Abstract
Heat stress is one of the major limitations to crop productivity. In the present study, an efficient method of screening was adopted for identification of heat tolerant Indian Mustard genotypes by applying 4-day cycle of heat stress to seedlings. Thirty-four genotypes were screened based upon lipid peroxidation and survival percentage and classified them into five different classes according to membership function value (MFV) for response against high temperature. The maximum and minimum value of mean MFV were 0.89 (highly heat tolerant, TPM1) and 0.12 (highly heat sensitive, JM2), respectively. The coefficient of determination (R2) between the mean MFV and the heat tolerance index (HTI) of MDA content, survival percentage was 0.914 and 0.808 suggesting that these parameters are reliable traits to evaluate the heat tolerance of Brassica juncea genotypes. The evaluation method was further validated using identified contrasting genotypes and assessment of heat stress associated biochemical parameters. Results showed efficient recovery of tolerant genotype as compared to sensitive genotype. Expression profiling of heat stress-related genes (HSP21 and HSFA7A) showed significant upregulation in the tolerant genotype (TPM1) (9.73- and 4.87-fold, respectively) as compared to the sensitive genotype (JM2) (4.18- and 1.73-fold, respectively) under heat stress condition. The results imply development of an efficient screening method which is useful for evaluation and breeding of thermo-tolerant B. juncea.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-2106-9) contains supplementary material, which is available to authorized users.
Keywords: Brassica juncea, Heat stress, Screening of germplasm, Heat shock protein 21, ROS scavenging
Introduction
Rise in global temperature (Sanchez et al. 2014) and frequent heat wave or extreme temperature (Hatfield and Prueger 2015) have been observed in the past decades. High temperature-induced stress is expressed as the increase in air temperature beyond an optimum level to the extent sufficient to cause injury or irreversible damage of crop plants (Teixeira et al. 2013). Heat stress impact on crop plants is complex which leads to high seedling mortality, decreased photosynthesis, leaf senescence, decreased pollen production and viability, seed abortion and consequently lower grain number and weight (Nadeem et al. 2018). However, critical temperature thresholds vary among crops, cultivars and phenological stages (Sanchez et al. 2014) and this results in differential plant responses.
Among critical stages in the development of the plant, the early stages of seedling growth constitute one of the critical periods and are of the greatest significance in stand establishment in many crops (Finch-Savage and Bassel 2016). High temperatures impact is also affected by local conditions like soil water content and evaporative water loss (Alfaro et al. 2006). Severe reduction in seedling emergence and leaf necrosis at seedling stage due to high air temperature and hot soil condition have been observed that lead to poor plant stand and eventually reduces the yield significantly (Azharudheen et al. 2013). The effective addressal of this issue requires breeding efforts to either create a new genotype or screen existing germplasm for thermo-tolerance. Screening acts as a filter to identify genetic variability existing in the available germplasm on the basis of different parameters (Sanghera et al. 2011). At the biochemical level, low membrane damage and ROS scavenging ability (Bailey-Serres and Mittler 2006) contribute to heat tolerance at multiple stages in plant life cycle; can be employed as a screening parameter. High-temperature stress is perceived and translated to intracellular signalling response through lipid peroxidation of poly unsaturated fatty acids present in cell membranes and is the most apparent symptom of oxidative stress in plants (Djanaguiraman et al. 2018). Since the accumulation of melondialdehyde, a stable compound is the outcome of the imbalance of pro-oxidants and defence mechanism, the differential accumulation after stress in plants could be correlated with susceptibly or tolerance of genotype.
Through genomics, transciptomic and proteomic studies numerous genes involved in heat tolerance mechanisms have been identified in various plant species (Bhardwaj et al. 2015). Heat shock transcription factors and heat shock proteins (HSFs/HSPs) constitute a master regulatory pathway involved in the response to heat stress (Scharf et al. 2012). For identification of suitable molecular determinants, heat stress related genes need to be validated through expression profiling and could be used for the development of genic markers for trait selection under crop improvement programme (Dong et al. 2015).
Indian mustard (Brassica juncea), an important oilseed crops, is grown in tropical and subtropical regions as a cold weather crop (6 °C to 27 °C) (Shekhawat et al. 2012). It is mainly grown under wide range of conditions like rainfed/irrigated, early/timely/late sown, and sole or mixed crop (Singh et al. 2016). Under rainfed conditions, early sowing of mustard will help to harvest maximum monsoon rain water along with avoiding disease infestation, aphid attack, and fruit shattering. But early sowing also affects the germination, seedling establishment and increasing seedling mortality due to high temperature and thus yield. So, it becomes very important to develop a screening method to identify the tolerant genotypes at the seedling stage. The objective of this study was to develop a screening method to identify Indian mustard germplasm for heat tolerance at the early stage of seedling development. The potential of the method was validated by different biochemical and molecular responses under heat stress. In this study, we have also tried to demonstrate that phenotypic traits in combination with biochemical and molecular level information could be exploited to generate useful information concerning the thermotolerance potential of genotypes.
Materials and methods
Plant material, seedling growth condition and stress treatment
A set of 34 B. juncea genotypes including 31 released well adapted varieties and 3 indigenous accessions was utilized for the study to exploit intra-species genetic diversity. The genotypes were screened for heat tolerance in a controlled plant growth chamber. Sowing was done in trays containing normal homogenized field soil and in each tray measured amount of water was given to bring the moisture levels to field capacity. Each germplasm was sown in triplicates. Seedlings were initially allowed to grow at 25 ± 1 °C for 5 days then subjected to the treatment. For heat treatment, seedlings were exposed to 45 °C (RH ~ 45–50%) for 4 h in light (the temperature was gradually elevated 0.3 °C/min). Then the temperature was decreased to 25 °C and RH ~ 70%) and the entire cycle was repeated for 4 days. A control experiment was carried out where optimum temperature (25 °C, RH ~ 70%) was maintained throughout the experiment. Water was not given during the period of heat stress. For recovery experiments, temperature was brought to the ambient (25 °C).
Survival rate
Survival rate is defined as a ratio of the total number of seedlings survived to the total number of seedlings at particular duration of time and area. It was calculated using the formula:
Histochemical assay
Superoxide (O2·−) anion is one of the most important ROS. Histochemical assay was performed by nitrotetrazolium blue chloride (NBT) staining as the chromogenic substrate for superoxide detection in seedlings. Assay was performed by vacuum infiltration with 0.5 mg/ml NBT solution in 10 mM K-PO4 (pH 7.8) (Jabs et al. 1996) to the seedlings. Seedlings were then incubated in 0.5 mg/ml NBT solution for 1 h at room temperature followed by rinsing in 90% ethanol at 70 °C until complete chlorophyll is removed. The seedlings were stored and examined in 70% glycerol.
Electrochemical conductivity
For measuring electrochemical conductivity (EC), intact plant seedlings were dipped in tubes containing unionized water and were stored at 4 °C overnight. After that, plants were kept at room temperature for normalization. Initial readings (EC1) were taken and tubes were autoclaved at 12 °C for 30 min, after which, final readings (EC2) were taken once the temperature of tubes came down. Electrolyte leakage was calculated using the formula:
Biochemical assays
Lipid peroxidation
Lipid peroxidation was determined by the method of Heath and Packer (1968) with slight modifications. The melondialdehyde (MDA) content was estimated by homogenizing 500 mg of tissue in 5 ml of 0.1% trichloroacetic acid (TCA). The homogenate was centrifuged at 10,000×g for 5 min. For every 1 ml of aliquot, 4 ml of 20% TCA containing 0.5% thiobarbituric acid was added. The mixture was then heated for 30 min at 95 °C and cooled on an ice-bath. The mixture was centrifuged for 15 min at 10,000×g, and supernatant was taken out. The absorbance of supernatant was measured at 532 and 600 nm. Correction of nonspecific turbidity was made by subtracting the absorbance measured at 600 nm from the absorbance at 532 nm. The lipid peroxidation level was expressed as mmol of MDA formed g−1 fw using the extinction coefficient of 155 mM−1 cm−1.
Protein estimation
Plant tissue (1 g) were homogenised in 3 ml of chilled buffer containing 50 mM phosphate buffer (pH 7.8), 2 mM EDTA, 1 mM DTT, 1 mM PMSF (Phenylmethylsulfonyl Fluoride), 0.5% (v/v) TritonX-100 and 10% (w/v) PVP-40 (Polyvinylpyrrolidone). The homogenate was centrifuged at 12,000 rpm for 20 min and the supernatant was further used for various enzymatic assays. The Bradford protein assay was used to measure the concentration of total protein in a sample with BSA as a standard.
Super oxide dismutase (SOD) assay
The SOD (EC 1.15.1.1) enzymatic activity was determined as per the protocol given by Beauchamp and Fridovich (1971). Reaction mixture was prepared in 50 mM potassium phosphate buffer using 2 μM riboflavin, 75 μM NBT, 100 μM EDTA, 13 mM DL-methionine and 15 μl of enzyme extract and the absorbance was taken at 560 nm. The reaction mix was illuminated at 25 °C for 30 min. Enzyme activity (1 unit) is expressed as the amount of enzymes required for 50% inhibition of NBT reduction at 25 °C.
Catalase assay
CAT (EC 1.11.1.6) activity was estimated at 25 °C based on the protocol given by Aebi (1974). In 1 ml of reaction mixture having 10 mM H2O2 and 60 μl of enzyme extract in 50 mM of potassium phosphate buffer (pH 7), decrease in absorbance of H2O2 was measured. Specific enzyme activity was expressed as l µmol of H2O2 decomposed mg protein−1 min−1.
Expression profiling of heat inducible marker genes
RNA extraction was performed using Trizol reagent (Sigma, T 9424) according to the manufacturer’s instructions. The quantity and quality (A260/A280; A260/A230) of RNA was measured using a NanoDrop 3300 spectrophotometer (Thermo Scientific, Waltham, MA) and RNA integrity was checked by electrophoresis on a 1.2% denaturing agarose gel. One µg of total RNA was reverse transcribed with Superscript TM III First-Strand Synthesis Super Mix for qRT-PCR using oligodT primers (Invitrogen, Carlsbad, CA, USA) as per the manufacturer’s instructions.
The qRT-PCR was conducted for the genes Heat Shock Protein (HSP21), Heat Shock Transcription Factor A7A (HSFA7A) and Tubulin (indigenous control) using the SyBr green based fluorescent dye. The details of the primers used for real time PCR is given in Table 1. PCR products were detected using SYBR Green 2× Master Mix kit (S 4320, Sigma) as per the manufacturer’s instructions. At the end of each PCR run, a melting curve was generated to check the specificity of the primers. Three biological replicates and two technical replicates were taken for each sample. Data Analysis was done using the Method (Livak and Schmittgen 2001).
Table 1.
Gene primer sequences used in quantitative real time PCR
| Gene name | Forward primer sequences (5′–3′) | Reverse primer sequences (5′–3′) |
|---|---|---|
| Tubulin | TCTGCTTCCGTACCCTCAAACTCA | GCAAAACCCACCATGAAGAAATGG |
| Heat shock protein (HSP21) | GGACGTCTCTCCTTTCGGATTGTTG | TGTCGAAACGCATCTTGATCTCGTG |
| Heat shock transcription factor A7A (HSFA7A) | GGAGATGCAAGGGCATGGAA | GGAGGTGGAAGCCAAACTCT |
Estimation of heat-tolerant index (HTI) and membership function value (MFV)
The HTI was calculated for survival percentage and MDA content of the 34 Brassica juncea genotypes as the ratio of the data derived from the heat-stressed (HS) and optimum temperature (OT) treatments of the same genotype for each trait using following the equation (Chen et al. 2012).
where HTIij is the heat-tolerant index and HIIij is heat injury index of the trait (j) for the genotype (i); and are the values of the trait (j) for the cultivar (i) evaluated under heat-stressed (HS) and optimum temperature (OT) treatments, respectively.
Brassica juncea thermo-tolerance was evaluated by the membership function value. The membership function value (MFV) was calculated following the equations (Chen et al. 2012; Liu et al. 2017). If an indicator is positively correlated with heat tolerance:
If an indicator is negatively correlated with heat tolerance
where Uij is the membership function value of the trait (j) for the cultivar (i) for heat tolerance; is the maximum value of the heat-tolerant index for the trait (j); is the minimum value of HTIij. Average value of the membership function of traits (Ui) for the genotypes was calculated. Heat tolerance was divided into five grades according to the average value (Ui) and standard deviation (SD) of MFV (Chen et al. 2012). A regression study was conducted to identify the fitness of the parameters.
Statistical analysis
The experimental design was randomized with three replicates for each of the treatments. Two way analysis of variance (ANOVA) was performed on the data to confirm the variability of data and validity of results in Microsoft Excel. Test of significance was performed at p 0.01 or as indicated.
Results
Screening of Brassica juncea germplasm for heat stress tolerance
A core set of 34 B. juncea germplasms were used in this study. Seedlings were screened for their heat stress tolerance on the basis of accumulation of MDA and survival percentage of the seedlings. After four cycles of heat stress, MDA level was in range of 1.58 (TPM1) to 8.47 (JM-2) fold higher as compared to their respective controls (Table 2). Heat stress treatments to the seedlings significantly decreased the survival percentage in all the accessions varying from the lowest of 23.33% for JM-2 to the highest of 78.33% for TPM1 (Table 2).
Table 2.
Survival percentage and fold change in MDA content of different genotypes in heat stress
| S. no. | Genotype | Fold change in MDA values | Survival (%) |
|---|---|---|---|
| 1 | JM-2 | 8.47 | 23.33 |
| 2 | IC-113037 | 6.68 | 33.33 |
| 3 | RGN-73 | 6.01 | 28.33 |
| 4 | RCC-4 | 5.74 | 30.00 |
| 5 | RL-1359 | 5.73 | 31.67 |
| 6 | RB-50 | 5.29 | 38.33 |
| 7 | JMM-927 | 4.41 | 50.00 |
| 8 | IC-264986 | 4.39 | 30.00 |
| 9 | NRHB-101 | 4.37 | 58.33 |
| 10 | LAXMI | 4.30 | 40.00 |
| 11 | GM-2 | 4.21 | 48.33 |
| 12 | BIO-902 | 4.02 | 31.67 |
| 13 | RGN-48 | 3.80 | 30.00 |
| 14 | RH-187 | 3.65 | 51.67 |
| 15 | RH-819 | 3.65 | 46.67 |
| 16 | KRANTI | 3.63 | 60.00 |
| 17 | NPJ-124 | 3.61 | 46.67 |
| 18 | GM-3 | 3.36 | 53.33 |
| 19 | TM-4 | 3.36 | 58.33 |
| 20 | RGNDR-02 | 3.22 | 43.33 |
| 21 | GEETA | 3.06 | 31.67 |
| 22 | ROHINI | 2.83 | 41.67 |
| 23 | NPJ-113 | 2.70 | 58.33 |
| 24 | MAHAK | 2.64 | 55.00 |
| 25 | NPJ-112 | 2.57 | 71.67 |
| 26 | IC-26513 | 2.40 | 60.00 |
| 27 | P. BOLD | 2.40 | 66.67 |
| 28 | P. JAIKISAN | 2.36 | 76.67 |
| 29 | P. BAHAR | 2.29 | 68.33 |
| 30 | MAHIAR | 2.05 | 41.67 |
| 31 | VARUNA | 1.88 | 58.33 |
| 32 | CG LOCAL | 1.85 | 51.67 |
| 33 | MAYA | 1.79 | 66.67 |
| 34 | TPM-1 | 1.58 | 78.33 |
Analysis of variance revealed significant genotypic differences for both the characters studied. Treatment effects were highly significant for both the characters. Genotypes × treatment interactions were also significant for the studied characters (Tables 3 and 4). Correlation analysis between MDA content and survival percentage showed correlation coefficient r = − 0.73 (p < 0.05).
Table 3.
ANOVA table for survival percentage
| Source of variation | SS | df | MS | F | p-value | F crit |
|---|---|---|---|---|---|---|
| Genotypes | 215.63 | 33 | 6.53 | 9.82 | 9.5E−33 | 1.47 |
| Treatments | 2702.21 | 1 | 2702.21 | 4059.28 | 4.3E−191 | 3.87 |
| Interaction | 215.63 | 33 | 6.53 | 9.82 | 9.5E−33 | 1.47 |
| Within | 226.33 | 340 | 0.67 | |||
| Total | 3359.79 | 407 |
Table 4.
ANOVA table for melondialdehyde (MDA) content
| Source of variation | SS | df | MS | F | p-value | F crit |
|---|---|---|---|---|---|---|
| Genotypes | 9186.4 | 33 | 278.38 | 67.27 | 6.7E−69 | 1.5 |
| Treatments | 21,537.5 | 1 | 21,537.5 | 5204.45 | 3E−110 | 3.91 |
| Interaction | 4533.3 | 33 | 137.37 | 33.19 | 4E−50 | 1.52 |
| Within | 562.8 | 136 | 4.14 | |||
| Total | 35,820.1 | 203 |
Heat tolerance evaluation
The MFV of parameter and mean MFV was calculated and used as an index to evaluate the thermo-tolerance of mustard genotypes. The estimated MFV values of the 34 mustard genotypes based on the studied traits under different temperature conditions was presented in Table S2 and genotype classification as shown in Fig. 1a with average value and standard deviation of 0.59 and 0.17, respectively. Among all the B. juncea genotypes analyzed, only one genotype was identified as highly heat tolerant (HHT) (Ui ≥ 0.88), 6 as heat tolerant (HT) (0.77 ≤ Ui < 0.88), 21 as moderately heat tolerant (MHT) (0.42 ≤ Ui < 0.77), 3 as heat sensitive (HS) (0.30 ≤ Ui < 0.42), and 3 as highly heat sensitive (HHS) (Ui < 0.30) (Fig. 1). The maximum and minimum value of mean MFV were 0.89 (TPM1) and 0.12 (JM2), respectively.
Fig. 1.
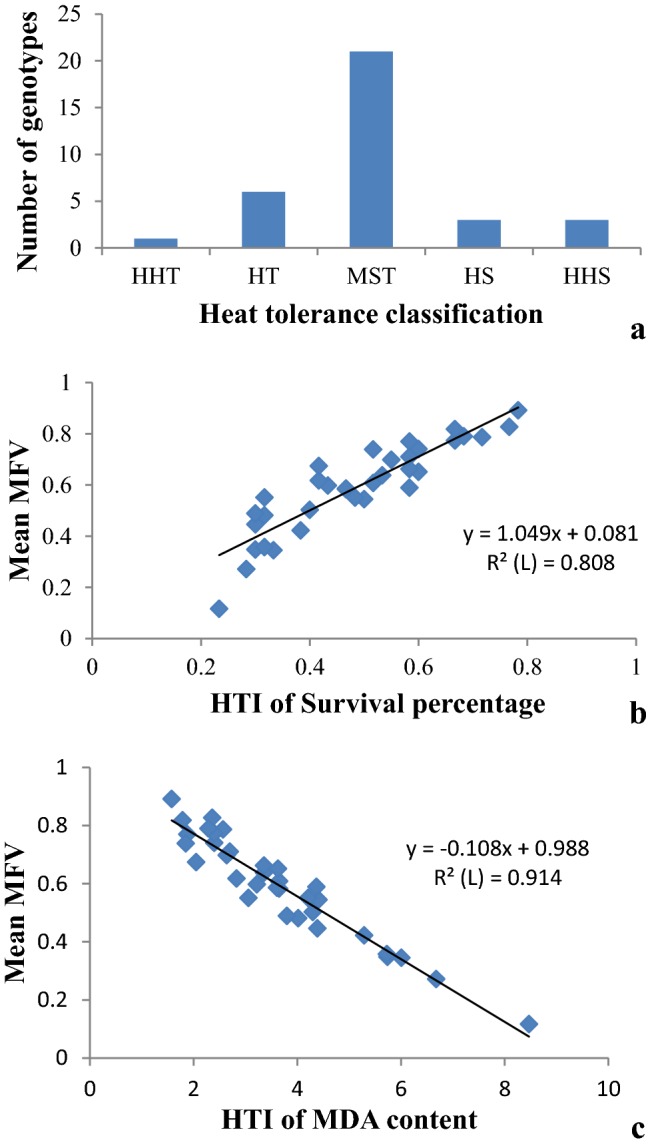
a Proportion of the 34 B. juncea genotypes with different heat tolerances. HHT: highly heat tolerant; HT: heat tolerant; MHT, moderately heat tolerant; HS: heat sensitive; HHS: Highly heat sensitive. The linear fit between mean MFV and the STI of survival percentage (b) and MDA content (c) of B. juncea genotypes
In our study, the mean MFV was derivatives of the STI of MDA content and survival percentage which suggested that the higher HTI or lower HII value of the parameter, the higher the MFV value. To determine whether the studied parameter is reliable in reflecting heat tolerance, a linear model between the HTI of parameter and the mean MFV was fitted. The R2 between the mean MFV and the HTI of MDA content, survival percentage were 0.914 and 0.808 (Fig. 1b, c). These results suggest that MDA content and survival percentage can be used as a reliable trait to evaluate the heat tolerance of B. juncea genotypes.
Four contrasting genotypes, TPM1 and Pusa Jaikisan (tolerant) whereas JM-2 and RGN-73 (sensitive) were selected out of 34 genotypes for heat stress tolerance analysis.
Assessment of heat stress in contrasting genotypes
Histochemical assay
To investigate the ROS accumulation during heat stress, seedlings of contrasting four genotypes were stained with NBT. The production of formazon compound in both genotypes under heat and control conditions was imaged (Fig. 2). As compared to genotypes Pusa Jaikisan and TPM-1 intense blue colour was observed in RGN-73 and JM-2 for 4 days of heat treated seedlings. When seedlings were allowed to recover staining intensity decreased in all the genotypes. The intensity of stain in heat and drought recovery however was lesser than that observed in only heat recovery (Fig. 2).
Fig. 2.
NBT dye assay for qualitative super oxide radical detection in heat tolerant contrasting genotypes (tolerant—TPM1, Pusa Jaikisan and sensitive—RGN-73, JM2)
Electrochemical conductivity
Electrolyte leakage was measured and results showed that EC was significantly higher in genotypes JM-2 and RGN-73 with respect to genotypes Pusa Jaikisan and TPM1 seedlings. The highest increase (> 3.1-fold) was found in JM-2, whereas the lowest (> 2.3-fold) was found in TPM1 as compared to control. A significantly reduced EC has been observed when the plants were subjected to recovery (Fig. 3a).
Fig. 3.

Fold change in electrolyte leakage (a) and MDA content (b) after heat treatment and recovery in heat tolerant contrasting genotypes (tolerant—TPM1, PusaJaikisan and sensitive—RGN-73, JM2)
Lipid peroxidation
MDA content was also found significantly high in all the contrasting genotypes. However, the cellular MDA content was more pronounced in sensitive genotypes as compared to tolerant ones. Maximum fold change was observed in JM-2 (8.47 times) whereas, minimum fold change was observed in TPM-1 (1.58 times). Furthermore, MDA content was significantly reduced in all the genotypes when recovery phase was given. After recovery, minimum fold change was observed in TPM-1 and maximum was in JM-2 (4 times) (Fig. 3b).
Antioxidant enzyme activities
To get more insight into the differential ROS expression, assay for SOD and CAT activities were performed in the contrasting only for TPM-1 and JM-2 genotypes. SOD and CAT activity was found maximum in heat stressed tolerant genotype with 2.4- and 2.8-fold activity induction, respectively, in comparison to non-stressed plant. In case of heat stressed sensitive genotype there was only 1.2- and 1.3-fold induction, respectively (Fig. 4a, b). Analysis of variance revealed no significant genotypic differences for both the characters (SOD and CAT). However treatment effects were highly significant for both the characters. Genotypes × treatment interactions were also significant for the SOD and CAT (Tables 5 and 6).
Fig. 4.

Effect of heat stress on superoxide dismutase (SOD) (a) and catalase (CAT) (b) activity in two contrasting heat tolerant genotype. All values are means of triplicates ± SE. Bars bearing asterisks are significantly different (p ≤ 0.01)
Table 5.
ANOVA for superoxide dismutase (SOD) assay
| Source of variation | SS | df | MS | F | p-value | F crit |
|---|---|---|---|---|---|---|
| Sample | 0.305544569 | 1 | 0.305544569 | 0.059990633 | 0.812676406 | 11.25862 |
| Columns | 226.6177389 | 1 | 226.6177389 | 44.49413573 | 0.000157447 | 11.25862 |
| Interaction | 77.31108628 | 1 | 77.31108628 | 15.17926171 | 0.004569997 | 11.25862 |
| Within | 40.74563718 | 8 | 5.093204648 | |||
| Total | 344.9800069 | 11 |
Table 6.
ANOVA for catalase (CAT) assay
| Source of variation | SS | df | MS | F | p-value | F crit |
|---|---|---|---|---|---|---|
| Sample | 11,383.3796 | 1 | 11,383.38 | 8.696876 | 0.018453 | 11.25862 |
| Columns | 57,622.17974 | 1 | 57,622.18 | 44.02321 | 0.000163 | 11.25862 |
| Interaction | 21,050.51502 | 1 | 21,050.515 | 16.08255 | 0.003893 | 11.25862 |
| Within | 10,471.23549 | 8 | 1308.9044 | |||
| Total | 100,527.3098 | 11 |
Expression profiling of HSP21 and HSFA7A
Expression profiling of genes HSP21 and HSFA7A in the two contrasting tolerant (TPM-1) and sensitive (JM-2) genotypes was performed to investigate heat stress responsive gene expression. Transcriptional up-regulation of both the genes was observed under heat stress while control condition showed no such expression (Fig. 5). In case of heat treatment given to seedlings for 4 days, significant increase in HSP21 gene expression was detected in both the varieties. However, maximum 9.73-fold increase was observed in TPM-1 as compared to JM-2 (4.18-fold increase) (Fig. 5). In case of HSFA7A, significant increase in expression was detected in both the varieties with maximum change was observed again in TPM-1 (4.87-fold) whereas in JM-2 a 1.73-fold change was observed.
Fig. 5.
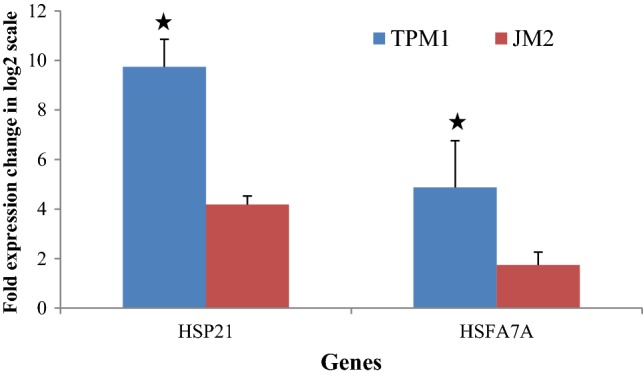
Effect of heat stress on the expression of HSP21 and HSFA7A. Tubulin was used as an internal control. The vertical column indicates the log-twofold expression level change. The x-axis represents the expression level of genes in control (untreated) sample. All values are means of triplicates ± SE. For each gene, bars bearing asterisks are significantly different (p ≤ 0.05)
Discussion
In India, the cultivation of Indian mustard is largely carried out under the rain-fed farming system. High temperature prevailing at the time of sowing reduces seed germination and causes seedling mortality, resulting in poor crop stand and reduced seed yield (Saxena et al. 1988). Thus, thermo tolerant germplasm/variety emerge as the primary requirement for an early maturing/season crop (AICORPO 2000). Current breeding efforts that are largely based on field selection are inefficient because temperature cannot be controlled to threshold limit and microclimatic conditions of different seedlings may vary in field area. In the laboratory, the seedlings are grown in Petri plates, in moistened blotting papers or boxes/trays containing soil. However, in such conditions, the relative humidity is very high, which again fails to mimic the natural field conditions where high temperature is accompanied by low relative humidity and water stress (Sharma et al. 2007). Thus there exists a need for a screening procedure which faithfully mimics the field conditions, yet is simple and scalable for a broader applicability at the early stages of crop. Therefore, development of a method to select heat tolerant lines quickly and quantitatively will contribute to the breeding and development of new heat tolerant crop varieties (Park et al. 2013).
In this study, a simple efficient screening methodology has been adopted which was comparable to natural field conditions since water was applied during heat stress treatment as soil temperature increases as a result of increase in air temperature associated with decline in soil moisture (Kumar et al. 2012; Gourdji et al. 2013). Survival percentage after 4-day cycle of heat stress reflects the recovery potential of the germplasm. MDA, a peroxidation products accumulate late in the stress response process, possibly during the propagation stage (Winston 1990) and then only in the affected membrane. The ROS-induced peroxidation of lipid membranes is a reflection of stress-induced damage at the cellular level. The ability of the plant to survive under heat conditions is dependent on its tolerance, which is the outcome of physiological, biochemical, and molecular changes under stress conditions (Bita and Gerats 2013). In rice, survival has been shown as selection criteria for thermal stress (Sarsu 2018). Thus for initial screening, we used the MDA content and survival percentage as parameters for identification of tolerant and sensitive genotype from a genotype pool. The levels of oxidative damage measured showed a general correlation with the survival of the plants. However, the two variables were not completely linked but showed strong negative correlation. In our screening experiments, we observed higher levels of TBARS in plants that survived, but the percentage survival did not always vary exactly with the level of TBARS (Table 2) as also shown by Larkindale and Knight (2002). Heat stress induced MDA accumulation were detected and quantified in different crop at seedling stage wheat (Sanghera and Thind 2016), chickpea (Kaushal et al. 2011) and Brassica (Wilson et al. 2014). ANOVA results showed that there is significant difference among genotypes under control and treatment condition. This suggests that the screening method has ability to discriminate the thermotolerance potential among genotypes used in study. In our study, analysis on the relations between the MFV of heat tolerance with the HTI of studied parameters indicated that heat tolerance was highly significant and correlated (p < 0.05) with variations of parameter investigated under heat stress treatments. Based upon MFV analysis classification of thermo-tolerance among genotypes was performed which showed that majority of genotypes are moderately thermo tolerant.
We further assessed other stress indicators to assess the thermo-tolerance of the contrasting genotype such as electrolyte leakage and superoxide radical through qualitative dye assay in heat stress as well as in recovery phase. Results showed lesser increase of EC and ROS in tolerant genotype as compared to sensitive genotype. After recovery, tolerant germplasm showed equal level of EC and ROS accumulation under non-treated condition (Figs. 2, 3). Contrary to this, in sensitive germplasm the electrolyte leakage and ROS accumulation were more as compared to the non-treated sample after recovery. This clearly suggests that fast recovery is one of the major components to repair oxidative damage and for the plant to become tolerant as also shown in wheat (Dash and Mohanty 2001).
Several studies suggest that thermal stress produces ROS such as superoxide radicals, hydroxyl radicals, and hydrogen peroxide at the chloroplastic PS II reaction centre, which are scavenged by antioxidants, including superoxide dismutase (SOD) (Bukhov and Mohanty 1999). We also performed the SOD and CAT activity assay to assess the ROS scavenging capability in tolerant (TPM1) and sensitive (JM2) lines which showed improved ROS scavenging capacity in TPM1 over JM2 implying that the tolerant genotype is endowed with better ROS scavenging system (Fig. 4). These components have been found in almost all cellular compartments, indicating the importance of ROS detoxification for cellular survival (Mittler et al. 2004; Asada 2006).
During heat stress, to sustain the intact cellular metabolism, functional protein conformation has to be maintained. Thus, in response to heat stress, there is an accumulation of group of proteins called “heat-shock proteins” (Hsps), (Gupta et al. 2010). Heat-shock proteins (HSPs) and heat-shock transcription factors (HSFs) are central components of the heat-shock regulatory network and are involved in cellular responses to various forms of stresses (Lee et al. 2005).With this viewpoint, we further carried out the expression analysis of chloroplast localised small heat shock protein (hsp21) and heat stress induced transcription factor (HSFA7A) in tolerant and sensitive germplasm based upon the screening method and results showed transcriptional up regulation of HSP21 and HSFA7A in tolerant genotype as compared to sensitive one. This indicates that HSPs are up-regulated because of the efficiency of upstream regulatory mechanisms like up regulation of transcription factors. HSFA7A genes role has been identified acquired thermotolerance in plant (Larkindale and Vierling 2008). In addition to chaperon activity, HSP21 is also required for chloroplast development under heat stress (Pfalz and Pfannschmidt 2013). In addition, HSP21 is localized in plastid nucleoids and is essential for proper chloroplast development by maintaining plastid-encoded RNA polymerase-dependent transcription (PEP) function (Zhong et al. 2013). Our results suggesting increased expression of the HSP21 and HSFA7A genes might be useful as molecular index to distinguish heat-tolerant Brassica lines.
In conclusion, a screening method is reported here which has the potential to identify thermotolerant genotypes, in a scalable lab setup. We found that the MDA content and survival percentage were reliable parameters for evaluating heat tolerance of B. juncea genotype. These results will significantly contribute to the evaluation and breeding of thermo-tolerant B. juncea.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
ANR conceived and designed the experiments; RY provided true to type genotype/ germplasm; ANR and NS conducted the experiments; ANR analysed the data; ANR and PS wrote the paper. All authors have read and approved the final manuscript.
Funding
Research was supported by Bhabha Atomic Research Centre.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Aebi H. Catalase. In: Bergmeyer HU, editor. Methods in enzymatic analysis. New York: Acadamic Press; 1974. pp. 674–684. [Google Scholar]
- AICORPO (2000) Screening Brassica species for abiotic stresses. In: Proceedings of the all India co-ordinated research projects on oilseeds, pp 128–132.
- Alfaro EJ, Gershunov A, Cayan D. Prediction of summer maximum and minimum temperature over the central and western United States: the roles of soil moisture and sea surface temperature. J Clin Investig. 2006;19:1407–1421. [Google Scholar]
- Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006;141:391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azharudheen TPM, Yadava DK, Singh N, Vasudev S, Singh R, Prabhu KV. A study on the thermo-tolerance at germination and seedling stage in Indian Mustard [Brassica Juncea (L.) Czern & Coss] Int J Agric Food Sci. 2013;4(6):589–594. [Google Scholar]
- Bailey-Serres J, Mittler R. The roles of reactive oxygen species in plant cells. Plant Physiol. 2006;141:311. doi: 10.1104/pp.104.900191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bhardwaj AR, Joshi G, Kukreja B, Malik V, Arora P, Pandey R, et al. Global insights into high temperature and drought stress regulated genes by RNA-Seq in economically important oilseed crop Brassica juncea. BMC Plant Biol. 2015;15:9. doi: 10.1186/s12870-014-0405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bita CE, Gerats T. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci. 2013;4:273. doi: 10.3389/fpls.2013.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhov N, Mohanty P. Elevated temperature stress effects on photosystems: characterization and evaluation of the nature of heat induced impairments. In: Singhal GS, Renger G, Sopory SK, Irrgang KD, Govindjee E, editors. Concepts in photobiology: photosynthesis and photomorphogenesis. New Delhi: Narosa Publishing House; 1999. pp. 617–648. [Google Scholar]
- Chen X, Min D, Yasir TA, Hu YG. Evaluation of 14 morphological, yield-related and physiological traits as indicators of drought tolerance in Chinese winter bread wheat revealed by analysis of the membership function value of drought tolerance (MFVD) Field Crops Res. 2012;137:195–201. doi: 10.1016/j.fcr.2012.09.008. [DOI] [Google Scholar]
- Dash S, Mohanty N. Evaluation of assays for the analysis of thermo-tolerance and recovery potentials of seedlings of wheat (Triticum aestivum L.) cultivars. Plant Physiol. 2001;158(9):1153–1165. doi: 10.1078/0176-1617-00243. [DOI] [Google Scholar]
- Djanaguiraman M, Boyle DL, Welti R, Jagadish SVK, Prasad PVV. Decreased photosynthetic rate under high temperature in wheat is due to lipid desaturation, oxidation, acylation, and damage of organelles. BMC Plant Biol. 2018;18(1):55. doi: 10.1186/s12870-018-1263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Yi H, Lee J, Nou S, Han C, Hur Y. Global gene expression analysis to identify differentially expressed genes critical for the heat stress response in Brassica rapa. PLoS ONE. 2015;10:0130451. doi: 10.1371/journal.pone.0130451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch-Savage WE, Bassel GW. Seed vigour and crop establishment: extending performance beyond adaptation. J Exp Bot. 2016;67:567–591. doi: 10.1093/jxb/erv490. [DOI] [PubMed] [Google Scholar]
- Gourdji SM, Sibley AM, Lobell DB. Global crop exposure to critical high temperatures in the reproductive period: historical trends and future projections. Environ Res Lett. 2013;8:024041. doi: 10.1088/1748-9326/8/2/024041. [DOI] [Google Scholar]
- Gupta SC, Sharma A, Mishra M, Mishra R, Chowdhuri DK. Heat shock proteins in toxicology: how close and how far? Life Sci. 2010;86:377–384. doi: 10.1016/j.lfs.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Hatfield JL, Prueger JH. Temperature extremes: effect on plant growth and development. Weather Clim Extrem. 2015;10:4–10. doi: 10.1016/j.wace.2015.08.001. [DOI] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Jabs T, Dietrich RA, Dangl JL. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science. 1996;273(5283):1853–1856. doi: 10.1126/science.273.5283.1853. [DOI] [PubMed] [Google Scholar]
- Kaushal N, Gupta K, Bhandhari K, Kumar S, Thakur P, Nayyar H. Proline induces heat tolerance in chickpea (Cicer arietinum L.) plants by protecting vital enzymes of carbon and antioxidative metabolism. Physiol Mol Biol Plants. 2011;17(3):203–213. doi: 10.1007/s12298-011-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Gupta D, Nayyar H. Comparative response of maize and rice genotypes to heat stress: status of oxidative stress and antioxidants. Acta Physiol Plant. 2012;34:75–86. doi: 10.1007/s11738-011-0806-9. [DOI] [Google Scholar]
- Larkindale J, Knight MR. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002;128(2):682–695. doi: 10.1104/pp.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Vierling E. Core genome responses involved in acclimation to high temperature. Plant Physiol. 2008;146:748–756. doi: 10.1104/pp.107.112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee U, Wie C, Escobar M, Williams B, Hong SW, Vierling E. Genetic analysis reveals domain interactions of Arabidopsis Hsp100/ClpB and cooperation with the small heat shock protein chaperone system. Plant Cell Environ. 2005;17:559–571. doi: 10.1105/tpc.104.027540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li P, Xu GC, Xiao L, Ren ZP, Li ZB. Growth, morphological, and physiological responses to drought stress in Bothriochloa ischaemum. Front Plant Sci. 2017;24(8):230. doi: 10.3389/fpls.2017.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Nadeem M, Li J, Wang M, Shah L, Lu S, Wang X, Ma C. Unraveling field crops sensitivity to heat stress: mechanisms, approaches, and future prospects. Agronomy. 2018;8(7):128. doi: 10.3390/agronomy8070128. [DOI] [Google Scholar]
- Park CJ, Sharma R, Lefebvre B, Canlas PE, Ronald PC. The endoplasmic reticulum-quality control component SDF2 is essential for XA21-mediated immunity in rice. Plant Sci J. 2013;210:53–60. doi: 10.1016/j.plantsci.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Pfalz J, Pfannschmidt T. Essential nucleoid proteins in early chloroplast development. Trends Plant Sci. 2013;18:186–194. doi: 10.1016/j.tplants.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Sanchez B, Rasmussen A, Porter JR. Temperatures and the growth and development of maize and rice: a review. Global Change Biol. 2014;20:408–417. doi: 10.1111/gcb.12389. [DOI] [PubMed] [Google Scholar]
- Sanghera AK, Thind SK. Evaluation of seedling growth and MDA content of wheat genotypes in relation to heat tolerance. Indian J Sci Technol. 2016 doi: 10.17485/ijst/2016/v9i31/50284. [DOI] [Google Scholar]
- Sanghera GS, Wani SH, Hussain W, Singh NB. Engineering cold stress tolerance in crop plants. Curr Genom. 2011;12:30. doi: 10.2174/138920211794520178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsu Fatma. Pre-Field Screening Protocols for Heat-Tolerant Mutants in Rice. Cham: Springer International Publishing; 2018. Screening Protocols for Heat Tolerance in Rice at the Seedling and Reproductive Stages; pp. 9–24. [Google Scholar]
- Saxena MC, Saxena NP, Mohamed AK. High temperature stress. In: Summerfield RJ, editor. World crops: cool season food legumes, current plant science and biotechnology in agriculture. Dordrecht: Springer; 1988. [Google Scholar]
- Scharf KD, Berberich T, Ebersberger I, Nover L. The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochim Biophys Acta. 2012;1819:104–119. doi: 10.1016/j.bbagrm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Sharma R, Chhabra ML, Dhawan A, Dhawan K, Singh D (2007) Thermo tolerance in Brassica: refined rapid screening method to identify thermo tolerant genotypes in Brassica. In: Proceedings of the genetics and breeding: breeding for stress resistance, pp 430–432
- Shekhawat K, Rathore SS, Premi OP, Kandpal BK, Chauhan JS. Advances in agronomic management of Indian mustard (Brassica juncea (L.) Czernj.Cosson): an overview. Int J Agron. 2012 doi: 10.1155/2012/408284. [DOI] [Google Scholar]
- Singh D, Balota M, Collakova E, Isleib TG, Welbaum GE, Tallury SP. Heat stress related physiological and metabolic traits in peanut seedlings. Peanut Sci. 2016;43:24–35. doi: 10.3146/0095-3679-43.1.24. [DOI] [Google Scholar]
- Teixeira EI, Fischer G, Velthuizen HV, Walter C, Ewert F. Global hot-spots of heat stress on agricultural crops due to climate change. Agric For Meteorol. 2013;170:206–215. doi: 10.1016/j.agrformet.2011.09.002. [DOI] [Google Scholar]
- Wilson RA, Sangha MK, Banga SS, Atwal AK, Gupta S. Heat stress tolerance in relation to oxidative stress and antioxidants in Brassica juncea. J Environ Biol. 2014;35(2):383–387. [PubMed] [Google Scholar]
- Winston GW. Physicochemical basis for free radical formation in cells: production and defenses. In: Alscher RG, Cumming JR, editors. Stress response in plants: adaptation and acclimation mechanisms. New York: Wiley; 1990. [Google Scholar]
- Zhong L, Zhou W, Wang H, Ding S, Lu Q, Wen X, Peng L, Zhang L, Lu C. Chloroplast small heat shock protein HSP21 interacts with plastid nucleoid protein pTAC5 and is essential for chloroplast development in arabidopsis under heat stress. Plant Cell. 2013;25(8):2925–2943. doi: 10.1105/tpc.113.111229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



