Abstract
Congenital granular cell epulis (CGCE) is an uncommon lesion of unclear pathogenesis. It occurs predominantly in female newborns with a predilection site of the maxillary alveolar ridge. The mass enlarges prenatally but the growth arrests after birth. Histologically, CGCE is characterized by a proliferation of polygonal cells with eosinophilic, granular cytoplasm and eccentric, bland-appearing nuclei. It closely resembles adult granular cell tumor (GCT) microscopically and S-100 immunostain is often helpful in distinguishing the two (S-100-positive in GCT and S-100-negative in CGCE). Clinically, the lesion should also be distinguished from entities such as infantile myofibroma, rhabdomyoma, melanotic neuroectodermal tumor of infancy, peripheral odontogenic fibroma, and neurofibroma. CGCE demonstrates an excellent prognosis and has not been associated with any syndromes/genetic defects or malignant transformation. Clinicians and pathologists should be familiar with this rare entity and its differential diagnosis for accurate diagnosis and management.
Keywords: Epulis, Congenital granular cell tumor, Pediatric
History
An otherwise healthy female newborn was evaluated for a midline maxillary soft tissue mass. The lesion was noted since birth with no increase in size. The pregnancy was uneventful and the lesion was not detected prenatally. Delivery occurred at 39 weeks. There was no pertinent family medical history. The parents were worried that the lesion would interfere with feeding.
Clinical Findings
Clinical examination revealed a well-circumscribed, firm, sessile lesion arising in the maxillary alveolar ridge. The surface is pink and smooth without ulceration. The patient did not show any dysmorphic features and no other lesions were identified. Diagnostic considerations included infantile myofibroma, congenital granular cell epulis (CGCE), and melanotic neuroectodermal tumor of infancy. The patient subsequently underwent simple excision of the lesion (excised to the periosteum level) under general anesthesia at the age of 6 days.
Diagnosis
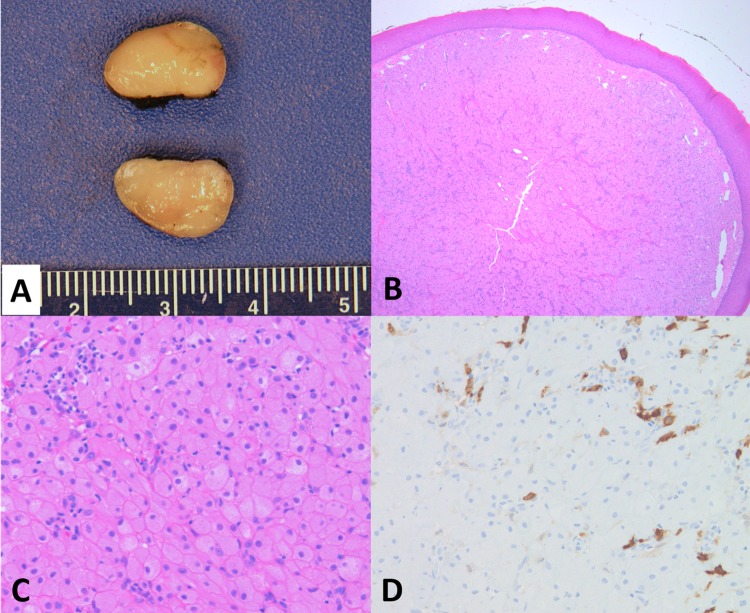
Gross examination revealed a well-circumscribed lesion measuring 1.2 × 0.9 × 0.8 cm (Fig. 1a). The cut surface was tan-yellow, smooth, and glistening. Histologically, the lesion was characterized by sheets of polygonal cells with an eosinophilic granular cytoplasm (Fig. 1b, c). The eccentric nuclei demonstrated a bland appearance. Angulated interstitial cells with scattered lymphocytic infiltrate were also noted. The lesional cells were immunoreactive for vimentin, while negative for S-100 immunostain (Fig. 1d). The pathologic findings were in keeping with congenital granular cell epulis. The resection margin was focally involved by the lesion histologically. Follow-up revealed alveolar ridges showing an unremarkable appearance with no recurrence at the time of this writing (6 months of post-excision follow-up period).
Fig. 1.
a Gross examination of CGCE shows a homogeneous, tan-yellow, and smooth cut surface. b Low power view of the lesion shows a lobulated mass with overlying, thin squamous epithelium (H&E, × 4). c The mass is characterized by proliferation of polygonal cells with eosinophilic, granular cytoplasm and eccentric, benign-appearing nuclei. Scattered interstitial and inflammatory cells are noted in the upper left of the image (H&E, × 10). d Unlike granular cell tumor in adults, lesional cells of CGCE are negative for S-100 immunostain; the S-100-positive cells in the image represent interstitial cells (× 10)
Discussion
CGCE is a benign lesion that is seen exclusively in newborn infants, often presenting as a mass on the anterior alveolar ridge of the maxilla and less commonly, on the mandible. While first described by Neumann in 1871, CGCE is uncommon with fewer than several 100 cases having been reported since then [1]. The histogenesis of CGCE has historically been unclear, although Schwann cells, fibroblasts, or mesenchymal cells have been hypothesized as strong candidates for the histological origin of CGCE [2]. Moreover, Childers et al. reported the presence of odontogenic epithelial rests in 20% of the cases, raising the possibility of odontogenic origin of CGCE [3].
Typically, the growth of the epulis manifests during the third trimester of pregnancy and completely arrests after birth [2]. As a result, the presence of the lesion has the potential to hinder proper breast feeding in newborns and is often resolved via surgical excision. Frequently, CGCE is detected in utero via the use of antepartum ultrasound or magnetic resonance imaging [4, 5]. The lesion presents overwhelmingly in females with a ratio of approximately 9–10:1 and because of this gender-specific predominance and its in utero origins, it has been theorized that the development of the CGCE may be closely linked to maternal hormones [2].
CGCE is usually an isolated finding without association with any syndromes or genetic abnormalities [2, 6]. It predominantly occurs as a solitary lesion, while multiple lesions are seen in 10% of the cases [2]. In multifocal cases, the lesions often involve both maxilla and mandible [7–9]. CGCE usually ranges from 1 to 2 cm in diameter, although larger masses up to 9 cm have been previously reported [10]. Clinically, it presents as a well-circumscribed, sessile or pedunculated lesion with a smooth, normal-colored surface (Fig. 2) [6].
Fig. 2.
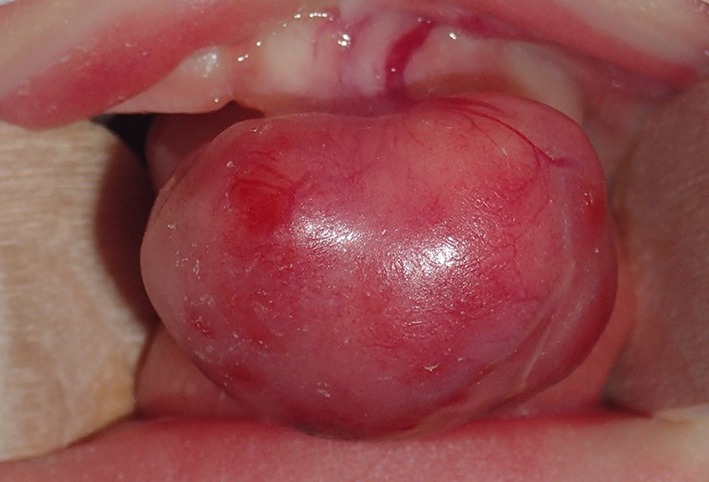
Epulis is characterized by a well-circumscribed lesion on the anterior alveolar ridge of a newborn infant showing a pink, smooth, and glistening external surface
Histologically, the lesion is comprised of sheets or nests of polygonal to slightly spindled cells with eosinophilic granular cytoplasm. The nuclei are eccentrically located with an overall bland appearance [2]. Angulated interstitial cells, cytoplasmic hyaline globules, odontogenic rests, and dilated vascular channels are occasionally identified in CGCE [2, 3]. The overlying squamous epithelium is usually thin without prominent rete ridges. A spindle cell variant of CGCE has been reported by Prigkos et al., characterized by the predominance of spindle cells, the lack of typical granular cells, and acanthotic overlying epithelium with broad rete ridges [11]. The origin of the interstitial cells is also unclear. Takahasi et al. demonstrated the neuroendocrine differentiation of these cells, while Vered et al. suggested that the interstitial cells represent the earlier stage of the granular cells [12, 13].
Recognizing the immunohistochemical profile of CGCE is helpful to render the diagnosis, particularly in cases with atypical morphology. CGCE tends to distinctively stain negative for S-100, laminin, CD34, CD68, NGFR/p75, inhibin-alpha, chromogranin, desmin, keratins, smooth muscle actin, CD31, and GLUT-1 and conversely stains positive for vimentin and neuron-specific enolase [2, 3, 14]. Proliferation index of CGCE determined by Ki-67 and PCNA immunostaining is reported to be 11.1–16.7% and 15.1–33.3%, respectively [15, 16].
Histologically, CGCE bears a great degree of similarity with adult granular cell tumors (GCT) and the two were once thought to be closely related. In most cases, GCT is distinguished from CGCE by its S-100 immunoreactivity. However, rare cases of S-100 negative GCT (so-called primitive polypoid non-neural GCT) of the oral cavity have also been reported in the literature [17, 18]. In addition, GCTs and CGCE can also be differentiated at both the anatomical and microscopic level. For instance, GCT occurs more frequently in the tongue, soft palate, and floor of the mouth [19] unlike CGCE. Furthermore, the overlying squamous epithelium of CGCE does not exhibit pseudoepitheliomatous hyperplasia, a trait which is commonly seen in GCT [13].
Clinically, major differential diagnoses of CGCE include rhabdomyoma, infantile myofibroma, melanotic neuroectodermal tumor of infancy, peripheral odontogenic fibroma, and neurofibroma, among others [2, 20, 21]. Many of these potential diagnoses also present in pre-natal stages but can be readily differentiated from CGCE due to their variation in anatomical location and histologic appearance. The clinicopathological features of CGCE and selected differential diagnoses are summarized in Table 1.
Table 1.
Clinicopathologic characteristics of congenital granular cell epulis and its differential diagnosis
| Lesion | Epidemiology | Predilection site | Histologic appearance | Immunohistochemistry |
|---|---|---|---|---|
| Congenital granular cell epulis |
Newborn F > M |
Alveolar ridge of the maxilla | Sheets of polygonal eosinophilic granular cells with overlying thin squamous epithelium | S-100 (−), vimentin (+) |
| Granular cell tumor [2] |
30–60 years F > M |
Tongue | Sheets of polygonal eosinophilic granular cells with overlying pseudoepitheliomatous hyperplasia of the squamous epithelium | S-100 (+), CD68 (+) |
| Rhabdomyoma [2] |
Extracardiac - adults M > F |
Extracardiac is rare (head and neck is the most common extracardiac site) |
Fetal subtype—spectrum of myocyte differentiation Adult subtype—polygonal eosinophilic cells with granular cytoplasm and cross striations |
Desmin (+), SMA (+) |
| Infantile myofibroma [2] |
Newborn-6 years M > F |
Tongue and buccal mucosa | Nodular proliferation with biphasic appearance: short fascicles of plump myofibroblasts in the periphery and central zones with hyperchromatic round-to-spindle cells and hemangiopericytoma-like vessels | Vimentin (+), SMA (+) |
| Melanotic neuroectodermal tumor of infancy [9] |
5 months (median) F = M |
Maxilla | Dual population of small neuroblastic cells and larger melanin-containing epithelial cells |
Epithelial cells: CK (+), EMA (+), vimentin (+), HMB-45 (+) Small neuroblastic cells: synaptophysin (+), NSE (+) |
| Peripheral odontogenic fibroma [10] |
Newborn-80 years F > M |
Mandible | Cellular connective tissue with multiple small islands and strands of odontogenic epithelium | CK (+) in the epithelium |
F female, M male, SMA smooth-muscle actin, CK cytokeratin, EMA epithelial membrane antigen, NSE neuron specific enolase
CGCE has an excellent prognosis with simple excision as the mainstay curative treatment [2]. Conservative monitoring is also acceptable in patients with smaller lesions as CGCE occasionally regresses spontaneously [6]. Recurrence after resection is uncommon and there has not been report of malignant transformation [2].
In conclusion, CGCE is a rare, benign growth on the alveolar ridge found exclusively in newborns with female predominance. The diagnosis is made based on a combination of characteristic clinical, histological, and immunohistochemical findings. Accurate diagnosis is important to achieve appropriate management and prognostication.
Acknowledgements
We thank Salim Afshar, DMD, MD, of Boston Children’s Hospital for providing the clinical image included in this article.
Compliance with Ethical Standards
Conflict of interest
No conflict of interest to disclose.
Research Involving Human Participants and/or Animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Neumann E. Ein fall von kongenitaler epulis. Arch Heilkd. 1871;12:189–190. [Google Scholar]
- 2.Conrad R, Perez MC. Congenital granular cell epulis. Arch Pathol Lab Med. 2014;138:128–131. doi: 10.5858/arpa.2012-0306-RS. [DOI] [PubMed] [Google Scholar]
- 3.Childres ELB, Fanburg-Smith JC. Congenital epulis of the newborn: 10 new cases of a rare oral tumor. Ann Diagn Pathol. 2011;15:157–161. doi: 10.1016/j.anndiagpath.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Kumar P, Kim HH, Zahtz GD, et al. Obstructive congenital epulis: prenatal diagnosis and perinatal management. Laryngoscope. 2002;112:1935–1939. doi: 10.1097/00005537-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Silva GC, Vieira TC, Vieira JC, et al. Congenital granular cell tumor (congenital epulis): a lesion of multidisciplinary interest. Med Oral Patol Oral Cir Bucal. 2007;12:E428-30. [PubMed] [Google Scholar]
- 6.Ritwik P, Brannon RB, Musselman RJ. Spontaneous regression of congenital epulis: a case report and review of the literature. J Med Case Rep. 2010;4:331. doi: 10.1186/1752-1947-4-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saki N, Araghi S. Multiple congenital epulis in alveolar ridges of maxilla and mandible in a newborn: a rare case report. Case Rep Otolaryngol. 2014;2014:606985. doi: 10.1155/2014/606985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang Y, Yang YS, Zhang Y. Multiple congenital granular cell epulis in a female newborn: a case report. J Med Case Rep. 2014;8:413. doi: 10.1186/1752-1947-8-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bianchi PR, de Araujo VC, Ribeiro JW, et al. Multiple congenital granular cell epulis: case report and immunohistochemical profile with emphasis on vascularization. Case Rep Dent. 2015;2015:878192. doi: 10.1155/2015/878192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eghbalian F, Monsef A. Congenital epulis in the newborn, review of the literature and a case report. J Pediatr Hematol Oncol. 2009;31:198–199. doi: 10.1097/MPH.0b013e31818ab2f7. [DOI] [PubMed] [Google Scholar]
- 11.Prigkos AC, Nikolakis MD, Kyriakopoulos VF. Spindle cell epulis in an 8-month-old child: a histologic variant of congenital granular cell epulis? Head Neck Pathol. 2012;6:467–470. doi: 10.1007/s12105-012-0351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi H, Fujita S, Satoh H, et al. Immunohistochemical study of congenital gingival granular cell tumor (congenital epulis) J Oral Pathol Med. 1990;19:492–496. doi: 10.1111/j.1600-0714.1990.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 13.Vered M, Dobriyan A, Buchner A. Congenital granular cell epulis presents an immunohistochemical profile that distinguishes it from the granular cell tumor of the adult. Virchows Arch. 2009;454:303–310. doi: 10.1007/s00428-009-0733-y. [DOI] [PubMed] [Google Scholar]
- 14.Souto GR, Caldeira PC, Johann AC. Evaluation of GLUT-1 in the granular cell tumor and congenital granular cell epulis. J Oral Pathol Med. 2013;42:450–453. doi: 10.1111/jop.12035. [DOI] [PubMed] [Google Scholar]
- 15.Zhang B, Tan X, Zhang K, et al. A study of cell proliferation using immunohistological staining: a case report of congenital granular cell epulis. Int J Pediatr Otorhinolaryngol. 2016;88:58–62. doi: 10.1016/j.ijporl.2016.06.047. [DOI] [PubMed] [Google Scholar]
- 16.Kato H, Nomura J, Matsumura Y, et al. A case of congenital granular cell epulis in the maxillary anterior ridge: a study of cell proliferation using immunoshitochemical staining. J Maxillofac Oral Surg. 2013;12:333–337. doi: 10.1007/s12663-011-0248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rawal YB, Dodson TB. S-100 negative granular cell tumor (so-called primitive polypoid non-neural granular cell tumor) of the oral cavity. Head Neck Pathol. 2017;11:404–412. doi: 10.1007/s12105-016-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solomon LW, Velez I. S-100 negative granular cell tumor of the oral cavity. Head Neck Pathol. 2016;10:3670–3673. doi: 10.1007/s12105-015-0673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narashimhan K, Arneja JS, Rabah R. Treatment of congenital epulis (granular cell tumor) with excision and gingivoperiosteoplasty. Can J Plast Surg. 2007;15:215–218. doi: 10.1177/229255030701500411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton S, MacRae D, Agrawal S, et al. Melanotic neuroectodermal tumour of infancy. Can J Plast Surg. 2008;16:41–44. doi: 10.1177/229255030801600108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martelli-Junior H, Mesquita RA, de Paula AM, et al. Peripheral odontogenic fibroma (WHO type) of the newborn: a case report. Int J Paediatr Dent. 2006;16:376–379. doi: 10.1111/j.1365-263X.2006.00738.x. [DOI] [PubMed] [Google Scholar]