Abstract
A spectrum of mesenchymal neoplasms occur in the sinonasal region. One of these is solitary fibrous tumor (SFT), a translocation-associated neoplasm characterized by NAB2-STAT6 gene fusion. Sinonasal SFTs characteristically display CD34 immunopositivity, which aids in diagnosis. However, a small proportion of SFTs may be negative for CD34, making diagnosis difficult. The availability of STAT6 immunohistochemistry (IHC) has helped to overcome this. Malignant SFTs, characterized by increased cellularity and mitoses > 4 per ten high power fields, are extremely unusual in the sinonasal region, with only ten such cases reported to date. We report a case of a CD34-negative malignant SFT that was diagnosed using STAT6 IHC and confirmed by demonstrating NAB2 ex 4-STAT6 ex 2 fusion, and recurred 8 months after complete excision, to highlight the aggressive nature of this tumor.
Keywords: Spindle cell tumor, Nose, Hemangiopericytoma, Solitary fibrous tumor, STAT6
Introduction
Solitary fibrous tumor (SFT) is a mesenchymal tumor of fibroblastic origin, first described in the pleura, but which also occurs at various extra-pleural sites [1]. It is a translocation-associated neoplasm characterized by NAB2-STAT6 gene fusion, resulting in specific nuclear expression of STAT6 protein, which may be detected immunohistochemically [2]. Exceedingly rare in the sinonasal region, SFTs have been reported to account for < 0.1% of all sinonasal neoplasms [3]. Majority of these tumors present as slow growing masses which display indolent behavior, and are cured on complete resection [4]. Only ten reports are available in literature of sinonasal SFTs which showed malignant histological features [5–12]. Of these, STAT6 positivity was described in only the three most recent cases. We report the case of an elderly male with sinonasal malignant SFT confirmed by STAT6 immunohistochemistry and molecular testing, who developed local recurrence after complete excision.
Methods
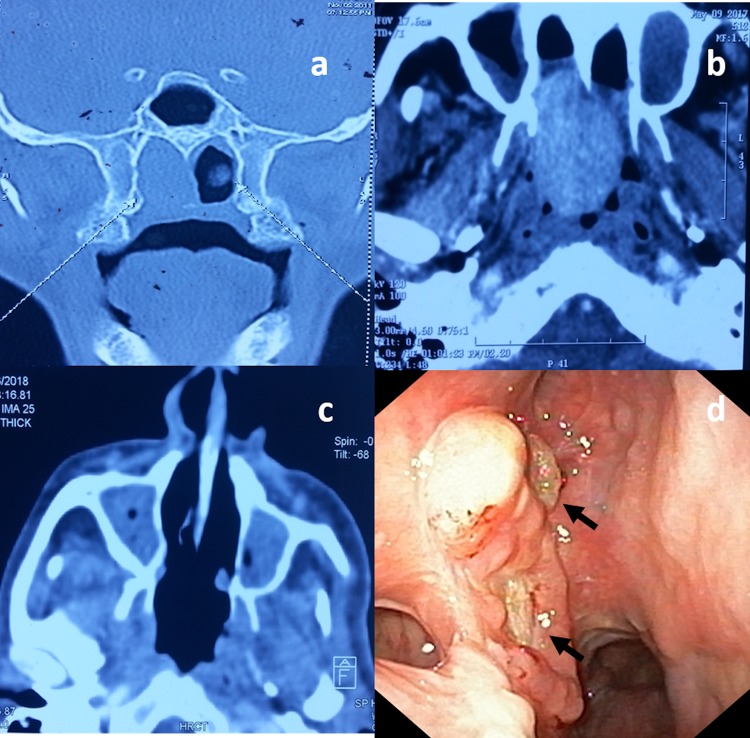
This 80 year-old male presented with complaints of nasal obstruction and occasional nasal bleeding from the right nostril for 7 years duration. He underwent an endoscopic surgery for the same complaints. Figure 1a demonstrates the radiological features at that time. A histopathological diagnosis of a spindle cell tumor was the only information available. Following this, his symptoms had subsided, until he presented to our centre 2 years ago with recurrence of earlier symptoms. Examination revealed a granular proliferative mass in the right posterior part of the nasal cavity extending into nasopharynx, which appeared to recur at the same site upon comparing with previous scans (Fig. 1b). HE stained slides from the first procedure were submitted for review.
Fig. 1.
Coronal CT scan revealing a mass filling the right posterior nasal cavity (a). Axial scans 5 years later revealing recurrence of the tumor at the same site (b). Axial scans showing no evidence of tumor at six months post-op (c). Nasal endoscopy at 8 months’ follow-up showing small granular lesions suggestive of local recurrence (d)
The patient underwent complete tumor excision via a transpalatal approach, and was asymptomatic after surgery. Radiology six months later revealed no evidence of tumor (Fig. 1c). At 8 months follow-up, endoscopy examination revealed a small granular lesion in right lateral wall of the nose close to pterygoid base (Fig. 1d), from which a biopsy was taken.
Results
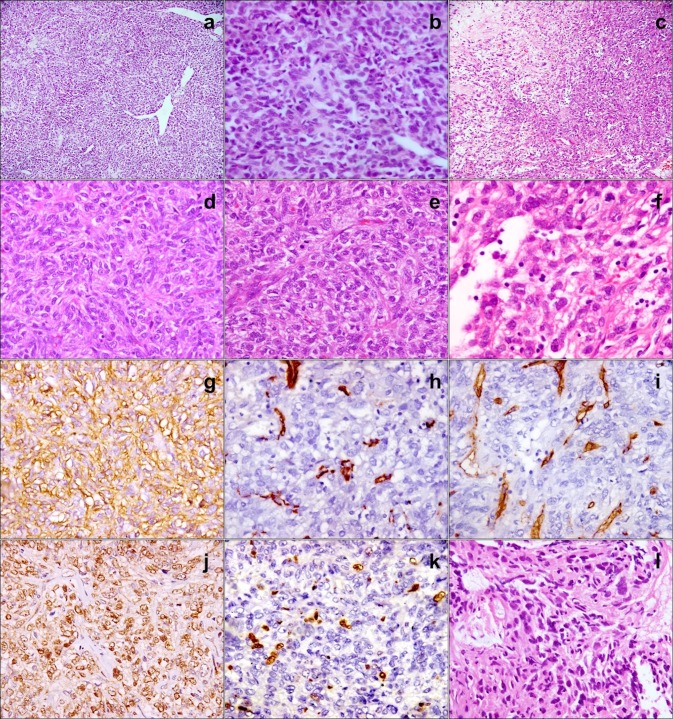
HE stained sections from the initial biopsy and the resection specimen showed similar features (Fig. 2), consisting of a cellular tumor composed of spindle shaped cells dispersed in a variably collagenous stroma. Occasional hypocellular areas were seen; however, most of the tumor was composed of areas with increased cellularity. Tumor cells were arranged in a pattern-less pattern. Individual cells were ovoid to spindle shaped, with indistinct cytoplasmic borders, elongated vesicular nuclei with moderate pleomorphism, and inconspicuous nucleoli. The initial biopsy showed 3–4 mitoses per 10 high power fields (hpf), while frequent mitotic figures were identified in the resected tumor (7–8/10 hpf). Variably sized, thin and thick-walled, including staghorn, vascular channels were interspersed between the tumor cells. Necrosis was not identified. Entrapped epithelial invaginations were absent. The excised tumor was infiltrating between bony trabeculae. Differential diagnoses included biphenotypic sinonasal sarcoma (BSS), SFT, synovial sarcoma, malignant glomangiopericytoma (GPC), spindle cell carcinoma and malignant peripheral nerve sheath tumor (MPNST) [4]. As paraffin blocks were not available, immunohistochemistry could not be performed on the first biopsy. The tumor cells from the resection specimen were immunopositive for vimentin, CD99, and bcl2. Smooth muscle actin, desmin and S-100 were negative, arguing against the diagnoses of BSS, GPC and MPNST. Pancytokeratin was negative, suggesting that synovial sarcoma and spindle cell carcinoma were remote possibilities. CD34 was also negative. Ki67 labeling index was 7–8%. A diagnosis of sinonasal malignant mesenchymal neoplasm was given.
Fig. 2.
Initial biopsy shows a spindle cell tumor with staghorn vasculature (a; HE, × 100) displaying increased cellularity and moderate pleomorphism (b; HE, × 400). Excised tumor shows hypo- and hypercellular areas (c; × 100); tumor cells with indistinct borders embedded in a collagenous stroma (d; HE, x) have enlarged vesicular nuclei with coarse chromatin and frequent mitoses (e; HE, × 400). Some large nuclei show prominent nucleoli (f; HE, 60 ×). CD99 is positive (g; × 400); SMA (h; x400) and CD34 (i; × 400) are negative; STAT6 shows diffuse nuclear positivity (j; × 400); Ki-67 labelling is increased (k; × 400). Recurrent tumor shows increased cellularity, nuclear hyperchromasia and pleomorphism (l; × 400)
Biopsy from the recurrent tumor showed similar features. The case was reviewed in its entirety by a head and neck pathologist. STAT6 IHC was performed on the excision specimen, which showed strong nuclear positivity clinching the diagnosis as SFT. Molecular confirmation was obtained by RT-PCR for NAB2-STAT6 fusion transcripts, performed as described previously [13], which revealed NAB2 ex 4- STAT6 ex 2 fusion (Fig. 3). The increased cellularity, pleomorphism, and elevated mitotic count argued in favor of this SFT being malignant.
Fig. 3.
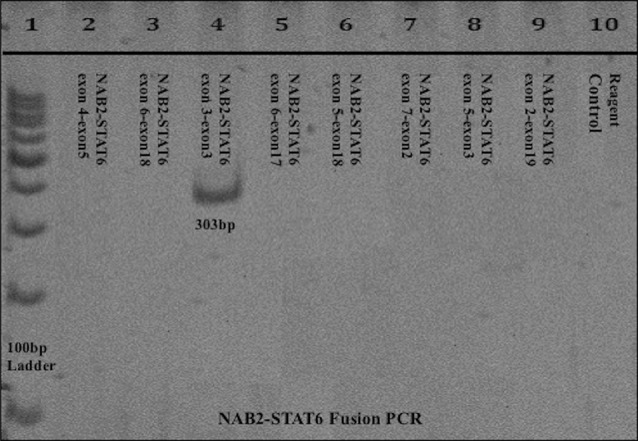
Conventional RT-PCR for NAB2-STAT6 fusion transcripts analyzed on 10% PAGE, Lane 1: 100 bp molecular weight marker; Lane 4: 303 bp band seen for NAB2-STAT6 exon 3-exon 3 fusion PCR (exon 4-exon 2 fusion type); none of the other lanes show bands; Lane 10: Reagent control
Whole body PET-CT revealed no evidence of metastasis. Due to the possible malignant behavior of the lesion, the patient was referred for adjuvant radiotherapy, but he refused further treatment. He is alive with disease 12 months after surgery.
Discussion
Extrapleural SFTs share histological, immunohistochemical, and molecular genetic features with pleural SFTs, indicating that they are all the same distinct entity. In the head and neck region, SFTs are usually described in the oral cavity, meninges and the orbit, with sinonasal SFTs being extremely infrequent [4–6]. The latter, with a little less than 100 cases reported till date, have been reported to account for < 0.1% of all upper respiratory tract neoplasms, and 0.021% of all sinonasal neoplasms [2, 4, 7]. Han et al. identified four sinonasal SFTs, including two malignant, in their series of 53 SFTs from all sites, i.e. 7.5% [12].
Sinonasal SFTs occur in adults, with equal distribution among males and females [7, 14]. Patients present with symptoms of nasal obstruction and epistaxis. On examination, SFTs appear as firm, rubbery, grey-white polypoid masses [7]. Histologically, SFTs are characterized by submucosal, pattern-less arrangement of spindle shaped tumor cells in a collagen-rich stroma, with alternating hypo- and hypercellular areas, and interspersed branching “staghorn-shaped” blood vessels [2]. Sinonasal malignant SFTs, characterized by mitotic count > 4/ 10 hpf, hypercellularity, cellular atypia and necrosis, are extremely unusual, with only ten such cases reported to date (Table 1) [12, 15]. The differential diagnosis of sinonasal spindle cell tumors is wide, and may be resolved by immunohistochemistry [16].
Table 1.
Reported cases of malignant solitary fibrous tumor
| Author, year | Age/sex | Location | Histopathology diagnosis | IHC | Molecular testing | Management | Follow-up and outcome |
|---|---|---|---|---|---|---|---|
| Ganly et al. 2006 [5] | 78/F | 6 cm left front ethmoid tumor | Malignant SFT with > 4 mitoses per 10 HPF | CD34, bcl-2 + ve | ND | Anterior craniofacial resection followed by RT | NED at 16 months |
| Ganly et al. 2006 [5] | 51/F | 6 cm right nasal mass | Malignant SFT with mitoses > 4/10 HPF | CD34, bcl-2 + ve | ND | Endoscopic resection | NED at 12 months |
| Zietler et al. 2007 [6] | 70/M | 6.5 cm left nasal mass extending into orbit, maxillary sinus, and through cribriform plate | Malignant SFT with mitoses > 10/10 HPF and bone infiltration | Vimentin, CD34, bcl-2, CD99 + ve; S100, desmin -ve | ND | Anterior craniofacial resection with en bloc removal | Planned for EBRT, but diagnosed with colon carcinoma 5 weeks later, underwent colectomy and died postoperatively |
| Papadakis et al. 2011 [7] | 53/M | Mass in left nasal cavity and maxillary sinus | Atypical SFT with necrosis and mitoses > 4/10 HPF | Vimentin, CD34, bcl-2 + ve; CD99, actin -ve | ND | Endoscopic medial maxillectomy, anterior and posterior ethmoidectomy with en bloc removal | NED at 10 months |
| Subramaniam et al. 2011 [8] | 55/M | Nasal and ethmoid mass infiltrating frontal, sphenoid sinuses, cribriform plate | Dedifferentiated SFT | bcl-2, CD34, CD99 + ve (reduced in dSFT); S100, desmin, myogenin, SMA –ve; Ki-67 10% in SFT and 30% in dSFT | TP53 mutation in exon 7 in dSFT | Craniofacial resection | N/a |
| Xue et al. 2014 [9] | 18/F | N/a | Malignant SFT with bone invasion; mitotic count n/a | Vimentin, bcl-2, CD99 + ve; CD34 in scattered cells; S100 –ve; Ki67 28% | ND | Cytoreductive surgery | 4.5 cm residual tumor weeks post-op; IMRT (60 Gy/24F) and SBRT 12 Gy/ 3F; AWD at 4.5 years |
| Roy et al. 2015 [10] | 56/F | Mass in nasal cavity, bilateral ethmoid and frontal sinuses, infiltrating the basi-frontal area and left orbit | Initial: SFT Recurrence: malignant SFT with mitoses > 10/10 HPF and bone infiltration | N/a | ND | Gross total excision | Local recurrence at 5 years: near total excision and 3D conformal RT (54 Gy/ 30 F); PD 37 months after completion: salvage chemotherapy (ifosfamide, epirubicin x 6 cycles); SD |
| Kao et al. 2015 [11] | 56/M | Nasal cavity mass | Malignant SFT with mitoses 5/10 HPF | STAT6 + ve | NAB2 ex 4- STAT6 ex 2 fusion | N/a | NED at months |
| Han et al. 2015 [12] | 37/F | 2 cm left nasal cavity mass | Malignant SFT | CD99, STAT6 + ve; CD34, bcl-2 -ve | ND | N/a | N/a |
| Han et al. 2015 [12] | 57/M | 2 cm left nasal cavity mass | Malignant SFT | CD34, bcl-2, CD99, STAT6 + ve | ND | N/a | N/a |
| Present case | 80/M | Right nasal cavity mass, extending to nasopharynx | Malignant SFT with mitoses 7–8/10 HPF | Vimentin, CD99, bcl2, STAT6 + ve; CD34, S100, SMA, desmin -ve; Ki67 8% | NAB2 ex 4- STAT6 ex 2 fusion | Complete excision | Local recurrence at 8 months, AWD at 12 months |
AWD alive with disease; dSFT dedifferentiated SFT; HPF high power fields; N/a not available; NED no evidence of disease; PD progressive disease; RT radiotherapy; SD stable disease; SFT solitary fibrous tumor; SMA smooth muscle actin; +ve positive; -ve negative
Immunohistochemically, SFTs stain with CD34, CD99 and bcl-2, while they are negative for S100, smooth muscle actin and desmin [2, 3, 12]. However, these are not specific, and negative staining does not exclude SFT [1]. Interestingly, lack of CD34 staining, encountered in 5–10% of all SFTs, has been associated with malignant and dedifferentiated SFTs [12, 15, 17]. In recent years, SFTs have been shown to demonstrate strong nuclear STAT6 positivity, considered as a surrogate marker for identification of NAB2-STAT6 fusion which is unique to SFTs at all sites, irrespective of benign or malignant histology [12]. The availability of STAT6 immunohistochemistry has helped overcome the diagnostic challenges presented by the overlapping morphological features of mesenchymal tumors in the sinonasal region. More importantly, it is of utility in CD34-negative cases. Of the previously reported sinonasal malignant SFTs, only three cases have been confirmed with STAT6 IHC, one of which was also confirmed by molecular testing [11, 12]. In the present case, CD34 was negative, leading away from the diagnosis of SFT; the diagnosis was later confirmed by strong STAT6 positivity, and further supported by molecular confirmation.
Clinically, SFTs are believed to be slow-growing neoplasms, cured by surgical excision [5]. Extent of resection is the most significant prognostic factor, and complete resection is considered the gold standard of treatment [6, 7]. Adjuvant radiation therapy (RT) has been suggested in cases with uncertain margin clearance and malignant histology; however, its role is controversial [7, 10]. Patient age older than 55 years, tumor size greater than 15 cm, areas of necrosis, and mitotic count > 4/10 hpf are indicative of aggressive behavior [3, 4]. The scant number of reported cases of malignant SFT has made it difficult to assess the prognostic significance of malignant histology. While it was earlier believed that atypical or malignant features on histology were not indicative of malignant behavior clinically [7], this may not always be true, as local recurrence has been documented following complete excision of malignant SFT, and occurred in the present case as well [10].
The present case demonstrates the utility of STAT6 IHC in accurate diagnosis of sinonasal SFTs, particularly when CD34 is negative. It highlights the need for pathologists to identify features such as increased mitoses and elevated mitotic count in order to accurately diagnose malignant sinonasal SFTs to institute appropriate management, and highlights the capacity of these neoplasms for aggressive behavior. Due to the potential for recurrence, patients with sinonasal malignant SFT should be kept under close follow up. STAT6 IHC is a valuable surrogate marker to identify SFT when molecular testing is unavailable, and should be employed routinely in the immunohistochemical work-up of sinonasal spindle cell neoplasms.
Acknowledgements
The authors are grateful to the Division of Molecular Pathology, Department of Pathology, Tata Memorial Centre, Mumbai, India for performing molecular testing for NAB2-STAT6 fusion.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article is a case report and does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from the patient included in the study.
References
- 1.Vogels RJ, Vlenterie M, Versleijen-Jonkers YM, Ruijter E, Bekers EM, Verdijk MA, Link MM, Bonenkamp JJ, van der Graaf WT, Slootweg PJ, Suurmeijer AJ, Groenen PJ, Flucke U. Solitary fibrous tumor—clinicopathologic, immunohistochemical and molecular analysis of 28 cases. Diagn Pathol. 2014;9:224. doi: 10.1186/s13000-014-0224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agaimy A, Barthelmeß S, Geddert H, Boltze C, Moskalev EA, Koch M, Wiemann S, Hartmann A, Haller F. Phenotypical and molecular distinctness of sinonasal haemangiopericytoma compared to solitary fibrous tumour of the sinonasal tract. Histopathology. 2014;65:667–673. doi: 10.1111/his.12452. [DOI] [PubMed] [Google Scholar]
- 3.Flucke U, Thompson LDR, Wenig BM. Solitary Fibrous Tumour. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumors. Lyon: IARC; 2017. [Google Scholar]
- 4.Thompson LDR, Lau SK. Sinonasal tract solitary fibrous tumor: a clinicopathologic study of six cases with a comprehensive review of the literature. Head Neck Pathol. 2017;12:471480. doi: 10.1007/s12105-017-0878-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganly I, Patel SG, Stambuk HE, Coleman M, Ghossein R, Carlson D, Edgar M, Shah JP. Solitary fibrous tumors of the head and neck: a clinicopathologic and radiologic review. Arch Otolaryngol Head Neck Surg. 2006;132:517–525. doi: 10.1001/archotol.132.5.517. [DOI] [PubMed] [Google Scholar]
- 6.Zeitler DM, Kanowitz SJ, Har-El G. Malignant solitary fibrous tumor of the nasal cavity. Skull Base. 2007;17:239–246. doi: 10.1055/s-2007-984489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papadakis I, Koudounarakis E, Haniotis V, Karatzanis A. Veleg- rakis G. Atypical solitary fibrous tumor of the nose and maxillary sinus. Head Neck. 2013;35:E77–E79. doi: 10.1002/hed.21909. [DOI] [PubMed] [Google Scholar]
- 8.Subramaniam MM, Lim XY, Venkateswaran K, Shuen CS, Soong R, Petersson F. Dedifferentiated solitary fibrous tumour of the nasal cavity: the first case reported with molecular characterization of a TP53 mutation. Histopathology. 2011;59:1269–1274. doi: 10.1111/j.1365-2559.2011.03997.x. [DOI] [PubMed] [Google Scholar]
- 9.Xue Y, Chai G, Xiao F, Wang N, Mu Y, Wang Y, et al. Post- operative radiotherapy for the treatment of malignant solitary fibrous tumor of the nasal and paranasal area. Jpn J Clin Oncol. 2014;44:926–931. doi: 10.1093/jjco/hyu100. [DOI] [PubMed] [Google Scholar]
- 10.Roy S, Mallick S, Kakkar A, Jana M, Julka PK. Recurrent malig- nant sino-nasal solitary fibrous tumor: eliminate the enemy at the first instance. J Cancer Res Ther. 2015;11:650. doi: 10.4103/0973-1482.138045. [DOI] [PubMed] [Google Scholar]
- 11.Kao YC, Lin PC, Yen SL, Huang SC, Tsai JW, Li CF, et al. Clin- icopathological and genetic heterogeneity of the head and neck solitary fibrous tumours: a comparative histological, immuno- histochemical and molecular study of 36 cases. Histopathology. 2016;68:492–501. doi: 10.1111/his.12772. [DOI] [PubMed] [Google Scholar]
- 12.Han Y, Zhang Q, Yu X, Han X, Wang H, Xu Y, et al. Immunohistochemical detection of STAT6, CD34, CD99 and BCL-2 for diagnosing solitary fibrous tumors/hemangiopericytomas. Int J Clin Exp Pathol. 2015;8:13166–13175. [PMC free article] [PubMed] [Google Scholar]
- 13.Rekhi B, Shetty O, Tripathi P, Bapat P, Ramadwar M, Bajpai J, et al. Molecular characterization of a series of solitary fibrous tumors, including immunohistochemical expression of STAT6 and NAB2-STAT6 fusion transcripts, using Reverse Transcriptase(RT)-Polymerase chain reaction(PCR) technique: an Indian experience. Pathol Res Pract. 2017;213:1404–1411. doi: 10.1016/j.prp.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Janjua A, Sklar M, Macmillan C, Vescan A, Witterick IJ. Endoscopic resection of solitary fibrous tumors of the nose and paranasal sinuses. Skull Base. 2011;21:129–134. doi: 10.1055/s-0031-1275259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fletcher CDM, Bridge JA, Lee JC. Extrapleural solitary fibrous tumor. In: Fletcher CDM, Bridge JA, Hogendoom PCW, Mertens F, editors. WHO Classification of Tumors of Soft Tissue and Bone. Lyon: IARC; 2013. pp. 80–82. [Google Scholar]
- 16.Kakkar A, Rajeshwari M, Sakthivel P, Sharma MC, Sharma SC. Biphenotypic sinonasal sarcoma: a series of six cases with evaluation of role of beta-catenin immunohistochemistry in differential diagnosis. Ann Diagn Pathol. 2018;33:6–10. doi: 10.1016/j.anndiagpath.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Geramizadeh B, Marzban M, Churg A. Role of immunohistochemistry in the diagnosis of solitary fibrous tumor, a review. Iran J Pathol. 2016;11:195–203. [PMC free article] [PubMed] [Google Scholar]