Abstract
In the present study, a potential newly isolated thermotolerant acetic acid bacteria (TH-AAB), Acetobacter pasteurianus FPB2-3, with ethanol and acetic acid-tolerant properties was found to be very effective in the production of vinegar from pineapple peels as an alternative, inexpensive raw material using simultaneous vinegar fermentation (SVF). The results showed that using whole pineapple peel with the addition of diammonium phosphate (DAP) and MgSO4 at an initial pH of 5.5 gave a slightly higher acetic acid content than that produced from the squeezed juice. Subsequently, the effects of sugar concentration and inoculation time of A. pasteurianus FPB2-3 on acetic acid production were examined. The results revealed that an increase in sucrose concentration led to the high production of ethanol, which resulted in the suppression of acetic acid production. Allowing for the inoculated yeast to ferment prior to inoculation of the AAB for 1 or 2 days resulted in a longer lag time for ethanol oxidation. However, acetic acid accumulation commenced after 5 days and gradually increased to the maximum concentration of 7.2% (w/v) within 16 days. Furthermore, scaled-up fermentation in 6 l vessels resulted in slower acetic acid accumulation but still achieved a maximum acetic acid concentration of up to 6.5% (w/v) after 25 days. Furthermore, the antioxidant capacity of the vinegar produced from pineapple peels (PPV) was slightly higher than that produced from the squeezed juice (PJV), which was consistent with the higher total phenolic compound content found in the PPV sample. In addition to acetic acid, a main volatile acid present in vinegars, other volatile compounds, such as alcohols (isobutyl alcohol, isoamyl alcohol, and 2-phenyl ethanol), acids (3-methyl-butanoic acid), and esters (ethyl acetate, 3-methyl butanol acetate, and 2-phenylethyl acetate), were also detected and might have contributed to the observed differences in the odour and aroma of the pineapple vinegars.
Keywords: Vinegar, Pineapple waste product, Thermotolerant acetic acid bacteria, Volatile acid, Antioxidant
Introduction
Vinegar, a traditional fermented product, can be produced from a variety of raw materials, each resulting in a product with a unique taste and flavour. Acetic acid is identified as the predominant volatile acid, giving a strong aroma and sour flavouring. Thus, it has become widely used as a food preservative and food seasoning (Ho et al. 2017). In addition, it can be considered a beverage in terms of healthy drinks due to having beneficial effects in terms of digestion, appetite stimulation, anti-oxidative properties, recovery from exhaustion, lowering lipid content and regulating blood pressure (Laranjinha et al. 1994; Salbe et al. 2009; Chou et al. 2015). Generally, there are two steps of fermentation, including the anaerobic conversion of fermentable sugars to ethanol by yeasts, followed by the aerobic oxidation of ethanol to acetic acid by acetic acid bacteria (AAB). Submerged fermentation using a semi-continuous process is commonly employed in current industrial vinegar fermentation with a relatively fast acidification process. Traditional balsamic vinegar (TBV) is produced by surface fermentation with the spontaneous formation of ethanol and acetic acid by undefined starter culture, the so-called mother of vinegar (Gullo and Giudici 2008). To achieve a starter culture for controlling the quality of TBV, the interaction of the mother vinegar has been investigated (Solieri and Giudici 2008; Gullo et al. 2009). Some yeast strains are assumed to have a commensalistic interaction with AAB in TBV (Solieri and Giudici 2008), although evidence suggests that the growth of AAB may contribute to the problem of inhibited or incomplete alcohol fermentation (Drysdale and Fleet 1989). On the other hand, a high concentration of alcohol at a relatively low pH could suppress the capability of AAB, resulting in low acetic acid production (Krusong and Vichitraka 2010). Simultaneous fermentation is not able to promote maximum productivity due to the antagonistic effect caused by the different optimal growth conditions of AAB and yeast (Krusong and Vichitraka 2010). Recently, a new type of vinegar made with discarded fruit or vegetable raw materials has been produced (Horiuchi et al. 1999; Ye et al. 2004; Lee et al. 2013; Ubeda et al. 2013; Elijah and Etukudo 2016; Roda et al. 2017; De Leonardis et al. 2018). In addition, vinegar is an inexpensive commodity, so economic considerations require that a relatively low-cost raw material, such as by-products from food processing, fruit waste, substandard fruit and agricultural surpluses, be used (Solieri and Giudici 2008). The waste may contain valuable substances, such as pigments, sugars, organic acids, flavours, and bioactive compounds, such as antioxidants, enzymes, antimicrobial compounds, and fibres, that could be applied to bioprocesses to generate products with higher added value (Ghosh et al. 2016). Pineapple is an abundant agricultural product in tropical regions of the world, including Thailand. The fruit juice from pineapple is the third most preferred worldwide, after orange and apple juices (Cabrera et al. 2000). During processing, pineapple waste is generated as a by-product, including residual pulp, peels, and skin, constituting approximately 50% (w/w) of the total pineapple weight (Ketnawa et al. 2012). Pineapple waste is found to be a potential raw material in various forms, which can be used as a nutrient substance in culture broth for microbes that can be concomitantly converted into value-added products. Recently, we attempted to isolate thermotolerant acetic acid bacteria (TH-AAB) from fermented plant beverages and revealed that several TH-AAB possess high tolerance to acetic acid and ethanol (manuscript submitted elsewhere). In this study, one of those TH-AAB, A. pasteurianus FPB2-3, was utilised to produce acetic acid through simultaneous vinegar fermentation (SVF) by co-culture with Saccharomyces cerevisiae var. montache using pineapple peels as the starting raw material. The effect of inoculation time on AAB and initial sugar concentration on acetic acid production was also investigated. In addition, several properties of the vinegar obtained from pineapple peels were also discussed.
Materials and methods
Microorganisms
Acetobacter pasteurianus FPB2-3 isolated from fermented plant beverages was cultured on potato medium (Soemphol et al. 2012). Saccharomyces cerevisiae var. montache was kindly provided by Asst. Prof. Noppon Lertwattanasakul, Department of Microbiology in the Faculty of Science, Kasetsart University, Thailand, which was cultured on yeast malt extract (YM) medium. The pre-culture of each strain was cultured at 30 °C for 18 h. Pineapple (Ananas comosus) was acquired locally from Nong Khai and used in this study. Pineapple peel was obtained from a local processing plant in Amphoe Si Chiang Mai in Nong Khai. After collection, samples were kept at − 20 °C until use.
Preparation of fermentation medium
Pineapple peels were manually cut into small pieces using a knife followed by chopping in an electric blender to obtain a homogeneous mixture. The pineapple peels were chopped using a blender with water at a ratio of 1:3 by weight. The filtrate was obtained by passing through three layers of cotton cloth and designated pineapple peel juice (PJ), moreover, the whole minced pineapple peel was also used directly for medium (PP). The media were adjusted to the appropriate sugar content by adding sucrose at a final concentration of 140 g/l. To examine the effect of the medium on acetic acid production, diammonium phosphate (DAP) and MgSO4 were supplemented at a final concentration of 2 g/l. The initial pH was adjusted by adding NH4OH. The media were then sterilised by autoclaving at 110 °C for 10 min.
Simultaneous fermentation conditions
Simultaneous fermentation was carried out under static conditions at 30 °C. For fermentation, we examined the effect of the pineapple peel medium, initial sugar concentration, and inoculation time for AAB on acetic acid production ability.
To investigate the effect of the pineapple medium, four kinds of media were prepared as mentioned above by including a medium prepared from squeezed juice that was supplemented with 2 g/l of DAP and MgSO4 (PJ+) or used alone without supplementation (PJ−). In addition, whole minced pineapple peel was used as a medium with (PP+) or without (PP−) added nutrients. The pre-cultures of the two microbes, S. cerevisiae and A. pasteurianus, were transferred into the culture medium at an equal volume of 5% (v/v), which allowed for cell viability to be started at approximately 107 CFU/ml. Fermentation was carried out under static conditions at 30 °C.
To investigate the effect of sugar concentration, the PP+ medium was used in this study by varying the sugar concentration with sucrose at final concentrations of 100, 140, and 200 g/l. The pre-cultures of the two microbes were then transferred to achieve the same concentration described above.
To determine the effect of the inoculation time of AAB on the acetic acid accumulation profile, the PP+ medium at a concentration of 140 g/l was used. The inoculation of the AAB seed culture was implemented simultaneously with the yeast at the beginning of fermentation and at 1 and 2 days after the initiation of fermentation with the yeast. To determine the fermentation profile on a larger scale, fermentation was carried out using a 6 l glass vessel containing 3 l PP+ medium. The inoculation of AAB was determined either the same day or 1 day after fermentation was started.
Total phenolic content (TPC) and DPPH radical scavenging in PP vinegar
The Folin–Ciocalteu assay was used to determine the total phenolic compound (TPC) content in the PP vinegar according to previous reports (Chandra and De Mejia 2004), with some modifications. Briefly, vinegar samples at different dilutions were mixed with 0.1 ml of 0.2 mM Folin–Ciocalteu reagent and incubated in the dark at room temperature for 10 min. Then, 0.3 ml of sodium carbonate solution was added and further incubated under the same conditions for 10 min. The absorbance change was measured at 760 nm using a spectrophotometer (UV-1800, Shimadzu, Japan). Gallic acid was used as a reference standard. The results were expressed as mg gallic acid equivalent per millilitre of vinegar (mg GAE/ml).
The free radical scavenging activity of PP vinegar samples was determined using a DPPH radical scavenging assay with slight modification (Kim et al. 2002). An aliquot (0.1 ml) of vinegar and 0.1 ml of a 0.2 mM DPPH (1,1-diphenyl 2-picrylhydrazyl) solution (freshly prepared in methanol) was added, followed by incubation in the dark for 30 min at room temperature. The absorbance changes were determined spectrophotometrically (UV1800, Shimadzu, Japan) at 517 nm. The results were expressed in mg trolox equivalent [(TEAC)/ml]. All measurements were carried out in triplicate. Statistical analysis was used for comparison between the data of the PP and PJ vinegar samples. Student’s t test was used to calculate the significant differences (P ≤ 0.05) between the samples using SPSS.
Analytical methods
The sugar, ethanol and acetic acid contents were analysed using high-pressure liquid chromatography (HPLC). After centrifugation, a culture supernatant sample was diluted to a suitable concentration and filtered through a 0.22 μm microporous membrane filter. The HPLC system (Shimadzu model LC-10AT Liquid Chromatogram, Shimadzu Corp., Kyoto, Japan) was equipped with a Bio-Rad Aminex HPX-87H column (300 mm × 7.8 mm, Bio-Rad Laboratories Inc., Hercules, CA) and Shimadzu RID-UV detector. The column was maintained at a temperature of 60 °C and the mobile phase was 5 mM H2SO4 at a flow rate of 0.6 ml/min. Volatile compounds in the vinegar sample were assessed using an Agilent 7890A gas chromatograph equipped with an Agilent 7000B mass spectrometer (Agilent Technologies, Inc., Palo Alto, CA, USA). The analyte separation was carried out on a DB-wax capillary column (length, 60 m; i.d., × 0.25 mm; film thickness, 0.25 µm) located at the Centre of Scientific and Technological Equipment, Suranaree University of Technology, Thailand.
Total acidity was determined by titration with 0.8 N NaOH using phenolphthalein as an indicator. Viability was determined by spread plate. The serially diluted suspension was spread on a PDA plate for counting yeast cells, while an YPGD agar plate was used for AAB.
Results and discussion
Simultaneous vinegar fermentation using pineapple peels
The pineapple peels obtained from a local processing plant in Sri Chiang Mai District were prepared as described above. Sugar analysis of the supernatant by HPLC revealed that there were three major sugars, sucrose, glucose and fructose, at concentrations of 3.56, 6.08, and 7.05 g/l, respectively. This total concentration may not be suitable for vinegar fermentation. Theoretically, the maximum ethanol production would not reach over 1% ethanol, which may cause lower acetic acid production and could be turned into acetate via over-oxidation by AAB. Consistently, Perumpuli et al. (2014) reported that an initial ethanol concentration below 4% may turn into acetate via over-oxidation and result in low acetic acid production, while a high level may suppress the capability of AAB to form acetic acid from ethanol (Perumpuli et al. 2014). Therefore, controlling the optimal initial ethanol level is an important factor of acetic acid production. In addition, ethanol and acetic acid resistance in the AAB strain were also required. In this study, we selected the TH-AAB A. pasteurianus, which was previously isolated from fermented plant beverages, a kind of healthy drink similar to fermented tea (Kombucha) made from various kinds of plants (fruit vegetables or herbs) in many households in some areas of Thailand (Kantachote et al. 2005). In addition to excellent growth at high temperatures, this strain was also able to tolerate up to 12% (v/v) ethanol (data not shown).
From the start of fermentation, the culture medium conditions were investigated by comparing the medium of whole minced pineapple peels and that of only juice. The sugar concentration was adjusted with sucrose to a final sugar concentration of 140 g/l with or without the addition of several nutrients, following a previously reported method (Krusong and Vichitraka 2010). As shown in Fig. 1, ethanol was produced rapidly within 2 days at a maximum concentration of 90 g/l, which then gradually decreased with the drastic increase in acetic acid until day 12 of fermentation. The preparation and acetic fermentation rarely exceeded 10% alcohol and it would be reasonable to not consider the ethanol concentration as a limit to the growth of acetic acid bacteria (Sossou et al. 2009). Overall, acetic acid accumulation revealed that the utilisation of the whole peel as a raw material was sufficient for acetic acid fermentation. The fermentation medium adjusted to the initial pH of 5–6 was a more favourable condition for both yeast and AAB, as shown by the high amount of ethanol and acetic acid. In addition, supplementing magnesium and mineral salt could enhance AAB to oxidize ethanol to acetic acid. It appears that preparation of a suitable medium for AAB would be preferable, since the accumulation of ethanol was comparable among the tested conditions. However, a simple medium without optimisation showed a larger amount of remaining ethanol and, consequently, a lower accumulation of acetic acid. It could also be observed that minced PP began to float on the top of the mixture in the early stage of fermentation, where AAB cells were able to receive more oxygen than at the bottom (data not shown). This speculation was supported by a previous report in which the cells of A. aceti WK were able to obtain higher oxygen by growing on the surface of a loofah sponge, resulting in the higher production of acetic acid than that of free cells (Krusong and Tantratian 2014). A moderately acidic pH (4.5–6.0) has been reported to be the optimum pH for the enzymatic activities of membrane-bound alcohol dehydrogenase (ADH) and acetaldehyde dehydrogenase (ALDH), which are responsible for the sequential oxidation of ethanol to acetic acid in AAB (Kanchanarach et al. 2010; Adachi et al. 1980). According to our previous result, at the beginning of fermentation, the accumulation of acetic acid in the range of 1–2% acidity gave a pH of 4.2–4.5, which was consistent with previous reports (Wang et al. 2013). At the later stage, a drastic accumulation of acetic acid was observed and resulted in a lower pH (3.1–3.2), which could suppress cell viability and thus ADH and ALDH activity (Wang et al. 2013). In addition, the simultaneous production of ethanol by yeast triggers the activation of ADH synthesis for ethanol oxidation to form acetic acid (Ohmori et al. 1980). The addition of up to 4% ethanol (v/v) in the culture medium was shown to activate the enzymatic activities of membrane-bound ADH and ALDH in the TH-AAB A. pasteurianus SKU1108 (Chinnawirotpisan et al. 2003). Pineapple peel has been reported to contain noticeable amounts of mineral elements (Ketnawa et al. 2012). Divalent metal cations have been reported to function as prosthetic groups for the enzyme activities of AAB, promoting growth and oxidation ability (Sainz et al. 2016) and enhancing the production of acetic acid at high temperatures (Krusong et al. 2015a, b).
Fig. 1.
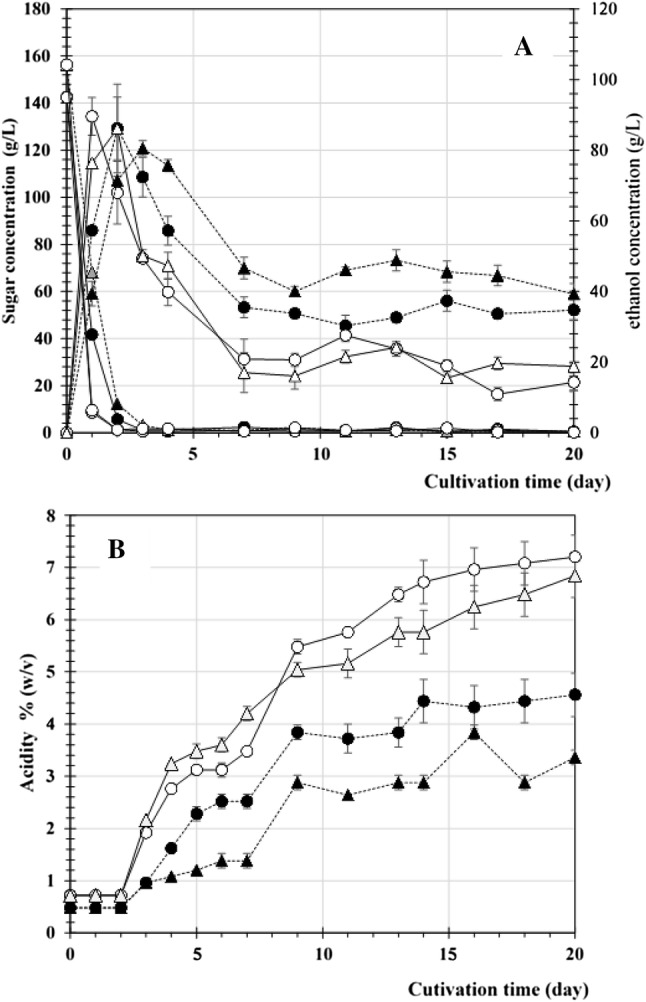
Time course of simultaneous vinegar fermentation using pineapple peels as raw materials. a Sugar utilisation (solid line) and ethanol production (dashed line) during fermentation in pineapple peel medium with (PP+, closed circle) or without (PP−, opened triangle) the addition of various nutrients, excluding sucrose. Additionally, panel B indicates acetic acid production in the PP and PJ media, as indicated by the same symbols as in panel A; data are expressed as the mean ± SD of three independent experiments
Effect of sugar concentration
Next, we carried out a preliminary investigation of the effect of sugar concentration on acetic acid production by varying the initial concentration at approximately 100, 140, and 200 g/l by sucrose. As shown in Fig. 2, increasing the sugar content resulted in a higher production of ethanol and, consequently, suppressed the action of AAB, as shown by the longer lag time in acetic acid production. After adaptation of the cell, however, AAB could produce acetic acid, though the residual ethanol was still high in the medium with an initial sugar concentration of 200 g/l. These results suggested that an initial sugar concentration of 140 g/l provided suitable conditions for AAB to produce high acetic acid amount, reaching approximately 7% (w/v). The initial sugar concentration seemed to be involved in the yeast cells for ethanol fermentation. A small proportion of initial sugar is required for the growth of AAB. In addition, enzymatic processing may take place for further oxidation to corresponding sugar acids, concomitantly generating bioenergy via the electron transport chain on the membrane, also known as oxidative fermentation (Matsushita et al. 1994). In addition, ethanol may also serve as a substrate and generate electron flow during the oxidation of ethanol to acetic acid. Unfortunately, AAB requires oxygen as an electron acceptor for complete ATP biosynthesis and the growth conditions in this study implemented a restricted oxygen supply. Therefore, oxygen turnover was limited, leaving a high concentration of ethanol in the culture medium. There are two terminal oxidases that have been reported in AAB, the former a cytochrome-containing oxidase that is sensitive to cyanide and mainly functions for energy generation and the latter an insensitive cyanide oxidase (CIO) with low affinity for oxygen (Saichana et al. 2015).
Fig. 2.
Effect of initial sugar concentration on SVF using pineapple peels (PP+). The sugar concentration was adjusted to 100 (∆), 140 (○), and 200 (◊) g/l, and ethanol production is indicated with the corresponding closed symbols that depict sugar concentration (a). b Acetic acid production from PP + medium at initial sugar concentrations of 100 (▲), 140 (●), and 200 (♦) g/l. Data are expressed as the mean ± SD of three independent experiments
In addition, increased aeration after the first stage of ethanol production increased the cell number of A. pasteurianus and, consequently, improved acetic acid production under co-culture with S. cerevisiae (Wang et al. 2013). However, increasing the ethanol concentration during acetic acid fermentation is not significant for the growth of acetic bacteria (Gullo et al. 2005). The gradual supplementation of glucose through fed-batch fermentation at the acetic acid fermentation stage during co-culture supported the growth of A. pasteurianus, thus producing a higher concentration of acetic acid than that produced by batch fermentation (Wang et al. 2013).
Effect of AAB inoculation time on acetic acid production
As described above, it can be speculated that the initial concentration of ethanol produced by yeast was an important factor for AAB to achieve high acetic acid production. The inoculation ratio between yeast and AAB has been reported and revealed that the inoculum amounts of AAB and yeast of 16% and 0.06%, respectively, were optimum for acetic acid production (Wang et al. 2013). In contrast, based on our previous study, inoculation of both strains at the same ratio with a total inoculum amount of 10% gave the highest amount of acetic acid. However, it was speculated that the optimal initial concentration of ethanol and initial accumulation of acetic acid would influence further growth and metabolism of both yeast and AAB under the same fermentation conditions. To investigate more details on the interaction between yeast and AAB, the inoculation time of AAB was changed by adding the inoculum 1 and 2 days after yeast fermentation to compare with its addition at the beginning of the fermentation.
As shown in Fig. 3, the first stage of fermentation was carried out using yeast and exhibited a drastic accumulation of ethanol within 2 days. The inoculation of AAB at the same time (sim) as yeast led to the initiation of ethanol oxidation to acetic acid earlier than when ABB were inoculated after 1 and 2 days of yeast fermentation. Commencing ethanol fermentation prior to acetic acid production may result in slightly higher ethanol accumulation, and the AAB cells require a longer adaptation period, which is caused by the high ethanol concentration at the initiation of fermentation. These effects resulted in a slightly longer lag time for acetic acid production. However, acetic acid eventually accumulated up to 7% (w/v) within 15 days. The simultaneous inoculation of these two microbes could be considered a biological interaction between yeast and AAB. As mentioned previously by Krusong et al. (2010), commensalism would typically be found in the beginning of fermentation when the yeast provides ethanol to AAB for growth. Later, an antagonistic effect occurred after acetic acid accumulation began (Krusong and Vichitraka 2010). As shown in Fig. 3, even yeast provided a positive effect on AAB growth. However, a longer period of high ethanol accumulation led to a suppression of AAB growth. This result indicated that inoculating two starters in the beginning would result in proper fermentation. AAB growth with the initial ethanol production may trigger the cells of AAB to adapt by activating the synthesis of the enzymes responsible for ethanol oxidation (Chinnawirotpisan et al. 2003), while staggered inoculation may result in an excessive concentration of ethanol, which suppresses cell growth and requires even longer adaptation times. Inoculation of AAB at the beginning of fermentation could generate the appropriate amount of acetic acid, which can affect ADH activity (Xia et al. 2016) and improve the ethanol oxidation rate for acetic acid bacteria (Krusong et al. 2015a, b).
Fig. 3.
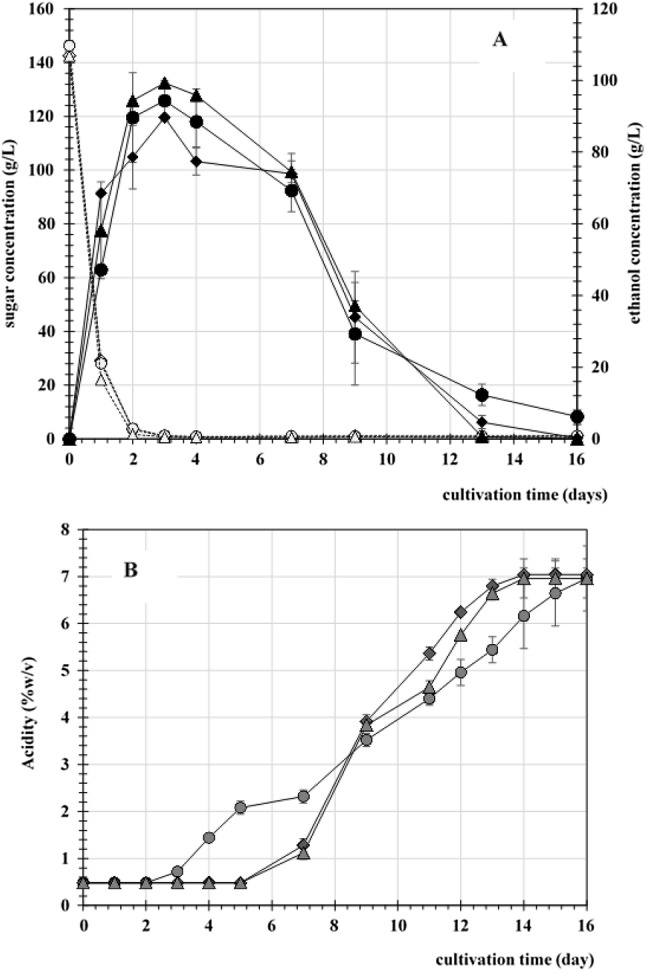
Effect of AAB inoculation time on SVF using pineapple peels (PP+). The inoculum of AAB was transferred into PP+ medium at the same time as yeast (sim, ●) or after 1 day (D1, ♦) or 2 days (D2, ▲) of fermentation. Sugar (dashed line with open symbols) and ethanol (solid line with closed symbols) were determined and are shown with corresponding symbols (a), and acetic acid production is shown in b. Data are expressed as the mean ± SD of three independent experiments
Decreased intracellular ATP as a result of acetic acid inhibition improved energy metabolism to produce more energy from the ethanol oxidation pathway instead of glucose and thus adapted to acetic acid fermentation conditions (Zheng et al. 2017).
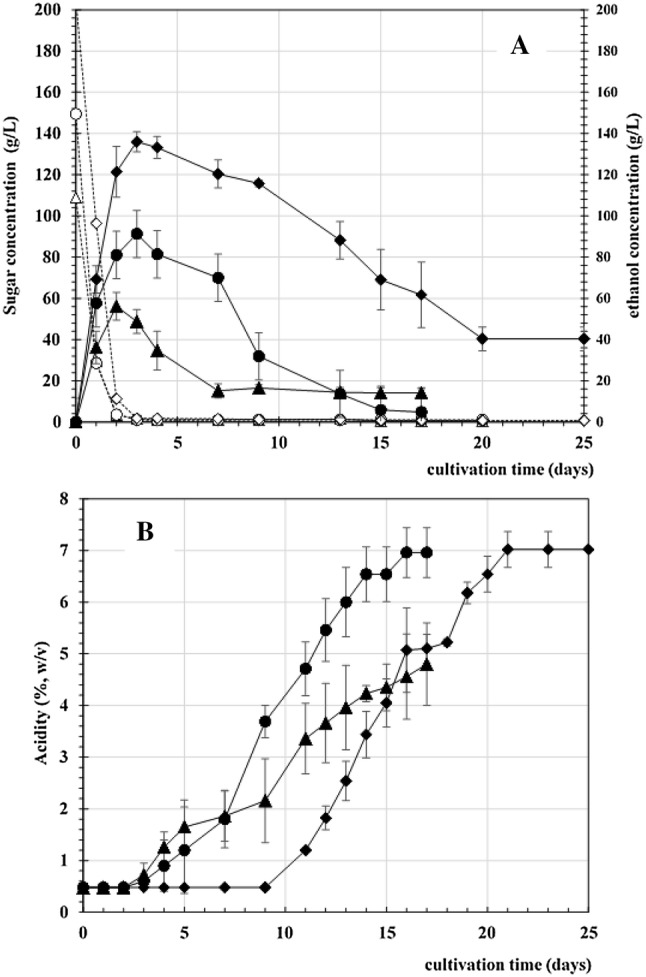
Scaled-up vinegar fermentation
When simultaneous fermentation was carried out in the 6 l vessel containing 3 l of PP+ medium, the fermentation profile was similar to that of the fermentation performed in a flask. However, the accumulation of acetic acid took longer than it did on the smaller scale. The maximum acetic acid accumulation was obtained after 25 days of fermentation (Fig. 4a). The restriction of oxygen available in the large vessel could possibly be one reason for the decreased production rate. However, a high acetic acid concentration of approximately 6.5% (w/v) was achieved at the end of fermentation, at which all of the ethanol was almost exhausted.
Fig. 4.
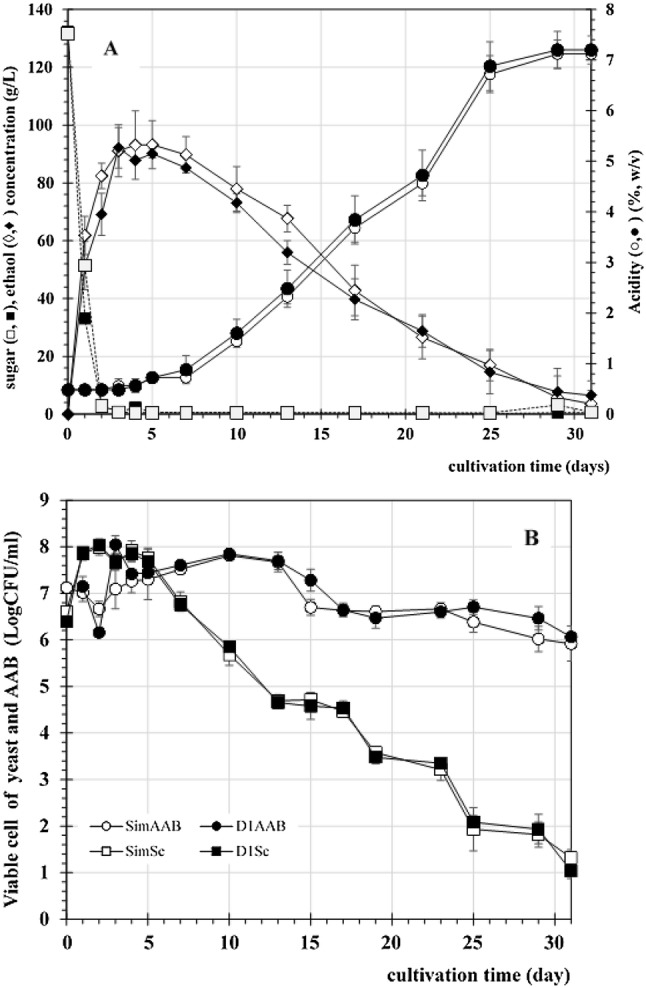
Scaled-up SVF fermentation. Fermentation was carried out in a 6 l vessel containing 3 l of PP+ medium. The inoculum of AAB was transferred at the same time as yeast (closed symbol) and added after 1 day of fermentation with yeast (open symbol). Sugar utilisation and ethanol and acetic acid production were determined as indicated with the symbols in the figure. Panel B shows the viable cell count of AAB (circle) and yeast (square) in the fermentation, which started with both yeast and AAB (open symbol), or with inoculation of AAB after 1 day of fermentation (closed symbol). Data are expressed as the mean ± SD of three independent experiments
As mentioned above, the beginning of ethanol formation by yeast supports the growth of AAB, as shown by the slightly increased cell viability up to 8 log CFU/ml (Fig. 4b). This indicated that commensalistic interaction was observed only during the phase of ethanol production by yeast, which provided ethanol as a substrate for AAB growth (Krusong and Vichitraka 2010). Yeast cell growth was promoted only during the first stage of fermentation within 3 days and then gradually decreased due to cell autolysis (Wang et al. 2013) after the accumulation of acetic acid. Consistently, Sossou et al. (2009) reported that there were three different phases of yeast growth corresponding to the stages of the viability, stress and death of yeast during vinegar production. The state of stress is a transitional stage leading to the death of yeast (Sossou et al. 2009). In addition to ethanol being a stressor for yeast cells, acetaldehyde, a first product of ethanol oxidation by AAB, is more related to the disruption of enzyme activities in yeast cells (Claro et al. 2007). In addition, acetic acid accumulation caused a change in the intracellular pH of yeast cells and influenced the production of ethanol (Valli et al. 2005). AAB remained steady and tended to decline during the last period of fermentation when high acetic acid levels were produced. Eventually, the concentration of acetic acid reached a level that was harmful to yeast cells and inhibited their growth. Only AAB tolerated such a high acetic acid concentration and prolonged further production. This could be explained by the fact that the AAB strain used in this experiment was a thermotolerant and acetic acid-resistant strain that could produce higher acetic acid than that produced by the mesophilic strain, similar to the other thermotolerant strains of AAB isolated previously (Chen et al. 2016; Ndoye et al. 2006; Perumpuli et al. 2014). These strains may carry adapted cells after successive growth (or fermentation) under sub-lethal growth conditions (Saichana et al. 2015). Similar phenomena were found in A. aceti WK, whose acetic acid-resistant properties occurred during stepwise adaptation caused by the increment of metabolic abilities to resist high acetic acid surroundings as well as the ability to produce large amounts of acetic acid (Krusong et al. 2014). The cellular protein response for acetic acid resistance analysed via transcriptomic and proteomic profiles has been extensively studied and found to be related to protein folding, stress response, oxidation–reduction, metabolism, protein biosynthesis, and membrane modification (Zheng et al. 2017).
In fact, this fermentation is similar to that of natural vinegar production (Othaman et al. 2014), but this procedure provides a shorter fermentation time. Nevertheless, in the two-step vinegar fermentation of pineapple waste hydrolysate, alcoholic fermentation was conducted for 7–10 days and the subsequent acetic acid production lasted for 30 days until the acetic acid concentration reached 5% (w/v) (Roda et al. 2017). In addition, this SVF provided no interference in terms of contaminants and can be standardised using certain microbes and easily implemented by local farmers and manufacturers.
Characteristics and properties of pineapple peel vinegar
After fermentation for 30 days, pineapple peel vinegar, which was yellow in colour, was obtained with a mild fruity acetic aroma. Two types of pineapple peel vinegar were collected to determine their properties, as shown in Table 1. Both contained an acetic acid concentration of approximately 6% (w/v), and only trace amounts of ethanol remained, which meets commercial vinegar standards, even though a variety of vinegars are marketed.
Table 1.
Physicochemical properties and antioxidant activity of pineapple peel vinegar from both whole peel (PPV) and juice (PJV)
| Vinegar sample | Sucrose (g/l) | Glucose (g/l) | Fructose (g/l) | Ethanol (g/l) | Acetic acid (g/l) | Total polyphenol (mg gallic acid equivalent/ml of vinegar) | Antioxidant activity by DPPH (mg TEAC/ml of vinegar) |
|---|---|---|---|---|---|---|---|
| PPV | 7.28 ± 0.43* | 2.17 ± 0.14* | 1.72 ± 0.03* | 1.78 ± 0.68* | 58.79 ± 0.69* | 3.211 ± 0.09* | 46.93 ± 0.76* |
| PJV | 0.95 ± 0.07** | 0.97 ± 0.07** | 0.50 ± 0.06** | 0.96 ± 0.11** | 58.93 ± 0.74* | 2.26 ± 0.12** | 41.54 ± 0.35** |
Data expressed as mean ± SD with at least triplicate measurement
Symbols with asterisks (*, **) indicated a significant difference (p < 0.05) between two types of vinegar (PPV and PPJ)
The total phenolic compound (TPC) content was reported as gallic acid equivalents by reference to the standard curve and showed that the TPC content in the pineapple peel vinegar (PPV) was higher than that of the pineapple juice vinegar (PJV). This may be due to the small pieces of peel that passed through a cotton cloth during filtration. Consistently, antioxidants were found to be slightly higher in the PPV than in the PJV. In contrast, it has been reported that the TPC content in pineapple fruit vinegar produced by two-step fermentation was almost three times lower than in peel vinegar (Jasmine Praveena and Estherlydia 2014). This might be due to bioactive substances such as organic acids or phenolic compounds derived from the raw materials in the peel. Major polyphenolic compounds in pineapple peels were previously identified, consisting of gallic acid, catechin, epicatechin, and ferulic acid (Li et al. 2014). These four polyphenolic compounds exhibited antioxidant capacities with structure–activity relationships and there were no synergistic effects (Li et al. 2014).
In addition, the phenolic profile in vinegar produced from pineapple waste was identified and composed of glycosylated and free compounds among the different sub-classes of flavonoids, hydroxycinnamic acids, phenolic acids, benzenediols and coumarins. The most represented compounds were gallic acid, p-hydroxy-benzaldehyde, caffeic acid, vanillic acid, syringic acid, p-coumaric acid, anisaldehyde, catechin, epicatechin, sinapic acid, and salicylaldehyde (Roda et al. 2017). Some phenolic constituents, such as syringaresinol and lariciresinol as the major lignans, have also been detected in pineapple fruit (Peñalvo et al. 2005).
These compounds can be produced and/or increased as a result of the overall vinegar fermentation process (Giudici et al. 2009), where phenolic compounds are transformed into new anti-oxidative molecules (Shahidi et al. 2008).
A wide variety of raw materials are used to produce vinegars around the world, which would be reflective of their different functional properties (Budak et al. 2014). During alcoholic and acetic fermentation, a wide range of modifications could be induced in the chemical compounds that are related to sensory properties. l-Lysine, mellein, and gallic acid were significantly more concentrated in pineapple vinegar than in pineapple juice (Roda et al. 2017). Consistently, numerous amino acids were detected in brewed vinegar and assumed to have been produced from cell autolysis phenomena (Maestre et al. 2008).
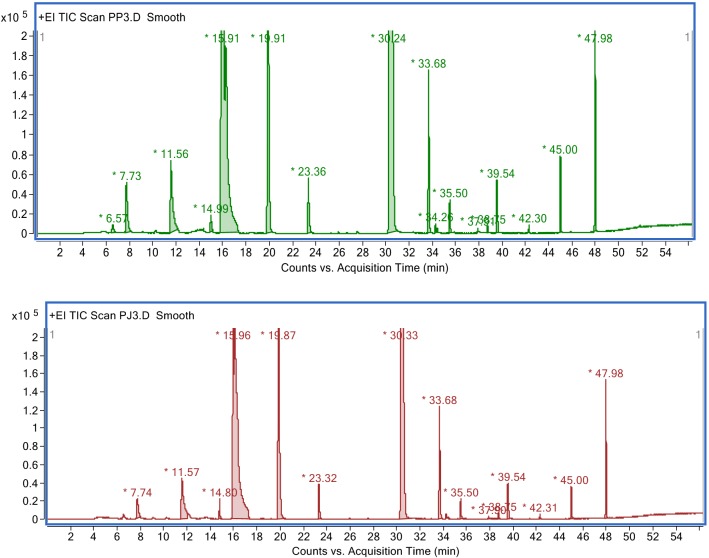
Aside from acetic acid, other fermentation products in vinegars, such as volatile compounds, also contribute to the organoleptic properties of vinegars (Ozturk et al. 2015) and were detected in vinegar samples, as shown in Table 2 and Fig. 5. Esters are the primary contributors to good-quality products. A balanced ester content is important for vinegar’s sensory characteristics (Callejón et al. 2010). The most important volatile ester by quantitation, particularly for vinegars produced using slow methods, is ethyl acetate (Boffo et al. 2009; Baena-Ruano et al. 2010). Acetic esters formed through a chemical condensation process slowly occur during the ageing process (Ubeda et al. 2012). In this study, however, 3-methyl butanol acetate, attributed to a banana odour, was significantly detected at higher concentrations than that of ethyl acetate (Table 2).
Table 2.
Volatile compounds detected in pineapple peel vinegar
| Volatile compound (acquisition time, min) | Composition ratio of volatile compounds in vinegar samples (% area)a | |
|---|---|---|
| PPV | PJV | |
| Acetic acid, methyl ester (6.5) | 0.150 | n.d.b |
| Ethyl acetate (7.7) | 1.088 | 0.589 |
| Isobutyl acetate (11.5) | 1.968 | 1.936 |
| Isobutanol (14.9) | 0.245 | 0.237 |
| Isopentyl alcohol (15.9) | 23.208 | 18.755 |
| Isoamyl alcohol (3-methyl butanol) (19.9) | 6.911 | 6.387 |
| Acetoin (23.3) | 0.701 | 0.729 |
| Acetic acid (30.3) | 60.726 | 66.015 |
| Benzaldehyde (33.6) | 1.630 | 2.031 |
| Propanoic acid (34.2) | 0.074 | n.d. |
| Isobutyric acid (35.5) | 0.287 | 0.326 |
| Butanoic acid (37.9) | 0.024 | 0.047 |
| 4-Methylbenzaldehyde (38.7) | 0.044 | 0.075 |
| Methylbutanoic acid (39.5) | 0.520 | 0.691 |
| Naphthalene (42.3) | 0.054 | 0.058 |
| Phenylethyl acetate (45.0) | 0.565 | 0.439 |
| Phenylethyl alcohol (47.9) | 1.805 | 1.683 |
n.d. not detected
aNumber expressed as % formulated from area detected of all compounds from Fig. 5
Fig. 5.
GC-MS chromatographic analysis of vinegar samples produced from pineapple peel using whole minced peel (PPV) (upper panel) or its juice (PJ) (lower panel)
Conversely, 2-phenylethyl acetate, giving a floral–fruity perception, was preserved from the pineapple juice in which it was detected (Table 2). According to Callejon et al. (2010), 2-phenylethyl acetate has one of the highest odour activity values (Callejón et al. 2010). Isobutyl alcohol and isoamyl alcohol were found to be in high quantities in balsamic vinegar (Del Signore 2001) and were detected in the vinegar samples in this study, being the most important alcohol aroma (Table 2). In addition, 2-phenyl ethanol, reminiscent of rose, was also significantly detected in the samples, which might be derived from the fermentation of the juice (Cejudo-Bastante et al. 2013).
In addition to acetic acid, several volatile acids were also detected, particularly, 3-methyl-butanoic acid, producing a ‘floral–sweaty’ odour (Ubeda et al. 2012), which was the most abundant of the acids assessed in the pineapple vinegar (Table 2) and was also found in juice vinegar, wine vinegar, and blueberry vinegar (Su and Chien 2010; Roda et al. 2017). The diversity of these compounds affects the potential impact of the distinct odour and aromas, which influence the overall odour impression of the pineapple vinegar (Ubeda et al. 2012).
Conclusions
In addition to submerged and two-step fermentation, which is preferable for industrial vinegar fermentation with high productivity, slow-process fermentation, such as traditional balsamic vinegar or traditional Chinese vinegar fermentation, is achieved naturally by the interaction of microbes, resulting in unique characteristics and flavour or aroma. From this point of view, we focused on a simple procedure and avoided contamination, which is suitable for local manufacturers. In fact, simultaneous vinegar fermentation (SVF) showed the biological interaction between yeast and AAB to be a mostly antagonistic effect due to their distinct growth conditions. This interaction leads to the difficulty of start-up acetification, which affects the minimum acidity value of the final product. This study revealed that a crucial factor is the use of an AAB strain that can tolerate ethanol and minimal oxygen requirements. In addition, an agricultural pineapple by-product was used as an alternative raw material with a low cost that produced vinegar with unique compounds. Pineapple peels can be used for vinegar fermentation and can produce a clear liquid without post-filtration. Vinegar fermentation resulted in reduction in sugar to ethanol, with concomitant increase in acidity and varieties of corresponding volatile compounds in accordance with standard requirements. The composition of the vinegar included higher alcohols, esters and several aldehydes (acetoin, 3-methyl butanol, ethyl acetate, 2-phenylethanol, etc.) as the major components, along with significantly low levels of compounds contributing off-flavours. This work demonstrated that SVF using pineapple by-products could be effective for obtaining alternative and value-added final products in compliance with food quality requirements.
Acknowledgements
This work was financial supported by National Research Council of Thailand (NRCT), allocated by Khon Kaen University (No. 590029).
Authors’ contributions
VT carried out the experiments, analysed the data and participated in drafting the manuscript. MC participated in the data analysis of the pineapple vinegar. WS contributed to the design of the experiments, conducted the experiments, analysed the data, participated in drafting the manuscript and revised the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interests
The authors declare no conflict of interests.
References
- Adachi O, Tayama K, Shinagawa E, Matsushita K, Ameyama M. Purification and characterization of membrane-bound aldehyde dehydrogenase from Gluconobacter suboxydans. Agric Biol Chem. 1980;44:503–515. [Google Scholar]
- Baena-Ruano S, Santos-Dueñas IM, Mauricio JC, Garcia-Garcia I. Relationship between changes in the total concentration of acetic acid bacteria and major volatile compounds during the acetic acid fermentation of white wine. J Sci Food Agric. 2010;90(15):2675–2681. doi: 10.1002/jsfa.4139. [DOI] [PubMed] [Google Scholar]
- Boffo EF, Tavares LA, Ferreira MMC, Ferreira AG. Classification of Brazilian vinegars according to their 1H NMR spectra by pattern recognition analysis. LWT Food Sci Technol. 2009;42(9):1455–1460. [Google Scholar]
- Budak NH, Aykin E, Seydim AC, Greene AK, Guzel-Seydim ZB. Functional properties of vinegar. J Food Sci. 2014;79(5):757–764. doi: 10.1111/1750-3841.12434. [DOI] [PubMed] [Google Scholar]
- Cabrera HAP, Menezes HC, Oliveira JV, Batista RFS. Evaluation of residual levels of benomyl, methyl parathion, diuron, and vamidothion in pineapple pulp and bagasse (smooth cayenne) J Agric Food Chem. 2000;48:5750–5753. doi: 10.1021/jf9911444. [DOI] [PubMed] [Google Scholar]
- Callejón RM, Torija MJ, Mas A, Morales ML, Troncoso AM. Changes of volatile compounds in wine vinegars during their elaboration in barrels made from different woods. Food Chem. 2010;113(4):1252–1259. [Google Scholar]
- Cejudo-Bastante MJ, Durán-Guerrero E, Natera-Marín R, Castro-Mejías R, García-Barroso C. Characterisation of commercial aromatised vinegars: phenolic compounds, volatile composition and antioxidant activity. J Sci Food Agric. 2013;93(6):1284–1302. doi: 10.1002/jsfa.5885. [DOI] [PubMed] [Google Scholar]
- Chandra S, De Mejia EG. Polyphenolic compounds, antioxidant capacity, and quinone reductase activity of an aqueous extract of Ardisia compressa in comparison to mate (llex paraguariensis) and green (Camellia sinensis) teas. J Agric Food Chem. 2004;52(11):3583–3589. doi: 10.1021/jf0352632. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bai Y, Li D, Wang C, Xu N, Hu Y. Screening and characterization of ethanol-tolerant and thermotolerant acetic acid bacteria from Chinese vinegar Pei. World J Microbiol Biotechnol. 2016;32(1):1–9. doi: 10.1007/s11274-015-1961-8. [DOI] [PubMed] [Google Scholar]
- Chinnawirotpisan P, Theeragool G, Limtong S, Toyama H, Adachi O, Matsushita K. Quinoprotein alcohol dehydrogenase is involved in catabolic acetate production, while NAD dependent alcohol dehydrogenase in ethanol assimilation in Acetobacter pasteurianus SKU1108. J Biosci Bioeng. 2003;96:564–571. doi: 10.1016/S1389-1723(04)70150-4. [DOI] [PubMed] [Google Scholar]
- Chou CH, Liu CW, Yang DJ, Wu YHS, Chen YC. Amino acid, mineral, and polyphenolic profiles of black vinegar, and its lipid lowering and antioxidant effects in vivo. Food Chem. 2015;168:63–69. doi: 10.1016/j.foodchem.2014.07.035. [DOI] [PubMed] [Google Scholar]
- Claro FB, Rijsbrack K, Soarese V. Flocculation onset in Saccharomyces cerevisiae: effect of ethanol heat and osmotic stress. J Appl Microbial. 2007;102:693–700. doi: 10.1111/j.1365-2672.2006.03130.x. [DOI] [PubMed] [Google Scholar]
- De Leonardis A, Macciola V, Iorizzo M, Lombardi SJ, Lopez F, Marconi E. Effective assay for olive vinegar production from olive oil mill wastewaters. Food Chem. 2018;240:437–440. doi: 10.1016/j.foodchem.2017.07.159. [DOI] [PubMed] [Google Scholar]
- Del Signore A. Chemometric analysis and volatile compounds of traditional balsamic vinegars from Modena. J Food Eng. 2001;50(2):77–90. [Google Scholar]
- Drysdale GS, Fleet GH. The effect of acetic acid bacteria upon the growth and metabolism of yeasts during the fermentation of grape juice. J Appl Bacteriol. 1989;67:471–481. [Google Scholar]
- Elijah AI, Etukudo MP. Quality evaluation of vinegar produced from banana peel using Saccharomyces cerevisiae and Acetobacter aceti isolated from Palm Wine Dreg. Niger J Agric Food Environ. 2016;12:205–211. [Google Scholar]
- Ghosh PR, Fawcett D, Sharma SB, Poinern GEJ. Progress towards sustainable utilisation and management of food wastes in the global economy. Int J Food Sci. 2016;2016:3563478. doi: 10.1155/2016/3563478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudici P, Gullo M, Solieri L. Vinegars of the World. Milan: Springer; 2009. Traditional balsamic vinegar; pp. 157–177. [Google Scholar]
- Gullo M, Giudici P. Acetic acid bacteria in traditional balsamic vinegar: phenotypic traits relevant for starter cultures selection. Int J Food Microbiol. 2008;125:46–53. doi: 10.1016/j.ijfoodmicro.2007.11.076. [DOI] [PubMed] [Google Scholar]
- Gullo M, Caggia C, Devero L, Giudici P. Characterization of acetic acid bacteria in traditional balsamic vinegar. Int J Food Microbiol. 2005;106:209–212. doi: 10.1016/j.ijfoodmicro.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Gullo M, De Vero L, Giudici P. Succession of selected strains of Acetobacter pasteurianus and other acetic acid bacteria in traditional balsamic vinegar. Appl Environ Microbiol. 2009;75:2585–2589. doi: 10.1128/AEM.02249-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CW, Lazim AM, Fazry S, Zaki UKHH, Lim SJ. Varieties, production, composition and health benefits of vinegars: A review. Food Chem. 2017;221:1631. doi: 10.1016/j.foodchem.2016.10.128. [DOI] [PubMed] [Google Scholar]
- Horiuchi JI, Kanno T, Kobayashi M. New vinegar production from onions. J Biosci Bioeng. 1999;88:107–109. doi: 10.1016/s1389-1723(99)80186-8. [DOI] [PubMed] [Google Scholar]
- Jasmine Praveena R, Estherlydia D. Comparative study of phytochemical screening and antioxidant capacities of vinegar made from peel and fruit of pineapple (Ananas comosus L) Int J Pharma Bio Sci. 2014;5:B394–B403. [Google Scholar]
- Kanchanarach W, Theeragool G, Yakushi T, Toyama H, Adachi O, Matsushita K. Characterization of thermotolerant Acetobacter pasteurianus strains and their quinoprotein alcohol dehydrogenases. Appl Microbiol Biotechnol. 2010;85:741–751. doi: 10.1007/s00253-009-2203-5. [DOI] [PubMed] [Google Scholar]
- Kantachote D, Charernjiratrakul W, Aussawariangpipop N. Characteristics of fermented plant beverages in Southern Thailand. Songklanakarin J Sci Technol. 2005;27:601–615. [Google Scholar]
- Ketnawa S, Chaiwut P, Rawdkuen S. Pineapple wastes: a potential source for bromelain extraction. Food Bioprod Process. 2012;90:385–391. [Google Scholar]
- Kim DO, Lee KW, Lee HJ, Lee CY. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J Agric Food Chem. 2002;50(13):3713–3717. doi: 10.1021/jf020071c. [DOI] [PubMed] [Google Scholar]
- Krusong W, Tantratian S. Acetification of rice wine by Acetobacter aceti using loofa sponge in a low-cost reciprocating shaker. J Appl Microbiol. 2014;117:1348–1357. doi: 10.1111/jam.12634. [DOI] [PubMed] [Google Scholar]
- Krusong W, Vichitraka A. An investigation of simultaneous pineapple vinegar fermentation interaction between acetic acid bacteria and yeast Warawut. Asian J Food Agro-Industry. 2010;3:192–203. [Google Scholar]
- Krusong W, Pornpukdeewatana S, Kerdpiboon S, Tantratian S. Prediction of influence of stepwise increment of initial acetic acid concentration in charging medium on acetification rate of semi-continuous process by artificial neural network. LWT- Food Sci Technol. 2014;56:383–389. [Google Scholar]
- Krusong W, Kerdpiboon S, Jindaprasert A, Yaiyen S, Pornpukdeewatana S, Tantratian S. Influence of calcium chloride in the high temperature acetification by strain Acetobacter aceti WK for vinegar. J Appl Microbiol. 2015;119:1291–1300. doi: 10.1111/jam.12930. [DOI] [PubMed] [Google Scholar]
- Krusong W, Yaiyen S, Pornpukdeewatana S. Impact of high initial concentrations of acetic acid and ethanol on acetification rate in an internal Venturi injector bioreactor. J Appl Microbiol. 2015;118:629–640. doi: 10.1111/jam.12715. [DOI] [PubMed] [Google Scholar]
- Laranjinha JAN, Almeida LM, Madeira VMC. Reactivity of dietary phenolic acids with peroxyl radicals: antioxidant activity upon low density lipoprotein peroxidation. Biochem Pharmacol. 1994;48:487–494. doi: 10.1016/0006-2952(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Cho HD, Jeong JH, Lee MK, Jeong YK, Shim KH, Il Seo K. New vinegar produced by tomato suppresses adipocyte differentiation and fat accumulation in 3T3-L1 cells and obese rat model. Food Chem. 2013;141:3241–3249. doi: 10.1016/j.foodchem.2013.05.126. [DOI] [PubMed] [Google Scholar]
- Li T, Shen P, Liu W, Liu C, Liang R, Yan N, Chen J. Major polyphenolics in pineapple peels and their antioxidant interactions. Int J Food Prop. 2014;17(8):1805–1817. [Google Scholar]
- Maestre O, Santos-Dueñas IM, Peinado R, Jiménez-Ot C, García-García I, Mauricio JC. Changes in amino acid composition during wine vinegar production in a fully automatic pilot acetator. Process Biochem. 2008;43(8):803–807. [Google Scholar]
- Matsushita K, Toyama H, Adachi O. Respiratory chains and bioenergetics of acetic acid bacteria. Adv Microb Physiol. 1994;36:247–301. doi: 10.1016/s0065-2911(08)60181-2. [DOI] [PubMed] [Google Scholar]
- Ndoye B, Lebeccque S, Dubois-Dauphin R, Tounkanra L, Guiro AT, Kere C, Diawara B, Thonart P. Thermoresistant properties of acetic acids bacteria isolated from tropical products of Sub-Saharan Africa and destined to industrial vinegar. Enz Microb Technol. 2006;39:916–923. [Google Scholar]
- Ohmori S, Masai H, Arima K, Beppu T. Isolation and identification of acetic acid bacteria for submerged acetic acid fermentation at high temperature. Agric Biol Chem. 1980;44:2901–2906. [Google Scholar]
- Othaman MA, Sharifudin SA, Mansor A, Kahar AA, Long K. Coconut water vinegar: new alternative with improved processing technique. J Eng Sci Technol. 2014;9(3):293–302. [Google Scholar]
- Ozturk I, Caliskan O, Tornuk F, Ozcan N, Yalcin H, Baslar M, Sagdic O. Antioxidant, antimicrobial, mineral, volatile, physicochemical and microbiological characteristics of traditional home-made Turkish vinegars. LWT Food Sci Technol. 2015;63(1):144–151. [Google Scholar]
- Peñalvo JL, Haajanen KM, Botting N. Quantification of lignans in food using isotope dilution gas chromatography/mass spectrometry. J Agric Food Chem. 2005;53:9342–9347. doi: 10.1021/jf051488w. [DOI] [PubMed] [Google Scholar]
- Perumpuli PABN, Watanabe T, Toyama H. Identification and characterization of thermotolerant acetic acid bacteria strains isolated from coconut water vinegar in Sri Lanka. Biosci Biotechnol Biochem. 2014;78:533–541. doi: 10.1080/09168451.2014.882758. [DOI] [PubMed] [Google Scholar]
- Roda A, Lucini L, Torchio F, Dordoni R, De Faveri DM, Lambri M. Metabolite profiling and volatiles of pineapple wine and vinegar obtained from pineapple waste. Food Chem. 2017;229:734–742. doi: 10.1016/j.foodchem.2017.02.111. [DOI] [PubMed] [Google Scholar]
- Saichana N, Matsushita K, Adachi O, Frébort I, Frebortova J. Acetic acid bacteria: a group of bacteria with versatile biotechnological applications. Biotechnol Adv. 2015;33:1260–1271. doi: 10.1016/j.biotechadv.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Sainz F, Navarro D, Mateo E, Torija MJ, Mas A. Comparison of D-gluconic acid production in selected strains of acetic acid bacteria. Int J Food Microbiol. 2016;222:40–47. doi: 10.1016/j.ijfoodmicro.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Salbe AD, Johnston CS, Buyukbese MA, Tsitouras PD, Harman SM. Vinegar lacks antiglycemic action on enteral carbohydrate absorption in human subjects. Nutr Res. 2009;29:846–849. doi: 10.1016/j.nutres.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Shahidi F, McDonald J, Chandrasekara A, Zhong Y. Phytochemicals of foods, beverages and fruit vinegars: chemistry and health effects. Asia Pac J Clin Nutr. 2008;17(1):380–382. [PubMed] [Google Scholar]
- Soemphol W, Saichana N, Yakushi T, Adachi O, Matsushita K, Toyama H. Characterization of genes involved in D-sorbitol oxidation in thermotolerant Gluconobacter frateurii. Biosci Biotechnol Biochem. 2012;76(8):1497–1505. doi: 10.1271/bbb.120227. [DOI] [PubMed] [Google Scholar]
- Solieri L, Giudici P. Yeasts associated to Traditional Balsamic Vinegar: ecological and technological features. Int J Food Microbiol. 2008;125:36–45. doi: 10.1016/j.ijfoodmicro.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Sossou SK, Ameyapoh Y, Karou SD, de Souza C. Study of pineapple peelings processing into vinegar by biotechnology. Pak J Biol Sci. 2009;12:859–865. doi: 10.3923/pjbs.2009.859.865. [DOI] [PubMed] [Google Scholar]
- Su MS, Chien PJ. Aroma impact components of rabbiteye blueberry (Vaccinium ashei) vinegars. Food Chem. 2010;119(3):923–928. [Google Scholar]
- Ubeda C, Callejón RM, Troncoso AM, Moreno-Rojas JM, Peña F, Morales ML. Characterization of odour active compounds in strawberry vinegars. Flavour Fragr J. 2012;27(4):313–321. [Google Scholar]
- Ubeda C, Callejón RM, Hidalgo C, Torija MJ, Troncoso AM, Morales ML. Employment of different processes for the production of strawberry vinegars: effects on antioxidant activity, total phenols and monomeric anthocyanins. LWT Food Sci Technol. 2013;52:139–145. [Google Scholar]
- Valli M, Sauer M, Branduardi P, Borth N, Porrot D, Mattanovich D. Intracellular pH distribution in Saccharomyces cerevisiae cell populations, analyzed by flow cytometry. Appl Environ Microbiol. 2005;71:1515–1521. doi: 10.1128/AEM.71.3.1515-1521.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yan M, Chen X, Li DS, Qin L, Li Z, Yao J, Liang X. Mixed culture of Saccharomyces cerevisiae, and Acetobacter pasteurianus, for acetic acid production. Biochem Eng J. 2013;79(2):41–45. [Google Scholar]
- Xia K, Zang N, Zhang J, Zhang H, Li Y, Liu Y, Liang X. New insights into the mechanisms of acetic acid resistance in Acetobacter pasteurianus using iTRAQ-dependent quantitative proteomic analysis. Int J Food Microbiol. 2016;238:241–251. doi: 10.1016/j.ijfoodmicro.2016.09.016. [DOI] [PubMed] [Google Scholar]
- Ye XJ, Morimura S, Han LS, Shigematsu T, Kida K. In vitro evaluation of physiological activity of vinegar produced from barley-, sweet potato-, and rice-shochu post-distillation slurry. Biosci Biotechnol Biochem. 2004;68:551–556. doi: 10.1271/bbb.68.551. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Zhang R, Yin H, Bai X, Chang Y, Xia M, Wang M. Acetobacter pasteurianus metabolic change induced by initial acetic acid to adapt to acetic acid fermentation conditions. Appl Microbiol Biotechnol. 2017;101(18):7007–7016. doi: 10.1007/s00253-017-8453-8. [DOI] [PubMed] [Google Scholar]