Abstract
Non-alcoholic steatohepatitis (NASH) is a complex disease consisting of various components including steatosis, lobular inflammation, and ballooning degeneration, with or without fibrosis. Therefore, it is difficult to diagnose NASH with only one imaging modality. This study was aimed to evaluate the feasibility of magnetic resonance imaging (MRI) for predicting NASH and to develop a non-invasive multiparametric MR index for the detection of NASH in non-alcoholic fatty liver disease (NAFLD) patients. This prospective study included 47 NAFLD patients who were scheduled to undergo or underwent ultrasound-guided liver biopsy within 2 months. Biopsy specimens were graded as NASH or non-NASH. All patients underwent non-enhanced MRI including MR spectroscopy (MRS), MR elastography (MRE), and T1 mapping. Diagnostic performances of MRS, MRE, and T1 mapping for grading steatosis, activity, and fibrosis were evaluated. A multiparametric MR index combining fat fraction (FF), liver stiffness (LS) value, and T1 relaxation time was developed using linear regression analysis. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic performance of the newly devised MR index. Twenty NASH patients and 27 non-NASH patients were included. Using MRS, MRE, and T1 mapping, the mean areas under the curve (AUCs) for grading steatosis, fibrosis, and activity were 0.870, 0.951, and 0.664, respectively. The multiparametric MR index was determined as 0.037 × FF (%) + 1.4 × LS value (kPa) + 0.004 × T1 relaxation time (msec) −3.819. ROC curve analysis of the MR index revealed an AUC of 0.883. The cut-off value of 6 had a sensitivity of 80.0% and specificity of 85.2%. The multiparametric MR index combining FF, LS value, and T1 relaxation time showed high diagnostic performance for detecting NASH in NAFLD patients.
Subject terms: Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a leading cause of chronic liver disease with increasing prevalence worldwide1. NAFLD encompasses a wide spectrum of diseases, ranging from simple steatosis and non-alcoholic steatohepatitis (NASH) to liver cirrhosis. NASH increases the risk of hepatocellular carcinoma (HCC) and death from cardiovascular disease2,3. Therefore, it is crucial to differentiate NASH from simple steatosis. Liver biopsy is regarded as the gold standard for the diagnosis of NASH4,5. However, liver biopsy is an invasive and costly procedure with the risk of pain, bleeding, and although extremely rarely, even death. As a liver biopsy specimen represents only about 0.0002% of the whole liver, sampling error with inter- and intra-observer variability is another potential problem. Therefore, there have been substantial clinical demands for alternative and noninvasive methods to diagnose NASH.
With recent advances in MRI, MR spectroscopy (MRS), and MR elastography (MRE) have emerged as promising methods for detecting and grading fat and fibrosis, respectively6–9. MRS enables the direct measurement of the fat proton signal fraction and is considered the method of choice for accurate non-invasive quantification of liver fat10. MRE has shown promising results for staging hepatic fibrosis and detecting NASH in NAFLD patients8,9. It has also been shown that T1 mapping can be used to differentiate patients with liver fibrosis and cirrhosis11 and to predict clinical outcomes in patients with chronic liver disease12.
Although MRS, MRE, and T1 mapping have shown good diagnostic performance in detecting and grading NASH components such as steatosis or fibrosis, it is difficult to diagnose NASH with one modality only because NASH is characterized by various components including steatosis, lobular inflammation, ballooning degeneration, and fibrosis. Therefore, we postulated that a non-invasive multiparametric MR index combining MRS, MRE, and T1 mapping may help diagnose NASH in NAFLD patients, thereby potentially reducing the need for liver biopsy.
The purpose of this study was to evaluate the feasibility of MRI for predicting NASH and to develop a non-invasive multiparametric MR index for the detection of NASH in NAFLD patients.
Results
Patients were classified into the NASH (n = 20) or non-NASH (n = 27) group based on the Steatosis, Activity, Fibrosis (SAF) scoring system. Mean age was significantly higher in the NASH group than in the non-NASH group (p = 0.007). Fasting glucose level was significantly higher and platelet count was significantly lower in the NASH group than in the non-NASH group (p = 0.012 and 0.001, respectively). There was no significant difference in other clinical and laboratory data between these two groups (Table 1). Based on histopathologic evaluation, steatosis was graded as S0 (n = 0), S1 (n = 25), S2 (n = 18), or S3 (n = 4); hepatocyte ballooning as B0 (n = 27), B1 (n = 10), or B2 (n = 10); lobular inflammation as L0 (n = 0), L1 (n = 15), or L2 (n = 32); and fibrosis as F0 (n = 14), F1 (n = 13), F2 (n = 12), F3 (n = 7), or F4 (n = 1).
Table 1.
Baseline characteristics of patients.
| Total patients (n = 47) | Non-NASH (n = 27) | NASH (n = 20) | p-value | |
|---|---|---|---|---|
| Age (years) | 51.0 ± 12.7 | 46.9 ± 12.7 | 56.6 ± 10.4 | 0.007 |
| Male: Female | 16:31 | 11:16 | 5:15 | 0.477 |
| Body mass index (kg/m2) | 28.3 ± 6.2 | 27.9 ± 7.7 | 28.7 ± 3.7 | 0.612 |
| ALT (IU/L) | 80.2 ± 43.1 | 82.2 ± 53.0 | 81.0 ± 28.4 | 0.926 |
| AST (IU/L) | 59.6 ± 26.5 | 55.8 ± 30.2 | 67.3 ± 18.8 | 0.134 |
| ALP (IU/L) | 88.2 ± 21.3 | 90.7 ± 24.4 | 86.1 ± 17.9 | 0.474 |
| GGT (IU/L) | 79.0 ± 61.1 | 95.0 ± 76.2 | 62.9 ± 30.9 | 0.059 |
| Total bilirubin (mg/dL) | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.986 |
| Total cholesterol (mg/dL) | 181.9 ± 36.4 | 190.1 ± 33.1 | 173.8 ± 40.0 | 0.133 |
| Triglycerides (mg/dL) | 154.9 ± 65.3 | 168.2 ± 68.9 | 144.8 ± 59.1 | 0.224 |
| HDL-cholesterol (mg/dL) | 43.5 ± 11.1 | 44.2 ± 11.1 | 42.8 ± 11.9 | 0.674 |
| LDL-cholesterol (mg/dL) | 112.6 ± 33.2 | 118.0 ± 34.0 | 106.3 ± 33.5 | 0.247 |
| Fasting glucose (mg/dL) | 117.0 ± 32.4 | 105.9 ± 16.3 | 133.7 ± 41.9 | 0.012 |
| Albumin (g/dL) | 4.2 ± 0.6 | 4.1 ± 0.8 | 4.2 ± 0.3 | 0.692 |
| Platelet count ( × 103/L) | 207.8 ± 54.1 | 228.0 ± 46.8 | 177.5 ± 49.9 | 0.001 |
Note. – ALT alanine aminotransferase, AST aspartate aminotransferase, ALP alkaline phosphatase, GGT γ-glutamyltransferase, HDL high density lipoprotein, LDL low density lipoprotein.
MR parameters and histopathologic components
Diagnostic performance of MRS for grading steatosis is summarized in Table 2. Using MRS, the mean area under the curve (AUC) for grading steatosis was 0.870. Diagnostic performances of MRE and T1 mapping for grading activity (ballooning and lobular inflammation) and fibrosis are summarized in Table 2. Using MRE, the mean AUCs for grading activity (ballooning and lobular inflammation) and fibrosis were 0.825 and 0.951, respectively. Using T1 mapping, the mean AUCs for grading activity and fibrosis were 0.664 and 0.615, respectively.
Table 2.
Diagnostic performance of MRS, MRE and T1 mapping for grading each histopathologic component.
| Mean AUC | AUC | Cut-off value* | Sensitivity (%) | Specificity (%) | |||
|---|---|---|---|---|---|---|---|
| MRS | Steatosis | ≥S2 | 0.870 | 0.862 | 12.88 | 81.8 | 92.0 |
| ≥S3 | 0.878 | 19.08 | 100.0 | 81.4 | |||
| MRE | Ballooning† | ≥B1 | 0.825† | 0.898 | 3.31 | 90.0 | 81.5 |
| ≥B2 | 0.811 | 3.47 | 90.0 | 67.6 | |||
| Lobular inflammation† | ≥L2 | 0.765 | 3.13 | 65.6 | 86.7 | ||
| Fibrosis | ≥F1 | 0.951 | 0.991 | 2.58 | 97.0 | 100.0 | |
| ≥F2 | 0.879 | 3.13 | 97.0 | 100.0 | |||
| ≥F3 | 0.984 | 4.34 | 100.0 | 92.3 | |||
| T1 mapping | Ballooning† | ≥B1 | 0.664† | 0.624 | 843.29 | 95.4 | 60.7 |
| ≥B2 | 0.682 | 921.57 | 90.0 | 56.4 | |||
| Lobular inflammation† | ≥L2 | 0.686 | 894.98 | 71.9 | 64.7 | ||
| Fibrosis | ≥F1 | 0.615 | 0.614 | 1064.37 | 34.3 | 92.9 | |
| ≥F2 | 0.659 | 921.57 | 69.6 | 61.5 | |||
| ≥F3 | 0.572 | 894.678 | 88.9 | 45.0 |
*Units of MRS, MRE, and T1 mapping are percentage (%), kilopascal (kPa), and millisecond (msec), respectively.
†The mean values of AUCs for grading activity (ballooning and lobular inflammation) are demonstrated.
In subgroup analysis, the mean T1 value was significantly higher in group B (mean FFMRS > 15%) than in group A (mean FFMRS ≤ 15%) for ≥ F2 (significant fibrosis) patients (1133.9 ± 97.0 vs. 935.9 ± 79.6 msec, p = 0.001). AUCs of T1 mapping for grading ≥ F2 (significant fibrosis) were 0.837 and 0.714 in group A and B, respectively. These results indicated that hepatic fat is a confounding factor of T1 value estimation.
MR index
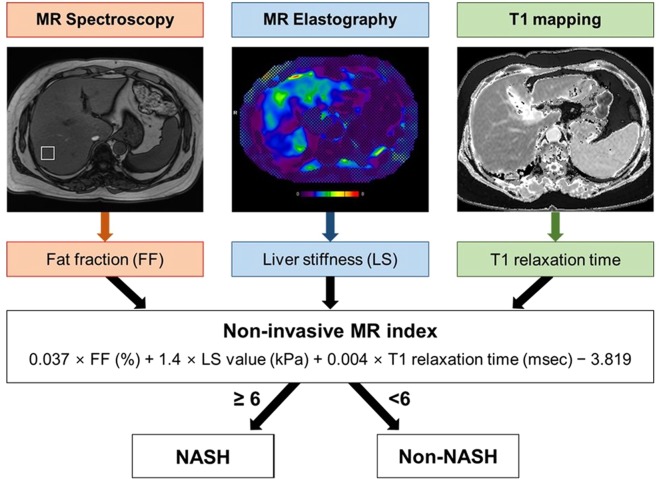
A multiparametric MR index combining fat fraction (FF) (measured on MRS), liver stiffness (LS) value (on MRE) and T1 relaxation time (on T1 mapping) was determined as 0.037 × FF (%) + 1.4 × LS value (kPa) + 0.004 × T1 relaxation time (msec) −3.819 (Fig. 1). Receiver operating characteristic (ROC) curve analysis revealed an AUC of 0.883 and an optimal cut-off value of 4.6 with a corresponding sensitivity of 95.0% and specificity of 77.8% (Table 3). The specificity was relatively low whereas the sensitivity was high. To exclude patients who do not need a liver biopsy, because specificity is more important than the sensitivity, a cut-off value of 6 exhibiting a relatively high specificity while maintaining the sensitivity was used (sensitivity of 80.0% and specificity of 85.2%) (Table 3, Fig. 2).
Figure 1.
Development of a non-invasive multiparametric MR index. Using linear regression analysis, a non-invasive multiparametric MR index combining fat fraction (FF), liver stiffness (LS) value, and T1 relaxation time measured on MRS, MRE, and T1 mapping was determined as 0.037 × FF (%) + 1.4 × LS value (kPa) + 0.004 × T1 relaxation time (msec) − 3.819.
Table 3.
Diagnostic performance of multiparametric MR index.
| AUC | Cut-off value | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|
| Entire group | 0.883 | 4.6 | 95.0 | 77.8 |
| 6.0 | 80.0 | 85.2 | ||
| Group A (mean FFMRS ≤ 15%) | 0.909 | 4.3 | 100.0 | 83.3 |
| Group B (mean FFMRS > 15%) | 0.901 | 4.6 | 100.0 | 66.7 |
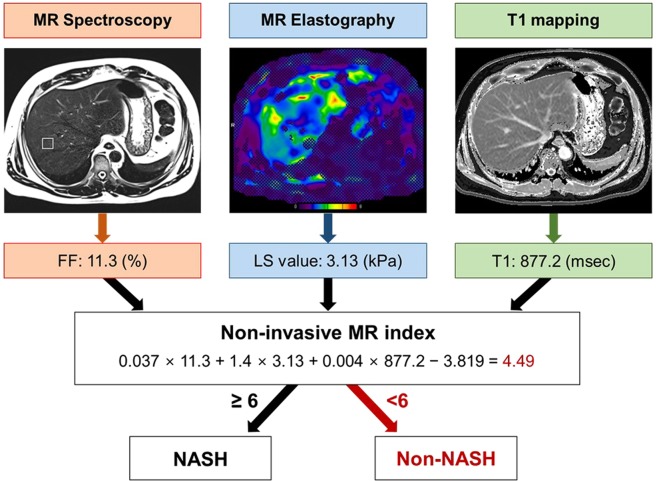
Figure 2.
An example of using the MR index to predict NASH. A 51-year-old man with clinically suspected NASH who underwent percutaneous liver biopsy and MR imaging. Fat fraction measured on MR spectroscopy was 11.3%, liver stiffness value measured on MR elastography was 3.13 kPa, and T1 relaxation time measured on T1 mapping was 877.2 msec. The non-invasive multiparametric MR index predicted that the patient was a non-NASH patient. Histopathologic analyses of the patient’s biopsy specimens revealed grade 1 steatosis, grade 0 balloon degeneration, grade 1 lobular inflammation, and grade 1 fibrosis. Therefore, he was classified as a non-NASH patient according to the SAF scoring system.
In subgroup analysis, the MR index showed higher diagnostic performances in both group A and B than in the entire group. In group A (mean FFMRS ≤ 15%), the MR index showed an AUC of 0.909 and an optimal cut-off value of 4.3 with a corresponding sensitivity of 100% and specificity of 83.3%. In group B (mean FFMRS > 15%), the MR index showed an AUC of 0.901 and an optimal cut-off value of 4.6 with a corresponding sensitivity of 100.0% and specificity of 66.7% (Table 3).
Discussion
In our study, MRS and MRE showed high diagnostic performance for staging steatosis (mean AUC 0.870) and fibrosis (mean AUC 0.951) in NAFLD patients, respectively. These results are in good agreement with those of previous studies in which MRS and MRE showed strong correlations with hepatic FF and hepatic fibrosis, respectively8,13–15. Although MRS and MRE are considered good modalities for diagnosing and grading steatosis and fibrosis, respectively, it is difficult to diagnose NASH with one modality only because NASH is a complex disease consisting of various components including steatosis, lobular inflammation, and ballooning degeneration, with or without fibrosis. Therefore, we devised a new multiparametric MR index combining FF, LS value, and T1 relaxation time. Our newly devised MR index predicted NASH in NAFLD patients with a sensitivity of 80.0% and a specificity of 85.2%.
NAFLD is a term used to describe fat accumulation in the liver with no history of excess alcohol intake or other liver disease. NASH is a subgroup of NAFLD, characterized by hepatocellular injury, inflammation, and ballooning with or without fibrosis. There are several staging and grading systems for NASH. The most widely used system is the NASH Activity Score (NAS), which was originally developed as a tool for evaluating therapeutic outcomes in NAFLD patient trials. It has proven useful for comparative or interventional studies. However, it is less beneficial as a diagnostic tool for NASH and has low prognostic value because it does not include fibrosis16. The more recently developed SAF scoring system may be more accurate for identifying NASH and be more relevant for long-term prognostication17,18.
Hepatic fat is an essential component for diagnosing NAFLD/NASH. There have been several efforts to identify hepatic steatosis using various imaging techniques including ultrasonography19, unenhanced computed tomography20,21, MRI22, and MRS23,24. MRS is considered the most precise non-invasive imaging technique for hepatic fat quantification. In the past, MRS was performed with free-breathing, resulting in long acquisition times and erroneous data acquisition due to motion artifacts25. Recently, high-speed T2-corrected multi-echo (HISTO) MRS was introduced for T2-corrected hepatic fat measurement with a single breath hold26,27. In our study, MRS showed high mean AUC of 0.870 for grading steatosis in NAFLD patients.
NAFLD ranges from simple steatosis to NASH, which can progress to cirrhosis and HCC. Although liver biopsy is considered the only reliable method for distinguishing NASH from simple steatosis, there is the potential risk of sampling error and complications such as pain, bleeding, and although extremely rarely, even death. These drawbacks of liver biopsy have increased the demand for alternative and noninvasive methods for diagnosis of NASH. These include non-invasive models composed of demographic and laboratory parameters, such as aspartate aminotransferase to platelet ratio index (APRI), fibrosis-4 index (FIB-4), BARD score, and NAFLD fibrosis score (NFS)18. Non-invasive imaging techniques such as acoustic radiation force impulse (ARFI), transient elastography (FibroScan), shear wave elastography (SWE), and MRE have also been studied to detect fibrosis in NAFLD patients18. Among them, MRE showed the highest diagnostic accuracy for the grading and diagnosis of fibrosis in NAFLD patients in meta-analysis and prospective studies28,29. In our study, MRE showed high mean AUC of 0.951 for grading fibrosis in NAFLD patients.
T1 mapping was originally used for non-invasive evaluation of myocardial fibrosis, which is characterized by edema (increase in tissue water) and increased extracellular matrix (ECM) remodeling (increase in collagen volume fraction) after an acute ischemic event30,31. Several studies have assessed this technique in chronic liver disease patients11,32,33. In liver fibrosis, increased edema and ECM remodeling occur after hepatocyte injury, resulting in lengthened T1 relaxation time. The T1 mapping technique directly measures T1 relaxation time in milliseconds. Banerjee et al. demonstrated that T1 relaxation time was strongly correlated with liver fibrosis in 79 patients who underwent liver biopsy11. T1 mapping had an AUC of 0.94 with a sensitivity of 86% and a specificity of 93% for differentiating healthy people and patients with no fibrosis from those with fibrosis. In our study, compared with MRE, T1 mapping showed relatively low diagnostic performances for grading fibrosis (mean AUC, 0.615 vs. 0.951) and activity (mean AUC, 0.664 vs. 0.825). This result may be due to the fact that T1 values are influenced by hepatic fat34. Therefore, subgroup analyses were performed to evaluate the diagnostic performance of T1 mapping for grading significant fibrosis (F2) and the diagnostic performance of MR index for detecting NASH. Diagnostic performance of T1 mapping improved in each subgroup (FFMRS ≤ 15% and > 15%) relative to the entire group. In particular, diagnostic performance was higher in group A (FFMRS ≤ 15%) than in group B (FFMRS > 15%). Diagnostic performance of the MR index also improved in each group relative to the entire group and was higher in group A than in group B. These results indicate that hepatic fat is a confounding factor of the T1 value, therefore the T1 value should be used with caution in patients with fatty liver of moderate or greater degree. Applying different MR indices according to hepatic fat component can overcome the limitation of T1 mapping. To the best of our knowledge, there is no new sequence yet to improve T1 mapping.
As noted above, MRI studies in NAFLD/NASH have focused primarily on the diagnosis of fat or fibrosis, which are components of NASH. Like non-invasive models using clinical data, if a non-invasive MR index is developed by combining MR parameters in NAFLD patients, it would provide an easily available prediction model in the clinical setting. Therefore, a new MR index was devised by combining FF, LS value, and T1 relaxation time measured by MRS, MRE, and T1 mapping, which are included in our MRI protocol for NAFLD patients. This new MR index predicted NASH as a SAF score with high diagnostic performance.
This study had several limitations. First, only a few patients limited to a single tertiary center using one MRI scanner were included. Although a small cross-vendor validation study comparing the reproducibility of two difference MR scanners from Philips and GE Healthcare in 13 subjects demonstrated that LS value measurements were reproducible and had good consistency across two vendors35, a multicenter study with a larger number of patients using several different MRI scanners is needed to develop an MR index that can be widely used in various clinical settings. Second, our new devised MR index was not validated using a validation group due to the small number of patients. Another prospective study using a validation group of NAFLD patients is needed to validate the results of this study. Third, it is well known that hepatic iron deposition can be increased associated with liver disease36, and age, gender, and menopause for female may affect hepatic iron deposition37,38, which in turn may affect MRI measurements. However, it was not possible to perform subgroup analyses according to age, gender, and menopause due to small number of patients. We hope to study the effect of hepatic iron deposition on MRI measurements in the future with a larger number of patients. Fourth, in our study, a non-invasive index was developed using only MR parameters and linear regression analysis with three fixed MR parameters (FF, LS value, and T1 relaxation time). However, the aim of the study was to develop a simple MR index for predicting NASH in NAFLD patients. In the future, it is expected to devise a NASH index through a prospective study using multivariate regression analysis of clinical (demographic and laboratory) data and MR parameters.
In conclusion, MRI showed high diagnostic performance for detecting and grading the NASH components of steatosis, activity (lobular inflammation and ballooning degeneration), and fibrosis. Our newly devised MR index combining MRS, MRE, and T1 mapping also demonstrated high diagnostic performance for detecting NASH in NAFLD patients. Therefore, this multiparametric MR index may help diagnose NASH in NAFLD patients and potentially reduce the need for liver biopsy.
Materials and Methods
Study design
This clinical trial was a prospective study conducted in accordance with the Declaration of Helsinki at the Korea University Guro Hospital in Korea. The study was approved by the Institutional Review Board of the Korea University Guro Hospial (approval no.: KUGH16184) and written informed consent was obtained from each patient. This trial was registered into ClinicalTrials.gov on 22 November 2017. The study followed CONSORT guidelines in the reporting of results.
Patients
Patients with clinically suspected NASH who were scheduled to undergo or underwent liver biopsy within 2 months were identified from October 2016 to June 2017. Patients 18 years and older who voluntarily participated in this study were included. Exclusion criteria were as follows: (a) other known causes of chronic liver disease such as chronic hepatitis B or C, autoimmune hepatitis, and primary sclerosing cholangitis; (b) use of steatogenic medications within the past six months; (c) significant alcohol consumption (more than 70 g per week for women and 140 g per week for men); (d) history of HCC; (e) pregnancy; and (f) contraindications to perform MRI. Finally, 47 patients were included in this study. Demographic and clinical data including age, gender, height, weight, and body mass index were obtained. Laboratory data within 1 month of the MR exam were also recorded. These are summarized in Table 1.
Histopathological evaluation
All patients underwent percutaneous liver biopsies and at least two cores of liver tissue were obtained from the right hepatic lobe by using the Tru-cut technique with an 18-gauge automatic needle device (TSK Laboratory, Tochigi, Japan). An experienced pathologist with more than 15 years of experience, who was blinded to the patients’ clinical and radiologic data, reviewed all liver biopsy specimens. Biopsy specimens were graded according to the SAF scoring system as NASH (steatosis score > = 1, lobular inflammation > = 1, ballooning > = 1, and any fibrosis score) or non-NASH. The SAF score is a semi-quantitative score of steatosis (0–3), activity [lobular inflammation (0–2) + ballooning (0–2)], and fibrosis (0–4). Steatosis score (S) was assessed according to the amount of large or medium-sized lipid droplets without foamy microvesicles, ranging from 0 to 3 (S0, <5%; S1, 5–33%; S2, 34–66%; S3, >67%). Activity score (A) is the unweighted sum of hepatocyte ballooning (0–2) and lobular inflammation (0–2), ranging from 0 to 4. Hepatocyte ballooning was graded as B0, none; B1, few balloon cells; or B2, many cells/ prominent ballooning. Lobular inflammation was graded as L0, none; L1, <2 foci/ 200 fields; or L2; ≥2 foci/ 200 fields. Fibrosis score was evaluated using the NASH-Clinical Research Network scoring system as follows: F0, no fibrosis; F1, perisinusoidal fibrosis in zone 3 (stage 1a or 1b) or portal/periportal fibrosis (stage 1c); F2, perisinusoidal and portal/periportal fibrosis; F3, bridging fibrosis; and F4, cirrhosis.
MR examination
All patients underwent MR imaging with a 3 T MR scanner (MAGNETOM Skyra, Siemens Healthineers, Erlangen, Germany). T2-weighted half-Fourier acquisition single-shot turbo spin-echo (HASTE) sequence, 3-dimensional T1-weighted gradient-recalled echo volumetric interpolated breath-hold examination (VIBE) sequence, MR spectroscopy, MR elastography, and T1 mapping were acquired.
MR spectroscopy
Single-voxel MRS was performed using a prototypical HISTO MR spectroscopic technique. The HISTO technique is a modified stimulated echo acquisition mode (STEAM) sequence27. MRS spectra were obtained as the same method described in our previously published study27. A single voxel (20 × 20 × 20 mm) was placed in the right hepatic posterior segment by an experienced technologist (more than 10 years of experience in MRS) while avoiding large bile ducts, vessels, and focal hepatic lesions (Fig. 3a). Parameters included repetition time (TR), 3000 msec; mixing time, 10 msec; and 5 echo times (TEs) of 12, 24, 36, 48, and 72 msec. Each MRS acquisition was performed within 15 seconds during one breath hold. This process was repeated three times. Post-processing of the MRS data was performed using inline software of the MR vendor.
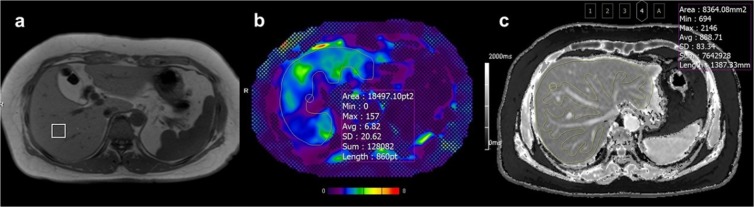
Figure 3.
Measurement of MR parameters using regions-of-interest (ROIs). (a) A square-shaped voxel on MR spectroscopy (b) A free-hand ROI on the stiffness map of MR elastography (c) A free-hand ROI on T1 mapping.
MR elastography
A pneumatic driver system (Resoundant, Rochester, MN, USA) was used to induce shear waves into the liver. A cylindrical passive driver was placed against the patient’s right anterior chest wall at the level of the xiphoid process8. Continuous acoustic vibrations of 60 Hz were transmitted from the active driver to the passive driver through a flexible vinyl tube8. Measurement parameters of a prototypical MR elastography 2D spin-echo based echo-planar sequence were as follows: TR/TE, 100/47 msec; SPAIR fat suppression; field of view (FOV), 380 mm × 380 mm; matrix size, 100 × 100; slice thickness, 6 mm; and interslice gap, 1.2 mm. Four MR elastography sections were obtained in each patient and it took 11 seconds to obtain four slices. Patients were requested to hold their breath at the end of expiration. After the acquisition was completed, wave images were processed inline by the MR scanner to generate stiffness maps (elastograms) in kilopascals (kPa). Elastograms were displayed with a scale corresponding to the stiffness value.
T1 mapping
For T1 mapping, a prototypical 2-dimensional Look-Locker inversion recovery T1 mapping sequence based on fast low-angle shot (FLASH) with inline construction was performed. Three axial slices were obtained at the level of confluence of the hepatic veins to the inferior vena cava, portal hilum, and gallbladder fossa within a breath hold. For each slice the Look-Locker T1 mapping sequence applies a non-selective inversion recovery pulse followed by low-flip angle FLASH acquisitions of multiple inversion contrasts. Imaging parameters were as follows: TR/TE, 3.0/1.32 msec; FA, 8°; number of excitations, 1.0; FOV, 380 × 309 mm; matrix size, 192 × 154; acceleration factor, 2; slice thickness, 8 mm; number of inversion contrasts, 16.
Image analysis
Measurement of fat fraction on MRS
Liver FFs were calculated automatically and displayed as a percentage (%). Mean FF values were used as representative values.
Measurement of liver stiffness on MRE
To measure liver stiffness (LS) values, two board-certified radiologists (C.H.L. and J.W.K. with 25 years and 6 years of experience in abdominal radiology, respectively), blinded to the clinical data and pathologic results, drew free-hand regions-of-interest (ROIs) along the liver margin by consensus, avoiding large vessels, liver edges, and motion artifacts. ROIs were manually drawn on wave images where wave propagations were regular and relatively free of reflections and interference patterns. ROIs drawn on wave images were copied and pasted onto the stiffness maps (Fig. 3b). One ROI per slice (total 4 slices) was measured, and mean LS values (in kilopascals) were used as representative values.
Measurement of T1 relaxation time on T1 mapping
To measure T1 relaxation time, the same two radiologists, blinded to the clinical data and pathologic results, drew free-hand ROIs along the liver margin on each slice of the T1 maps by consensus, avoiding major intrahepatic vessels and focal hepatic lesions (Fig. 3c). One ROI per slice (total 3 slices) was measured, and mean T1 relaxation times (in milliseconds) were used as representative values.
Statistical analysis
For clinical and laboratory data, the independent-sample t-test was used to compare continuous variables, and the chi-square (χ2) test was used to compare categorical variables between the NASH and non-NASH groups.
To evaluate the diagnostic performances of MRS, MRE, and T1 mapping for grading each histopathologic component (steatosis, ballooning, locular inflammation, and fibrosis), ROC curve analyses were performed and AUCs were obtained.
A previous study demonstrated that the T1 value estimates may be affected by liver fat34. Therefore, subgroup analyses were performed to evaluate the diagnostic performances of T1 mapping and the MR index using ROC curve analyses. Patients were assigned to one of two groups based on mean FFMRS: group A (mean FFMRS ≤ 15%) and group B (mean FFMRS > 15%).
A p-value < 0.05 indicated a statistically significant difference. All statistical analyses were performed using statistical software (SPSS version 20.0 for Windows, SPSS, Chicago, IL; and MedCalc for Windows, version 9.3.2.0, MedCalc Software, Mariakerke, Belgium).
Development of the MR index
A multiparametric MR index as a weighted linear combination of liver signal FF, LS value, and T1 relaxation time was developed using linear regression analysis to predict the SAF score (Fig. 1). ROC curve analysis was performed to evaluate the diagnostic performance of the MR index.
Acknowledgements
Mun Young Paek (Siemens Healthineers Limited, Seoul, Korea), Marcel Dominik Nickel (Siemens Healthcare GmbH, Erlangen, Germany), and Stephan Kannengiesser (Siemens Healthcare GmbH, Erlangen, Germany) contributed to design of MRI sequence and protocols. This study received funding from Korea University Grant (K1609801) and Korea University Guro Hospital (O1700501).
Author contributions
C.H.L. had full access to all of the data and take responsibility for the integrity of the data. J.W.K. and Y.S.P. acquired imaging data. Y.L. and J.E.Y. acquired clinical data. J.W.K. and Y.L. performed statistical analysis, interpreted data, and drafted the manuscript. B.K. and S.Y.L. acquired pathologic data. C.H.L., Y.S.P., and J.E.Y. reviewed and revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jeong Woo Kim and Young-Sun Lee.
References
- 1.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 2.Ekstedt M, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 3.Ascha MS, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 4.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N. Engl. J. Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 5.Lee SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014;20:7392–7402. doi: 10.3748/wjg.v20.i23.7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang A, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422–431. doi: 10.1148/radiol.12120896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J. Magn. Reson. Imaging. 2011;34:729–749. doi: 10.1002/jmri.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim BH, et al. MR elastography for noninvasive assessment of hepatic fibrosis: experience from a tertiary center in Asia. J. Magn. Reson. Imaging. 2011;34:1110–1116. doi: 10.1002/jmri.22723. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, et al. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259:749–756. doi: 10.1148/radiol.11101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.d’Assignies G, et al. Noninvasive quantitation of human liver steatosis using magnetic resonance and bioassay methods. Eur. Radiol. 2009;19:2033–2040. doi: 10.1007/s00330-009-1351-4. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee R, et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J. Hepatol. 2014;60:69–77. doi: 10.1016/j.jhep.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavlides M, et al. Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. J. Hepatol. 2016;64:308–315. doi: 10.1016/j.jhep.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang BK, et al. Hepatic fat quantification: a prospective comparison of magnetic resonance spectroscopy and analysis methods for chemical-shift gradient echo magnetic resonance imaging with histologic assessment as the reference standard. Invest. Radiol. 2012;47:368–375. doi: 10.1097/RLI.0b013e31824baff3. [DOI] [PubMed] [Google Scholar]
- 14.Kuhn JP, et al. Effect of multipeak spectral modeling of fat for liver iron and fat quantification: correlation of biopsy with MR imaging results. Radiology. 2012;265:133–142. doi: 10.1148/radiol.12112520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huwart L, et al. Liver fibrosis: noninvasive assessment with MR elastography versus aspartate aminotransferase-to-platelet ratio index. Radiology. 2007;245:458–466. doi: 10.1148/radiol.2452061673. [DOI] [PubMed] [Google Scholar]
- 16.Ekstedt M, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 17.Bedossa P, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56:1751–1759. doi: 10.1002/hep.25889. [DOI] [PubMed] [Google Scholar]
- 18.Stal P. Liver fibrosis in non-alcoholic fatty liver disease - diagnostic challenge with prognostic significance. World J. Gastroenterol. 2015;21:11077–11087. doi: 10.3748/wjg.v21.i39.11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SH, et al. Appropriateness of a donor liver with respect to macrosteatosis: application of artificial neural networks to US images–initial experience. Radiology. 2005;234:793–803. doi: 10.1148/radiol.2343040142. [DOI] [PubMed] [Google Scholar]
- 20.Park SH, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–112. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 21.Lee SW, et al. Unenhanced CT for assessment of macrovesicular hepatic steatosis in living liver donors: comparison of visual grading with liver attenuation index. Radiology. 2007;244:479–485. doi: 10.1148/radiol.2442061177. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, et al. Hepatic macrosteatosis: predicting appropriateness of liver donation by using MR imaging–correlation with histopathologic findings. Radiology. 2006;240:116–129. doi: 10.1148/radiol.2393042218. [DOI] [PubMed] [Google Scholar]
- 23.Hajek M, et al. MR spectroscopy as a tool for in vivo determination of steatosis in liver transplant recipients. MAGMA. 2011;24:297–304. doi: 10.1007/s10334-011-0264-9. [DOI] [PubMed] [Google Scholar]
- 24.Koelblinger C, et al. Hepatic steatosis assessment with 1H-spectroscopy and chemical shift imaging at 3.0 T before hepatic surgery: reliable enough for making clinical decisions? Eur. J. Radiol. 2012;81:2990–2995. doi: 10.1016/j.ejrad.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 25.Guiu B, et al. Quantification of liver fat content: comparison of triple-echo chemical shift gradient-echo imaging and in vivo proton MR spectroscopy. Radiology. 2009;250:95–102. doi: 10.1148/radiol.2493080217. [DOI] [PubMed] [Google Scholar]
- 26.Pineda N, et al. Measurement of hepatic lipid: high-speed T2-corrected multiecho acquisition at 1H MR spectroscopy–a rapid and accurate technique. Radiology. 2009;252:568–576. doi: 10.1148/radiol.2523082084. [DOI] [PubMed] [Google Scholar]
- 27.Park YS, et al. Effect of Gd-EOB-DTPA on hepatic fat quantification using high-speed T2-corrected multi-echo acquisition in (1)H MR spectroscopy. Magn. Reson. Imaging. 2014;32:886–890. doi: 10.1016/j.mri.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Xiao G, et al. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. 2017;66:1486–1501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 29.Cui J, et al. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment. Pharmacol. Ther. 2015;41:1271–1280. doi: 10.1111/apt.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhuva AN, et al. T1 mapping: non-invasive evaluation of myocardial tissue composition by cardiovascular magnetic resonance. Expert. Rev. Cardiovasc. Ther. 2014;12:1455–1464. doi: 10.1586/14779072.2014.986098. [DOI] [PubMed] [Google Scholar]
- 31.Clique H, Cheng HL, Marie PY, Felblinger J, Beaumont M. 3D myocardial T1 mapping at 3T using variable flip angle method: pilot study. Magn. Reson. Med. 2014;71:823–829. doi: 10.1002/mrm.24688. [DOI] [PubMed] [Google Scholar]
- 32.Cassinotto C, et al. MR relaxometry in chronic liver diseases: Comparison of T1 mapping, T2 mapping, and diffusion-weighted imaging for assessing cirrhosis diagnosis and severity. Eur. J. Radiol. 2015;84:1459–1465. doi: 10.1016/j.ejrad.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, et al. Assessment of liver fibrosis by variable flip angle T1 mapping at 3.0T. J. Magn. Reson. Imaging. 2016;43:698–703. doi: 10.1002/jmri.25030. [DOI] [PubMed] [Google Scholar]
- 34.Mozes FE, Tunnicliffe EM, Pavlides M, Robson MD. Influence of fat on liver T1 measurements using modified Look-Locker inversion recovery (MOLLI) methods at 3T. J. Magn. Reson. Imaging. 2016;44:105–111. doi: 10.1002/jmri.25146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serai SD, Yin M, Wang H, Ehman RL, Podberesky DJ. Cross-vendor validation of liver magnetic resonance elastography. Abdom. Imaging. 2015;40:789–794. doi: 10.1007/s00261-014-0282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang YXJ, Chen W, Deng M. How liver pathologies contribute to T1rho contrast require more careful studies. Quant. Imaging Med. Surg. 2017;7:608–613. doi: 10.21037/qims.2017.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwenzer NF, et al. T2* relaxometry in liver, pancreas, and spleen in a healthy cohort of one hundred twenty-nine subjects-correlation with age, gender, and serum ferritin. Invest. Radiol. 2008;43:854–860. doi: 10.1097/RLI.0b013e3181862413. [DOI] [PubMed] [Google Scholar]
- 38.Wang YXJ, et al. Age- and Gender-Associated Liver Physiological T1rho Dynamics Demonstrated with a Clinically Applicable Single-Breathhold Acquisition. SLAS Technol. 2018;23:179–187. doi: 10.1177/2472630317747198. [DOI] [PubMed] [Google Scholar]