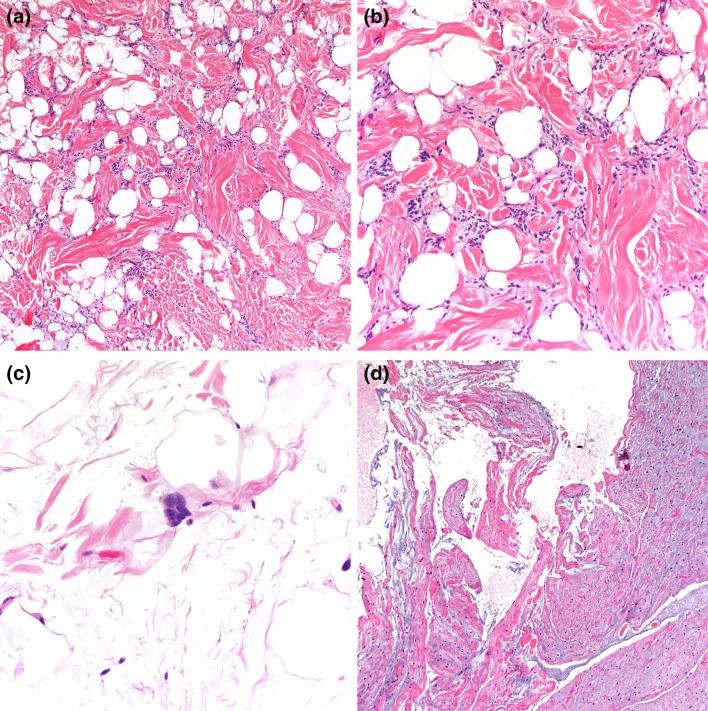
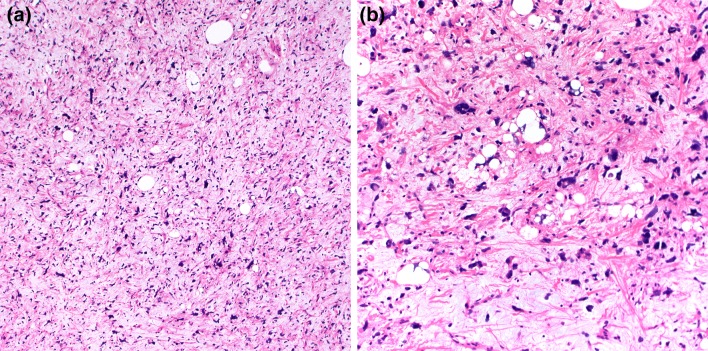
The size of the tumors ranges between 3–5 cm, although larger lesions (up to 14 cm) are not uncommon [3]. Grossly, spindle cell/pleomorphic lipomas resemble ordinary lipomas. The cut surface varies from yellow to white–gray and myxoid, depending on the proportion of adipocytic, spindle/pleomorphic cell and myxoid stromal components. Microscopically, the classic spindle lipoma is composed of a mixture of spindle cells and mature adipocytes (Fig. 1a, b). These spindle cells are uniform and have a single elongated nucleus with bipolar cytoplasm. They tend to be arranged in short parallel bundles or fascicles (“school of fish” appearance), but may have a prominent nuclear palisading or random distribution. The cellularity is extremely variable and mast cells are usually prominent. The stroma consists of a fibromyxoid matrix mixed with variable amounts of brightly eosinophilic, ropy collagen fibers. Secondary changes of fat necrosis or perivascular hyalinization can be seen. In addition to the histologic features of spindle cell lipoma, pleomorphic lipoma is characterized by the presence of scattered, hyperchromatic pleomorphic cells and bizarre giant cells that frequently have a concentric floret-like arrangement (Fig. 1c). Lipoblasts can be seen, but mitoses are exceptional. Rarely, myxoid stroma can be prominent resulting in angiectoid spaces with a pseudoangiomatous growth pattern (Fig. 1d). Cases with mixed features of spindle cell and pleomorphic lipoma are frequent, and the distinction between these two benign lesions is somewhat arbitrary. In some cases mature adipocytes form a distinct minority of the cellular constituens, so-called “low-fat” and “fat-free” spindle cell lipomas [8].
Fig. 1.
Spindle cell lipoma is composed of a proliferation of adipocytes and bland spindle cells with characteristic ropey collagens (a, b). Scattered mast cells are also frequent. Pleomorphic lipomas also contain floret-like multinucleated giant cells (c). Extensive myxoid stromal change with pseudoangiomatous pattern (d)
Immunohistochemically, the lesional cells in spindle cell and pleomorphic lipoma are positive for CD34 and negative for actins and desmin. Although S-100 protein highlights mature adipocytes, the constituent spindle and pleomorphic cells are negative for this marker. Molecular genetic studies support the notion that spindle cell and pleomorphic lipomas are closely related; the loss of chromosomes 13q (13q12 and 13q14-q22) and/or 16q (16q13-qter) is characteristic of this family of lipomas [9, 10]. Interestingly, this same gene deletion is also present in mammary-type myofibroblastoma, cellular angiofibromas, and superficial acral fibromyxomas [11–13]. RB1 (retinoblastoma protein) immunohistochemical expression is usually lost in these entities, and this marker may be a valuable adjunct diagnostic tool [14].
Spindle cell and pleomorphic lipomas have various histologic patterns and the differential diagnosis can be broad. Morphologically, spindle cell lipoma closely resembles (lipomatous) solitary fibrous tumor, which is easily excluded by lack of STAT6 protein expression. Myxoid stromal change may lead to differential diagnosis with myxoid liposarcoma. However, the typical plexiform vascular network is lacking in spindle cell lipoma. Additional molecular analysis for DDIT3 and/or FUS rearrangement can be helpful in difficult cases. Due to cytologic atypia, pleomorphic lipoma may be confused with pleomorphic sarcomas (including pleomorphic liposarcomas). Pleomorphic lipoma, however, is generally less cellular, and mitotic figures are rare in comparison to pleomorphic sarcomas. While the clinical presentation may be sufficiently characteristic to assist in the diagnosis, spindle cell/pleomorphic lipoma may be difficult to distinguish from ALT/WDL based soley on morphologic features. The characteristic ropey collagens of spindle cell/pleomorphic lipoma are a very useful diagnostic feature, as this collagen pattern is typically absent in ALT/WDL. Moreover, spindle cell/pleomorphic lipoma lacks MDM2 amplification and protein overexpression. In small biopsy specimens, spindle cell lipoma may also mimic dermatofibrosarcoma protuberans. However, dermatofibrosarcoma protuberans tends to occur in younger patients and forms a nodule or ill-defined dermal plaque that extends into subcutis with a characteristic honeycomb pattern. An additional important differential diagnosis is benign peripheral nerve sheath tumors, such as neurofibroma and schwannoma, which may be identified by positive S100 protein and SOX10 expression.
Liposarcoma
Despite being one of the most common soft tissue sarcomas, liposarcoma rarely involves the head and neck region; larynx and hypopharynx are the most common sites, followed by the neck [15, 16]. Recent advances in the molecular genetic characterization of soft tissue tumors have led to the classification of liposarcoma into three main subtypes: ALT/WDL, myxoid liposarcoma, and pleomorphic liposarcoma [17].
ALT is the preferred designation for the ALT/WDL spectrum when it occurs in the extremities or superficial locations. However, a recent study found that those tumors arising in the head and neck show high rates of adverse events, suggesting that classification as WDL rather than ALT is more appropriate [18]. The term dedifferentiated liposarcoma (DDL) describes a (usually) high-grade non-lipogenic sarcoma occurring in association with an ALT/WDL. ALT/WDL is characterized by a 30 to 50% risk of local recurrence but is incapable of metastasis unless dedifferentiation occurs [17]. In general, the anatomic location (superficial vs. deep) is the most important prognostic factor and the key predictor of relapse [17]. Complete surgical excision with negative margins is the treatment choice of ALT/WDL. The metastatic rate of DDL ranges from 15 to 30%. However, it generally exhibits a better prognosis than do most high-grade pleomorphic sarcomas [19]. If possible, DDL is treated by wide surgical resection with or without adjuvant radiation therapy and/or chemotherapy. Several novel agents, including MDM2 and CDK4 inhibitors, are being tested in various clinical trials [20, 21].
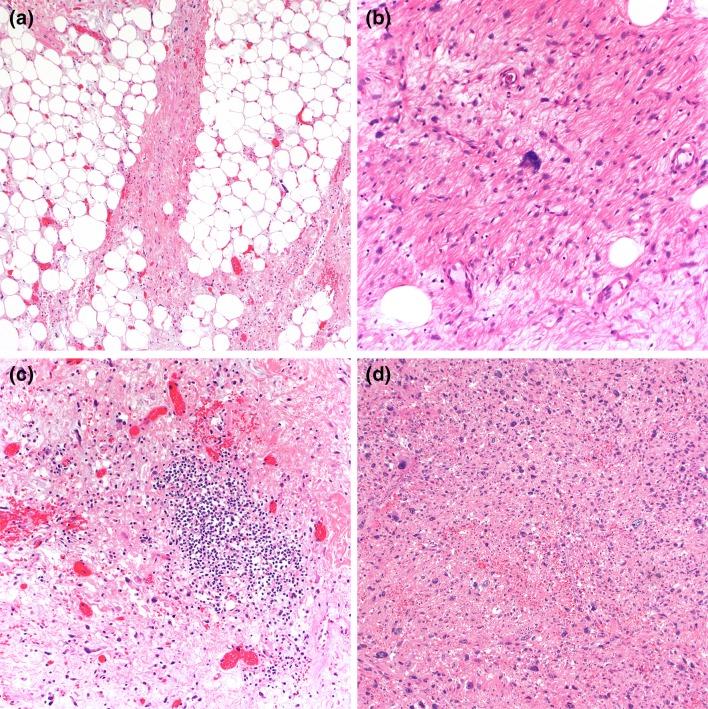
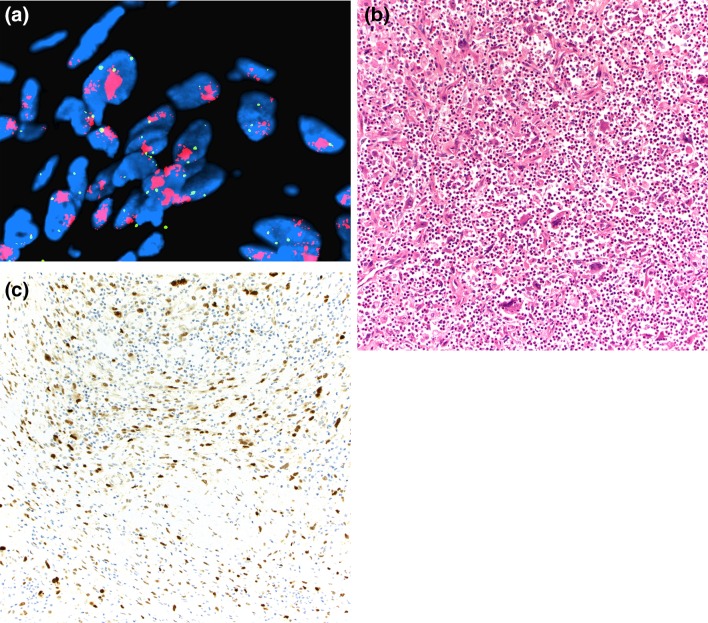
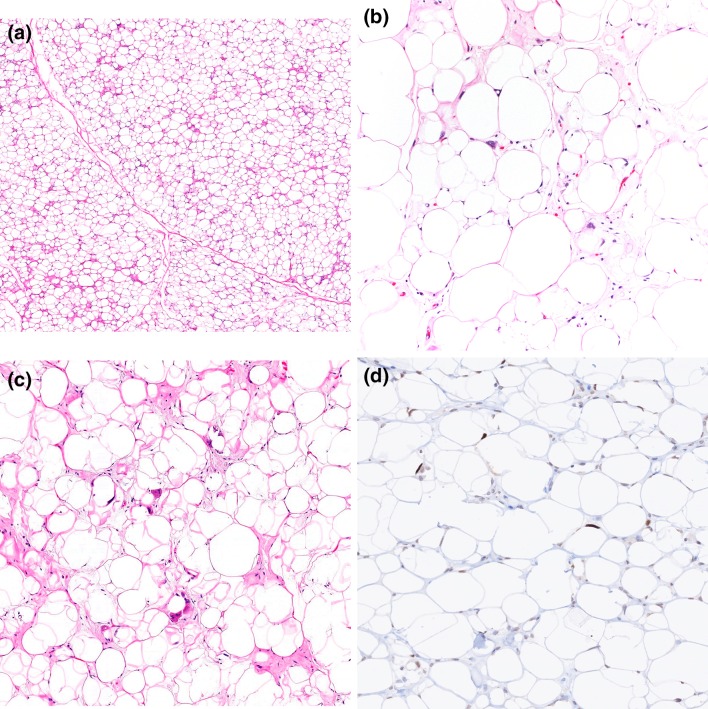
Grossly, ALT/WDL are typically multilobular and appear well-circumscribed. Three main microscopic variants (lipoma-like, sclerosing, and inflammatory) have been described, but the subtypes are not prognostically different. Lipoma-like WDL is composed of adipocytes of varying sizes and shapes, atypical hyperchromatic cells, and rare lipoblasts (Fig. 2a). Lipoblasts may be entirely absent and their identification is not required for diagnosis. Mitotic figures are rare. Sclerosing WDL often shows scattered hyperchromatic pleomorphic and floret-like cells within wide bands and sheets of collagen (Fig. 2b). The presence of dense chronic inflammatory infiltrate with lymphoid follicles can obscure the adipocytic nature of the tumor in the inflammatory type (Fig. 2c). DDL are usually large, and the cut surface typically reveals a distinct firm nodule in the context of adipose tissue. The transition from ALT/WDL to DDL is usually abrupt. Rarely, the two components are intermingled and may show gradual transition [3]. Morphologically, dedifferentiated areas are most often composed of non-lipogenic, high-grade undifferentiated pleomorphic and/or spindle cells arranged in sheets and fascicles (Fig. 2d). Less commonly, DDL with “low-grade dedifferentiation” exhibit relatively uniform fibroblast-like spindle cells with mild cytologic atypia and minimal mitotic activity, mimicking fibromatosis or low-grade fibromyxoid sarcoma [3]. Heterologous differentiation toward leiomyosarcoma, rhabdomyosarcoma, and/or osteosarcoma occurs in about 5 to 10% of cases [22, 23]. Meningothelial-like whorls (often associated with metaplastic bone formation) have also been documented [24, 25]. Rarely, DDL contain pleomorphic lipoblasts within the dedifferentiated component (so-called “homologous dedifferentiation”) [26, 27]. Cytogenetically, ALT/WDL and DDL demonstrate giant ring and long marker chromosomes containing an amplified sequence of chromosome 12 region 12q13-15, detectable by fluorescence in situ hybridization (FISH) for genes, such as MDM2, CDK4, and CPM [17] (Fig. 3a). Expression of MDM2 and CDK4 immunohistochemistry may play a diagnostic role (Fig. 3b, c), although caution is needed as both these markers can be positive in histiocytes, which may be prominent in areas of fat necrosis [28, 29]. A subset of DDL can also show nuclear expression of STAT6, which could be a diagnostic pitfall [30]. DDL variably express CD34, actins, and desmin.
Fig. 2.
Lipoma-like WDL is composed mature adipose tissue and hyperchromatic pleomorphic cells, which is most often seen in the fibrous stroma (a). Sclerosing WDL shows fibrillary or sclerotic collagenous stroma containing similar scattered atypical cells (b). Inflammatory WDL displays a prominent chronic inflammatory infiltrate (c). DDL can have a variety of morphologic appearances. High-grade DDL with the appearance of undifferentiated pleomorphic sarcoma (d)
Fig. 3.
MDM2 amplification (red signal) compared with centromere reference probe (green signal) in DDL demonstrated by fluorescence in situ hybridization (a). Immunohistochemical stain for MDM2 selectively highlights the lesional tumor cells (nuclear staining) in DDL with prominent inflammation (b, c)
Myxoid liposarcoma represents the second most common liposarcoma subtype, accounting for 30 to 35% of all liposarcomas [3]. Myxoid and round cell liposarcoma are low and high-grade forms of the same neoplasm. Therefore, the most recent WHO classification eliminated the “round cell” terminology [17]. Clinically, myxoid liposarcoma occurs in the lower limbs, particularly the thigh. Previous studies have also demonstrated that it rarely develops in the head and neck region [15, 16]. The highest prevalence is between the third and fifth decades of life, and it affects both sexes equally. Most patients complain of a slow-growing, painless soft tissue mass. While the prognosis is related to grade, which, in turn, is based on the fraction of round cell component, myxoid liposarcoma tends to recur repeatedly and to metastasize to both bone and soft tissue sites, including the retroperitoneum. The fraction of high-grade round cell component should be documented in all specimens, although this can be difficult in small core biopsies. The mainstay of treatment is wide surgical resection with or without adjuvant therapy (radiotherapy, chemotherapy, or both). Clinical trials have demonstrated that two agents, trabectedin and eribulin, are extremely effective in the treatment of advanced myxoid liposarcoma [31].
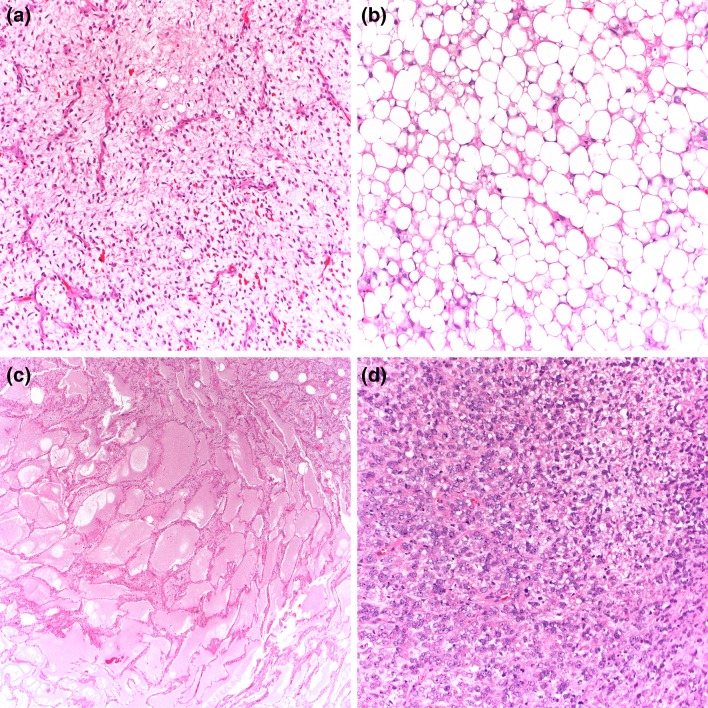
Myxoid liposarcomas are usually multinodular and may appear encapsulated. The cut surface varies from gelatinous to tan-white with hemorrhagic areas. Microscopically, low-grade myxoid liposarcomas are composed of uniform, round to oval, spindle cell proliferation, and small lipoblasts (usually univacuolated), accompanied by abundant myxoid background and the presence of a thin-walled, capillary-sized vascular network, organized in a distinctive plexiform pattern (Fig. 4a). Prominent nucleoli and mitotic figures are not typically present. Tumors rarely exhibit extensive adipocytic differentiation (differentiated myxoid liposarcoma) (Fig. 4b) [32]. In high-grade myxoid liposarcoma (round cell liposarcoma), the characteristic myxoid matrix and plexiform capillary networks are diminished by tightly packed tumor cells showing increased nuclear size, frequent mitotic activity, and nuclear overlapping (Fig. 4d). Immunohistochemically, myxoid liposarcoma often demonstrates S-100 protein expression, similar to other adipocytic tumors. Nearly all low and high-grade myxoid liposarcomas contain a specific t(12;16) or t(12;22), resulting in fusion of FUS-DDIT3 or EWSR1-DDIT3, respectively, supporting a molecular basis for morphologic continuum of tumor progression [17]. Molecular testing is not always necessary for diagnosis, but can be helpful to differentiate low-grade myxoid liposarcoma from lipoblastoma (showing PLAG1 gene aberrations or, very rarely, HMGA2 gene rearrangement [33, 34]) and in the diagnosis of high grade myxoid liposarcoma when the characteristic vasculature and myxoid stroma is less apparent. Molecular testing may also be necessary with small biopsies.
Fig. 4.
Low-grade myxoid liposarcoma is composed of relatively uniform, round to oval cells and small lipoblasts embedded in a prominent myxoid stroma with a delicate arborizing capillary vascularture (a). Myxoid liposarcoma with extensive adipocytic differentiation (differentiated myxoid liposarcoma) (b). Myxoid matrix showing characteristic pulmonary edema-like pattern (c). High-grade myxoid liposarcoma also contains areas of primitive round cells in solid sheets (d)
Pleomorphic liposarcoma is the least common variant of liposarcoma (< 15% of all liposarcomas [3]). It appears in adulthood and typically occurs in the deep soft tissues of the limbs. Approximately 25% of the cases develop in the skin or the subcutaneous tissue [35]. There are only a small number of reports of pleomorphic liposarcoma in the head and neck region [15, 16]. By definition, it is a high-grade, pleomorphic sarcoma with at least focal lipogenic differentiation. Tumors are typically treated by wide surgical resection with or without adjuvant radiation therapy or chemotherapy. The metastatic rate is between 30 and 50%, and the overall mortality rate ranges from 40 to 50%; adverse prognostic factors include central location, origin in deep soft tissue, and large size [36, 37]. Cutaneous and superficial pleomorphic liposarcomas have a favorable prognosis [35].
Microscopically, pleomorphic liposarcoma is characterized by the presence of areas resembling undifferentiated pleomorphic sarcoma with univacuolated and/or multivacuolated lipoblasts (Fig. 5a, b). It shows an infiltrative growth pattern at the periphery. The mitotic index is usually high and areas of necrosis may be found. Some examples also show prominent epithelioid morphology (epithelioid variant) [38] or myxoid stromal changes. In general, immunohistochemistry may have a limited role in the diagnosis of pleomorphic liposarcoma. Lipogenic areas are often immunoreactive for S-100 protein, and the background pleomorphic neoplastic cells may show variable expression of actins, desmin, CD34, and CD68. In the epithelioid variant, the tumor cells are frequently positive for keratins and Melan-A [37]. Both MDM2 and CDK4 are characteristically negative in pleomorphic liposarcomas, which is diagnostically helpful in separating them from WDL/DDL. There are no consistent or specific cytogenetic abnormalities within this subgroup of liposarcoma [39], and they display complex karyotypes similar to other high-grade pleomorphic sarcomas. More recent NGS-based analyses have shown the presence of TP53 (17%) and NF1 (8%) mutations [39, 40]. Molecular characteristics of liposarcoma subtypes are summarized in Table 1.
Fig. 5.
Histopathologic features of pleomorphic liposarcoma showing sheets of high-grade undifferentiated pleomorphic tumor cells (a). Pleomorphic lipoblasts are a defining feature (b)
Table 1.
Key genetic alterations in liposarcoma subtypes
| Tumor type | Cytogenetic alterations | Associated genes |
|---|---|---|
| Atypical lipomatous tumor/well-differentiated liposarcoma | Ring or giant marker chromosomes (12q13-15) | Amplification of MDM2, CDK4, HMGA2, YEATS4, FRS2, PTPRQ, DDIT3 and others |
| Dedifferentiated liposarcoma | Ring or giant marker chromosomes (12q13-15) | Amplification of MDM2, CDK4, HMGA2, YEATS4, FRS2, PTPRQ, DDIT3 and others |
| Myxoid liposarcoma |
t(12;16)(q13;p11) t(12;22)(q13;q12) |
FUS-DDIT3 EWSR1-DDIT3 |
| Pleomorphic liposarcoma | Complex karyotypic alterations | Modification of TP53, NF1, and RB1 |
Recently Described Adipocytic Tumors
Anisometric Cell Lipoma (Dysplastic Lipoma)
In 2015, subcutaneous minimally atypical lipomatous tumor with various fat cell size was first described by Evans [41]. In that seminal report, he reported 13 cases of a peculiar subcutaneous adipocytic lesion (predominantly located in the head and neck region) that were often misdiagnosed as ALT/WDL. Evans subsequently used the term “anisometric cell lipoma” to avoid confusion with other lipomatous tumors [42]. More recently, Michal et al. highlighted its additional immunohistochemical and genetic features and coined the term “dysplastic lipoma” [43].
Clinically, anisometric cell lipoma develops in middle-aged adults with a strong male predilection. The tumor typically presents as a subcutaneous mass on the posterior neck, upper back, or shoulders. However, involvement of the breast, axilla, and genital areas has also been reported [42, 43]. The majority of cases are solitary, but approximately 20% are multifocal [42, 43]. Rare cases have been reported in patients with a known history of retinoblastoma [42, 43]. The clinical course is typically benign and there is no documented dedifferentiation. Given that local recurrences have been observed, complete surgical excision is generally recommended.
These tumors are often well-circumscribed and show a lobulated cut surface. The median size is approximately 5 cm, but cases exceeding 10 cm have been reported [43]. Microscopically, anisometric cell lipomas demonstrate a constellation of distinct morphologic features, including highly variable adipocyte size and shape, frequent microscopic foci of fat necrosis, minimal nuclear atypia, and Lochkern change (Fig. 6a–c). Enlarged hyperchromatic stromal cells and thickened fibrous septa, as seen in ALT/WDL, are absent. Immunohistochemistry often shows a diffuse loss of RB1 nuclear expression in the adipocytic cell nuclei, correlating with RB1 gene deletion. Occasional cells with nuclear atypia are positive for p53 (Fig. 6d), but there was no evidence of TP53 mutations in the previously tested cases [43]. The tumor cells are negative for both MDM2 and CDK4 protein overexpression and gene amplification, although weak focal MDM2 or CDK4-positive histiocytic cells may be present, particularly in areas of fat necrosis (Table 2).
Fig. 6.
Anisometric cell lipoma (dysplastic lipoma). The tumor demonstrates significant adipocytic variation with minimal cytologic atypia (a), Lochkern change (b), and areas of mild fat necrosis and histiocytic cells (c). Occasional atypical adipocytic nuclei with p53 expression (d)
Table 2.
Recently described adipocytic tumors
| Clinical features | Morphologic features | Immunophenotype | Genetic abnormality | |
|---|---|---|---|---|
| Anisometric cell lipoma (dysplastic lipoma) | Adults; M > F; subcutis or superficial soft tissue; often posterior neck and upper trunk | Mature adipocytes with significant variation in size and shape, accompanied by frequent microscopic foci of fat necrosis, minimal nuclear atypia, and Lochkern change | Loss of RB1 expression (complete or partial), +p53 | Heterozygous RB1 deletion |
| Atypical spindle cell/pleomorphic lipomatous tumor | Adults; M > F; superficial or deep soft tissue; extremities (mainly distal) and limb girdles, but can involve any part of the body (including body cavities and viscera) | Various proportions of spindle cells, pleomorphic (multinucleated) cells, adipocytes, and/or lipoblasts with collagenous to myxoid stoma and infiltrative borders | +CD34 (majority), loss of RB1 (about 50%), +S-100 (variable), +desmin (variable) | Deletions/losses of 13q14 (RB1, RCBTB2, ITM2B, and DLEU1), monosomy 7 |
Atypical Spindle Cell/Pleomorphic Lipomatous Tumor
According to the last WHO Classification of Tumours of Soft Tissue and Bone of 2013, spindle cell liposarcoma is merely a variant of well-differentiated liposarcoma [17]. However, this concept has undergone significant change over the past few decades, and it remains a controversial designation that encompasses tumors showing various morphologic features. More recently, Marino-Enriquez et al. and Creytens et al. coined the term “atypical spindle cell/pleomorphic lipomatous tumor” to describe a large series of atypical low-grade adipocytic neoplasms without MDM2 amplification, characterized by atypical spindle/pleomorphic cells, frequent lipoblasts, and an infiltrative growth pattern [44, 45].
Atypical spindle cell/pleomorphic lipomatous tumors occur primarily in middle-aged adults, but can occur at any age. Lesions show a predilection for the extremities, mainly distal, and present in both subcutis and subfascial tissue [44–46]. Less common locations are the head and neck, trunk, and genital areas. Rare cases occur in the body cavities and viscera [44]. Approximately 15% of cases recur locally, but they have virtually no capacity for metastasis or dedifferentiation [44–46]. Complete surgical resection is the therapy of choice.
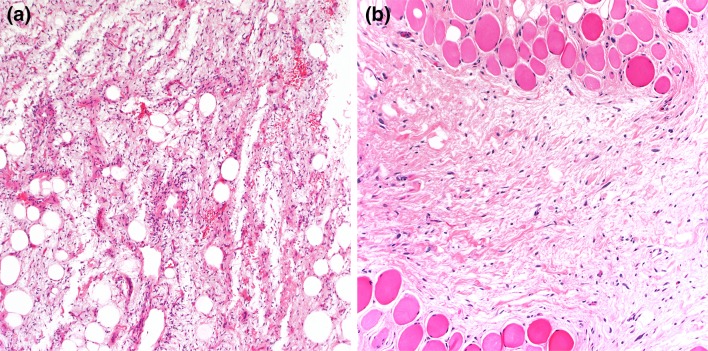
Grossly, atypical spindle cell/pleomorphic lipomatous tumors are unencapsulated, solitary nodular masses of variable size, 0.5 to 28 cm (mean, 5 cm). Microscopically, tumors are composed of various proportions of spindle cells, pleomorphic (multinucleated) cells, adipocytes, lipoblasts, and a collagenous to myxoid stroma (Fig. 7a, b). Cytologic atypia is often conspicuous within the lesional cells; in some areas, the lipoblasts have “bizarre” nuclei with various cytoplasmic vacuolization. Mitotic figures can be seen, but necrosis is absent. Within the adipocytic component, there is greater variability in adipocyte size. By immunohistochemistry, the lesional cells express CD34 and may also exhibit S-100 protein and desmin expression. Notably, loss of RB1 nuclear expression is seen in approximately 50–70% of cases. Although focal and weak MDM2 or CDK4 protein overexpression may be observed, the tumor cells are consistently negative for MDM2/CDK4 amplification. Cytogenetically, the majority have been shown to contain the presence of 13q14 deletions, including RB1 and its flanking genes RCBTB2, DLEU1, and ITM2B, suggesting a potential relationship with conventional spindle cell/pleomorphic lipoma [44]. Monosomy 7 also has been documented in some cases [47] (Table 2).
Fig. 7.
Atypical spindle cell lipomatous tumor presents as a large deep soft tissue. The tumor is composed of scattered adipocytes and mildly atypical spindle cells in a myxoid stroma (a). It often exhinits an infiltrative growth pattern (b)
Funding
This study has no funding source.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Enzinger FM, Harvey DA. Spindle cell lipoma. Cancer. 1975;36:781–788. doi: 10.1002/1097-0142(197511)36:5<1852::AID-CNCR2820360542>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 2.Shmookler BM, Enzinger FM. Pleomorphic lipoma: a benign tumor simulating liposarcoma. A clinicopathologic analysis of 48 cases. Cancer. 1981;47(1):126–133. doi: 10.1002/1097-0142(19810101)47:1<126::AID-CNCR2820470121>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.Goldblum JR, Folpe AL, Weiss SW, Enzinger FM. Weiss’s soft tissue tumors. Philadelphia: Mosby; 2014. pp. 703–732. [Google Scholar]
- 4.Billings SD, Henley JD, Summerlin DJ, et al. Spindle cell lipoma of the oral cavity. Am J Dermatopathol. 2006;28:28–31. doi: 10.1097/01.dad.0000189615.13641.4b. [DOI] [PubMed] [Google Scholar]
- 5.Lau SK, Bishop JA, Thompson LD. Spindle cell lipoma of the tongue: a clinicopathologic study of 8 cases and review of the literature. Head Neck Pathol. 2015;9(2):253–259. doi: 10.1007/s12105-014-0574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheah A, Billings SD, Goldblum JR, et al. Spindle cell/pleomorphic lipomas of the face: an under-recognized diagnosis. Histopathology. 2015;66:430–437. doi: 10.1111/his.12548. [DOI] [PubMed] [Google Scholar]
- 7.Fanburg-Smith JC, Devaney KO, Miettinen M, et al. Multiple spindle cell lipomas: a report of 7 familial and 11 nonfamilial cases. Am J Surg Pathol. 1998;22:40–48. doi: 10.1097/00000478-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Billings SD, Folpe AL. Diagnostically challenging spindle cell lipomas: a report of 34 “low-fat” and “fat-free” variants. Am J Dermatopathol. 2007;29(5):437–442. doi: 10.1097/DAD.0b013e31813735df. [DOI] [PubMed] [Google Scholar]
- 9.Mandahl N, Hoglund M, Mertens F, et al. Cytogenetic aberrations in 188 benign and borderline adipose tissue tumors. Genes Chromosom Cancer. 1994;9:207–215. doi: 10.1002/gcc.2870090309. [DOI] [PubMed] [Google Scholar]
- 10.Dal Cin P, Sciot R, Polito P, et al. Lesions of 13q may occur independently of deletion of 16q in spindle cell/pleomorphic lipomas. Histopathology. 2013;31:222–225. doi: 10.1046/j.1365-2559.1997.2450851.x. [DOI] [PubMed] [Google Scholar]
- 11.Maggiani F, Debiec-Rychter M, Vanbockrijck M, et al. Celluar angiofibroma: another mesenchymal tumour with 13q14 involvement, suggesting a link with spindle cell lipoma and (extra)-mammary myofibroblatoma. Histopathology. 2007;51:410–412. doi: 10.1111/j.1365-2559.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 12.Pauwels P, Sciot R, Croiset F, et al. Myofibroblastoma of the breast: genetic link with spindle cell lipoma. J Pathol. 2000;191:282–285. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH635>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 13.Agaimy A, Michal M, Giedl J, et al. Superficial acral fibromyxoma: clinicopathological, immunohistochemical, and molecular study of 11 cases highlighting frequent Rb1 loss/deletions. Hum Pathol. 2017;60:192–198. doi: 10.1016/j.humpath.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Chen BJ, Marino-Enriquez A, Fletcher CD, et al. Loss of retinoblastoma protein expression in spindle cell/pleomorphic lipomas and cytogenetically related tumros: an immunohistochemical study with diagnostic implications. Am J Surg Pathol. 2012;36:1119–1128. doi: 10.1097/PAS.0b013e31825d532d. [DOI] [PubMed] [Google Scholar]
- 15.Gerry D, Fox NF, Spruill LS, et al. Liposarcoma of the head and neck: analysis of 318 cases with comparison to non-head and neck sites. Head Neck. 2014;36:393–400. doi: 10.1002/hed.23311. [DOI] [PubMed] [Google Scholar]
- 16.Gritli S, Khamassi K, Lachkhem A, et al. Head and neck liposarcomas: a 32 years experience. Auris Nasus Larynx. 2010;37:347–351. doi: 10.1016/j.anl.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F (2013) World Health Organization Classification of Tumours of Soft Tissue and Bone
- 18.Waters R, Horvai A, Greipp P, et al. Atypical lipomatous tumour/well-differentiated liposarcoma and de-differentiated liposarcoma in patients aged ≤ 40 years: a study of 116 patients. Histopathology. 2019;75(6):833–842. doi: 10.1111/his.13957. [DOI] [PubMed] [Google Scholar]
- 19.Henrick WH, Chu YC, Goldblum JR, et al. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol. 1997;21:271–281. doi: 10.1097/00000478-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Dickson MA, Tap WD, Keohan ML, et al. Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J Clin Oncol. 2013;31:2024–2028. doi: 10.1200/JCO.2012.46.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Constantinidou A, Pollack SM, Jones RL. MDM2 inhibition in liposarcoma: a step in the right direction. Lancet Oncol. 2012;13:1070–1071. doi: 10.1016/S1470-2045(12)70457-6. [DOI] [PubMed] [Google Scholar]
- 22.Agaimy A, Michal M, Hadravsky L, et al. Dedifferentiated liposarcoma composed predominantly of rhabdoid/epithleioid cells: a frequently misdiagnosed highly aggressive variant. Hum Pathol. 2018;77:20–27. doi: 10.1016/j.humpath.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 23.Evans HL, Khurana KK, Kemp BL, et al. Heterologous elements in the dedifferentiated component of dedifferentiated liposarcoma. Am J Surg Pathol. 1994;18:1150–1157. doi: 10.1097/00000478-199411000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Fanburg-Smith JC, Miettinen M. Liposarcoma with meningothelial-like whorls: a study of 17 cases of a distinctive histological pattern associated with dedifferentiated liposarcoma. Histopathology. 1998;33:141–424. doi: 10.1046/j.1365-2559.1998.00536.x. [DOI] [PubMed] [Google Scholar]
- 25.Nascimento AG, Kurtin PJ, Guillou L, et al. Dedifferentiated liposarcoma: a report of nine cases with a peculiar neurallike whorling pattern asscoaited with metaplastic bone formation. Am J Surg Pathol. 1998;22:945–955. doi: 10.1097/00000478-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Boland JM, Weiss SW, Oliveira AM, et al. Liposarcomas with mixed well-differentiated and pleomorphic features: a clinicopathologic study of 12 cases. Am J Surg Pathol. 2010;34:837–843. doi: 10.1097/PAS.0b013e3181dbf2f7. [DOI] [PubMed] [Google Scholar]
- 27.Marino-Enriquez A, Fletcher CD, Dal Cin P, et al. Dedifferentiated liposarcoma with “homologous” lipoblastic (pleomorphic liposarcoma-like) differentiation: clinicopathologic and moelcular analysis of a series suggesting revised diagnostic criteria. Am J Surg Pathol. 2010;34:1122–1131. doi: 10.1097/PAS.0b013e3181e5dc49. [DOI] [PubMed] [Google Scholar]
- 28.Ng W, Messiou C, Smith M, et al. p16 expression in fat necrosis: a potential diagnostic pitfall in the evaluation of differentiated adipocytic neoplasms. Int J Surg Pathol. 2015;23:544–548. doi: 10.1177/1066896915595465. [DOI] [PubMed] [Google Scholar]
- 29.Stojanov IJ, Marino-Enriquez A, Bahri N, et al. Lipomas of the oral cavity: utility of MDM2 and CDK4 in avoiding overdiagnosis as atypical lipomatous tumor. Head Neck Pathol. 2019;13:169–176. doi: 10.1007/s12105-018-0928-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doyle LA, Tao D, Marino-Enriquez A. STAT6 is amplified in a subset of dedifferentiated liposarcoma. Mod Pathol. 2014;27:1231–1237. doi: 10.1038/modpathol.2013.247. [DOI] [PubMed] [Google Scholar]
- 31.Ratan R, Patel SR. Trabectedin and eribulin: where do they fit in the management of soft tissue sarcoma? Curr Treat Options Oncol. 2017;18:34. doi: 10.1007/s11864-017-0477-x. [DOI] [PubMed] [Google Scholar]
- 32.Iwasaki H, Ishiguro M, Nishio J, et al. Extensive lipoma-like changes of myxoid liposarcoma: morphologic, immunohistochemical, and molecular cytogenetic analyses. Virchows Arch. 2015;466:453–464. doi: 10.1007/s00428-015-1721-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dadone B, Refae S, Lemarie-Delaunay C, et al. Molecular cytogenetics of pediatric adipocytic tumors. Cancer Genet. 2015;208:469–481. doi: 10.1016/j.cancergen.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Pedeutour F, Deville A, Steyaert H, et al. Rearrangement of HMGA2 in a case of infantile lipoblastoma without Plag1 alteration. Pediatr Blood Cancer. 2012;58:798–800. doi: 10.1002/pbc.23335. [DOI] [PubMed] [Google Scholar]
- 35.Gardner JM, Dandekar M, Thomas D, et al. Cutaneous and subcutaneous pleomorphic liposarcoma: a clinicopathologic study of 29 cases with evaluation of MDM2 gene amplification in 26. Am J Surg Pathol. 2012;36:1047–1051. doi: 10.1097/PAS.0b013e3182517b96. [DOI] [PubMed] [Google Scholar]
- 36.Gebhard S, Coindre JM, Michels JJ, et al. Pleomorphic liposarcoma: clinicopathologic, immunohistochemical, and follow-up analysis of 63 cases: a study from the French Federation of Cancer Centers Sarcoma Group. Am J Surg Pathol. 2002;26:601–616. doi: 10.1097/00000478-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Hornick JL, Bosenberg MW, Mentzel T, et al. Pleomorphic liposarcoma: clinicopathologic analysis of 57 cases. Am J Surg Pathol. 2004;28:1257–1267. doi: 10.1097/01.pas.0000135524.73447.4a. [DOI] [PubMed] [Google Scholar]
- 38.Miettinen M, Enzinger FM. Epithelioid variant of pleomorphic liposarcoma: a study of 12 cases of a distinctive variant of high-grade liposarcoma. Mod Pathol. 1999;12:722–728. [PubMed] [Google Scholar]
- 39.Barretina J, Taylor BS, Banerji S, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42:715–721. doi: 10.1038/ng.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cancer Genome Atlas Research Network Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell. 2017;171(4):950. doi: 10.1016/j.cell.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evans HL (2015) Subcutaneous minimally atypical lipomatous tumors with variable fat cell size—a study of 13 cases. Mod Pathol 28 (suppl 2_:17A
- 42.Evans HL. Anisometric cell lipoma: a predominantly subcutaneous fatty tumor with notable variation in fat cell size but not more than slight nuclear enlargement and atypia. AJSP. 2016;21(4):195–199. [Google Scholar]
- 43.Michal M, Agaimy A, Contreras AL, et al. Dysplastic lipoma: a distinctive atypical lipomatous neoplasm with anisocytosis, focal nuclear atypia, p53 overexpression, and a lack of MDM2 gene amplification by FISH: a report of 66 cases demonstrating occasional multifocality and a rare association with retinoblastoma. Am J Surg Pathol. 2018;42:1530–1540. doi: 10.1097/PAS.0000000000001129. [DOI] [PubMed] [Google Scholar]
- 44.Marino-Enriquez A, Nascimento AF, Ligon AH, et al. Atypical spindle cell lipomatous tumor: clinicopathologic characterization of 232 cases demonstrating a morphologic spectrum. Am J Surg Pathol. 2017;41:234–244. doi: 10.1097/PAS.0000000000000770. [DOI] [PubMed] [Google Scholar]
- 45.Creytens D, Mentzel T, Ferdinande L, et al. “Atypical” pleomorphic lipomatous tumor: a clinicopathologic, immunohistochemical and molecular study of 21 cases, emphasizing its relationship to atypical spindle cell lipomatous tumor and suggesting a morphologic spectrum (atypical spindle cell/pleomorphic lipomatous tumor) Am J Surg Pathol. 2017;41:1443–1455. doi: 10.1097/PAS.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 46.Bahadir B, Behzatoglu K, Hacihasanoglu E, et al. Atypical spindle cell/pleomorphic lipomatous tumor: a clinicopathologic, immunohistochemical, and molecular study of 20 cases. Pathol Int. 2018;68:550–556. doi: 10.1111/pin.12719. [DOI] [PubMed] [Google Scholar]
- 47.Italiano A, Chambonniere ML, Attias R, et al. Monosomy 7 and absence of 12q amplification in two cases of spindle cell liposarcoma. Cancer Genet Cytogenet. 2008;184:99–104. doi: 10.1016/j.cancergencyto.2008.04.004. [DOI] [PubMed] [Google Scholar]