Significance
Meniscus damage is a common musculoskeletal injury and a major risk factor for knee osteoarthritis (OA). An important knowledge gap exists about meniscus cell homeostasis mechanisms and how their impairment contributes to meniscus pathology following trauma and in aging. Forkhead Box O (FoxO) transcription factors are essential regulators of cellular homeostasis. We performed analysis of expression patterns and function of FoxO in human and mouse menisci. Our results show that FoxOs are suppressed in degenerated meniscus and that FoxO1 regulates meniscus growth and maturation, while both FoxO1 and 3 have protective functions in meniscus during aging and OA. These discoveries provide insight into mechanisms of meniscus degeneration and targets for therapeutic intervention.
Keywords: FoxO, meniscus, osteoarthritis, fibroblasts, chondrocytes
Abstract
The objective of this study was to examine FoxO expression and FoxO function in meniscus. In menisci from human knee joints with osteoarthritis (OA), FoxO1 and 3 expression were significantly reduced compared with normal menisci from young and old normal donors. The expression of FoxO1 and 3 was also significantly reduced in mouse menisci during aging and OA induced by surgical meniscus destabilization or mechanical overuse. Deletion of FoxO1 and combined FoxO1, 3, and 4 deletions induced abnormal postnatal meniscus development in mice and these mutant mice spontaneously displayed meniscus pathology at 6 mo. Mice with Col2Cre-mediated deletion of FoxO3 or FoxO4 had normal meniscus development but had more severe aging-related damage. In mature AcanCreERT2 mice, the deletion of FoxO1, 3, and 4 aggravated meniscus lesions in all experimental OA models. FoxO deletion suppressed autophagy and antioxidant defense genes and altered several meniscus-specific genes. Expression of these genes was modulated by adenoviral FoxO1 in cultured human meniscus cells. These results suggest that FoxO1 plays a key role in meniscus development and maturation, and both FoxO1 and 3 support homeostasis and protect against meniscus damage in response to mechanical overuse and during aging and OA.
The menisci play an important role in the biomechanics of the knee joint. Meniscus damage is one of the most common musculoskeletal injuries. Meniscal tears can be caused by trauma or degenerative disease. Most meniscus lesions are of nontraumatic origin. Traumatic meniscus injury is frequent among high school athletes, with an incidence of 5.1 per 100,000 in the United States (1, 2). Approximately 35% of the general population between 19 and 50 y of age has meniscus tears identified by MRI, with incidence increasing with age (3), and up to 91% among symptomatic osteoarthritis (OA) patients (4, 5). Traumatic meniscal tears are risk factors for the development of OA and meniscus lesions are predictors of OA progression (6, 7). While epidemiological and joint imaging data demonstrate that meniscus damage is a risk factor for OA, the mechanisms of meniscus damage are poorly understood, and in OA drug development there is an inadequate consideration of how drugs affect meniscus.
Current treatment strategies for meniscus lesions are primarily aimed at pain relief and improvement of joint function. New arthroscopic approaches for meniscal repair are limited to application in young patients with a traumatic tear, and still have high failure rates (8). A critical information deficit exists about mechanisms of meniscus cell homeostasis and why these mechanisms fail with aging or after trauma, leading to the development of meniscus degeneration (9–11).
Among the various levels in the signal transduction cascade, the activation of transcription factors is a critical process that integrates different extracellular and intracellular inputs to regulate cell differentiation, activation, and survival. Only a few transcription factors have been examined for expression and function in meniscus. Early growth response 1 (EGR1), a mediator of TGF-β3–induced fibrosis (12) showed the largest differential expression in the meniscus versus cruciate ligament during mouse embryonic development (13).
Members of the FoxO family of transcription factors activate defense mechanisms against various forms of stress and extend life span and protect against diseases of aging (14). FoxOs are downstream targets of phosphoinositide-3 kinase (PI3K)/Akt signaling, and modulate cell proliferation, growth, apoptosis, and the expression of antioxidant and autophagy proteins (15, 16). FoxOs also control the self-renewal of stem cell populations (17, 18) and are involved in the regulation of cell differentiation (19–23).
We reported earlier that the expression of FoxO is reduced in aging and in OA-affected human and mouse cartilage (24) and that FoxO deletion in mice leads to more severe cartilage damage (25). This study used human tissues and cells and mouse models to elucidate FoxO expression patterns in normal meniscus, during aging, and in OA. We also tested FoxO functions using gene deletions in mice and studies with human meniscus cells.
Results
FoxO Expression in Menisci from Normal and OA-Affected Human Knees.
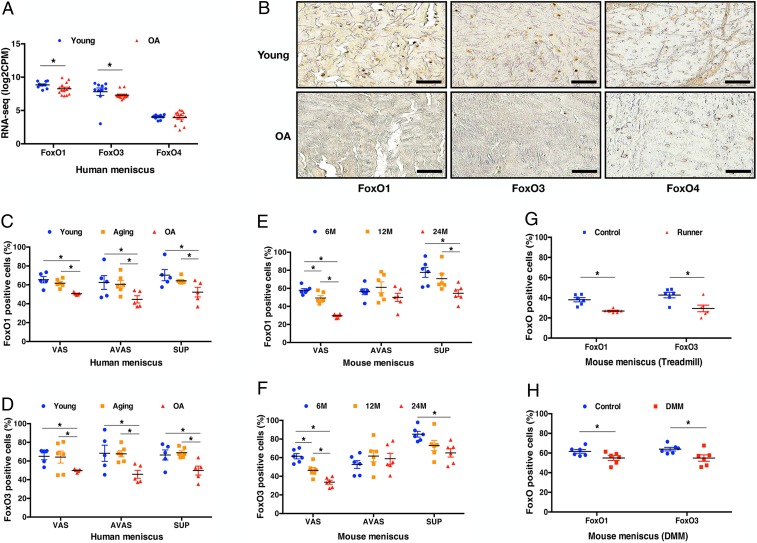
RNA-sequencing (RNA-seq) of five normal human meniscus samples and seven OA human meniscus samples, showed that FoxO1 and FoxO3 mRNA levels were lower in OA menisci while FoxO4 was not significantly different (Fig. 1A). Immunohistochemistry showed that FoxO1 and FoxO3 were expressed in normal menisci and decreased in OA menisci (Fig. 1 B–D). FoxO4 expression was not different between human young and OA menisci (Fig. 1B).
Fig. 1.
FoxO expression in human and mouse meniscus. (A) RNA-seq of young (n = 5, 19 ± 1 y), and OA (n = 7, 68 ± 4 y) human menisci. (B) Immunohistochemistry of FoxO1, FoxO3, and FoxO4 in human young and OA menisci. (Scale bar: 50 μm.) FoxO expression percentage in young (25 ± 1 y), aging (56 ± 1 y), and OA (72 ± 6 y) human menisci; (C) FoxO1; and (D) FoxO3 (n = 5 to 6 per group, *P < 0.05) VAS, vascular; AVAS, avascular; SUP, superficial). FoxO expression percentage in normal mouse meniscus; (E) FoxO1 expression at different ages (6, 12, and 24 mo) in different meniscus regions; (F) FoxO3 expression at different ages (6, 12, and 24 mo) in different meniscus regions (n = 6 per group, *P < 0.05). FoxO expression percentage in mouse meniscus after 6-wk treadmill running of 6-mo-old mice; (G) comparison of FoxO1 and FoxO3 in the entire meniscus between control and treadmill running model; (H) comparison of FoxO1 and FoxO3 expression in the entire meniscus between control and DMM model (n = 6 per group, *P < 0.05).
Additional menisci were analyzed by qPCR. FoxO1 and FoxO3 mRNA levels in human degenerated meniscus were significantly decreased in vascular and avascular zones as compared to young normal menisci (SI Appendix, Fig. S1). Only low levels of FoxO4 mRNA were detected in normal menisci and there were no differences compared with degenerated menisci (SI Appendix, Fig. S1).
Immunohistochemistry of FoxO expression in specific zones (SI Appendix, Fig. S2) of normal human menisci (25 ± 1 y) showed that FoxO1-expressing cells were present throughout the vascular, avascular, and superficial zones (Fig. 1C and SI Appendix, Figs. S3A and S4 A–C). Similar expression patterns were observed for FoxO3 in vascular, avascular, and superficial zones (Fig. 1D and SI Appendix, Figs. S3A and S4 D–F).
FoxO1 and FoxO3 expression were not different between young and normal aging groups (56 ± 1 y) with no history of OA and low-grade histological changes in cartilage and macroscopically normal menisci (SI Appendix, Fig. S4 A–F). However, FoxO1 and FoxO3 protein expression were lower in OA (72 ± 6 y) than in young and normal aging groups (Fig. 1 C and D and SI Appendix, Fig. S4 G–L).
FoxO Expression in Normal Mouse Menisci and in Experimental OA Models.
FoxO expression was analyzed in mouse menisci in specific zones and regions (SI Appendix, Fig. S2). In 4-mo-old wild-type (WT) mouse menisci FoxO1 and FoxO3 were highly expressed while levels of FoxO4 were much lower than FoxO1 and FoxO3 (SI Appendix, Fig. S5 A–C). In normal menisci of mature 6-mo-old wild-type mice, there were differences in FoxO expression in specific meniscus zones. FoxO1 and FoxO3 were most highly expressed in cells in the superficial zone, and FoxO3 was higher in the vascular zone than in the avascular zone (SI Appendix, Fig. S3B).
In the C57BL/6 wild-type mouse joint aging model (26), FoxO1 and FoxO3 were significantly decreased in the vascular zone at 12 mo compared to 6 mo (Fig. 1 E and F). Significant further decreases in the vascular and superficial zones were observed at 24 mo for FoxO1 and FoxO3 (Fig. 1 E and F and SI Appendix, Fig. S6). Moreover, the superficial zone in the anterior region and the vascular zone in the posterior region showed the largest decreases at 24 mo (SI Appendix, Fig. S6 A and B). There were no differences between posterior avascular zone and posterior vascular zone at 6 mo but FoxO1 and FoxO3 expression in the posterior vascular zone was significantly decreased at 24 mo (SI Appendix, Fig. S6 C and D).
In the mechanical overuse model (6 wk of treadmill running) in C57BL/6 wild-type mice (25), histopathological scores of meniscus damage in anterior and posterior regions were significantly increased (SI Appendix, Fig. S7 A and B). FoxO1 and FoxO3 were significantly decreased in the superficial and posterior vascular zones (SI Appendix, Fig. S7 C and D). FoxO1 was significantly decreased in the superficial zone after 6 wk of treadmill running, and FoxO3 was significantly decreased in both vascular and superficial zones after running (Fig. 1G).
In the destabilization of the medial meniscus (DMM) model (27), meniscus histopathological scores were significantly increased in anterior and posterior regions 8 wk after surgery compared to the sham control group (SI Appendix, Fig. S8 A and B). FoxO1 and FoxO3 were significantly decreased in the meniscus superficial zone in mice with DMM (Fig. 1H). In the anterior region, FoxO1 and FoxO3 were significantly increased in the anterior region but decreased in the posterior region (SI Appendix, Fig. S8 C and D).
Postnatal Meniscus Development, Maturation, and Degeneration in Col2Cre FoxO Knockout Mice.
To determine the function of FoxO in meniscus, we examined mice with Col2Cre-mediated individual deletion of FoxO1, FoxO3, or FoxO4 (SI Appendix, Fig. S5 D–F) and mice with Col2Cre-mediated deletion of all three FoxO isoforms (FoxO triple knockout; TKO, SI Appendix, Fig. S5 G and H). FoxO 1, 3, and 4 gene expression in meniscus was decreased in the FoxO knockout (KO) mice (SI Appendix, Fig. S9).
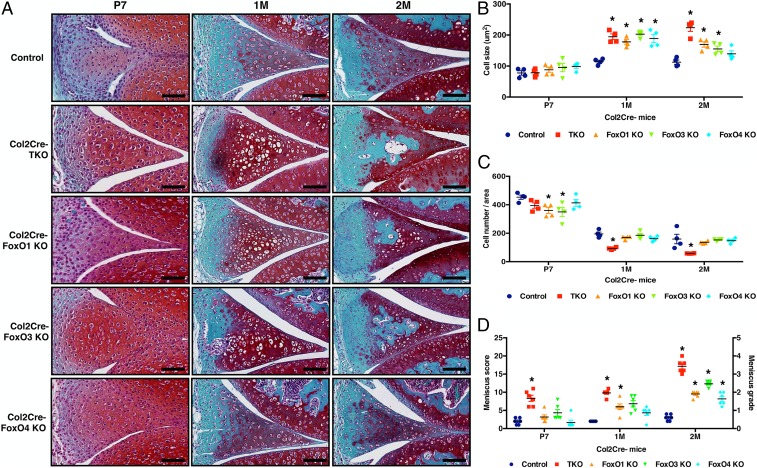
There were no apparent meniscus structural or cellular abnormalities at birth and postnatal day 1 (P1) in the FoxO KO mice. However, from P7 to 2 mo, cell sizes (Fig. 2 A and B) were increased in the FoxO TKO, FoxO1 KO, and FoxO3 KO mice. There were acellular regions in FoxO TKO, FoxO1 KO, FoxO3 KO mice at 2 mo (Fig. 2A), and cell numbers per entire area of the meniscus (Fig. 2C) were decreased in FoxO TKO mice. Meniscus histopathology scores were already increased during the early postnatal period in FoxO TKO mice at P7. FoxO TKO and FoxO single KO mice showed significantly higher meniscus histopathology scores than control mice at 2 mo (Fig. 2D).
Fig. 2.
Changes in postnatal meniscus development in mice with Col2Cre-mediated conditional FoxO deletion. (A) Safranin-O staining of control, Col2Cre-FoxO TKO, FoxO1, 3, and 4 single KO mice at P7 and 1 and 2 mo old; (B) Cell size (μm2); (C) cell numbers per meniscus area; (D) meniscus histopathology scoring and grading (n = 6 per group, *P < 0.05).
Meniscus Changes in Mature AcanCreERT2 FoxO KO Mice.
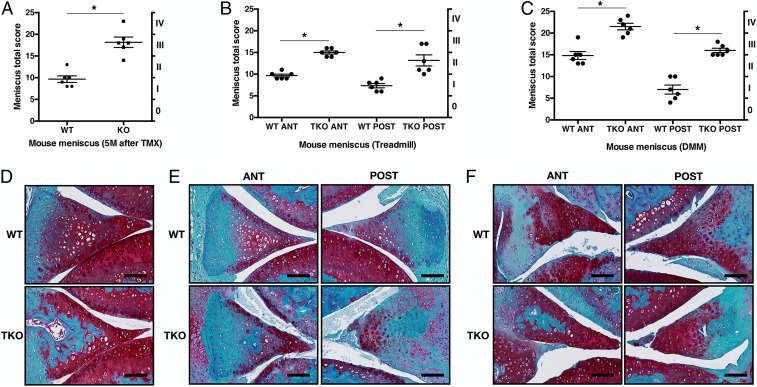
Since FoxO conditional deletion using Col2Cre (28, 29) resulted in defects in postnatal meniscus maturation, we used the Aggrecan-CreERT2 (AcanCreERT2) knockin mice (30) to analyze the role of FoxO in the maintenance of mature meniscus. Four-month-old FoxO1lox/lox;FoxO3lox/lox;FoxO4lox/loxAcanCreERT2 mice received five injections of tamoxifen (TMX). Five months after tamoxifen injection, menisci in AcanCreERT2-FoxO TKO mice spontaneously developed more severe histopathological changes than the menisci in control mice (Fig. 3 A and D). Six weeks after treadmill running, AcanCreERT2-FoxO TKO mice showed significantly increased histopathology scores in the anterior and posterior meniscus regions (Fig. 3 B and E). In the surgical model 8 wk after DMM surgery, menisci in AcanCreERT2-FoxO TKO mice also showed significantly increased histopathology scores in the anterior and posterior meniscus (Fig. 3 C and F).
Fig. 3.
Changes in mature meniscus of AcanCreERT2-FoxO TKO mice. (A) Meniscus scores (0 to 25) and grading (0 to IV) of AcanCreERT2 TKO mice at 5 mo after tamoxifen (TMX) injection. WT: Littermate AcanCreERT2-negative mice that also received tamoxifen injections. (B) Meniscus scores and grading of 6-mo-old AcanCreERT2 TKO mice in specific meniscus regions after 6-wk treadmill running. (C) Meniscus scores and grading of 6-mo-old AcanCreERT2 TKO mice in specific meniscus regions 8 wk after DMM surgery. (n = 6 per groups, *P < 0.05). (D) Safranin-O staining of AcanCreERT2 TKO mice at 5 mo after TMX injection. (E) Safranin-O staining of AcanCreERT2 TKO mice in specific meniscus regions after 6-wk treadmill running. (F) Safranin-O staining of 6-mo-old AcanCreERT2 TKO mice in specific meniscus regions after DMM surgery (PO 8 wk). (Scale bar: 100 μm.)
Relationship of Meniscus and Cartilage Changes in Mice with FoxO Deletion.
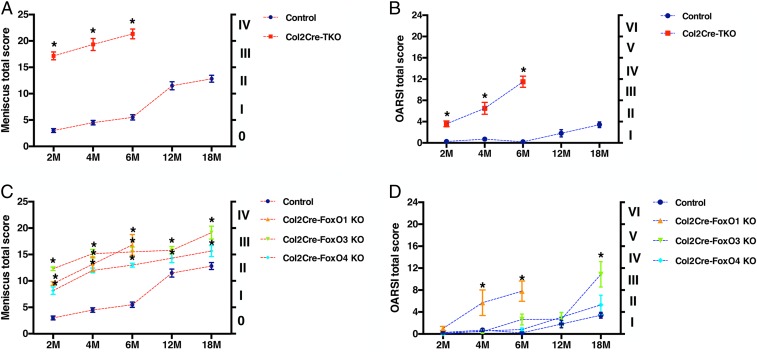
To examine the temporal relationship between cartilage and meniscus lesions in the FoxO KO mice, we compared the histopathology scores of cartilage and meniscus from the same mice in the different experimental models. In Col2Cre-FoxO TKO mice, meniscus and cartilage histopathology scores were already significantly higher than in control mice at 2 mo, with the severity of meniscus damage being relatively higher than in cartilage (Fig. 4 A and B). At 2 mo, the FoxO single KO mice also had significantly increased meniscus scores while the cartilage scores were still normal (Fig. 4 C and D). At 4 and 6 mo, meniscus scores in all FoxO KO mice increased further and cartilage scores became significant in the FoxO TKO and FoxO1 KO mice. Col2Cre-FoxO TKO mice and Col2Cre-FoxO1 KO mice were not maintained beyond 6 mo of age due to the severe changes in the joints. FoxO3 KO mice had significantly increased meniscus scores by 12 mo but cartilage scores became only significant at 18 mo. These results suggest that degenerative changes caused by FoxO gene deletion occurred earlier in meniscus than in articular cartilage.
Fig. 4.
Histopathology of meniscus and articular cartilage in mice with FoxO deletion. Meniscus total scores (0 to 25) and grading (0 to IV) and articular cartilage OARSI total scores (0 to 24) and grades (I through VI) of Col2Cre-FoxO TKO, Col2Cre-FoxO single KO1, KO3, KO4. (A) Meniscus scores and grades of Col2Cre-FoxO TKO mice at 2, 4, 6, 12, and 18 mo old; (B) OARSI scores and grades of Col2Cre-FoxO TKO mice at 2, 4, 6, 12, and 18 mo old; (C) meniscus scores and grades of Col2Cre-FoxO conditional KO mice at 2, 4, 6, 12, and 18 mo old; and (D) OARSI scores and grades of Col2Cre-FoxO conditional KO mice at 2, 4, 6, 12, and 18 mo old (n = 6 per group, *P < 0.05).
Changes in the Expression of FoxO Target Genes in Menisci from FoxO KO Mice.
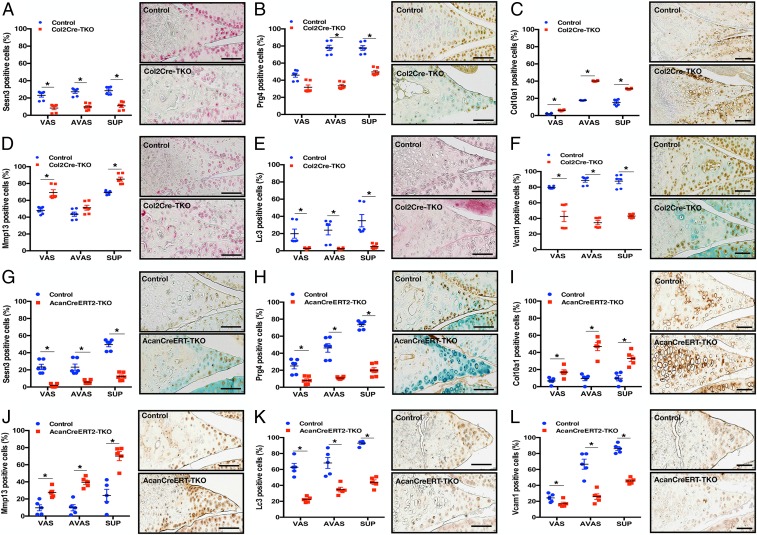
Immunohistochemistry showed that the FoxO target gene Sesn3 was decreased in all meniscus regions of Col2Cre-FoxO TKO mice (2 mo old) (Fig. 5A). Prg4 was highly expressed in avascular and superficial zones of WT mice and significantly decreased in all regions in TKO mice (Fig. 5B). The meniscus hypertrophy marker Col10a1 was increased in all meniscus regions and Mmp13 was increased in vascular and superficial zones (Fig. 5 C and D). However, the autophagy marker Lc3, and Vcam-1 an angiogenesis and neovascularization marker which in damaged meniscus is part of the repair process (31–33), were decreased in entire the meniscus (Fig. 5 E and F). In the AcanCreERT2-FoxO TKO mice (6 mo old), Sesn3, Prg4, Lc3, and Vcam1 were decreased in all meniscus regions (Fig. 5 G, H, K, and L) while Col10a1 and Mmp13 were increased in all meniscus regions (Fig. 5 I and J).
Fig. 5.
Immunohistochemistry of FoxO TKO mouse meniscus. Sesn3, Prg4, Col10a1, Mmp13, Lc3, and Vcam1 immunohistochemistry of Col2Cre TKO mouse meniscus at 2 mo old (A–F) and AcanCreERT2 TKO at 6 mo old (G–L). (n = 6 per group, *P < 0.05.) (Scale bar: 100 μm.)
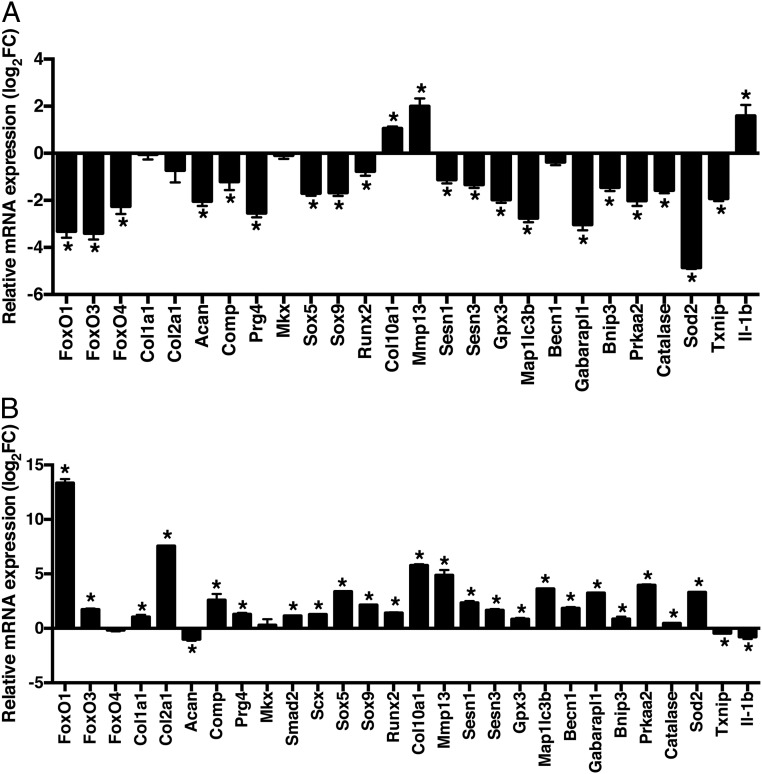
Menisci were collected from AcanCreERT2-FoxO TKO and WT mice for RNA isolation and gene expression analysis. Menisci from AcanCreERT2-FoxO TKO mice showed significantly decreased expression of meniscus extracellular matrix (ECM) genes (Acan, Comp, and Prg4), decreased transcription factors (Sox5, Sox9, and Runx2), decreased antioxidant defense-related genes (Sesn1, Sesn3, Gpx3, Sod2, and Catalase), redox regulatory gene (Txnip), adaptation to energy stress (Prkaa2), and also decreased autophagy genes (Maplc3b, Becn1, Gabarapl1, and Bnip3). The expression of Il-1β was increased (Fig. 6A).
Fig. 6.
Changes in mature meniscus by FoxO knockout and FoxO overexpression. (A) Gene expression in AcanCreERT2-FoxO TKO mice at 6 mo old: FoxO genes (FoxO1, FoxO3, and FoxO4), meniscus-specific genes (Col1a1, Col2a1, Acan, Comp, Prg4, Mkx, Sox5, and Sox9), hypertrophy genes (Runx2, Col10a1, and Mmp13), antioxidant defense genes (Sesn1, Sesn3, and Gpx3), autophagy genes (Map1lc3b, Becn1, Gabarapl1, Bnip3, and Prkaa2), and redox regulation (Catalase, Sod2, and Txnip). Relative gene expression was normalized by expression values of each gene in comparison between data from AcanCreERT2 TKO mice littermate AcanCreERT2-negative mice that also received tamoxifen injections. RNA was collected from 6-mo-old mice (medial and lateral menisci from both joints of each mouse were pooled) for qRT-PCR analysis (n = 6 mice per group, *P < 0.05). (B) Cells from human OA menisci were transduced with adenovirus encoding FoxO1-AAA, a constitutively active form of FoxO1. RNA was collected after 48 h for qRT-PCR analysis (n = 6 per group, *P < 0.05).
FoxO Function in Cultured Human Meniscus Cells.
As an additional approach to test a direct function of FoxO in meniscus cells, we transduced human normal avascular meniscus cells with adenovirus encoding a constitutively active form of FoxO1 (Ad-FoxO1) (34). FoxO1 overexpression in human meniscus cells significantly increased meniscus ECM genes (Comp, Col1a1, Col2a1, and Prg4), antioxidant defense-related genes (Sesn1, Sesn3, Gpx3, Sod2, and Catalase), and autophagy-related genes (Maplc3b, Becn1, Gabarapl1, and Bnip3) while Il-1β was decreased (Fig. 6B).
Discussion
This study reports on patterns of FoxO expression in normal, aged, and degenerated menisci from humans and several mouse models. To investigate FoxO function we used mice with conditional FoxO deletion and in vitro models with human meniscus cells. The focus of the present study on FoxO is based on the important role of these transcription factors in regulating essential cellular homeostasis mechanisms (16). We performed a prior analysis of FoxO in articular cartilage using the same mouse models and reported that FoxOs are essential in protecting against cartilage damage (25). In the present study, we examined menisci from the same mice that were previously studied for changes in cartilage, thus allowing simultaneous analysis of changes in these two interacting joint tissues.
Our results on FoxO expression in normal human and mouse menisci show that FoxO1 and 3 are more abundant than FoxO4. While there is similar expression of FoxO1 and 3 in all meniscus regions in normal human and mouse menisci, in mature mice there are significantly higher FoxO1 and 3 expressions in the superficial zone as compared to the other meniscus regions. In mice, the posterior region showed higher FoxO1 and 3 expressions than the anterior region.
Aging is a main risk factor for meniscus degeneration (26). From our large autopsy collection of human knee joints (35), we selected menisci from older donors who had macroscopically normal articular cartilage and menisci with minimal histopathological changes. Interestingly, these menisci did not show reduced FoxO1 or FoxO3 expression. Mice, however, showed aging-related meniscus damage and reduced FoxO1 and FoxO3 expression already at 12 mo with further reduction at 24 mo. At 12 mo there is already aging-related meniscus damage (36) and FoxO1 and 3 expression were most reduced in the posterior vascular zone. Degenerative changes were also most severe in the posterior vascular zone.
In human degenerated menisci, FoxO1 and 3 expressions were significantly reduced. In the mouse DMM and treadmill models there was also an association between reduced FoxO expression and meniscus degeneration. FoxO1 and 3 suppressions were most profound in the superficial and vascular zones in the aging and treadmill models, while reduced FoxO in the DMM model was only seen in the superficial zone. In the anterior region, FoxO1 and 3 expressions were strongest in the superficial zone. However, FoxO1 and 3 expressions were significant decreased in the posterior avascular and vascular zone in the DMM model.
To examine the role of FoxO in meniscus function in vivo, we used mice with conditional FoxO deletion. Mice with conditional gene deletion in cartilage have been widely and successfully used in OA research. A challenge in using mutant mice for meniscus research is that there is no gene known to be exclusively expressed in this tissue that is not also expressed in other joint tissues such as cartilage, tendons, and ligaments and could be used as a driver for gene deletion in meniscus. Here we used mice with FoxO deletion mediated by the Col2A1-CRE or Acan-CRE-ERT drivers. Col2a1 and Acan are predominantly expressed in the superficial and avascular meniscus (28, 29). In the inner third of the meniscus, Col2a1 and Acan are stained more prominently than in the outer third (30). Col2a1 is also expressed in fibrochondrocytes of anterior and posterior meniscus horns (37). Col2a1 is not expressed in mouse meniscus between embryonic day 13.5 and 1 wk of age (38). Consistent with this, we did not observe any developmental abnormalities in the meniscus, even in the Col2Cre FoxO TKO mice. In mature meniscus, the inner part is more cartilage-like and expresses higher levels of collagen II, whereas the outer part is more fibrous and expresses more collagen I (38). By the age of 2 mo we observed significantly increased histopathology scores in the Col2Cre FoxO triple and single KO mice.
We used a second Cre driver, the tamoxifen-inducible Acan-CreERT2 to more accurately assess the consequences of FoxO deletion in joints from 4-mo-old mice that had reached maturity without any abnormalities in cartilage or meniscus. Acan-CreERT2 drives gene expression or deletion predominantly in the inner zone and in the superficial zone of the mouse meniscus (31). Acan-CreERT2 also has the advantage that it mediates more efficient recombination in joint tissues when tamoxifen is administered during postnatal periods (39). The Acan-CreERT2 FoxO TKO mice spontaneously developed more severe histopathological changes than the menisci in control mice by the age of 9 mo, and treadmill running-induced meniscus damage was also more severe. The results from the AcanCreERT2-FoxO TKO mice show that FoxO are required for the maintenance of meniscus integrity during adulthood.
FoxO TKO and FoxO1 KO mice at 1 mo showed significantly increased meniscus cell size, indicative of hypertrophic cells. These cellular changes preceded degenerative changes, which started at 2 mo and progressed to severe damage by 4 mo. These results from the Col2a1Cre FoxO KO mice indicate that FoxO, in particular FoxO1, is indispensable for normal postnatal meniscus growth and maturation.
We used two independent approaches to investigate FoxO target genes in meniscus, including gene expression analysis in WT and FoxO KO mice and in cultured human meniscus cells with FoxO1 overexpression. These studies revealed that FoxO1 regulates genes involved in several important functions, including autophagy, ECM, transcription factors, antioxidant defense, ECM degradation, and inflammation. We previously reported that there is compromised autophagy in meniscus in models of joint injury (40). The present findings suggest that FoxO suppression is one candidate mechanism for deficient autophagy and protection against oxidants in meniscus. These results also reveal a function of regulating meniscus expression of PRG4, an essential joint lubricant (41). The protein is produced by cells in synovium, the superficial zone of articular cartilage, and cells in the superficial zone of the meniscus. We show here that the expression of PRG4 protein is significantly reduced in menisci of the FoxO KO mice. In human meniscus cells, adenoviral FoxO increased PRG4. Thus, like in cartilage, PRG4 is a FoxO target gene in meniscus. Reduced expression of PRG4 as a consequence of FoxO suppression may lead to damage in the meniscus superficial zone. If this protective layer is not maintained by FoxO, meniscus tissue is more susceptible to damage as indicated by the more severe disease in the KO mice in the DMM and treadmill models. FoxO-deficient mice present increased numbers of cells with hypertrophic morphology, associated with increased abnormal expression of hypertrophy-related genes. In this regard, FoxO suppression in meniscus might contribute to meniscal calcification which is associated with meniscus degeneration and predisposes to cartilage lesions (42). MMP13 and Col10a1 are markers of chondrocyte hypertrophy and both are increased in menisci from FoxO KO mice. However, we also observed that expression of these genes was increased after Ad-FoxO1 transduction of cultured human meniscus cells. In the in vitro studies with human meniscus cells and Ad-FoxO1, MMP13 appears to be an exception as a catabolic mediator that is not suppressed by FoxO. Most catabolic factors are suppressed by FoxO or increased under FoxO deficiency so that the overall effect of FoxO deficiency is to aggravate tissue destruction, predominantly by controlling genes involved in cellular defense and homeostasis mechanisms.
While our results demonstrate a role of FoxO in meniscus cells, there is an experimental challenge in distinguishing the role of a gene in meniscus versus cartilage, as there are no Cre drivers that selectively delete a gene only in meniscus or cartilage. FoxO deficiency occurs with aging and in OA in cartilage and meniscus and is likely to impact cartilage and vice versa. Both tissues closely interact through biomechanical (43, 44) as well as biochemical mechanisms (7, 45).
In conclusion, FoxO expression is reduced in meniscus aging and following meniscus injury in humans and mice. FoxO suppression is a risk factor for the development of meniscus degeneration and restoring FoxO expression or activity is a candidate approach to prevent or treat meniscus damage.
Methods
Human Meniscus Tissues from Normal, Aging, and OA Knees.
Normal human knee joints were obtained from tissue banks (approved by Scripps Institutional Review Board). Knees were collected by resection of femur, tibia, and fibula 15 cm above and below the joint line. The knees were received within 48 h postmortem. Subjects with a history of knee trauma were excluded. Macroscopic and microscopic grading of the articular cartilage in all knee compartments was performed with a modified Outerbridge system as described (46–48). Menisci were also obtained from OA joints at the time of knee arthroplasty. We reported previously on macroscopic and histopathologic analysis of human knee menisci in aging and OA (35). In the previous study, six cases in three groups (young, old, and osteoarthritis) were randomly selected for analysis. In this study, young (average age 25 ± 1, n = 6), aging (average age 56 ± 1, n = 6), and OA (average age 72 ± 6, n = 6) were analyzed with the same methods of the previous study (35).
RNA-Sequencing.
Full thickness human normal meniscus tissue from the central region was harvested for RNA isolation from five male donors (ages 18 to 20, mean 19 ± 1). OA-affected menisci were harvested from the tissue removed during knee replacement surgery from four female and three male donors (ages 57 to 85, mean 68 ± 4).
RNA was isolated from a minimum of 150 mg of meniscus (dry weight) as previously described (49). RNA integrity numbers (RINs) were calculated using a 2100 Bioanalyzer (Agilent). Average RIN numbers were 6.08 ± 0.95.
Library preparation and sequencing.
Total RNA (150 ng per sample) from five normal and seven OA meniscus donors was sequenced to generate a total of 8 to 30 million 100-bp reads as described (50).
The number of reads sequenced per sample ranged from 19 to 24 million reads, which should be sufficient for gene level quantification, but only 2 to 12 million reads per sample mapped to protein coding genes. To account for this issue, we applied high stringency to the filtering of lowly expressed genes (log counts per million [cpm] >3) so that only the differential expression analysis included only genes that were expressed in high enough abundances to be confident in their relative gene expression values.
Read mapping, quantification, and differential expression were performed as described (56). Only genes with counts with log2 cpm greater than 3.0 in one or more samples were considered expressed. Genes with an adjusted P value of <0.05 (based on the moderated t statistic using the Benjamini–Hochberg [BH] method to control the false discovery rate) (50) and a |log2FC| > 1 were considered significantly differentially expressed (DE).
Mouse Aging Model.
All animal studies were performed with approval by the Scripps Institutional Animal Care and Use Committee. Pathogen-free C57BL/6J mice were purchased from the Scripps Research Institute breeding facility. The mice were sacrificed at various ages and knee joints were collected for analysis. Both male and female mice were included in this study. A total of 18 mice at three different ages were assessed: 6 (n = 6), 12 (n = 6), and 24 (n = 6) month-old mice. We performed histological and immunohistochemical analyses in the three age groups and quantified changes in different zones of the anterior horn and posterior horn of the menisci as illustrated in SI Appendix, Fig. S2.
Mice with Conditional Postnatal FoxO Deletion.
Col2a1Cre/+ transgenic mice (28, 29) and Aggrecan (Acan)CreERT2 knockin mice (30) on a C57BL6/J background were obtained from The Jackson Laboratory. FoxO1lox/lox, FoxO3lox/lox, and FoxO4lox/lox were obtained from R. DePinho (The University of Texas MD Anderson Cancer Center, Houston, TX) (51). Col2a1 (28, 29) and Acan (30) are predominantly expressed in the hyaline cartilage, pericellular and extracellular locations of the meniscus (52). In the inner third of the meniscus, Col2a1 and Acan are stained prominently (53). Also, Col2a1 is expressed in fibrochondrocytes of anterior and posterior meniscus horns (44). With Col2Cre, we generated mice with deletion of individual FoxO 1, 3, or 4, and mice with deletion of FoxO1, 3, and 4 (FoxO triple KO, FoxO TKO). In the AcanCreERT2 model, we bred AcanCreERT2/+ knockin mice with FoxO1lox/lox; FoxO3lox/lox; FoxO4lox/lox triple transgenic mice to obtain AcanCreERT2 TKO mice. Tamoxifen (Sigma-Aldrich) was intraperitoneally injected at a dose of 1.5 mg/10 g body weight on 5 consecutive days in 4-mo-old mice. Genotyping was performed by PCR using tail DNA. Littermates homozygous for the floxed FoxO not expressing Cre recombinase were used as controls of Col2Cre-FoxO KO mice, and AcanCreERT2−/− littermates were injected with tamoxifen as controls for AcanCreERT2 TKO mice. Knee joints were collected from 1-, 2-, 4-, 6-, and 12-mo-old control, Col2Cre TKO, and Col2Cre-FoxO1/3/4 single KO mice. In addition, knee joints were collected from control and AcanCreERT2 TKO mice 5 mo after tamoxifen injection.
Surgical and Treadmill Running-Induced OA Models.
The surgical OA model was created by DMM (27) in 6-mo-old mice. In the treadmill-induced OA model, 6-mo-old mice were placed on a treadmill (Columbus Instruments Exer 3/6 treadmill) at a 10° incline for 45 min at a speed of 15 m/min, including 2 min of warming up (54). Mice were killed 8 wk after DMM surgery and 6 wk after treadmill exercise, and six knee joints from each group were collected.
Histological Analyses of Mouse Joints.
A total of 36 WT mice (aging, n = 18; surgical model, n = 6; treadmill-induced OA, n = 12) and 108 transgenic mice (Col2Cre-FoxO WT, n = 24; Col2Cre-FoxO KO, n = 24; AcanCreERT2-FoxO WT, n = 18; AcanCreERT2-FoxO KO, n = 18; surgical, n = 12; and treadmill, n = 12) were graded by Kwok’s meniscus scoring system (score 0 to 25) (36).
The entire knee joints were fixed in 10% zinc buffered formalin for 2 d and decalcified in TBD-2 Decalcifier (Thermo Fisher Scientific) for 24 h. Sections of the mouse knee joints were stained with Safranin-O Fast Green for further analysis. To calculate cell numbers, among the mouse genotypes and treatment conditions, we normalized the measured area for each meniscus. Each meniscus size was divided by the smallest P7 size to set the ratio. The ratio after normalization was used for calculating cell numbers per area.
Histological scoring of OA-like changes on the medial femoral condyle and the medial tibial plateau was performed using the Osteoarthritis Research Society International (OARSI) cartilage OA histopathology semiquantitative scoring system (score 0 to 24) (55).
Immunohistochemistry.
Knee joint sections were deparaffinized, washed, and blocked with 10% goat serum for 1 h at room temperature. Primary antibodies against FoxO1A (1:250, Abcam), FoxO3A (1:500, Abcam), FoxO4 (1:250, Abcam), Sesn3 (1:200, Proteintech), Prg4 (1:300, Abcam), and VCAM1 (1:100, Abcam) were applied in 0.1% Tween 20 and incubated overnight at 4 °C, followed by secondary antibody using ImmPRESS regents (Vector Laboratories). All antibodies were of rabbit origin and rabbit IgG staining was used as negative control (SI Appendix, Fig. S10). The signal was developed with diaminobenzidine (DAB, Sigma-Aldrich) and counterstained with methyl green or hematoxylin.
Quantification of Immunohistochemistry.
For quantification of changes in the mouse or human tissues, menisci were divided into vascular, avascular, and superficial zones (SI Appendix, Fig. S2). The number of positive cells per field was counted under a microscope at the 40× magnification for each of the three zones from each meniscus section. The percent positive cells per field was determined as the ratio of the total number of positive cells to the total cell number of meniscus in the respective zone.
FoxO1 Overexpression Using Adenoviral Vector.
Recombinant adenoviral vector encoding constitutively active FoxO1-AAA was constructed using pAd/CMV/V5-DEST Gateway vector with pcDNA3-FLAG-FoxO1-AAA (Addgene plasmid 13508). Human meniscus cells were infected with Ad-FoxO1-AAA or control Ad-GFP (Sanford-Burnham-Prebys Medical Discovery Institute) at 10 multiplicity of infection (MOI). The cells were collected for RNA isolation after 48 h.
RNA Isolation from Menisci.
RNA was collected from 6-mo-old mice (medial and lateral menisci from both joints of each mouse were pooled) for qRT-PCR analysis (n = 6 mice per group). For human menisci, vascular and avascular zones were separated from OA patients (70 ± 4 y; 4 females, 2 males) and young normal cadavers (19 ± 1 y; 6 males) for RNA extraction. Total RNA was extracted from mouse meniscus tissues or cultured meniscus cells using TRIzol (Invitrogen), followed by Zymo Direct-zol RNA MiniPrep kits (Zymo Research). Human meniscus tissue was resuspended in RNA-later (Qiagen) immediately after harvest and stored at −20 °C until RNA extraction. For RNA isolation, meniscus tissues were homogenized in Qiazol Lysis Reagent (Qiagen) at a concentration of 25 mg tissue sample per 700 μL Qiazol. RNA was extracted from meniscus using the fibrous tissue RNA extraction kit (Qiagen). RNA was isolated using RNAqueous kit (Ambion) and then, on-column DNase treatment was performed using the DNase I (Qiagen) and the RNeasy MinElute Cleanup kit (Qiagen).
PCR.
Quantitative-PCR analysis was conducted on a LightCycler 480 Real-Time PCR System (Roche Diagnostics) with up to 45 cycles using TaqMan Gene Expression Assay probes (Life Technologies) (SI Appendix, Table S1). The levels of mRNA were calculated as relative quantities in comparison to Gapdh (SI Appendix, Fig. S11).
Statistical Analyses.
Results were analyzed using Prism version 5.2 (GraphPad Software, Inc.). A Mann–Whitney t test (unpaired and nonparametric tests) was used to establish statistical significance between two groups in the qRT-PCR results. Statistical analysis of qRT-PCR was based on delta Ct values and graphs present log2 fold change. Two-way ANOVA test was used for multiple comparisons between groups in the histopathology analyses. Variance was used to compare multiple groups, with subsequent pairwise (group) comparisons assessed at an experimentwise error level of 0.05. P values less than 0.05 were considered statistically significant.
Data Availability.
All data relevant to the conclusions of the manuscript are included in SI Appendix. Any additional data are available upon request.
Supplementary Material
Acknowledgments
We thank Josan Chung for technical support. This study was supported by the Shaffer Family Foundation and NIH grants AG049617 and AG059418.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918673117/-/DCSupplemental.
References
- 1.McDermott I. D., Amis A. A., The consequences of meniscectomy. J. Bone Joint Surg. Br. 88, 1549–1556 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Mitchell J., et al. , Epidemiology of meniscal injuries in US high school athletes between 2007 and 2013. Knee Surg. Sports Traumatol. Arthrosc. 24, 715–722 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Englund M., et al. , Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N. Engl. J. Med. 359, 1108–1115 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharyya T., et al. , The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J. Bone Joint Surg. Am. 85, 4–9 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Lamplot J. D., Brophy R. H., The role for arthroscopic partial meniscectomy in knees with degenerative changes: A systematic review. Bone Joint J. 98-B, 934–938 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Neogi T., Zhang Y., Epidemiology of osteoarthritis. Rheum. Dis. Clin. North Am. 39, 1–19 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Englund M., Roos E. M., Lohmander L. S., Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: A sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum. 48, 2178–2187 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Nepple J. J., Dunn W. R., Wright R. W., Meniscal repair outcomes at greater than five years: A systematic literature review and meta-analysis. J. Bone Joint Surg. Am. 94, 2222–2227 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brophy R. H., Rai M. F., Zhang Z., Torgomyan A., Sandell L. J., Molecular analysis of age and sex-related gene expression in meniscal tears with and without a concomitant anterior cruciate ligament tear. J. Bone Joint Surg. Am. 94, 385–393 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brophy R. H., Sandell L. J., Cheverud J. M., Rai M. F., Gene expression in human meniscal tears has limited association with early degenerative changes in knee articular cartilage. Connect. Tissue Res. 58, 295–304 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brophy R. H., Sandell L. J., Rai M. F., Traumatic and degenerative meniscus tears have different gene expression signatures. Am. J. Sports Med. 45, 114–120 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharyya S., et al. , Smad-independent transforming growth factor-beta regulation of early growth response-1 and sustained expression in fibrosis: Implications for scleroderma. Am. J. Pathol. 173, 1085–1099 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pazin D. E., Gamer L. W., Capelo L. P., Cox K. A., Rosen V., Gene signature of the embryonic meniscus. J. Orthop. Res. 32, 46–53 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Martins R., Lithgow G. J., Link W., Long live FOXO: Unraveling the role of FOXO proteins in aging and longevity. Aging Cell, 15, 196–207 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantley L. C., The phosphoinositide 3-kinase pathway. Science 296, 1655–1657 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Webb A. E., Brunet A., FOXO transcription factors: Key regulators of cellular quality control. Trends Biochem. Sci. 39, 159–169 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paik J. H., et al. , FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell 5, 540–553 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X., et al. , FOXO1 is an essential regulator of pluripotency in human embryonic stem cells. Nature Cell Biol. 13, 1092–1099 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakae J., et al. , The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev. Cell 4, 119–129 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Iyer S., et al. , FOXOs attenuate bone formation by suppressing Wnt signaling. J. Clin. Invest. 123, 3409–3419 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munekata K., Sakamoto K., Forkhead transcription factor Foxo1 is essential for adipocyte differentiation. In Vitro Cell. Dev. Biol. Anim. 45, 642–651 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Kitamura T., et al. , A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J. Clin. Invest. 117, 2477–2485 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talchai S. C., Accili D.. Legacy effect of Foxo1 in pancreatic endocrine progenitors on adult beta-cell mass and function. Diabetes 64, 2868–2679 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akasaki Y., et al. , Dysregulated FOXO transcription factors in articular cartilage in aging and osteoarthritis. Osteoarthritis Cartilage 22, 162–170 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuzaki T., et al. , FoxO transcription factors modulate autophagy and proteoglycan 4 in cartilage homeostasis and osteoarthritis. Sci. Transl. Med. 10, eaan0746 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsujii A., Nakamura N., Horibe S., Age-related changes in the knee meniscus. Knee 24, 1262–1270 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Glasson S. S., Blanchet T. J., Morris E. A., The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage 15, 1061–1069 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Hyde G., Boot-Handford R. P., Wallis G. A., Col2a1 lineage tracing reveals that the meniscus of the knee joint has a complex cellular origin. J. Anat. 213, 531–538 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagao M., Cheong C. W., Olsen B. R., Col2-Cre and tamoxifen-inducible Col2-CreER target different cell populations in the knee joint. Osteoarthritis Cartilage 24, 188–191 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henry S. P., et al. , Generation of aggrecan-CreERT2 knockin mice for inducible Cre activity in adult cartilage. Genesis 47, 805–814 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulsatelli L., et al. , Increased serum vascular cell adhesion molecule (VCAM)-1 levels in patients with erosive hand osteoarthritis. Rheumatology (Oxford) 52, 400–402 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Mapp P. I., Walsh D. A., Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat. Rev. Rheumatol. 8, 390–398 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Bray R. C., et al. , Vascular response of the meniscus to injury: Effects of immobilization. J. Orthop. Res. 19, 384–390 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Ouyang W., et al. , Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature 491, 554–559 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pauli C., et al. , Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage 19, 1132–1141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwok J., et al. , Histopathological analyses of murine menisci: Implications for joint aging and osteoarthritis. Osteoarthritis Cartilage 24, 709–718 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kambic H. E., McDevitt C. A., Spatial organization of types I and II collagen in the canine meniscus. J. Orthop. Res. 23, 142–149 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Gamer L. W., Xiang L., Rosen V., Formation and maturation of the murine meniscus. J. Orthop. Res. 35, 1683–1689 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Chambers M. G., Kuffner T., Cowan S. K., Cheah K. S., Mason R. M., Expression of collagen and aggrecan genes in normal and osteoarthritic murine knee joints. Osteoarthritis Cartilage 10, 51–61 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Meckes J. K., et al. , Compromised autophagy precedes meniscus degeneration and cartilage damage in mice. Osteoarthritis Cartilage 25, 1880–1889 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jay G. D., Waller K. A., The biology of lubricin: Near frictionless joint motion. Matrix Biol. 39, 17–24 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Sun Y., Mauerhan D. R., Meniscal calcification, pathogenesis and implications. Curr. Opin. Rheumatol. 24, 152–157 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Verdonk R., et al. , The role of meniscal tissue in joint protection in early osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 24, 1763–1774 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Rongen J. J., Hannink G., van Tienen T. G., van Luijk J., Hooijmans C. R., The protective effect of meniscus allograft transplantation on articular cartilage: A systematic review of animal studies. Osteoarthritis Cartilage 23, 1242–1253 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Hellio Le Graverand M. P., et al. , Matrix metalloproteinase-13 expression in rabbit knee joint connective tissues: Influence of maturation and response to injury. Matrix Biol. 19, 431–441 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Mankin H. J., Dorfman H., Lippiello L., Zarins A., Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Joint Surg. Am. 53, 523–537 (1971). [PubMed] [Google Scholar]
- 47.Pritzker K. P., et al. , Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthritis Cartilage 14, 13–29 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Outerbridge R. E., The etiology of chondromalacia patellae. J. Bone Joint Surg. Br. 43-B, 752–757 (1961). [DOI] [PubMed] [Google Scholar]
- 49.Fisch K. M., et al. , Identification of transcription factors responsible for dysregulated networks in human osteoarthritis cartilage by global gene expression analysis. Osteoarthritis Cartilage 26, 1531–1538 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benjamini Y., Drai D., Elmer G., Kafkafi N., Golani I., Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 125, 279–284 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Paik J. H., et al. , FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128, 309–323 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salminen H., Vuorio E., Säämänen A. M., Expression of Sox9 and type IIA procollagen during attempted repair of articular cartilage damage in a transgenic mouse model of osteoarthritis. Arthritis Rheum. 44, 947–955 (2001). [DOI] [PubMed] [Google Scholar]
- 53.Smith S. M., Shu C., Melrose J., Comparative immunolocalisation of perlecan with collagen II and aggrecan in human foetal, newborn and adult ovine joint tissues demonstrates perlecan as an early developmental chondrogenic marker. Histochem. Cell Biol. 134, 251–263 (2010). [DOI] [PubMed] [Google Scholar]
- 54.Lu Y., et al. , Col10a1-Runx2 transgenic mice with delayed chondrocyte maturation are less susceptible to developing osteoarthritis. Am. J. Transl. Res. 6, 736–745 (2014). [PMC free article] [PubMed] [Google Scholar]
- 55.Glasson S. S., Chambers M. G., Van Den Berg W. B., Little C. B., The OARSI histopathology initiative–Recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage 18 (suppl. 3), S17–S23 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the conclusions of the manuscript are included in SI Appendix. Any additional data are available upon request.