Abstract
This manuscript provides an overview of pleomorphic spindle cell tumors presenting on sun-damaged skin of the elderly and includes discussions of atypical fibroxanthoma, pleomorphic dermal sarcoma, spindle cell and metaplastic squamous cell carcinoma, spindle cell and dedifferentiated melanoma and poorly differentiated cutaneous angiosarcoma. These tumors share many of the clinical presenting and histological features, making confident diagnosis challenging. A reliable and robust diagnosis is necessary to predict behavior as the biologic potential of these tumors ranges from benign (e.g. atypical fibroxanthoma) to outright malignant with poor survival rates (e.g. cutaneous angiosarcoma). The salient clinical, histologic and immunohistochemical characteristics are discussed in detail with emphasis on distinguishing features and differential diagnosis to provide the reader with a better understanding of these entities and helpful clues for a more robust diagnosis.
Keywords: AFX, SCC, Melanoma, Tumor, Immunohistochemistry
Introduction
Pleomorphic spindle cell tumors in the head and neck area typically present on chronically sun-damaged skin of elderly individuals, and they are more common in males than females. This tumor group includes mainly atypical fibroxanthoma, pleomorphic dermal sarcoma, spindle cell, desmoplastic and metaplastic squamous cell carcinoma, spindle cell, desmoplastic and dedifferentiated melanoma and poorly differentiated angiosarcoma. The tumors show significant morphologic overlap and immunohistochemical overlap and reliable separation is therefore challenging with potential for misdiagnosis [1]. The behavior of these tumors varies significantly and ranges from benign (atypical fibroxanthoma) to high-grade malignant with poor survival rates (poorly differentiated angiosarcoma). The following manuscript provides an overview of the individual entities with emphasis on differentiating features and differential diagnosis.
Atypical Fibroxanthoma and Pleomorphic Dermal Sarcoma
Definition
Atypical fibroxanthoma (AFX) and pleomorphic dermal sarcoma (PDS) are closely related tumors and likely represent a disease spectrum. They share many of the clinical presenting features as well as the morphologic, immunohistochemical and genetic findings but the presence of invasion of subcutis, tumor necrosis, lymphovascular invasion or perineurial infiltration confers risk for more aggressive behavior with increased recurrence rates and risk for distant metastasis and mortality characteristic for PDS [2].
Clinical Features
Both AFX and PDS are confined to chronically sun-damaged skin and show a strong predilection for the head and neck area of elderly adults (median age: 80 years) with a marked male predilection [3–5]. The tumors present as solitary, rapidly growing and frequently ulcerated nodules. AFX are well-defined nodules and measure less than 2 cm. In contrast, PDS tend to be larger (median size: 2.5 cm) and less circumscribed, and occasionally plaque-like [2, 6].
Histologic Features
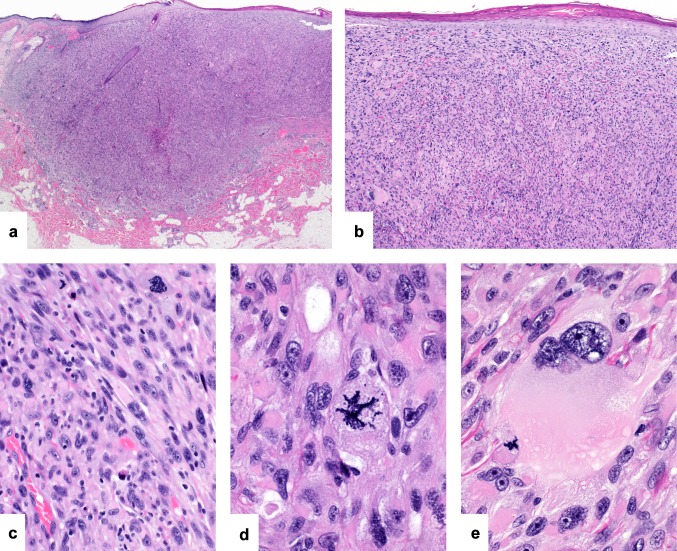
AFX are well-demarcated nodular or polypoid tumors within dermis (Fig. 1a). They are frequently ulcerated and show surrounding epidermal collarettes. The cellular tumors extend to the overlying epidermis and are arranged in sheets and intersecting fascicles of pleomorphic epithelioid and spindle cells in varying proportions (Fig. 1b and c) [3–6]. The tumor cells contain moderate amounts of occasionally frothy cytoplasm and hyperchromatic to vesicular pleomorphic nuclei with prominent and often multiple nucleoli (Fig. 1d). Multinucleated tumor giant cells are frequently admixed (Fig. 1e). Mitoses are numerous and include atypical forms. By definition, AFX are confined to the dermis and lack tumor necrosis, lymphovascular invasion or perineural infiltration [2, 6]. PDS are ill-defined and often ulcerated, frequently with a diffusely infiltrative growth with invasion of subcutaneous adipose tissue, skeletal muscle and underlying fibrous tissues including fascia or galea (Fig. 2a). The cellular population is identical to that seen in AFX but the tumors may show additional tumor necrosis, lymphovascular invasion or perineural infiltration [2].
Fig. 1.
Atypical fibroxanthoma/pleomorphic dermal sarcoma: atypical fibroxanthomas are relatively circumscribed nodular tumors confined to the dermis (a). They are highly cellular and extend to the overlying epidermis (b). The tumors are composed of pleomorphic spindle and epithelioid cells arranged in fascicles and sheets (c). Nuclear pleomorphism is marked and there is atypical mitotic activity (d). Admixed multinucleated tumor giant cells are present (e)
Fig. 2.
Atypical fibroxanthoma/pleomorphic dermal sarcoma: pleomorphic dermal sarcoma shows a deeply invasive growth into subcutaneous tissues (a). The presence of marked intratumoral hemorrhage may lead to an erroneous diagnosis of angiosarcoma (b). Atypical fibroxanthoma and pleomorphic dermal sarcoma are strongly and diffusely positive for CD10 (c)
Both AFX and PDS show a significant morphologic spectrum including spindle cell predominant tumors and those with pseudoangiomatous features characterized by areas of hemorrhage and hemosiderin deposition (Fig. 2b) [3–7]. A storiform growth, myxoid or keloidal stromal change, clear or granular cytoplasmic alterations and admixed osteoclast-like giant cells may rarely be encountered.
Immunohistochemistry
The diagnosis of AFX and PDS is one of exclusion and requires immunohistochemical work-up to rule out poorly differentiated squamous cell carcinoma, melanoma, angiosarcoma and leiomyosarcoma. The tumors are positive for CD10 and frequently stain with CD68 and SMA (Fig. 2c) [4–6, 8]. These stains are however entirely non-specific and non-contributory to the diagnosis. Rare tumors show focal expression of melan a, HMB45, MiTF, EMA, p63, FLI1 and CD31 [6, 7, 9, 10]. Importantly, they are consistently negative for S100, SOX10, cytokeratins, CD34, ERG and desmin.
Molecular Genetics
AFX and PDS show similar genetic abnormalities with UV signature mutations in TP53, TERT promoter mutations and frequent mutation in NOTCH1/2 and FAT1 and similar DNA methylation profile and DNA copy number aberrations [11–13].
Behavior and Prognosis
If strict diagnostic criteria are applied, AFX shows an almost invariably benign clinical course with rare local recurrence estimated at around 5% [6, 14]. In contrast, tumors classified as PDS show significant (around 30%) risk for local recurrence and a metastatic potential of 10 to 20% to skin, soft tissue, lymph nodes and lung [2, 15]. The presence of metastatic disease is associated with poor prognosis and high mortality rates [16].
Differential Diagnosis
Poorly differentiated and spindle cell squamous cell carcinoma may show identical histologic features to AFX and PDS. Reliable separation requires the identification of areas of better differentiated squamous cell carcinoma morphologically or demonstration of cytokeratin expression by immunohistochemistry. A junctional in situ component and immunohistochemical expression of S100 or SOX10 are helpful in the diagnosis of melanoma. Poorly differentiated cutaneous angiosarcoma shows many overlapping morphologic features with AFX/PDS. ERG immunohistochemistry is the most sensitive and specific tool in this setting to confirm the endothelial cell differentiation of these tumors. Leiomyosarcoma rarely occurs on sun-damaged skin of the head and neck and is characterized by desmin and h-caldesmon expression. Similarly, dermatofibrosarcoma protuberans is rare on sun-damaged skin of the elderly. It lacks the marked nuclear pleomorphism and the tumors consistently show CD34 staining. Atypical fibrous histiocytoma shows many overlapping morphologic features, but the tumors are centered in mid dermis and there is sparing of the papillary dermis. Recognition of entrapment of peripheral collagen bundles and areas of more conventional fibrous histiocytoma are further morphologic clues. The immunohistochemical staining pattern of atypical fibrous histiocytoma is similar to that of AFX and PDS, and immunohistochemistry is not helpful in this setting.
Spindle Cell and Desmoplastic Squamous Cell Carcinoma
Definition
Spindle cell and desmoplastic variants of squamous cell carcinoma are rare with potential for an aggressive disease course [17, 18]. They pose a significant diagnostic challenge as they bear little, if any, morphologic resemblance to conventional squamous cell carcinoma.
Clinical Features
The tumors present as erythematous or flesh colored nodules and plaques measuring few to multiple centimeters [17, 18]. Ulceration is common. The tumors show a predilection for sun-damaged skin of the head and neck area of elderly males in the 7th and 8th decade [17, 18]. Like other forms of squamous cell carcinoma, the spindle cell variant may arise on sites of chronic scarring, and there is a link with immunosuppression, particularly in the setting of solid organ transplants.
Histologic Features
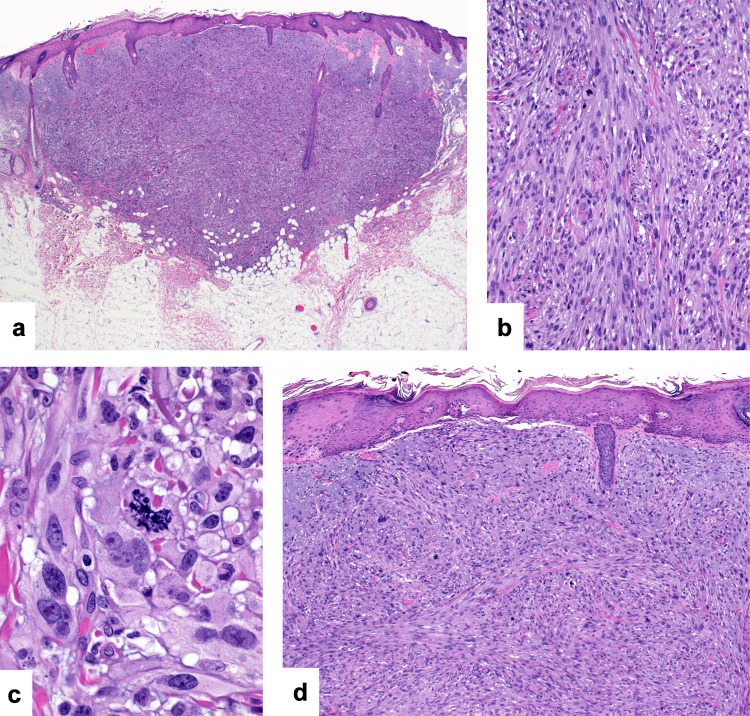
The tumors are based within dermis with nodular or diffusely infiltrative growth patterns and invasion of subcutaneous structures (Fig. 3a). They are composed of intersecting fascicles of variably pleomorphic spindle cells containing moderate amounts of cytoplasm and hyperchromatic to vesicular nuclei with prominent nucleoli (Fig. 3b and c) [17, 18]. Mitotic activity is present including atypical mitoses. Additional findings include perineurial infiltration and rarely tumor necrosis and lymphovascular invasion. The tumors extend to the overlying epidermis and an epidermal connection may be identified (Fig. 3d). A background of actinic keratoses or squamous cell carcinoma in situ is frequently noted. The desmoplastic variant is characterized by a proliferation of atypical spindle cells arranged singly or in small groups in a densely sclerotic or desmoplastic stroma (Fig. 4a and b) [17, 18]. A background of more conventional squamous cell carcinoma is only rarely seen in these tumors.
Fig. 3.
Spindle cell squamous cell carcinoma: This nodular tumor is centered within dermis and shows invasion of superficial subcutaneous adipose tissue (a). It is composed of intersecting fascicles of pleomorphic spindle cells (b) Higher magnification demonstrating nuclear pleomorphism and an atypical mitotic figure (c). The tumor extends to the overlying epidermis (d)
Fig. 4.
Desmoplastic squamous cell carcinoma: This large tumor is characterized by an abundant eosinophilic desmoplastic stroma (a). Pleomorphic tumor cells are arranged singly or in small groups in the densely sclerotic stroma (b). By immunohistochemistry, tumor cells in this spindle cell squamous cell carcinoma express cytokeratins multifocally (c) and there is strong and diffuse expression of CD10 (d)
Immunohistochemistry
The tumor cells stain positive for cytokeratins with a preference for those of high molecular weight, such as MNF116, AE1/3 or CK5/6 (Fig. 4c) [19]. Often, multiple cytokeratin preparations need to be used to detect cytokeratin staining which may be focal. In addition, there is staining for p63 and p40 in a significant subset of cases [20, 21]. The tumors frequently also express CD10 and SMA but S100, SOX10, desmin and the endothelial cell markers CD34, CD31 and ERG are consistently negative (Fig. 4d).
Behavior and Prognosis
Spindle cell and desmoplastic squamous cell carcinomas have potential for aggressive behavior, which is largely dictated by tumor size and depth of invasion. The desmoplastic variant and tumors arising in chronic scars or following radiation may pursue an aggressive disease course [17, 18].
Differential Diagnosis
The tumors closely resemble AFX/PDS and spindle cell or desmoplastic melanoma. Demonstration of cytokeratin expression by immunohistochemistry is necessary to separate these tumors from AFX/PDS while S100 or SOX10 staining is supportive of spindle cell or desmoplastic melanoma. Spindle cell angiosarcoma shows immunoreactivity for the endothelial cell markers ERG, CD31 or CD34. Expression of SMA may lead to an erroneous diagnosis of cutaneous leiomyosarcoma, which can be excluded by desmin staining.
Cutaneous Metaplastic Carcinoma
Definition
Metaplastic carcinomas, also known as carcinosarcoma, are characterized by the presence of malignant epithelial and mesenchymal components. In the skin, these tumors most commonly arise in association with epidermal derived malignant epithelial components, such as squamous or basal cell carcinoma. A background of a skin adnexal carcinoma or Merkel cell carcinoma is less common.
Clinical Features
The majority of tumors arise on sun-damaged skin of the head and neck, chest or upper extremities with a predilection for elderly males. Tumors arising in association with skin adnexal carcinomas affect a slightly younger patient population, typically middle-aged adults, without significant gender bias [22]. The tumors present as nodules and plaques with rapid growth and frequent ulceration, typically measuring multiple centimeters.
Histologic Features
Cutaneous metaplastic carcinoma are large and frequently ulcerated dermal-based tumors with ill-defined borders, an infiltrative growth pattern and frequent invasion of subcutaneous tissues (Fig. 5a). A recognizable malignant epithelial component is necessary for the correct diagnosis (Fig. 5b). The malignant epithelial component is most frequently epidermal derived, including squamous or basal cell carcinoma [22, 23]. Rarely, these tumors arise in the setting of skin adnexal carcinoma, such as porocarcinoma, hidradenocarcinoma, spiradenocarcinoma, pilomatrical carcinoma, trichoblastic carcinoma or Merkel cell carcinoma [22–24]. In the majority of tumors, the malignant mesenchymal aspect consists of undifferentiated sarcoma, resembling atypical fibroxanthoma (Fig. 5c and d). Osteoclast like giant cells may be present and other sarcomatous elements include osteo-, chondro-, rhabdo- and leiomyosarcomatous differentiation. The mitotic activity is brisk and atypical. Tumor necrosis and lymphovascular and perineurial invasion may be present.
Fig. 5.
Metatypical squamous cell carcinoma: This large tumor shows an expansile growth within dermis and subcutis (a). In areas, there is a background of poorly differentiated squamous cell carcinoma characterized by a sheet-like growth of atypical epithelioid cells (b). The remainder of the tumor is composed of cellular intersecting fascicles of spindle cells with extension to the epidermis (c). The fascicles are composed of pleomorphic spindle cells with brisk and atypical mitotic activity (d)
Immunohistochemistry
Cytokeratin expression is limited to the malignant epithelial component (Fig. 6a). The sarcomatous components are negative for cytokeratins but express immunohistochemical markers according to their lines of differentiation (Fig. 6b). The AFX-like undifferentiated components commonly express CD10 and SMA (Fig. 6c and d).
Fig. 6.
Metatypical squamous cell carcinoma: By immunohistochemistry, the areas of poorly differentiated squamous cell carcinoma express cytokeratins (a). Cytokeratin expression is entirely absent in the spindle cell population (b), which shows expression of CD10 (c) and SMA (d)
Behavior and Prognosis
Metaplastic carcinoma may show potential for aggressive behavior, especially in tumors arising from skin adnexal carcinoma with reported local recurrence rates of 22%, distant metastatic rates of 50% and a mortality of around 50%. The prognosis for tumors associated with squamous or basal cell carcinoma appears much more favorable with distant metastases in 8% and a 10% mortality [22].
Differential Diagnosis
Adequate sampling with recognition of the carcinomatous component is necessary for the correct diagnosis. On partial biopsies, the tumors are readily mistaken for AFX/PDS or other sarcomas unless the carcinomatous part is included in the biopsy.
Dedifferentiated Melanoma
Definition
Dedifferentiated melanoma or melanoma with sarcomatoid differentiation is a rare but diagnostically challenging variant of melanoma showing areas of sarcomatous differentiation with loss of expression of melanocytic markers on immunohistochemistry.
Clinical Features
The tumors present as large tumors which may ulcerate. A wide range of anatomic locations is affected but there may be a predilection for sun-damaged skin of the head and neck of elderly adults [25, 26].
Histologic Features
The dermal based tumors are large with nodular or diffusely infiltrative growth patterns and frequent invasion of subcutaneous tissues (Fig. 7a). Ulceration is common. The tumors are cellular and composed of intersecting fascicles of mitotically active pleomorphic spindle cells, reminiscent of AFX (Fig. 7b and c). An additional phenomenon is the presence of heterologous or divergent differentiation in the form of rhabdomyosarcomatous, osteo- or chondroblastic or epithelial elements [27–29]. The clue to the correct diagnosis is recognition of adjacent or admixed areas of more conventional melanoma or a desmoplastic melanoma. The presence of an overlying in situ melanoma or lentigo maligna is helpful (Fig. 7d).
Fig. 7.
Dedifferentiated Melanoma: This nodular tumor is based within dermis (a) and shows ulceration of the overlying epidermis (b). There is a background of adjacent melanoma in situ (d). Melan a immunohistochemistry highlights the in-situ melanoma and small focus of conventional invasive melanoma. The spindle cell component is melan a negative (e) and show strong and diffuse expression of CD10
Immunohistochemistry
The expression of the melanocytic markers S100 and SOX10 is lost in the dedifferentiated areas and confined to the in-situ component or the areas of conventional or desmoplastic invasive melanoma. Additional expression of melan a and HMB45 may be seen in the junctional and invasive components of epithelioid melanoma but their expression is typically absent in spindle cell and desmoplastic melanoma (Fig. 7e). Areas of dedifferentiation may show expression of SMA and CD10 but, by definition, they are negative for all melanocytic markers (Fig. 7f). Melanoma with heterologous or divergent differentiation expresses immunohistochemical markers according to the line of differentiation. Similar to dedifferentiated melanoma, there is no expression of melanocytic markers.
Behavior and Prognosis
Due to the rarity of the disease little is known about its behavior. In view of the large tumor size and depth of invasion the tumors show potential for an aggressive disease course.
Differential Diagnosis
Dedifferentiated melanoma shows identical features to and may be impossible to separate from AFX or PDS unless the area of more conventional melanoma is sampled. In the past, dedifferentiated melanoma was frequently mistaken as a collision tumor of melanoma and AFX [30]. A recent study with molecular analysis of dedifferentiated melanoma identified the same driver mutation in the areas of conventional melanoma and the sarcomatous aspects of the tumor, emphasizing that these are different elements of the same neoplasm [26].
Cutaneous Angiosarcoma
Definition
Angiosarcoma affects the skin almost exclusively in one of the following clinical settings: (1) on sun-damaged skin of the elderly; (2) following radiation treatment, most frequently in the setting of breast conserving therapy for carcinoma of the breast; (3) in a background of long-standing and severe lymphedema [31]. Given the scope of this review article only poorly differentiated tumors presenting on sun-damaged skin are discussed below.
Clinical Features
Angiosarcoma presenting on chronically sun-damaged skin is the most frequent form of angiosarcoma of the skin. The tumors show a strong predilection for the head and neck area of elderly adults and a male predominance [32, 33]. They present as erythematous to violaceous patches, plaques or nodules, typically measuring multiple centimeters.
Histologic Features
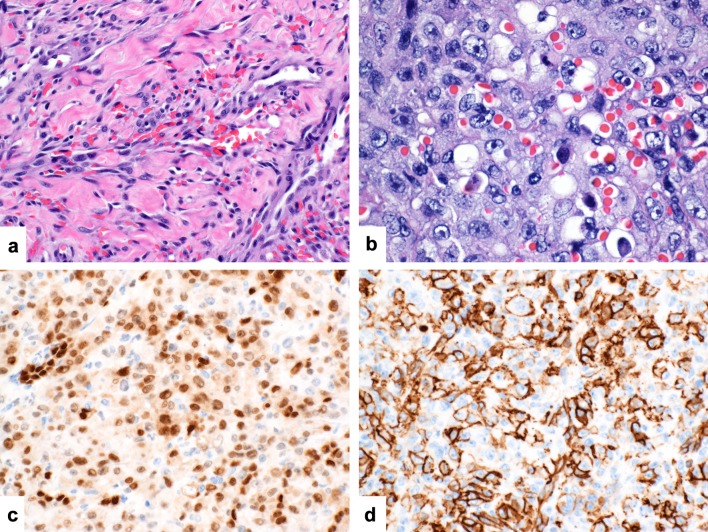
Cutaneous angiosarcomas are poorly circumscribed with a diffusely infiltrative growth in dermis and frequent invasion of underlying subcutaneous tissues (Fig. 8a and b). Morphologically well-differentiated tumors are characterized by overtly vasoformative elements giving rise to anastomosing vascular channels lined by atypical and multilayered endothelial cells. The vascular channels show a dissecting growth around pre-existing structures within dermis and subcutis. Poorly differentiated tumors show a sheet-like or fascicular arrangement of pleomorphic epithelioid or spindled cells with brisk and atypical mitotic activity (Fig. 8c). Cytoplasmic vacuolization and intracytoplasmic erythrocytes may be seen as evidence of early vasoformative elements (Fig. 8d). An at least focal background of better differentiated angiosarcoma is a helpful diagnostic finding (Fig. 9a). Tumors composed predominantly of epithelioid tumor cells are also referred to as epithelioid angiosarcoma (Fig. 9b). Tumor necrosis may also be noted.
Fig. 8.

Poorly differentiated cutaneous angiosarcoma: This multinodular tumor is based within dermis and invades subcutaneous adipose tissue (a and b). It is composed of atypical spindle cells with hyperchromatic nuclei (c). There are intracytoplasmic vacuoles and numerous intracytoplasmic erythrocytes are present as evidence of early vasoformative elements (d)
Fig. 9.
Poorly differentiated cutaneous angiosarcoma: A background of more overtly vasoformative areas provide a good diagnostic clue. Note the anastomosing growth of vascular channels lined by multiple layers of atypical endothelial cells (a). The epithelioid variant is characterized by a sheet-like growth of pleomorphic epithelioid cells containing intracytoplasmic lumina and erythrocytes (b). Poorly-differentiated angiosarcoma shows strong and diffuse nuclear expression of ERG (c) and strong membranous expression of CD31 (d)
Immunohistochemistry
Immunohistochemical staining with endothelial markers is necessary to confirm the diagnosis of poorly differentiated angiosarcoma. Of the available markers, ERG shows the highest sensitivity and specificity rates approaching 100% in this clinical setting (Fig. 9c) [34]. CD31 also show relatively good sensitivity and specificity rates (Fig. 9d). Other available markers of lesser sensitivity and specificity are CD34, factor VIII and podoplanin (D2–40). Cytokeratins may be expressed in epithelioid angiosarcoma. MYC amplification as demonstrated by FISH is typical of secondary angiosarcoma in the setting of radiation or lymphedema [35–38]. In these tumors MYC overexpression can also be demonstrated immunohistochemically. In tumors arising on sun-damaged skin MYC amplification and overexpression is less commonly seen [39].
Behavior and Prognosis
Cutaneous angiosarcomas are aggressive tumors with high metastatic rates and poor 5-year survival of less than 50%. Tumor size, depth of invasion, epithelioid cell change and the presence of necrosis correlate negatively with outcome [32, 33].
Differential Diagnosis
Poorly differentiated angiosarcoma closely mimics other poorly differentiated cutaneous neoplasms on sun-damaged skin. AFX/PDS with pseudoangiomatous differentiation poses a particular pitfall morphologically and immunohistochemically. The tumors share the expression of FLI1 and CD31. In contrast to AFX, cutaneous angiosarcoma is consistently ERG positive. ERG staining is also helpful in excluding poorly differentiated carcinoma. Spindle cell melanoma can be separated by S100 expression.
Conclusion
Despite the often non-specific clinical presenting features and the significant histologic overlap of tumors presenting on sun-damaged skin of the head and neck of elderly, a confident diagnosis and prediction of behavior is typically possible. This requires adequate biopsies and sampling with awareness of the diagnostic criteria and associated pitfalls and judicious use of immunohistochemistry.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brenn T. Pleomorphic dermal neoplasms: a review. Adv Anat Pathol. 2014;21(2):108–30. doi: 10.1097/PAP.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 2.Miller K, Goodlad JR, Brenn T. Pleomorphic dermal sarcoma: adverse histologic features predict aggressive behavior and allow distinction from atypical fibroxanthoma. Am J Surg Pathol. 2012;36(9):1317–26. doi: 10.1097/PAS.0b013e31825359e1. [DOI] [PubMed] [Google Scholar]
- 3.Beer TW, Drury P, Heenan PJ. Atypical fibroxanthoma: a histological and immunohistochemical review of 171 cases. Am J Dermatopathol. 2010;32(6):533–40. doi: 10.1097/DAD.0b013e3181c80b97. [DOI] [PubMed] [Google Scholar]
- 4.Luzar B, Calonje E. Morphological and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls: a review. J Cutan Pathol. 2010;37(3):301–9. doi: 10.1111/j.1600-0560.2009.01425.x. [DOI] [PubMed] [Google Scholar]
- 5.Mirza B, Weedon D. Atypical fibroxanthoma: a clinicopathological study of 89 cases. Aust J Dermatol. 2005;46(4):235–8. doi: 10.1111/j.1440-0960.2005.00190.x. [DOI] [PubMed] [Google Scholar]
- 6.Mentzel T, Requena L, Brenn T. Atypical fibroxanthoma revisited. Surg Pathol Clin. 2017;10(2):319–35. doi: 10.1016/j.path.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Thum C, Husain EA, Mulholland K, Hornick JL, Brenn T. Atypical fibroxanthoma with pseudoangiomatous features: a histological and immunohistochemical mimic of cutaneous angiosarcoma. Ann Diagn Pathol. 2013;17(6):502–7. doi: 10.1016/j.anndiagpath.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Kanner WA, Brill LB, 2nd, Patterson JW, Wick MR. CD10, p63 and CD99 expression in the differential diagnosis of atypical fibroxanthoma, spindle cell squamous cell carcinoma and desmoplastic melanoma. J Cutan Pathol. 2010;37(7):744–50. doi: 10.1111/j.1600-0560.2010.01534.x. [DOI] [PubMed] [Google Scholar]
- 9.Tallon B, Beer TW. MITF positivity in atypical fibroxanthoma: a diagnostic pitfall. Am J Dermatopathol. 2014;36(11):888–91. doi: 10.1097/DAD.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 10.Thum C, Hollowood K, Birch J, Goodlad JR, Brenn T. Aberrant Melan-A expression in atypical fibroxanthoma and undifferentiated pleomorphic sarcoma of the skin. J Cutan Pathol. 2011;38(12):954–60. doi: 10.1111/j.1600-0560.2011.01798.x. [DOI] [PubMed] [Google Scholar]
- 11.Griewank KG, Schilling B, Murali R, Bielefeld N, Schwamborn M, Sucker A, et al. TERT promoter mutations are frequent in atypical fibroxanthomas and pleomorphic dermal sarcomas. Mod Pathol. 2014;27(4):502–8. doi: 10.1038/modpathol.2013.168. [DOI] [PubMed] [Google Scholar]
- 12.Griewank KG, Wiesner T, Murali R, Pischler C, Muller H, Koelsche C, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma harbor frequent NOTCH1/2 and FAT1 mutations and similar DNA copy number alteration profiles. Mod Pathol. 2018;31(3):418–28. doi: 10.1038/modpathol.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koelsche C, Stichel D, Griewank KG, Schrimpf D, Reuss DE, Bewerunge-Hudler M, et al. Genome-wide methylation profiling and copy number analysis in atypical fibroxanthomas and pleomorphic dermal sarcomas indicate a similar molecular phenotype. Clin Sarcoma Res. 2019;9:2. doi: 10.1186/s13569-019-0113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wollina U, Schonlebe J, Ziemer M, Friedling F, Koch A, Haroske G, et al. Atypical fibroxanthoma: a series of 56 tumors and an unexplained uneven distribution of cases in southeast Germany. Head Neck. 2015;37(6):829–34. doi: 10.1002/hed.23673. [DOI] [PubMed] [Google Scholar]
- 15.Tardio JC, Pinedo F, Aramburu JA, Suarez-Massa D, Pampin A, Requena L, et al. Pleomorphic dermal sarcoma: a more aggressive neoplasm than previously estimated. J Cutan Pathol. 2016;43(2):101–12. doi: 10.1111/cup.12603. [DOI] [PubMed] [Google Scholar]
- 16.Wang WL, Torres-Cabala C, Curry JL, Ivan D, McLemore M, Tetzlaff M, et al. Metastatic atypical fibroxanthoma: a series of 11 cases including with minimal and no subcutaneous involvement. Am J Dermatopathol. 2015;37(6):455–61. doi: 10.1097/DAD.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 17.Breuninger H, Schaumburg-Lever G, Holzschuh J, Horny HP. Desmoplastic squamous cell carcinoma of skin and vermilion surface: a highly malignant subtype of skin cancer. Cancer. 1997;79(5):915–9. doi: 10.1002/(SICI)1097-0142(19970301)79:5<915::AID-CNCR7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 18.Velazquez EF, Werchniack AE, Granter SR. Desmoplastic/spindle cell squamous cell carcinoma of the skin. A diagnostically challenging tumor mimicking a scar: clinicopathologic and immunohistochemical study of 6 cases. Am J Dermatopathol. 2010;32(4):333–9. doi: 10.1097/DAD.0b013e3181c1d428. [DOI] [PubMed] [Google Scholar]
- 19.Sigel JE, Skacel M, Bergfeld WF, House NS, Rabkin MS, Goldblum JR. The utility of cytokeratin 5/6 in the recognition of cutaneous spindle cell squamous cell carcinoma. J Cutan Pathol. 2001;28(10):520–4. doi: 10.1034/j.1600-0560.2001.281005.x. [DOI] [PubMed] [Google Scholar]
- 20.Dotto JE, Glusac EJ. p63 is a useful marker for cutaneous spindle cell squamous cell carcinoma. J Cutan Pathol. 2006;33(6):413–7. doi: 10.1111/j.0303-6987.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 21.Henderson SA, Torres-Cabala CA, Curry JL, Bassett RL, Ivan D, Prieto VG, et al. p40 is more specific than p63 for the distinction of atypical fibroxanthoma from other cutaneous spindle cell malignancies. Am J Surg Pathol. 2014;38(8):1102–10. doi: 10.1097/PAS.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 22.Tran TA, Muller S, Chaudahri PJ, Carlson JA. Cutaneous carcinosarcoma: adnexal vs. epidermal types define high- and low-risk tumors. Results of a meta-analysis. J Cutan Pathol. 2005;32(1):2–11. doi: 10.1111/j.0303-6987.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- 23.Clark JJ, Bowen AR, Bowen GM, Hyngstrom JR, Hadley ML, Duffy K, et al. Cutaneous carcinosarcoma: a series of six cases and a review of the literature. J Cutan Pathol. 2017;44(1):34–44. doi: 10.1111/cup.12843. [DOI] [PubMed] [Google Scholar]
- 24.Martin B, Poblet E, Rios JJ, Kazakov D, Kutzner H, Brenn T, et al. Merkel cell carcinoma with divergent differentiation: histopathological and immunohistochemical study of 15 cases with PCR analysis for Merkel cell polyomavirus. Histopathology. 2013;62(5):711–22. doi: 10.1111/his.12091. [DOI] [PubMed] [Google Scholar]
- 25.Erstine EM, Tetzlaff MT, Ko JS, Prieto VG, Cheah AL, Billings SD. Living on the edge: diagnosing sarcomatoid melanoma using histopathologic cues at the edge of a dedifferentiated tumor: a report of 2 cases and review of the literature. Am J Dermatopathol. 2017;39(8):593–8. doi: 10.1097/DAD.0000000000000716. [DOI] [PubMed] [Google Scholar]
- 26.Kiuru M, McDermott G, Berger M, Halpern AC, Busam KJ. Desmoplastic melanoma with sarcomatoid dedifferentiation. Am J Surg Pathol. 2014;38(6):864–70. doi: 10.1097/PAS.0000000000000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52(2):119–29. doi: 10.1111/j.1365-2559.2007.02823.x. [DOI] [PubMed] [Google Scholar]
- 28.Gharpuray-Pandit D, Coyne J, Eyden B, Banerjee SS. Rhabdomyoblastic differentiation in malignant melanoma in adults: report of 2 cases. Int J Surg Pathol. 2007;15(1):20–5. doi: 10.1177/1066896906295775. [DOI] [PubMed] [Google Scholar]
- 29.Laskin WB, Knittel DR, Frame JN. S100 protein and HMB-45 negative "rhabdoid" malignant melanoma: a totally dedifferentiated malignant melanoma? Am J Clin Pathol. 1995;103(6):772–3. doi: 10.1093/ajcp/103.6.772. [DOI] [PubMed] [Google Scholar]
- 30.Wilsher MJ. Collision tumour: atypical fibroxanthoma and invasive melanoma. Pathology. 2009;41(7):699–701. doi: 10.3109/00313020903305746. [DOI] [PubMed] [Google Scholar]
- 31.Brenn T, Fletcher CD. Postradiation vascular proliferations: an increasing problem. Histopathology. 2006;48(1):106–14. doi: 10.1111/j.1365-2559.2005.02293.x. [DOI] [PubMed] [Google Scholar]
- 32.Morgan MB, Swann M, Somach S, Eng W, Smoller B. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50(6):867–74. doi: 10.1016/j.jaad.2003.10.671. [DOI] [PubMed] [Google Scholar]
- 33.Deyrup AT, McKenney JK, Tighiouart M, Folpe AL, Weiss SW. Sporadic cutaneous angiosarcomas: a proposal for risk stratification based on 69 cases. Am J Surg Pathol. 2008;32(1):72–7. doi: 10.1097/PAS.0b013e3180f633a3. [DOI] [PubMed] [Google Scholar]
- 34.McKay KM, Doyle LA, Lazar AJ, Hornick JL. Expression of ERG, an Ets family transcription factor, distinguishes cutaneous angiosarcoma from histological mimics. Histopathology. 2012;61(5):989–91. doi: 10.1111/j.1365-2559.2012.04286.x. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez AP, Sun Y, Tubbs RR, Goldblum JR, Billings SD. FISH for MYC amplification and anti-MYC immunohistochemistry: useful diagnostic tools in the assessment of secondary angiosarcoma and atypical vascular proliferations. J Cutan Pathol. 2012;39(2):234–42. doi: 10.1111/j.1600-0560.2011.01843.x. [DOI] [PubMed] [Google Scholar]
- 36.Guo T, Zhang L, Chang NE, Singer S, Maki RG, Antonescu CR. Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes Chromosomes Cancer. 2011;50(1):25–33. doi: 10.1002/gcc.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ko JS, Billings SD, Lanigan CP, Buehler D, Fernandez AP, Tubbs RR. Fully automated dual-color dual-hapten silver in situ hybridization staining for MYC amplification: a diagnostic tool for discriminating secondary angiosarcoma. J Cutan Pathol. 2014;41(3):286–92. doi: 10.1111/cup.12278. [DOI] [PubMed] [Google Scholar]
- 38.Mentzel T, Schildhaus HU, Palmedo G, Buttner R, Kutzner H. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological, immunohistochemical and molecular analysis of 66 cases. Mod Pathol. 2012;25(1):75–85. doi: 10.1038/modpathol.2011.134. [DOI] [PubMed] [Google Scholar]
- 39.Shon W, Sukov WR, Jenkins SM, Folpe AL. MYC amplification and overexpression in primary cutaneous angiosarcoma: a fluorescence in-situ hybridization and immunohistochemical study. Mod Pathol. 2014;27(4):509–15. doi: 10.1038/modpathol.2013.163. [DOI] [PubMed] [Google Scholar]