Significance
Odor detection in mammals depends on the largest family of G protein-coupled receptors, the odorant receptors, which represent ∼2% of our protein-coding genes. The vast majority of odorant receptors are trapped within the cell when expressed in nonolfactory cells. The underlying causes of why odorant receptors cannot be functionally expressed in nonolfactory cells have remained enigmatic for over 20 years. Our study points to divergence from a consensus sequence as a key factor in a receptor’s inability to function in nonolfactory cells, which, in turn, helps explain odorant receptors’ exceptional functional diversity and rapid evolution. We also show the success of protein engineering strategies for promoting odorant receptor cell surface expression.
Keywords: GPCR, protein trafficking, olfaction
Abstract
Mammalian odorant receptors are a diverse and rapidly evolving set of G protein-coupled receptors expressed in olfactory cilia membranes. Most odorant receptors show little to no cell surface expression in nonolfactory cells due to endoplasmic reticulum retention, which has slowed down biochemical studies. Here we provide evidence that structural instability and divergence from conserved residues of individual odorant receptors underlie intracellular retention using a combination of large-scale screening of odorant receptors cell surface expression in heterologous cells, point mutations, structural modeling, and machine learning techniques. We demonstrate the importance of conserved residues by synthesizing consensus odorant receptors that show high levels of cell surface expression similar to conventional G protein-coupled receptors. Furthermore, we associate in silico structural instability with poor cell surface expression using molecular dynamics simulations. We propose an enhanced evolutionary capacitance of olfactory sensory neurons that enable the functional expression of odorant receptors with cryptic mutations.
Mammalian olfactory receptors (ORs) are the largest and most diverse family of G protein-coupled receptors (GPCRs) (1, 2). ORs are expressed on the cell surface of olfactory sensory neurons (OSNs) to detect and discriminate the vast number of odors in the environment (3, 4). ORs are rapidly evolving with gene duplications and deletions, in addition to functional modifications between homologs presumably for species-specific environmental adaptation (2, 5, 6). ORs are usually retained in the endoplasmic reticulum (ER) when expressed alone in nonolfactory cells, including neurons (7–14).
Receptor transporting protein (RTP) 1 and RTP2, which are proposed to act as chaperones in the OSNs, enhance the cell surface expression of many ORs when cotransfected in heterologous cells (15–20). In RTP1 and RTP2 double knockout mice (RTP DKO), the majority of ORs are significantly underrepresented (uORs) due to the absence of mature OSNs expressing them, suggesting that these ORs require RTP1 and RTP2 in order to function (21). Interestingly, a small subset of ORs is overrepresented (oORs), suggesting that a minor subset of ORs function without RTP1 and RTP2. Accordingly, some oORs show cell surface expression when expressed without RTPs in heterologous cells (21).
The underlying causes of OR retention in the ER in cells other than mature OSNs as well as how RTP1 and RTP2 promote OR trafficking are not well understood. Adding export signals or making OR chimeras with canonical GPCRs have been shown to enhance functional expression of some ORs. However, previous structure-functional analysis using model ORs based on the assumption of OR-specific ER retention signals did not identify common residues that are involved in the cell surface expression of ORs (9, 22, 23).
In this study, we approach the mechanistic understanding of OR trafficking with the goals of identifying specific residues underlying ER retention and, using this knowledge, engineering ORs with increased expression in heterologous cells similar to that of nonolfactory GPCRs. To achieve these goals, we have used interdisciplinary strategies. First, we used a pair of closely related ORs that show differential cell surface expression in heterologous cells to identify specific amino acid residues that influence cell surface expression. We performed molecular dynamics (MD) simulations on a set of ORs and mutants with differential cell surface expression to estimate protein stability and its possible relationship to expression. Second, we conducted a large-scale analysis of the cell surface expression of 210 ORs. We used the dataset to identify critical residues from which we built a machine-learning model to predict cell surface expression. Third, we synthesized ORs based on insights from the model to demonstrate the role of conserved residues in OR trafficking. Fourth, stabilization strategies commonly used on GPCRs and other proteins (24–27) were applied to ORs. We improved the stability of the most promising consensus ORs by inserting salt bridges in their structure and obtained mutated consensus ORs that show surface expression levels comparable to a canonical GPCR. Together, our data suggest that divergence from conserved residues results in the retention of ORs inside the cells, which may be caused by structural instability. We hypothesize that an enhanced evolutionary capacitance in the OSNs with olfactory-specific chaperones would enable rapid functional evolution of ORs (28–32).
Results
A TM4 Residue, G4.53, Is Crucial for Cell Surface Trafficking of Model ORs.
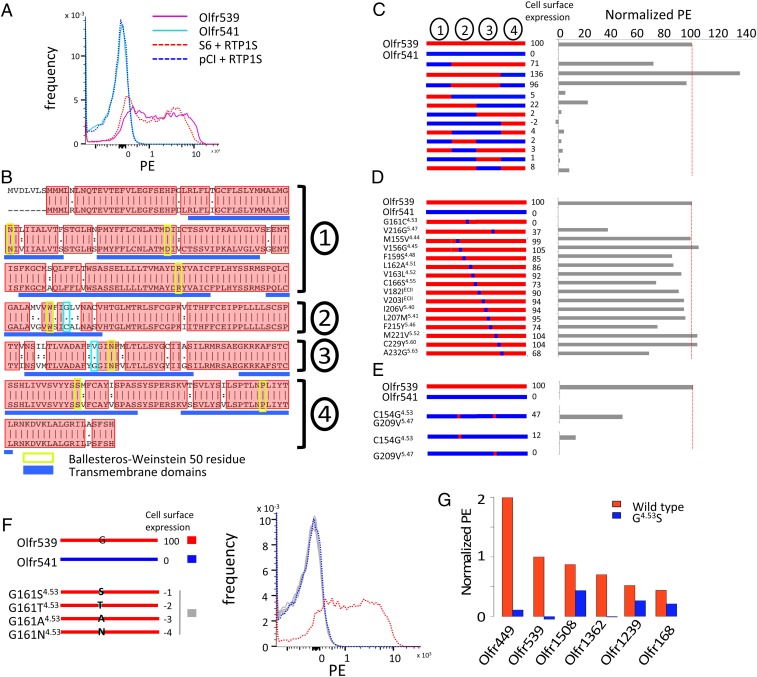
All OR cell surface expressions have been evaluated by flow cytometry (Methods). We chose Olfr539 and Olfr541 with 90% amino acid identity as a model system to study OR trafficking. Olfr539 is an oOR, an indication of high representation in the absence of RTP1 and RTP2 in vivo, that shows robust cell surface expression in human embryonic kidney (HEK) 293T cells. In contrast, Olfr541 is a uOR, which requires RTP1 and RTP2 for its cell surface expression in vivo, that shows no detectable cell surface expression in HEK293T cells (Fig. 1 A and B). We generated a series of chimeric ORs by intermingling parts of the amino acid sequence of Olfr539 with that of Olfr541 (Fig. 1C). Cell surface expression levels of the chimeric ORs were measured by flow cytometry. Mutant ORs which had the middle region (from 152th residue to 247th residue) of Olfr539 showed high surface expression levels, indicating an important domain for cell surface expression.
Fig. 1.
G1614.53 and V2165.47 are critical to the cell surface expression of Olfr539 and Olfr541. (A) Olfr539 robustly but Olfr541 poorly expresses in the cell surface in heterologous cells in the absence of RTP1 and RTP2. The expression is evaluated by flow cytometry with OR S6 and pCI as positive and negative controls, respectively, by recording the frequency of PE fluorescence (Methods). (B) Alignment of protein sequences of Olfr539 (Top) and Olfr541 (Bottom). Ninety percent of the amino acid residues are shared between the two ORs and are shown in red boxes. Ballesteros-Weinstein 50 residue for each TM domain is boxed in green (33). G/C1614.53 and G/V2165.47 are highlighted in cyan boxes. (C–F) (Left) Designs of chimeric ORs and (Right) cell surface expression results (normalized PE). (C) Chimeric ORs created by replacing parts of Olfr539 (red) with those of Olfr541 (blue). (D) Single amino acid mutants created by substituting amino acids of Olfr539 in regions 2 and 3 for those of Olfr541. (E) Reciprocal Olfr541 mutants created by substituting single or double amino acids of Olfr541 with those of Olfr539. (F) Olfr539 mutants created by substituting G1614.53 with S, T, A, or N, which are altogether conserved in almost 30% of the mouse ORs, lose cell surface expression. (G) G4.53S mutants of RTP-independent ORs (Olfr449, Olfr539, Olfr1508, Olfr1362, Olfr1239, and Olfr168) (blue) show less cell surface expression levels compared with the WT (red) in the absence of RTP1 and RTP2.
We next generated single amino acid mutants of each middle region residue of Olfr539 by substituting each amino acid of Olfr539 with that of Olfr541 (Fig. 1D). Olfr539 G161C4.53 [4.53 refers to the Ballesteros-Weinstein residue numbering system used for GPCRs (33); Fig. 1B] and V216G5.47 showed abolished or diminished Olfr539 cell surface expression. Accordingly, we expressed the reciprocal single mutants Olfr541 C154G4.53 and Olfr541 G209V5.47 and the double mutant Olfr541 C154G4.53/G209V5.47 to test if these residues are sufficient for promoting cell surface expression. Olfr541 C154G4.53 showed a moderate level of cell surface expression, although Olfr541 G209V5.47 did not show any improvements (Fig. 1E). Olfr541 C154G4.53/G209V5.47 showed a more enhanced cell surface expression than C154G4.53 alone, suggesting a synergistic interaction between the two critical residues in facilitating cell surface trafficking. This indicated that these two residues are critical in determining cell surface expression levels. Other residues had little or no effect on cell surface expression.
We further investigated which properties of G4.53 were controlling the cell surface trafficking. We generated mutants by substituting G1614.53 of Olfr539 for less frequently used amino acids (S, T, A, or N), which were altogether present in almost 30% of mouse ORs (Fig. 1F). None of these mutants exhibited cell surface expression, indicating a requirement of G4.53 for the cell surface trafficking of Olfr539. We next tested whether G4.53 controlled the trafficking of other ORs that are trafficked to the cell surface. We chose five ORs (Olfr1362, Olfr1508, Olfr449, Olfr168, and Olfr1239) that show cell surface expression in HEK293T cells (RTP-independent ORs; see below for details). All of the G4.53S mutants showed abolished or diminished cell surface expression (Fig. 1G) supporting the importance of this residue in facilitating cell surface trafficking of ORs.
Contribution of G4.53 in the in Silico Structural Stability of Olfr539 and Olfr541.
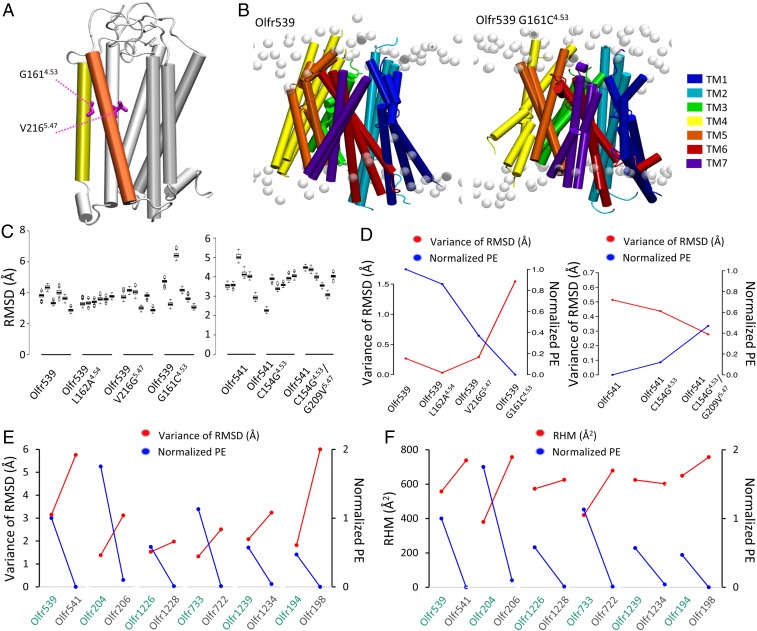
In order to understand how the two critical residues, G4.53 and V5.47, influence the cell surface trafficking of Olfr539 and Olfr541, we localized the two residues in 3D structural models of Olfr539 and Olfr541. OR models were constructed using a homology modeling method based on known GPCR experimental structures in the inactive state (34, 35). G4.53 and V5.47 are in the middle of the fourth and the fifth transmembrane (TM) domains, respectively (Fig. 2A). Following the structural model, G4.53 is unlikely to directly interact with odorants as TM4 is not involved in the binding cavity. However, V5.47 is part of TM5 and located at the cradle of the binding site. To evaluate if our mutations change the selectivity of Olfr539, we performed an activation screening on Olfr539 wild type (WT) and G4.53 (C, S, T, A, and N) and V5.47 (G) mutants against 22 odorants (SI Appendix, Fig. S1). We saw that if the receptor responds to any of the tested odorants, the response profiles of the mutated receptors are similar to those of the WT Olfr539, suggesting that the selectivity of the receptor is not grossly changed, and the residues 4.53 and 5.47 were not directly involved in odorant binding.
Fig. 2.
In silico structural stability of ORs correlates with the cell surface expression level. (A) A 3D homology model of Olfr539. TM4 (yellow) and TM5 (orange) are represented in colored tubes, and the remaining structure is in white. G1544.53 and V2095.47 are developed in pink licorice. (B) Superposed images of triplicate WT (Left) Olfr539 and (Right) Olfr539 G161C4.53 models after 500 ns MD simulations in an explicit model of plasma membrane (white). (C) RMSDs of six individual MD simulations of (Left) Olfr539 systems (WT, L162A4.54 G161C4.53, and V216G5.47) and (Right) Olfr541 systems (WT, C154G4.53, and C154G4.53/G209V5.47). The models are placed in descending order based on cell surface expression levels for Olfr539 systems and in ascending order for Olfr541 systems. (D) Plots of the variance of mean RMSDs (left axis, red plots) and the cell surface expression levels (right axis, blue plots) of (Left) Olfr539 systems and (Right) Olfr541 systems. (E) Plots of the variance of RMSDs (left axis, red plots) and the cell surface expression levels (right axis, blue plots) of Olfr pairs sharing a high sequence identity but showing different cell surface expression levels (high in green, low in gray). (F) Plots of the RHM (left axis, blue plots) and the cell surface expression levels (right axis, blue plots) of Olfr pairs sharing a high sequence identity but showing different cell surface expression levels (high in green, low in gray).
We hypothesized that diminished structural stability of ORs might cause ER retention due to general quality control mechanisms and that G4.53 and V5.47 are important for the stabilization of ORs. To test this, we built Olfr539, Olfr541, Olfr539 G161C4.53, Olfr539 V216G5.47, Olfr541 C154G4.53, and Olfr541 C154G4.53/G209V5.47 homology models. We also included a control mutant Olfr539 L162A4.54 that shows a similar cell surface expression to Olfr539. We ran six independent MD simulations of 500 ns for all of the systems embedded in an explicit lipid bilayer. All six simulations of Olfr539 showed well-packed structures, while Olfr539 G161C4.53 showed flexibility (Fig. 2 B and C) as quantified by the root mean square deviations (RMSDs) of atomic positions of TM domains for each MD simulation. Olfr539 and L162A4.54 showed similar RMSD across multiple simulations, all converging to equivalent equilibrated structures. However, Olfr539G161C4.53 and V216G5.47, which are poorly expressed, showed variability in structures. This suggests that poorly trafficked ORs have more flexible structures and are unstable when inserted into a cell membrane model (Fig. 2C and SI Appendix, Fig. S2). In contrast, Olfr541 showed a wide range of RMSD, whereas its mutants C154G4.53 and C154G/G209V showed less variation in RMSD (Fig. 2C). To further examine whether structural stability is associated with cell surface expression level, we plotted the cell surface expression levels and variances of RMSDs for both Olfr539 and Olfr541 systems. Indeed, the mutants with lower cell surface expression showed a larger variance of RMSDs. Conversely, the mutants with higher cell surface expression showed a smaller variance of RMSDs (Fig. 2D). This trend is more pronounced in RMSDs calculated for the extracellular (EC) side of the TM domains, suggesting that stabilities of this part may be especially important for cell surface expression (SI Appendix, Fig. S3). We also plotted the cell surface expression levels and variances of RSMD for five other OR pairs that show a high percentage of sequence identity but different cell surface expression levels (Fig. 2E and SI Appendix, Fig. S3). We found again a clear anticorrelation between the variance of RMSD and cell surface expression. To evaluate further the structural correlation with cell surface expression between these pairs, we calculated the residual hydrophobic mismatch (RHM) between the OR and the membrane during our MD simulations (Fig. 2F). We found that five out of six OR pairs show an anticorrelation between RHM and the cell surface expression. Further, we identified a linear correlation between differences of RHM and differences of cell surface expression in each pair (SI Appendix, Fig. S3; R2 = 0.9035). All together, these data support the hypothesis that structural stability contributes to the cell surface expression of ORs.
A Comprehensive Evaluation of Cell Surface Expression Levels of ORs in Heterologous Cells.
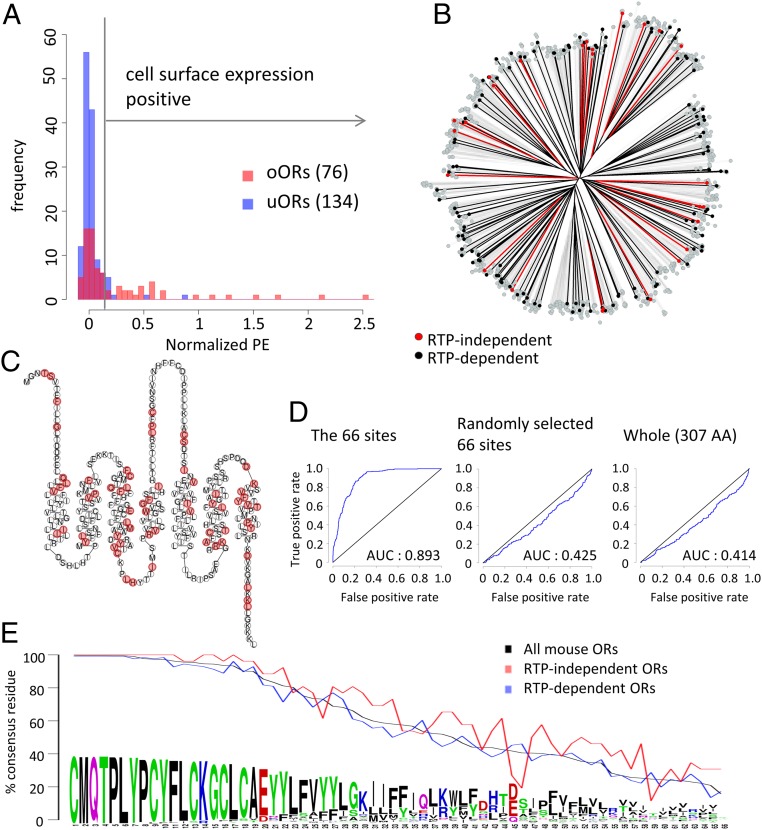
So far, we showed that the TM4 residue at the position 4.53 plays a crucial role in OR trafficking. However, it is unlikely that this position is the sole determinant, as G4.53 is present in 66% of mouse ORs, yet the vast majority of ORs do not show cell surface expression in heterologous cells. To gain a more comprehensive understanding of OR cell surface expression, we selected 210 ORs (76 oORs and 134 uORs in vivo) and tested which ORs exhibit cell surface expression in heterologous cells. Consistent with previous findings (21), oORs, as a group, showed more robust OR surface expression than uORs (P < 0.05, u test) (Fig. 3A). Using the approximation that surface expression levels are the overlap of two normal distributions of positive ORs and negative ORs, we defined the ORs with expression levels of more than 0.144 (A.U.) as positive ORs (cutoff is the top 0.001 of negative ORs). Under this criterion, 34/210 ORs (14.0%) were positive. Consistent with the more robust cell surface expression of oORs, 26/76 (34.2%) of oORs and only 8/134 (6.0%) of uORs showed positive cell surface expression. We defined the 26 ORs that are oORs and cell surface expression positive as RTP-independent ORs and the 126 ORs that are uORs and cell surface expression negative as RTP-dependent ORs.
Fig. 3.
Cell surface expression analyses for a large repertoire of mouse ORs in heterologous cells in the absence of RTP1 and RTP2. (A) Histogram of cell surface expression levels of 76 oORs (red) and 134 uORs (blue). The vertical line at 0.144 is a cutoff for positive/negative cell surface expression. (B) Phylogenetic tree of protein sequences of mouse ORs. Red, black, and gray indicate RTP-independent ORs, RTP-dependent ORs, and the other ORs, respectively. (C) Snake plot of the consensus protein sequence of mouse ORs. The 66 sites with less diverse residues in RTP-independent ORs (P < 0.05, Bonferroni corrected) are colored in red. (D) RTP dependence of tested ORs is predicted using SVM-based classifiers based on amino acid properties in 10-fold cross-validation, and the accuracy is validated by the ROC curves. The SVM-based classifier has been built using the 66 sites of interest, 66 random amino acid positions, and the entire 307 amino acid positions in the tested ORs. (E) Usage rate of consensus residues at the 66 sites. RTP-independent ORs more frequently use consensus residues than RTP-dependent ORs at 58 out of 66 sites.
Critical Residues Predict Cell Surface Expression of ORs.
We hypothesized that residues specific to RTP-independent or RTP-dependent ORs are critical for OR trafficking in the absence of RTP1 and RTP2. To investigate whether overall amino acid sequence similarities are associated with cell surface trafficking, we asked whether RTP-independent and/or RTP-dependent ORs are clustered in a phylogenetic tree (Fig. 3B and SI Appendix, Fig. S4). Both are distributed on multiple branches, indicating that overall similarities do not determine their ability to be trafficked to the cell surface.
Next, we hypothesized that amino acid residues at specific positions create a network that controls cell surface trafficking. To investigate this idea, we aligned 26 RTP-independent and 126 RTP-dependent ORs (total of 152 ORs) and calculated Grantham distances (36) of amino acid properties at individual sites. We identified 66 positions with lower Grantham distances between amino acids for RTP-independent ORs than for all of the 152 tested ORs (cutoff P < 0.05, t test with Bonferroni correction). As expected, the position 4.53 is one of these 66 sites; 80.8% of RTP-independent ORs possess a G residue at this position against only 61.1% in the RTP-dependent ORs.
Contrary to the initial assumption that specific domains control OR cell surface expression, the 66 sites were scattered throughout the OR sequence. Moreover, there was no specific site that was exclusively present in one of the groups, suggesting that there are no trafficking promotion or inhibition signals that are shared among all ORs (Fig. 3C). To investigate whether these 66 sites can predict the RTP dependence of ORs, we classified tested ORs by support-vector machine-based classifiers in 10-fold cross-validation. The support vector machine model generated by the 66 sites of amino acid residues discriminated RTP-independent ORs (P = 1.70 × 10−92, Wilcoxon signed rank test; area under the curve [AUC] = 0.893). However, those generated by the 66 randomly selected sites (P = 0.999, Wilcoxon signed rank test; AUC = 0.425) and those generated by all sites (P = 0.999, Wilcoxon signed rank test; AUC = 0.414) failed to discriminate RTP-independent ORs. This demonstrates that these 66 sites robustly predict whether an OR shows cell surface expression in heterologous cells (Fig. 3D).
What properties of these 66 residues are associated with cell surface expression? When we looked at the degree of conservation of these residues, many of these sites were conserved among ORs (Fig. 3E and SI Appendix, Fig. S5). Twenty-nine percent of the sites (19/66) are conserved in more than 90% of the mouse ORs, whereas only 13% of all amino acid sites (41/307) are conserved in more than 90% of full-length ORs (P = 0.0048, Fisher’s exact test). RTP-independent ORs have the most common amino acid residues much more frequently present than RTP-dependent ORs (58 out of the 66 sites, P = 6.35 × 10−6, χ2 test), suggesting that ORs that are in line with consensus amino acids in these positions are more likely to show cell surface expression.
Engineered Consensus ORs Robustly Express on the Cell Surface in Heterologous Cells.
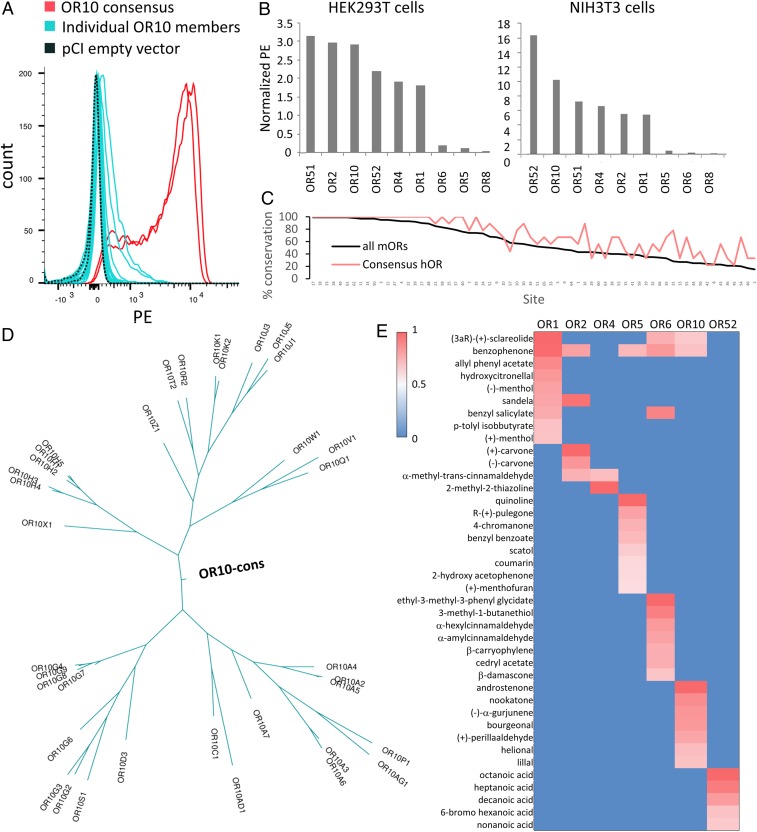
The above results suggest the importance of the most frequently occurring amino acid at a given site in cell surface expression. This observation led us to predict that ORs that are designed based on consensus amino acids for each site would be efficiently trafficked to the cell surface. The consensus strategy has already been applied to proteins or codons to improve their thermostability or function in other proteins (24, 25, 37, 38). The success of this strategy relies on the number of proteins available to build the consensus sequence and their sequence similarity (24, 25). Here we used the unique diversity of the OR family among GPCRs to apply the consensus strategy, aiming to obtain stable ORs. We aligned amino acid sequences of human OR families and determined the consensus sequences as the most frequently occurring amino acid residue at each position (supplementary dataset, ref. 39). We first chose the OR10 family to measure the cell surface expression levels of the consensus OR in HEK293T cells in comparison with each member of the OR10 family. Strikingly, the consensus OR10 robustly expressed on the cell surface, more than any of the individual OR10 family members tested (Fig. 4A). We generated consensus human ORs for eight other families (OR1, OR2, OR4, OR5, OR6, OR8, OR51, and OR52) and measured their cell surface expression levels in the absence of RTPs. Six out of nine consensus ORs show more robust cell surface expression than any tested natural ORs (Fig. 4B).
Fig. 4.
Potential of consensus ORs for robust cell surface expression in the absence of RTP1S. (A) Thirty-two human OR10 subfamily members (cyan) and the consensus OR (red, duplicate) were transfected into HEK293T cells, and their cell surface expression levels were measured by flow cytometry. (B) Cell surface expression levels of 9 consensus ORs (OR1, OR2, OR4, OR5, OR6, OR8, OR10, OR51, and OR52) were evaluated in HEK293T or NIH/3T3 cells. The PE fluorescence is normalized by setting Olfr539 response to 1 and Olfr541 to 0. (C) Usage rate of consensus residues at the 66 sites for consensus human ORs (consensus hOR; red) and all mouse ORs (black). (D) Ancestral tree of OR10 family member including OR10 consensus. (E) Heat map of ORs’ responses to 50 µM of odorants selected from a previously screened panel of 320 compounds. Luciferase activity was normalized for each OR by setting 1.0 as the highest response value and 0 as the lowest response value. Consensus OR1, OR2, OR4, OR5, OR6, OR10, and OR52 are functionally expressed in Hana3A cells.
To exclude the possibility that this effect was specific only to HEK293T cells, we expressed the consensus ORs in NIH 3T3 cells, which are derived from mouse fibroblasts. Again, most of the consensus ORs showed robust cell surface expression (Fig. 4B). We evaluated the level of conservation of the 66 amino acid sites that we showed to be important for RTP-independent OR expression between our consensus ORs and the mouse OR repertoire (Fig. 3E). Consensus ORs have the most common amino acid residues much more frequently represented than natural ORs (59 out of the 66 sites, P = 1.24 × 10−9, Wilcoxon signed rank test) (Fig. 4C). We built phylogenetic trees using parsimony criterion for each OR family including the corresponding consensus OR and found that the consensus OR is always located at the origin of the tree (Fig. 4D and SI Appendix, Fig. S6).
Finally, we verified that the consensus receptors are functional in their response to odorants (19). We used cAMP-mediated luciferase reporter gene assay (16) to screen active ligands from a set of 320 diverse odorants at 50 μM. We identified robust ligands for OR1, OR2, OR4, OR5, OR6, OR10, and OR52, each of which shows responses to specific subsets of the tested odorants (Fig. 4E). Our data show that these consensus ORs are indeed functional, suggesting a proper folding.
Stabilization of Consensus ORs Structure by Introduction of Salt Bridges.
The consensus ORs already show a clear increase in cell surface expression in comparison to naturally occurring ORs. Since we observed that the expression of an OR seems to be correlated with its stability and rigidity when inserted in a membrane using MD simulations (Fig. 2 B–D), we attempted to further improve the expression level of the consensus ORs by engineering salt bridges in the structures guided by 3D homology models (Fig. 5A). Thermostabilizing studies on β1-adrenergic receptor (40) and chemokine receptor (26) showed that tightening of interactions between intracellular loop 1 (ICL1) and the helix 8 improved the GPCR stabilization. Another study (41) showed that enriching the number of basic residues in the ICL of an OR enhances its expression. Based on these premises, we inserted triple Arg mutations in ICL1 and a negatively charged residue in helix 8 to promote salt bridge interactions between ICL1 and helix 8 in four human consensus ORs, namely, OR1, OR10, OR51, and OR52 (Fig. 5B). We evaluated the cell surface expression of the consensus ORs with their corresponding mutants, including a well-studied nonolfactory class A GPCR, the muscarinic 3 receptor (M3), as a positive control for high expression (Fig. 5C). We observed enhanced expression of OR10 and OR52 mutants that are comparable to the expression level of the M3 receptor, even when we decreased the amount of DNA used in transfection by 100-fold. However, we did not observe any enhancement in the expression level of OR1 and OR51 mutants. The insertion of such stabilizing interactions might improve the rigidity of the OR structure that seems to aid expression. We tested their functionality in comparison with their corresponding consensus OR (Fig. 5D). Mut-OR10 and Mut-OR52 both responded to their agonists (androstenone and nonanoic acid, respectively). Mut-OR52 showed activity similar to the consensus OR52, but the response of Mut-OR10 was diminished compared to OR10. We optimized the luciferase assay protocol to these unusually highly expressed ORs by testing different DNA concentrations for cell transfection. For both OR10/Mut-OR10 and OR52/Mut-OR52, decreasing the amount of transfected DNA by 10- to 100-fold compared with the optimized amount for natural ORs resulted in robust responses against tested odorants, showing the capacity of these consensus ORs in supporting high levels of functional expression.
Fig. 5.
Improvement of OR expression by mutations in TM1 and helix 8. (A) Homology model of OR10. Each TM is highlighted in a colored tube, and residues D511.60, S521.61, H531.62, and E294H8 are represented in licorice (pink). (B) Zoom on the residues 1.60, 1.61, 1.62, H8 mutated for OR1 (red), OR10 (blue), OR51 (green), and OR52 (pink). Ionic interactions between the residues are shown by dotted lines. (C) Expression analysis of OR1, OR10, OR51, OR52, and their mutants and the muscarinic receptor 3 (M3) at 1, 10, and 100 pg/µL of DNA in the transfection mix. (D) Dose–response curve of OR10 and OR52 and their mutants at different DNA concentration (from 0.001 to 100 in pg/µL of transfection mix) to androstenone and nonanoic acid, respectively. The y axis represents the luciferase luminescence normalized to the basal activity of each DNA concentration.
Discussion
The mammalian OR family is a unique protein family with its large size, rapid evolution, and poor expression in heterologous cells, which makes their function notoriously difficult to study. Here we investigated the underlying mechanisms by which OR trafficking is regulated in heterologous cells. Using chimeras and point mutations with a pair of highly similar ORs, we identified critical residues that regulate cell surface expression. MD simulations suggest that these residues may affect the flexibility and stability of ORs. We also conducted a large-scale cell-based screening to comprehensively identify ORs that are expressed on the cell surface, leading to the identification of specific residues associated with their cell surface expression. Cell surface expression-positive ORs tend to have conserved residues at the critical sites. We investigated if consensus ORs express on the cell surface in heterologous cells and demonstrated that most of the consensus ORs show robust cell surface expression.
Which residues or domains make OR trafficking difficult? Previous studies suggest different residues, domains, or features of ORs are responsible for intracellular retention (9, 23). Among them, a study proposed that fewer charged residues and more hydrophobic residues distributed throughout ORs might underlie intracellular retention (22). We tested whether RTP-independent ORs possess fewer charged and more hydrophobic residues compared to RTP-dependent ORs, as suggested by Bubnell et al. (22). We found no significant differences between tested ORs (P = 0.74 and P = 0.71), suggesting that these features do not explain the cell surface trafficking of ORs as a group. Our current study identified two sites (G4.53 and V5.47) contributing to cell surface expression in model ORs. In nonolfactory class A GPCRs, position 4.53 is conserved as S39%, A33%, V8%, T4%, C3.5%, P3%, G3%, I2%, and M1.5%, and position 5.47 is conserved as F65%, Y11%, L10%, N3.5%, V3%, G2%, I2%, and C2%. Position 4.53, as well as position 2.47, has been identified as a conserved packing cluster center in class A GPCRs, and its mutation in leucine (A4.53L) in Rhodopsin disrupts the structure of the receptor (42). In the β2-adrenergic receptor, S4.53 belong to the motif S4.53xxxS4.57 which does not participate to the functionality of the receptor and seems to be more involved in helix packing, maintaining the stability of the receptor (43). The position 5.47 appears to play a role in cannabinoid receptor (CB1R) stabilization as the crystal structure reported (Protein Data Bank [PDB] ID 5TGZ) containing T2103.46A+E2735.37K+T2835.47V+R3406.32E mutations shows enhanced protein homogeneity and thermostability (44). However, these residues were not identified in previous studies on ORs; it seems that different residues regulate the trafficking of different ORs. This raised a question of whether there are any common mechanisms that regulate OR trafficking. Our statistical analysis based on a large-scale cell surface expression analysis of hundreds of ORs identified 66 residues scattered throughout the receptor that play a critical role in OR cell surface expression. Although there was no single residue or domain that solely determined cell surface expression, we succeeded in building a machine learning model that reliably predicts OR cell surface expression based on these residues. This suggests that these 66 residues differentially contribute to the trafficking efficiency of individual ORs.
What features of ORs cause their ER retention when expressed in nonolfactory cells? It was long hypothesized that ORs may possess specific and conserved ER retention signals (8, 9, 15, 22). However, a previous report shows that mutating the most highly conserved OR-specific amino acids into one of a nonolfactory GPCR that shows high cell surface expression did not enhance the trafficking of a model OR (22). Our data are also inconsistent with the idea that conserved OR-specific residues or motifs cause ER retention. In contrast, our study demonstrated that consensus ORs show robust cell surface expression, suggesting that such ER retention signals, if any, would not be shared by OR members. How do ORs (aside from a minor subset) possess the common feature of being retained inside the cells? Our study supports the model that the intracellular retention of ORs is caused by the structural instability of ORs, which is caused by divergence from conserved residues at the critical sites. In other words, the majority of ORs do not fold correctly in heterologous cells due to a divergence from conserved residues; thus, they are trapped by the general protein quality control in the ER and cannot be trafficked to the plasma membrane. This model explains why many consensus ORs exhibit robust cell surface expression, while the vast majority of natural ORs do not show detectable cell surface expression in heterologous cells (45).
Why are ORs functional in OSNs but not in heterologous cells when expressed alone? Our study supports the idea that accessory proteins or chaperones expressed in the OSNs assist the folding of structurally unstable ORs (Fig. 6). In addition to RTP1 and RTP2, a previous study showed that an Hsp70 homolog enhanced the cell surface expression of an OR, suggesting the importance of chaperones for the cell surface expression of ORs (46). A possibility is that the unstable nature of ORs may be integral to proper OSN development (21, 47). Initiation of OR expression induces unfolded protein response (UPR) in developing OSNs, suggesting inefficient folding and ER accumulation of ORs in these cells (47, 48). RTP1 and RTP2 are induced by UPR signaling in developing OSNs, allowing OR proteins to exit the ER and down-regulate UPR (21, 47, 48). There is also the possibility that OSNs lack specific quality control proteins that are common in other cell types, allowing less stable ORs to be trafficked to the cell membrane where they are finally functional. This may resemble the case of the cystic fibrosis TM conductance regulator (CFTR) where a single amino acid deletion (ΔF508 CFTR) causes its ER retention to be regulated by, among others, calreticulin or the Hsp90 cochaperone Aha1. The down-regulation of these chaperones results in the functional plasma membrane expression of ΔF508 CFTR (49). Outside of the OSNs, ORs are found to be ectopically expressed in tissues as diverse as heart, gut, and testis (50, 51). These ectopic ORs are unlikely to need RTPs to be functionally expressed in many of these nonolfactory tissues where no or very low RTP1 and RTP2 expressions are detected. We looked at the nature of the 66 positions in a set of 4 mouse and 19 human ORs detected ectopically (SI Appendix, Fig. S7) (50, 51). The 66 positions are not significantly more conserved in ectopic ORs than all mouse ORs (38 out of the 66 sites, P = 0.398, Wilcoxon signed rank test). However, these results need to be taken with caution as the functional role of most of the ectopic ORs remains to be determined and their functional expression might depend on other types of chaperone yet to be discovered.
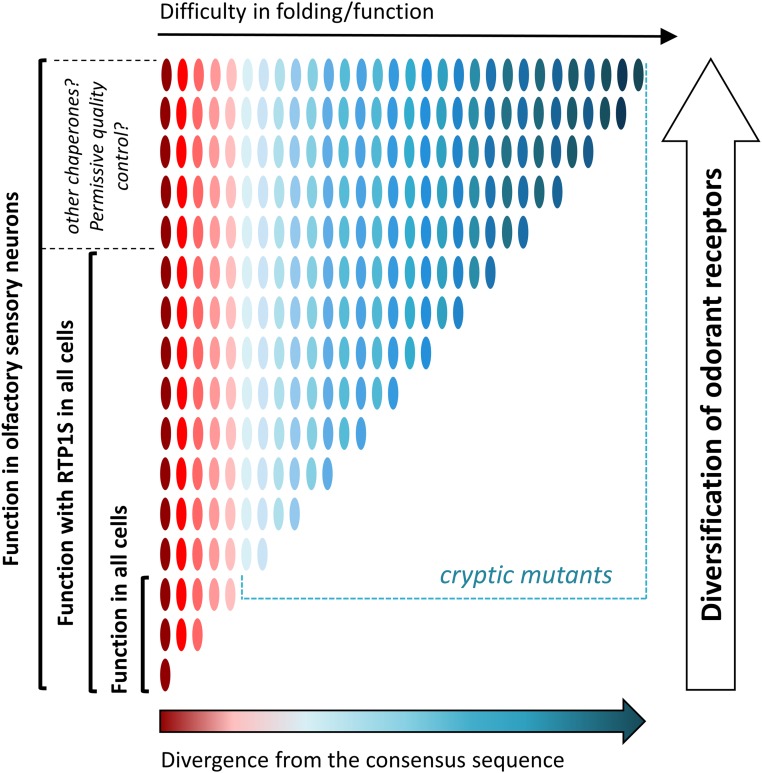
Fig. 6.
Proposed model. RTPs are evolutionary capacitors involved in enhanced diversification of ORs. OR genes are represented in oval shapes colored from dark red (ORs that are more aligned with the consensus) to dark blue (ORs that are more divergent from the consensus). Divergence of ORs from the consensus sequence results in difficulties in OR folding and function. Cryptic OR mutants are functional in the OSNs with RTPs and other capacitors. OR diversification and rapid evolution rely on the presence of olfactory-specific evolutionary capacitors.
Our study parallels with those conducted by Lindquist and others in deciphering the role of Hsp90, a chaperone that helps fold many proteins, as an evolutionary capacitor (Fig. 6) (29, 52, 53). Evolutionary capacitors, proteins that suppress deteriorating phenotypic variation under normal conditions, facilitate adaptation during evolution (31, 54). Our study suggests that RTP1, RTP2, and other chaperones yet to be discovered support functional expression of ORs that do not fold correctly in other cell types, conferring a unique capacity of the OSN. We speculate that olfactory-specific evolutionary capacitors play a role in the rapid evolution of ORs to facilitate sensory adaptation to detect and discriminate a vast number of odorants and natural odor mixtures (Fig. 6). Our model also implies that ORs in the ancestral species before expansion may resemble the consensus ORs; thus, they may not show similar levels of difficulties in cell surface expression in nonolfactory cells.
Last, despite some successes (10, 11), biochemical and structural studies of ORs have been challenging with the currently available expression systems due to the relatively poor expression of ORs when compared to canonical GPCRs. Our study shows the success of the application of protein engineering strategies to enhance OR stability and cell surface expression. We have identified a set of engineered ORs that show robust cell surface expression comparable to the M3 muscarinic acetylcholine receptor, a canonical GPCR. The consensus ORs can serve as candidate ORs for future biochemical studies such as the large-scale production of ORs and purification. In addition, the residues regulating trafficking identified in this study will point to further improvements in protein production, which will accelerate our effort toward determining OR structures. Altogether, our study provides insights into how various residues in ORs regulate the receptor expression on the cell membrane.
Methods
DNA and Vector Preparation.
ORFs of OR genes were subcloned into pCI (Promega) with a Rho tag at the N terminus. DNA fragments of OR genes were amplified by Phusion polymerase (Thermo Fisher Scientific). To generate chimeras and mutants of ORs, DNA fragments of OR genes were amplified by Phusion polymerase (Thermo Fisher Scientific). The fragments were mixed and amplified by PCR to obtain full sequences. All plasmid sequences were verified using Sanger sequencing (3100 Genetic Analyzer; Applied Biosystems).
Cell Culture.
HEK293T and Hana 3A cells (15) were grown in MEM containing 10% FBS (vol/vol) with penicillin-streptomycin and amphotericin B. Hana 3A cells were authenticated using polymorphic short tandem repeat (STR) at the Duke DNA Analysis Facility using GenePrint 10 (Promega) and shown to share profiles with the reference (ATCC). NIH/3T3 cells were grown in high-glucose Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% CS, penicillin-streptomycin, and amphotericin B. All cell lines were incubated at 37 °C, saturating humidity, and 5% CO2. No mycoplasma infection was detected in all cell cultures.
Flow Cytometry Analyses.
The principle of the method can be found in SI Appendix, Fig. S8. HEK293T cells were grown to confluency, resuspended, and seeded onto 35-mm plates at 25% confluency. The cells were cultured overnight. A Rho-tagged OR in the plasmid pCI and GFP expression vector were transfected using Lipofectamine 2000 (SI Appendix, Fig. S8 A and B). After 18 to 24 h, the cells were resuspended by cell stripper and then kept in 5 mL round bottom polystyrene (PS) tubes (Falcon 2052) on ice. The cells were spun down at 4 °C and resuspended in PBS containing 15 mM NaN3 and 2% FBS to wash the cell stripper. They were incubated in primary antibody [mouse anti Rho4D2 (55)] (SI Appendix, Fig. S8C) and then washed and stained with phycoerythrin (PE)-conjugated donkey anti-mouse F(ab′)2 Fragment antibody (Jackson Immunologicals: 715-116-150) (SI Appendix, Fig. S8D) in the dark. To stain dead cells, 7-Amino-actinomycin D (Calbiochem) was added (SI Appendix, Fig. S8E). The cells were analyzed using BD FACSCanto II FACS with gating allowing for GFP positive, single, spherical, viable cells (SI Appendix, Fig. S8F), and the measured PE fluorescence intensities were analyzed and visualized using Flowjo (56). We normalized the surface expression levels by cells expressing Olfr539, which was robustly expressed on the cell surface, and cells expressing Olfr541, which showed no detectable cell surface expression.
Homology Model Building.
The protocol follows a previously published method (57). Aligned protein sequences of mouse 1092 ORs are manually aligned to prealigned protein sequences of 11 GPCRs including bovine rhodopsin (PDB ID 1U19), human chemokine receptors CXCR4 (PDB ID 3ODU) and CXCR1 (PDB ID 2LNL), and human adenosine a2A receptor (PDB ID 2YDV) using Jalview (34).
Four experimental GPCR structures (1U19, 3ODU, 2YDV, and 2LNL) are used as templates to build Olfr539 and its mutants (G154C, V209G, and L155A) and Olfr541 and its mutants (C154G and C154G/G209V) by homology modeling with Modeler. Five models are obtained, and the one fulfilling several constraints (binding cavity sufficiently large, no large folded structure in extracellular loops, all TMs folded as α-helices, and a small α-helix structure between TM3 and TM4) is kept for further MD simulations.
MD Simulations.
Olfr539/Olfr541 and their mutants systems.
The models were embedded in a model membrane made up of POPC lipids solvated by TIP3P water molecules using Maestro. The total system is made up of ∼48,650 atoms in a periodic box of 91 × 89 × 98 Å3.
MD simulations are performed with sander and pmemd.cuda modules of AMBER12 with the ff03 force field for the protein and the gaff.lipid for the membrane. Hydrogen atoms are constrained by SHAKE algorithm and long-range electrostatic interactions are handled with particle mesh Ewald (PME). The cutoff for nonbonded interactions is set at 8 Å. Temperature and pressure are maintained constant with a Langevin thermostat with a collision frequency of 2 ps−1. In addition, a weak coupling anisotropic algorithm with a relaxation time of 1 ps−1 is applied. Snapshots are saved every 20 ps.
Two energy minimizations are performed during 10,000 steps with the 5,000 first steps using a conjugate gradient algorithm. The first one is run with a restraint of 200 kcal⋅mol−1 applied on all atoms of the membrane and water and the second one with the same restraint on all atoms of the receptor. This last constraint is kept for the heating phase of 20 ps (NTP, 100 to 310 K, Langevin thermostat with a collision frequency of 5 ps−1) and equilibration of 15 ns (NTP, 310 K). Restraints are then reduced by 5 kcal⋅mol−1⋅Å−2 and another cycle of minimization-equilibration is performed. The systems (Olfr539 models [WT, G154C, V209G, and L155A] and Olfr541 models [WT, C154G, and C154G/G209V]) are replicated six times, and 525-ns-long production MD are performed after an equilibration period of 50 ns. RMSDs of seven TM domains were calculated using CPPTRAJ in AmberTools. The RMSDs are between initial positions and each frame in the production step. The 3D structures were visualized using VMD.
Other Olfr pairs.
We used the Membrane Builder (58) utility of CHARMM-GUI (59) for embedding each of the receptors into a preequilibrated simulation box of a membrane composed by POPC lipids. Each of these protein–lipid complexes was solvated in explicit TIP3P water molecules in a dodecahedron box (approximate dimension of 7.80 nm × 7.80 nm × 10.68 nm) separately, and sodium and chloride counterions were added for maintaining the physiological salt concentration of each system at 150 mM. We used the software GROMACS (60) (version 2019.4) in combination with the all-atom CHARMM36 (61) force field for performing MD simulations at 310 K coupled to a temperature bath with a relaxation time of 0.1 ps (62). Pressure was calculated using molecular virial and held constantly by weak coupling to a pressure bath with a relaxation time of 0.5 ps. Each system was first subjected to a 5,000-step steepest descent energy minimization for removing bad contacts (63). Then, the systems were heated for 100 ps in steps of ramping up the temperature to 310 K under constant temperature–volume ensemble (NVT). Equilibrium bond length and geometry of water molecules were constrained using the SHAKE algorithm (64). We used a time step of 2 fs. The short-range electrostatic and van der Waals (VDW) interactions were estimated per time step using a charge group pair list with cutoff radius of 8 Å between the centers of geometry of the charged groups. Long-range VDW interactions were calculated using a cutoff of 14 Å, and long-range electrostatic interactions were treated using the PME method (65). Temperature was kept constant by applying the Nose-Hoover thermostat (66). Parrinello-Rahman barostat (67) with a pressure relaxation time of 2 ps was used for attaining the desired pressure for all simulations. The simulation trajectories were saved each 200 ps for analysis. The protein atoms were position restrained using a harmonic force constant of 1,000 kJ mol⋅−1⋅nm−2 during the NVT equilibration stage while the lipid and water molecules were allowed to repack around the protein. The system was further equilibrated at NPT by reducing the force constant on protein atoms from 5 kJ⋅mol−1⋅nm−2 to 0 in a stepwise manner for 3 ns each while having the pressure coupling on. We also performed an additional 10 ns of unrestrained simulation before starting the actual production run. This accounts for a total 25 ns of NPT equilibration prior to the production run. We performed three productions runs each 400 ns long starting from three independent sets of initial velocities for each system. Three independent simulations (each 400 ns long) were performed for each system.
Calculation of RMSD.
RMSD of a particular OR from its initial structure was computed using the gmx rms utility of GROMACS. For this, only the C-alpha atoms of the seven TM domains were considered, and the flexible loop regions were omitted. The variance in RMSD was calculated using the block averaging method as implemented in the gmx analyze utility.
Calculation of RHM.
For calculating the unfavorable hydrophobic interactions between protein and lipid, we took into account the TM hydrophobic residues not making sustained contacts (<20% of the total simulation time) with either membrane tail or head groups using the module gmx select along with -om utility. The RHM between receptor and membrane was expressed in terms of the cumulative solvent accessible surface area (SASA) of TM hydrophobic residues (Gly, Ala, Pro, Val, Met, Cys, Ile, Trp, Phe, Tyr, and Leucine) following the above criteria (68). Per residue SASA was calculated using the gmx sasa tool along with -res option as implemented in GROMACS.
OR Protein Sequence Analyses.
Protein sequences of 1,092 mouse ORs were aligned by Clustal Omega with default parameters. Conservation degree of amino acid residues was visualized by WebLogo (69).
To identify the amino acid residues involved in RTP dependence, Grantham distances (36) were calculated for all pairs of ORs at each position. Grantham distances consist of attributing distances numbers between two amino acids that are proportional to their evolutionary distance. The positions where Grantham distances of RTP-independent ORs were significantly shorter than those of all ORs were searched by one-sided t tests followed by Bonferroni correction.
Designing Consensus ORs.
Protein sequences of human ORs were downloaded from The Human Olfactory Data Explorer webpage (https://genome.weizmann.ac.il/horde/). The protein sequences were aligned using multiple alignment using fast Fourier transformation. The most frequently used amino acid residues were defined as consensus residues at each position. The consensus amino acid sequences were translated into DNA sequences using Codon Optimization Tool on Integrated DNA Technologies webpage.
Luciferase Assay in Hana3A Cells.
The Dual-Glo Luciferase Assay (Promega) was used to determine the activities of firefly and Renilla luciferase in Hana3A cells as previously described (57). Briefly, firefly luciferase, driven by a cAMP response element promoter (CRE-Luc; Stratagene), was used to determine OR activation levels. For each well of a 96-well plate, 5 ng SV40-RL, 10 ng CRE-Luc, 5 ng mouse RTP1s, 2.5 ng M3 receptor3, and 5 ng of Rho-tagged receptor plasmid DNA were transfected. Normalized activity for each well was further calculated as (Luc-400)/(Rluc-400), where Luc is luminescence of firefly luciferase and Rluc is Renilla luminescence. The basal activity of an OR was averaged from six wells in the absence of odorants and further corrected by subtracting that of the control empty vector. An odorant-induced activity was averaged from at least three wells and further corrected by subtracting the basal activity of that receptor. Odorant-induced responses were normalized to that of WT.
Data Availability Statement.
All data discussed in the paper are available in the manuscript or SI Appendix. The homology models used in this study and the supplementary dataset (39) are available as PDB format and table files, respectively, at https://doi.org/10.7924/r40867k2k (39).
Supplementary Material
Acknowledgments
We thank Mengjue Jessica Ni for expert technical assistance and Conan Juan, Sahar Kaleem, Aashutosh Vihani, Kevin Zhu, and Yen Dinh for manuscript editing. This work was supported by grants from NIH (DC014423 and DC016224 to H.M. and NIH R01-GM097261 to N.V.). M.H.N. received financial support from São Paulo Research Foundation (FAPESP grant no. 2017/00726-2). K.I. stayed at Duke University with financial support from Tokyo University of Agriculture and Technology as a student of the program for leading graduate schools in Japan. Y.F. stayed at Duke University with financial support from Japan Society for the Promotion of Science (JSPS) Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers.
Footnotes
Competing interest statement: K.I., C.A.d.M., M.H.N., and H.M. filed a provisional patent application relevant to this work. H.M. receives royalties from Chemcom. The remaining authors declare no competing interests.
This article is a PNAS Direct Submission. A.C.K. is a guest editor invited by the Editorial Board.
Data deposition: The homology models used in this study and the supplementary dataset are available as PDB format and table files, respectively, at https://doi.org/10.7924/r40867k2k.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1915520117/-/DCSupplemental.
References
- 1.Buck L., Axel R., A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 65, 175–187 (1991). [DOI] [PubMed] [Google Scholar]
- 2.Niimura Y., Matsui A., Touhara K., Extreme expansion of the olfactory receptor gene repertoire in African elephants and evolutionary dynamics of orthologous gene groups in 13 placental mammals. Genome Res. 24, 1485–1496 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Firestein S., How the olfactory system makes sense of scents. Nature 413, 211–218 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Saraiva L. R., et al. , Combinatorial effects of odorants on mouse behavior. Proc. Natl. Acad. Sci. U.S.A. 113, E3300–E3306 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adipietro K. A., Mainland J. D., Matsunami H., Functional evolution of mammalian odorant receptors. PLoS Genet. 8, e1002821 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhuang H., Chien M.-S., Matsunami H., Dynamic functional evolution of an odorant receptor for sex-steroid-derived odors in primates. Proc. Natl. Acad. Sci. U.S.A. 106, 21247–21251 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClintock T. S., et al. , Functional expression of olfactory-adrenergic receptor chimeras and intracellular retention of heterologously expressed olfactory receptors. Brain Res. Mol. Brain Res. 48, 270–278 (1997). [DOI] [PubMed] [Google Scholar]
- 8.Lu M., Echeverri F., Moyer B. D., Endoplasmic reticulum retention, degradation, and aggregation of olfactory G-protein coupled receptors. Traffic 4, 416–433 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Gimelbrant A. A., Stoss T. D., Landers T. M., McClintock T. S., Truncation releases olfactory receptors from the endoplasmic reticulum of heterologous cells. J. Neurochem. 72, 2301–2311 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Kaiser L., et al. , Efficient cell-free production of olfactory receptors: Detergent optimization, structure, and ligand binding analyses. Proc. Natl. Acad. Sci. U.S.A. 105, 15726–15731 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belloir C., Miller-Leseigneur M. L., Neiers F., Briand L., Le Bon A. M., Biophysical and functional characterization of the human olfactory receptor OR1A1 expressed in a mammalian inducible cell line. Protein Expr. Purif. 129, 31–43 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Von Dannecker L. E. C., Mercadante A. F., Malnic B., Ric-8B promotes functional expression of odorant receptors. Proc. Natl. Acad. Sci. U.S.A. 103, 9310–9314 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noe F., et al. , IL-6-HaloTag® enables live-cell plasma membrane staining, flow cytometry, functional expression, and de-orphaning of recombinant odorant receptors. J. Biol. Methods 4, e81 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shepard B. D., Natarajan N., Protzko R. J., Acres O. W., Pluznick J. L., A cleavable N-terminal signal peptide promotes widespread olfactory receptor surface expression in HEK293T cells. PLoS One 8, e68758 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saito H., Kubota M., Roberts R. W., Chi Q., Matsunami H., RTP family members induce functional expression of mammalian odorant receptors. Cell 119, 679–691 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Zhuang H., Matsunami H., Evaluating cell-surface expression and measuring activation of mammalian odorant receptors in heterologous cells. Nat. Protoc. 3, 1402–1413 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L., Pan Y., Chen G. Q., Matsunami H., Zhuang H., Receptor-transporting protein 1 short (RTP1S) mediates translocation and activation of odorant receptors by acting through multiple steps. J. Biol. Chem. 287, 22287–22294 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunham J. H., Hall R. A., Enhancement of the surface expression of G protein-coupled receptors. Trends Biotechnol. 27, 541–545 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krautwurst D., Yau K.-W., Reed R. R., Identification of ligands for olfactory receptors by functional expression of a receptor library. Cell 95, 917–926 (1998). [DOI] [PubMed] [Google Scholar]
- 20.Fukutani Y., et al. , The N-terminal region of RTP1S plays important roles in dimer formation and odorant receptor-trafficking. J. Biol. Chem. 294, 14661–14673 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma R., et al. , Olfactory receptor accessory proteins play crucial roles in receptor function and gene choice. eLife 6, e21895 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bubnell J., et al. , In vitro mutational and bioinformatics analysis of the M71 odorant receptor and its superfamily. PLoS One 10, e0141712 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamet S., et al. , In vitro mutational analysis of the β2 adrenergic receptor, an in vivo surrogate odorant receptor. PLoS One 10, e0141696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehmann M., Pasamontes L., Lassen S. F., Wyss M., The consensus concept for thermostability engineering of proteins. Biochim. Biophys. Acta 1543, 408–415 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Porebski B. T., Buckle A. M., Consensus protein design. Protein Eng. Des. Sel. 29, 245–251 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh S., et al. , Engineering salt bridge networks between transmembrane helices confers thermostability in G-protein-coupled receptors. J. Chem. Theory Comput. 14, 6574–6585 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tate C. G., A crystal clear solution for determining G-protein-coupled receptor structures. Trends Biochem. Sci. 37, 343–352 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Zhang J., Evolution by gene duplication: An update. Trends Ecol. Evol. 18, 292–298 (2003). [Google Scholar]
- 29.Rutherford S. L., Lindquist S., Hsp90 as a capacitor for morphological evolution. Nature 396, 336–342 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Dickinson W. J., Seger J., Cause and effect in evolution. Nature 399, 30 (1999). [DOI] [PubMed] [Google Scholar]
- 31.Masel J., Q&A: Evolutionary capacitance. BMC Biol. 11, 103 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson P., Masel J., Evolutionary capacitance emerges spontaneously during adaptation to environmental changes. Cell Rep. 25, 249–258 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Ballesteros J. A., Weinstein H., Analysis and refinement of criteria for predicting the structure and relative orientations of transmembranal helical domains. Biophys. J. 62, 107–109 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de March C. A., Kim S.-K., Antonczak S., Goddard W. A. 3rd, Golebiowski J., G protein-coupled odorant receptors: From sequence to structure. Protein Sci. 24, 1543–1548 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park J. H., et al. , Opsin, a structural model for olfactory receptors? Angew. Chem. Int. Ed. Engl. 52, 11021–11024 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Grantham R., Amino acid difference formula to help explain protein evolution. Science 185, 862–864 (1974). [DOI] [PubMed] [Google Scholar]
- 37.Rauscher F. J. 3rd, Morris J. F., Tournay O. E., Cook D. M., Curran T., Binding of the Wilms’ tumor locus zinc finger protein to the EGR-1 consensus sequence. Science 250, 1259–1262 (1990). [DOI] [PubMed] [Google Scholar]
- 38.Blatt L. M., Davis J. M., Klein S. B., Taylor M. W., The biologic activity and molecular characterization of a novel synthetic interferon-alpha species, consensus interferon. J. Interferon Cytokine Res. 16, 489–499 (1996). [DOI] [PubMed] [Google Scholar]
- 39.Ikegami K., et al. , Data from “Structural instability and divergence from conserved residues underlie intracellular retention of mammalian odorant receptors.” Duke Digital Repository. 10.7924/r40867k2k. Deposited 9 December 2019. [DOI] [PMC free article] [PubMed]
- 40.Warne T., Edwards P. C., Leslie A. G., Tate C. G., Crystal structures of a stabilized β1-adrenoceptor bound to the biased agonists bucindolol and carvedilol. Structure 20, 841–849 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiefer H., et al. , Expression of an olfactory receptor in Escherichia coli: Purification, reconstitution, and ligand binding. Biochemistry 35, 16077–16084 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Sanchez-Reyes O. B., et al. , G protein-coupled receptors contain two conserved packing clusters. Biophys. J. 112, 2315–2326 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chelikani P., et al. , Role of group-conserved residues in the helical core of β2-adrenergic receptor. Proc. Natl. Acad. Sci. U.S.A. 104, 7027–7032 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hua T., et al. , Crystal structure of the human cannabinoid receptor CB1. Cell 167, 750–762.e14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCaffrey K., Braakman I., Protein quality control at the endoplasmic reticulum. Essays Biochem. 60, 227–235 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Neuhaus E. M., Mashukova A., Zhang W., Barbour J., Hatt H., A specific heat shock protein enhances the expression of mammalian olfactory receptor proteins. Chem. Senses 31, 445–452 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Dalton R. P., Lyons D. B., Lomvardas S., Co-opting the unfolded protein response to elicit olfactory receptor feedback. Cell 155, 321–332 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y. R., Matsunami H., Unfolding the mystery of olfactory receptor gene expression. Dev. Cell 27, 128–129 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farinha C. M., Canato S., From the endoplasmic reticulum to the plasma membrane: Mechanisms of CFTR folding and trafficking. Cell. Mol. Life Sci. 74, 39–55 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee S.-J., Depoortere I., Hatt H., Therapeutic potential of ectopic olfactory and taste receptors. Nat. Rev. Drug Discov. 18, 116–138 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Shepard B. D., Koepsell H., Pluznick J. L., Renal olfactory receptor 1393 contributes to the progression of type 2 diabetes in a diet-induced obesity model. Am. J. Physiol. Renal Physiol. 316, F372–F381 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Queitsch C., Sangster T. A., Lindquist S., Hsp90 as a capacitor of phenotypic variation. Nature 417, 618–624 (2002). [DOI] [PubMed] [Google Scholar]
- 53.Rohner N., et al. , Cryptic variation in morphological evolution: HSP90 as a capacitor for loss of eyes in cavefish. Science 342, 1372–1375 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergman A., Siegal M. L., Evolutionary capacitance as a general feature of complex gene networks. Nature 424, 549–552 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Laird D. W., Molday R. S., Evidence against the role of rhodopsin in rod outer segment binding to RPE cells. Invest. Ophthalmol. Vis. Sci. 29, 419–428 (1988). [PubMed] [Google Scholar]
- 56.Dey S., Matsunami H., Calreticulin chaperones regulate functional expression of vomeronasal type 2 pheromone receptors. Proc. Natl. Acad. Sci. U.S.A. 108, 16651–16656 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bushdid C., de March C. A., Matsunami H., Golebiowski J., “Numerical models and in vitro assays to study odorant receptors” in Olfactory Receptors: Methods and Protocols, Simoes de Souza F. M., Antunes G., Eds. (Springer, New York,, 2018), pp. 77–93. [DOI] [PubMed] [Google Scholar]
- 58.Jo S., Lim J. B., Klauda J. B., Im W., CHARMM-GUI membrane builder for mixed bilayers and its application to yeast membranes. Biophys. J. 97, 50–58 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jo S., Kim T., Iyer V. G., Im W., CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 29, 1859–1865 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Hess B., Kutzner C., van der Spoel D., Lindahl E., GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Best R. B., et al. , Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone φ, ψ and side-chain χ(1) and χ(2) dihedral angles. J. Chem. Theory Comput. 8, 3257–3273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berendsen H. J., Postma J. P. M., van Gunsteren W. F., DiNola A., Haak J. R., Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984). [Google Scholar]
- 63.Petrova S. S., Solov’ev A. D., The origin of the method of steepest descent. Hist. Math. 24, 361–375 (1997). [Google Scholar]
- 64.Andersen H. C., Rattle: A “velocity” version of the shake algorithm for molecular dynamics calculations. J. Comput. Phys. 52, 24–34 (1983). [Google Scholar]
- 65.Darden T., York D., Pedersen L., Particle mesh Ewald: An N⋅ log (N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993). [Google Scholar]
- 66.Evans D. J., Holian B. L., The nose–hoover thermostat. J. Chem. Phys. 83, 4069–4074 (1985). [Google Scholar]
- 67.Parrinello M., Rahman A., Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 52, 7182–7190 (1981). [Google Scholar]
- 68.Mondal S., et al. , Membrane driven spatial organization of GPCRs. Sci. Rep. 3, 2909 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crooks G. E., Hon G., Chandonia J.-M., Brenner S. E., WebLogo: A sequence logo generator. Genome Res. 14, 1188–1190 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data discussed in the paper are available in the manuscript or SI Appendix. The homology models used in this study and the supplementary dataset (39) are available as PDB format and table files, respectively, at https://doi.org/10.7924/r40867k2k (39).